化工进展 ›› 2023, Vol. 42 ›› Issue (6): 2954-2962.DOI: 10.16085/j.issn.1000-6613.2022-1453
H2-SCR改性铂系催化剂低温脱硝的应用及性能强化挑战
张巍1,2( ), 秦川1,2, 谢康1,2, 周运河1,2, 董梦瑶1,2, 李婕1,2, 汤云灏1,2, 马英3, 宋健4
), 秦川1,2, 谢康1,2, 周运河1,2, 董梦瑶1,2, 李婕1,2, 汤云灏1,2, 马英3, 宋健4
- 1.长沙理工大学能源与动力工程学院,湖南 长沙 410114
2.可再生能源电力技术湖南省重点实验室,湖南 长沙 410114
3.永清环保股份有限公司,湖南 长沙 410330
4.湖南大唐节能科技有限公司,湖南 长沙 410007
-
收稿日期:2022-08-03修回日期:2022-12-25出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:张巍 -
作者简介:张巍(1974—),男,博士,副教授,硕士生导师,研究方向为高效清洁燃烧与污染物控制。E-mail:weizhang@csust.edu.cn。 -
基金资助:国家留学基金(201808430112);湖南省自然科学基金(2020JJ4098);湖南省教育厅科学研究项目重点项目(21A0216);长沙理工大学青年教师成长计划(2019QJCZ044);“可再生能源电力技术”湖南省重点实验室开放基金(2018ZNDL004)
Application and performance enhancement challenges of H2-SCR modified platinum-based catalysts for low-temperature denitrification
ZHANG Wei1,2( ), QIN Chuan1,2, XIE Kang1,2, ZHOU Yunhe1,2, DONG Mengyao1,2, LI Jie1,2, TANG Yunhao1,2, MA Ying3, SONG Jian4
), QIN Chuan1,2, XIE Kang1,2, ZHOU Yunhe1,2, DONG Mengyao1,2, LI Jie1,2, TANG Yunhao1,2, MA Ying3, SONG Jian4
- 1.School of Energy and Power Engineering, Changsha University of Science and Technology, Changsha 410114, Hunan, China
2.Key Laboratory of Renewable Energy and Electric Power Technology of Hunan Province, Changsha 410114, Hunan, China
3.Yonker Environmental Protection Company Limited, Changsha 410330, Hunan, China
4.Hunan Datang Energy-saving Technology Company Limited, Changsha 410007, Hunan, China
-
Received:2022-08-03Revised:2022-12-25Online:2023-06-25Published:2023-06-29 -
Contact:ZHANG Wei
摘要:
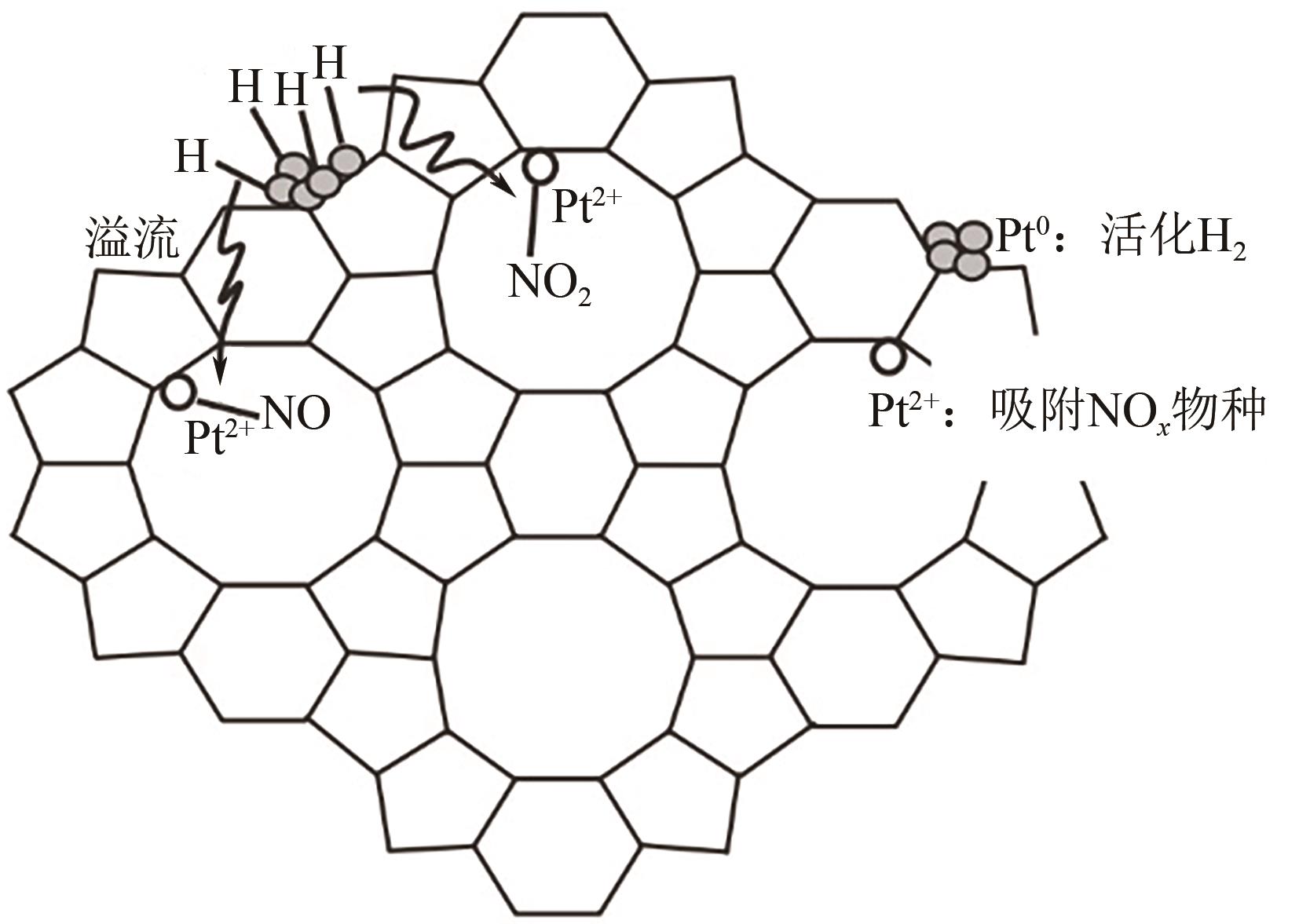
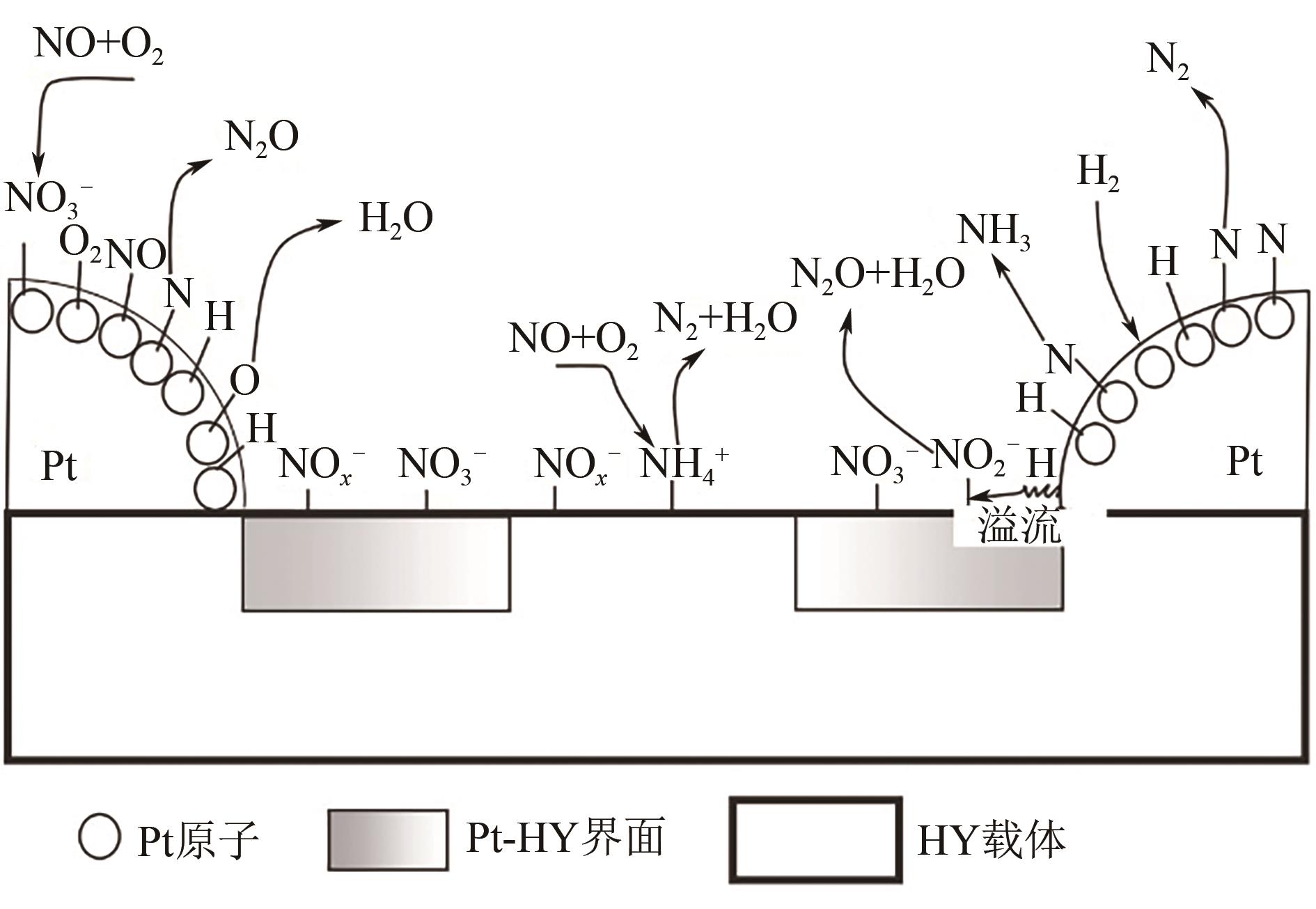
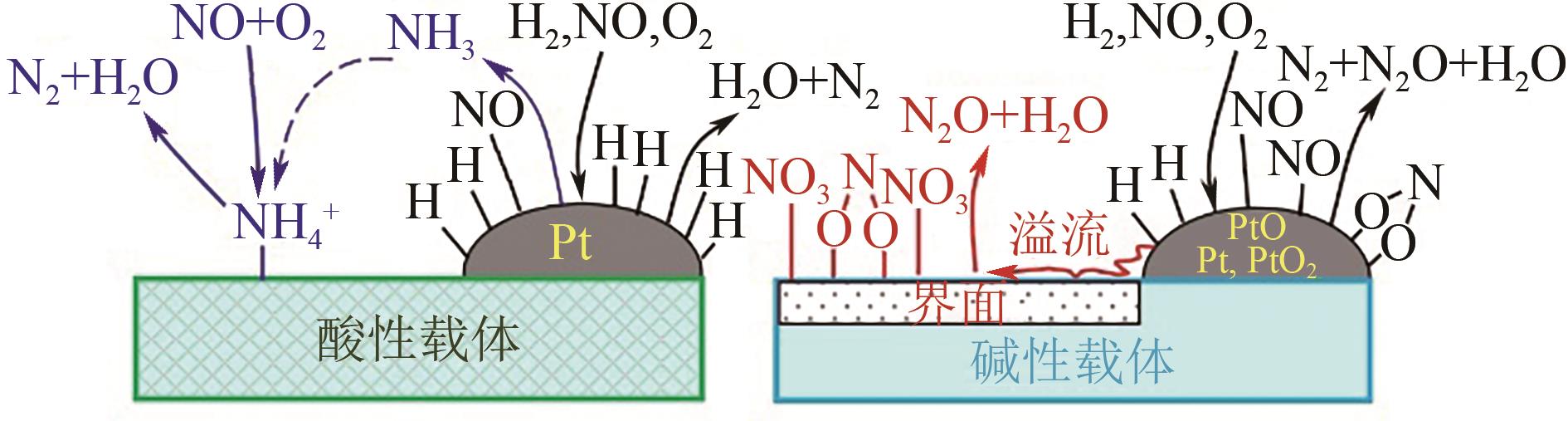
氮氧化物(NO x )的排放严重危害了生态环境和人类健康,其主要来源有固定源烟气排放和移动源尾气排放。近年来,利用氢气选择性催化还原(H2-SCR)改性铂系催化剂控制移动源NO x 的相关研究引起了广泛的关注,由于不同的改性方法可以促进铂与载体之间的电子迁移,从而形成双功能反应机制酸位点,因而通过深入认识铂系H2-SCR催化剂的NO x 催化反应机制并获得良好的改性方法对于开发高效移动源脱硝催化剂具有深远的意义。本文综述了铂系H2-SCR脱硝催化剂的种类,阐述了其表面的H2/NO吸附机制、NO氧化还原机制和双功能反应机制,总结了提高铂系H2-SCR催化剂的稳定性、抗硫性和选择性的强化方法。进一步阐述了金属氧化物和分子筛负载型铂系H2-SCR催化剂可变的多价态,电子迁移能力,NO、H2和O2在催化剂表面的反应机理,并对铂负载减量化降低成本的相关研究进行了展望。
中图分类号:
引用本文
张巍, 秦川, 谢康, 周运河, 董梦瑶, 李婕, 汤云灏, 马英, 宋健. H2-SCR改性铂系催化剂低温脱硝的应用及性能强化挑战[J]. 化工进展, 2023, 42(6): 2954-2962.
ZHANG Wei, QIN Chuan, XIE Kang, ZHOU Yunhe, DONG Mengyao, LI Jie, TANG Yunhao, MA Ying, SONG Jian. Application and performance enhancement challenges of H2-SCR modified platinum-based catalysts for low-temperature denitrification[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2954-2962.
| 催化剂 | 制备方法 | 最佳反应温度/最佳脱硝 效率/最佳N2选择性 | 反应条件 | 参考 文献 |
|---|---|---|---|---|
| Pt/MnO x | 共沉淀法 | 100℃/64%/30% | 0.048mL/min NO,5%(体积分数)O2,He平衡,空速(GHSV)为78000h-1 | [ |
| Pt/ZrO2 | 浸渍法 | 150℃/71%/75% | 0.2mL/min NO x,1mL/min H2,10%(体积分数)O2,N2为平衡气,温度60~280℃, 总流量200mL/min | [ |
| Pt/MgO | 浸渍法 | 150℃/71.4%/62% | 0.2mL/min NO x,1mL/min H2,10%(体积分数)O2,N2为平衡气,温度60~280℃, 总流量200mL/min | [ |
| Pt/Al2O3 | 浸渍法 | 91℃/88%/90% | 4.8mL/min NO,1vol% H2, 5vol% O2,N2为平衡气,温度20~350℃,总流量2400mL/min,GHSV为12000h-1 | [ |
| Pt/Mg3Al1O x | 共沉淀法 | 200~220℃/92%/60% | 0.125mL/min NO x,0.5%~2%(体积分数)H2,0~10%(体积分数)O2,Ar为平衡气, 总流量200~300mL/min,GHSV为12000h-1 | [ |
| Pt/Ce0.5Zr0.5O2 | 溶胶-凝胶法 | 120~220℃/92%/78% | 0.03mL/min NO,2.5%(体积分数)O2,0.8%(体积分数)H2,10%(体积分数)CO2,15%(体积分数)H2O,0.5%(体积分数)C3H6,总流量200mL/min,GHSV为33000h-1 | [ |
| Pt/MgO-CeO2 | 湿浸渍法 | 150℃/94%/80% | 0.25%(摩尔分数)NO,1.0%(摩尔分数)H2,5%(摩尔分数)O2,He为平衡气体,总流量100mL/min,GHSV为80000h-1 | [ |
表1 近年来文献中报道的金属氧化物负载铂系H2-SCR催化剂
| 催化剂 | 制备方法 | 最佳反应温度/最佳脱硝 效率/最佳N2选择性 | 反应条件 | 参考 文献 |
|---|---|---|---|---|
| Pt/MnO x | 共沉淀法 | 100℃/64%/30% | 0.048mL/min NO,5%(体积分数)O2,He平衡,空速(GHSV)为78000h-1 | [ |
| Pt/ZrO2 | 浸渍法 | 150℃/71%/75% | 0.2mL/min NO x,1mL/min H2,10%(体积分数)O2,N2为平衡气,温度60~280℃, 总流量200mL/min | [ |
| Pt/MgO | 浸渍法 | 150℃/71.4%/62% | 0.2mL/min NO x,1mL/min H2,10%(体积分数)O2,N2为平衡气,温度60~280℃, 总流量200mL/min | [ |
| Pt/Al2O3 | 浸渍法 | 91℃/88%/90% | 4.8mL/min NO,1vol% H2, 5vol% O2,N2为平衡气,温度20~350℃,总流量2400mL/min,GHSV为12000h-1 | [ |
| Pt/Mg3Al1O x | 共沉淀法 | 200~220℃/92%/60% | 0.125mL/min NO x,0.5%~2%(体积分数)H2,0~10%(体积分数)O2,Ar为平衡气, 总流量200~300mL/min,GHSV为12000h-1 | [ |
| Pt/Ce0.5Zr0.5O2 | 溶胶-凝胶法 | 120~220℃/92%/78% | 0.03mL/min NO,2.5%(体积分数)O2,0.8%(体积分数)H2,10%(体积分数)CO2,15%(体积分数)H2O,0.5%(体积分数)C3H6,总流量200mL/min,GHSV为33000h-1 | [ |
| Pt/MgO-CeO2 | 湿浸渍法 | 150℃/94%/80% | 0.25%(摩尔分数)NO,1.0%(摩尔分数)H2,5%(摩尔分数)O2,He为平衡气体,总流量100mL/min,GHSV为80000h-1 | [ |
| 1 | SHENG Liping, MA Zhaoxia, CHEN Shiyuan, et al. Mechanistic insight into N2O formation during NO reduction by NH3 over Pd/CeO2 catalyst in the absence of O2 [J]. Chinese Journal of Catalysis, 2019, 40(7): 1070-1077. |
| 2 | FENG Shuo, LI Zhaoming, SHEN Boxing, et al. An overview of the deactivation mechanism and modification methods of the SCR catalysts for denitration from marine engine exhaust[J]. J Environ Manage, 2022, 317: 115457. |
| 3 | 中华人民共和国国民经济和社会发展第十四个五年规划和2035年远景目标纲要[N]. 人民日报, 2021-03-13(001). |
| The Fourteenth Five-Year Plan for the national economic and social development of the People's Republic of China and the outline of the vision for 2035[N]. People's Daily, 2021-03-13(001). | |
| 4 | XU Haidi, LIN Qingjin, FENG Xi, et al. Grain size effect on the high-temperature hydrothermal stability of Cu/SAPO-34 catalysts for NH3-SCR[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 2213-3437. |
| 5 | VIVEK K P, SWETA S. Effect of oxide supports on palladium based catalysts for NO reduction by H2-SCR[J]. Catalysis Today, 2020, 375: 591-600. |
| 6 | YANG Shufang, WANG Xinping, CHU Wenling, et al. An investigation of the surface intermediates of H2-SCR of NO x over Pt/H-FER[J]. Applied Catalysis B: Environmental, 2011, 107 (3-4): 380-385. |
| 7 | 武鹏, 于青, 严晶晶, 等. 富氧条件下氢气选择催化还原氮氧化物研究的进展[J]. 催化学报, 2010, 31(8): 912-918. |
| WU Peng, YU Qing, YAN Jingjing, et al. Research progress in selective catalytic reduction of nitrogen oxides with hydrogen under oxygen-enriched conditions[J]. Journal of Catalysis, 2010, 31(8): 912-918 | |
| 8 | KYUNGSEOK L, BYUNGCHUL C, CHUNBEOM L. Effects of SiO2/Al2O3 ratio, reaction atmosphere and metal additive on de-NO x performance of HC-SCR over Cu-based ZSM-5[J]. Journal of Industrial and Engineering Chemistry, 2020, 90: 132-144. |
| 9 | RAJBALA Bhatia Divesh. Crystallite-scale model for NO x reduction by hydrogen spillover on SBA-15 and MCM-41[J]. Catalysis Today, 2021, 360: 263-274. |
| 10 | DENG D, DENG S J, He D, et al. A comparative study of hydrothermal aging effect on cerium and lanthanum doped Cu/SSZ-13 catalysts for NH3-SCR[J]. Journal of Rare Earths, 2021, 39 (8): 969-978. |
| 11 | DENG Y Q, ZHANG T, AU C T, et al. Oxidation of p-chlorotoluene to p-chlorobenzaldehyde over manganese-based octahedral molecular sieves of different morphologies[J]. Catalysis Communications, 2014, 43: 126-130. |
| 12 | AGLIULLIN M R, KOLYAGIN Y G, SEREBRENNIKOV D V, et al. Acid properties and morphology of SAPO-11 molecular sieve controled by silica source[J]. Microporous and Mesoporous Materials, 2022, 338. |
| 13 | 刘东升, 邹志强, 姚成, 等. 低温氧化型过渡金属氧化物催化剂研究进展[J]. 化学工业与工程, 2010, 27(6): 556-564. |
| LIU Dongsheng, ZHOU Zhiqiang, YAO Cheng, et al. Research progress of low temperature oxidative transition metal oxide catalysts[J]. Chemical Industry and Engineering, 2010, 27 (6): 556-564. | |
| 14 | YANG Chen, JIAO Qingrui, ZHAO Dong, et al. Promotion effect and mechanism of MnO doped CeO2 nano-catalyst for NH3-SCR[J]. Ceramics International, 2020, 46 (4): 4394-4401. |
| 15 | LIU Xuesong, JIANG Peng, CHEN Yong, et al. A basic comprehensive study on synergetic effects among the metal oxides in CeO2-WO3/TiO2 NH3-SCR catalyst[J]. Chemical Engineering Journal, 2020, 421: 1385-8947. |
| 16 | ZHANG Yihuan, HAO Cuicui, ZHANG Jia, et al. Ratio of adsorptive abilities for NH3 and NO x determined SCR activity of transition-metal catalyst[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 635: 0927-7757. |
| 17 | MASAHIRO I, MAKOTO S, MASAHIKO T, et al. Influence of supported-metal characteristics on deNO x catalytic activity over Pt/CeO2 [J]. Journal of Molecular Catalysis A: Chemical, 2009, 304(1/2): 159-165. |
| 18 | LI Xinyan, ZHANG Xiaoxiao, XU Yongzhen, et al. Influence of support properties on H2 selective catalytic reduction activities and N2 selectivities of Pt catalysts[J]. Chinese Journal of Catalysis, 2015, 36 (2): 197-203. |
| 19 | CHRISTOS M, KALAMARAS, GEORGE G, et al. Selective catalytic reduction of NO by H2/C3H6 over Pt/Ce1- x Zr x O2- δ : the synergy effect studied by transient techniques[J]. Applied Catalysis B: Environmental, 2017, 206: 308-318. |
| 20 | SUNG S K, SUNG C H. Relationship between the surface characteristics of Pt catalyst and catalytic performance on the H2 SCR[J]. Journal of Industrial and Engineering Chemistry, 2010, 16 (6): 992-996. |
| 21 | LIU Zhiming, LU Yunan, YUAN Lei, et al. Selective catalytic reduction of NO x with H2 over WO3 promoted Pt/TiO2 catalyst[J]. Applied Catalysis B: Environmental, 2016, 188: 189-197. |
| 22 | ZHANG Xiaoxiao, WANG Xinping, ZHAO Xiaojuan, et al. Promotion effect of tungsten on the activity of Pt/HZSM-5 for H2-SCR[J]. Chemical Engineering Journal, 2015, 260: 419-426. |
| 23 | HONG Xiaowei, Ye SUM, ZHU Tianle, et al. Promoting effect of TiO2 on the catalytic performance of Pt-Au/TiO2(x)-CeO2 for the co-oxidation of CO and H2 at room temperature[J]. Applied Surface Science, 2017, 396: 226-234. |
| 24 | SE M P, MI-YOUNG K, EUN S K, et al. H2-SCR of NO on Pt-MnO x catalysts: reaction path via NH3 formation[J]. Applied Catalysis A: General, 2011, 395 (1/2): 120-128. |
| 25 | YANG J I, HEON J. The effect of temperature on NO x reduction by H2 in the presence of excess oxygen on a Pt/Al2O3 monolithic catalyst[J]. Chemical Engineering Journal, 2009, 146 (1): 11-15. |
| 26 | ZHANG Cheng, GAO Yanshan, YAN Qinghua, et al. Fundamental investigation on layered double hydroxides derived mixed metal oxides for selective catalytic reduction of NO x by H2 [J]. Catalysis Today, 2020, 355: 450-457. |
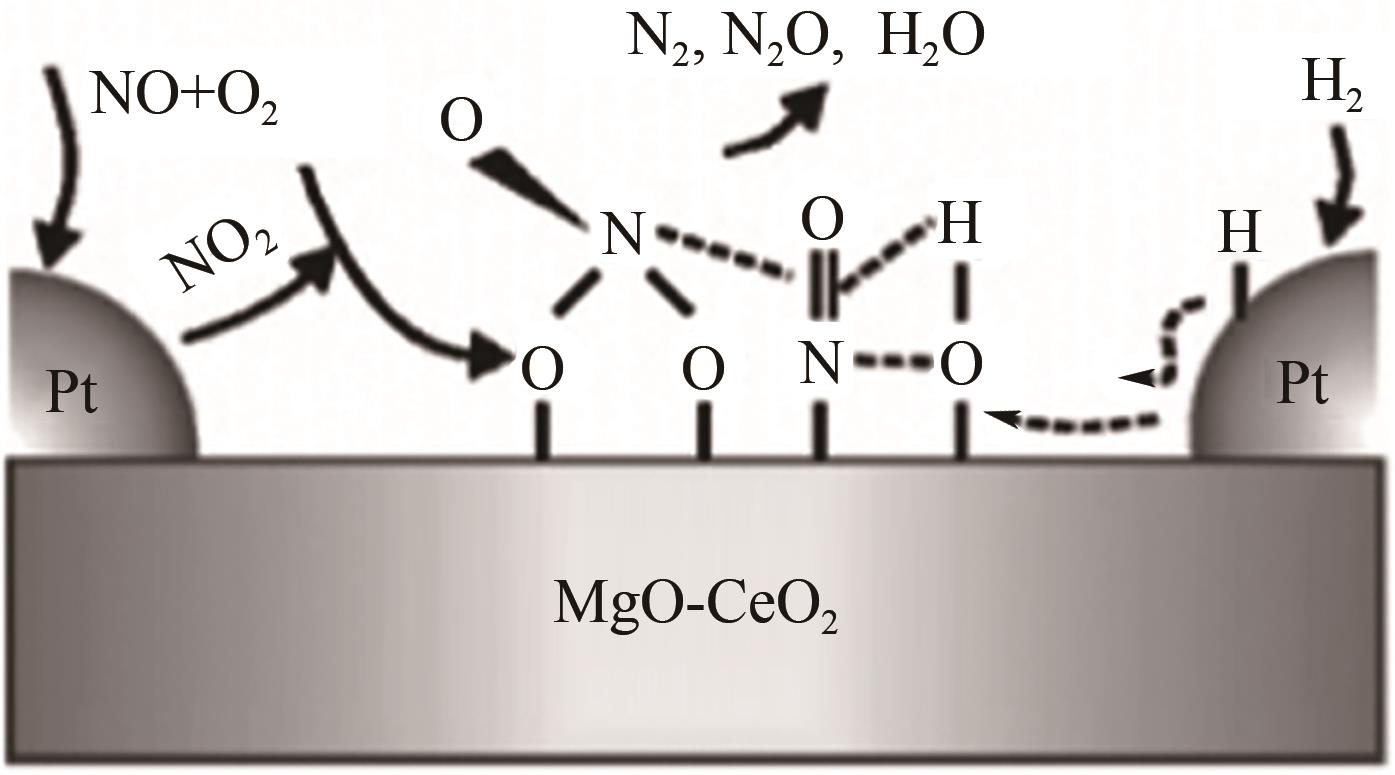
| 27 | COSTAS N, EFSTATHION A M. Low-temperature H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst[J]. Applied Catalysis B: Environmental, 2007, 72 (3/4): 240-252. |
| 28 | WANG Xinping, WANG Xuejing, YU Haibiao, et al. The functions of Pt located at different positions of HZSM-5 in H2-SCR[J]. Chemical Engineering Journal, 2019, 355: 470-477. |
| 29 | WU Peng, LIU Yunxia, ZHANG Fuxiang, et al. Influences of mesoporous structure on the NO+H2+O2 low temperature reaction over Pt/Si-MCM-41 catalyst[J]. Acta Physico-Chimica Sinica, 2008, 24 (3): 369-374. |
| 30 | WU Peng, LI Landong, YU Qing, et al. Study on Pt/Al-MCM-41 for NO selective reduction by hydrogen[J]. Catalysis Today, 2010, 158 (3/4): 228-234. |
| 31 | LI Landong, WU Peng, YU Qing, et al. Low temperature H2-SCR over platinum catalysts supported on Ti-containing MCM-41[J]. Applied Catalysis B: Environmental, 2010, 94 (3/4): 254-262. |
| 32 | YU Q, RICHTER M, KONG F X, et al. Selective catalytic reduction of NO by hydrogen over Pt/ZSM-35[J]. Catalysis Today, 2010, 158 (3/4): 452-458. |
| 33 | YU Q, RICHTER M, LI L D, et al. The promotional effect of Cr on catalytic activity of Pt/ZSM-35 for H2-SCR in excess oxygen[J]. Catalysis Communications, 2010, 11 (11): 955-959. |
| 34 | WU Yaohui, LIU Hao, LI Guoying, et al. Tuning composition on B sites of LaM0.5Mn0.5O3 (M = Cu, Co, Fe, Ni, Cr) perovskite catalysts in NO x efficient reduction[J]. Applied Surface Science, 2020, 508. |
| 35 | PAPP H, SABDE D P. An investigation on the mechanism of NO decomposition over Rh/SiO2 catalysts in presence of pulse injected H2 [J]. Applied Catalysis B: Environmental, 2005, 60 (1/2): 65-71 |
| 36 | BRIGITTA F, GERHARF E, ALBERT R. Kinetics and mechanism of the reduction of nitric oxides by H2 under lean-burn conditions on a Pt±Mo±Co/-Al2O3 catalyst[J]. Applied Catalysis B: Environmental, 2017. |
| 37 | ZHANG Xiaoxiao, WANG Xinping, ZHAO Xiaojuan, et al. An investigation on N2O formation route over Pt/HY in H2-SCR[J]. Chemical Engineering Journal, 2014, 252: 288-297. |
| 38 | GIORIA E, MARCHESINI F A, SOLDATI A, et al. Green synthesis of a Cu/SiO2 catalyst for efficient H2-SCR of NO[J]. Applied Sciences, 2019, 9(19): 4075. |
| 39 | COSTA C N, EFSTATHIOU A M. Mechanistic aspects of the H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst[J]. The Journal of Physical Chemistry C, 2007, 111: 3010-3020. |
| 40 | COSTAS N, COSTA P G S, JOSE L G, et al. Industrial H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst[J]. Applied Catalysis B: Environmental, 2007, 75 (3/4): 147-156. |
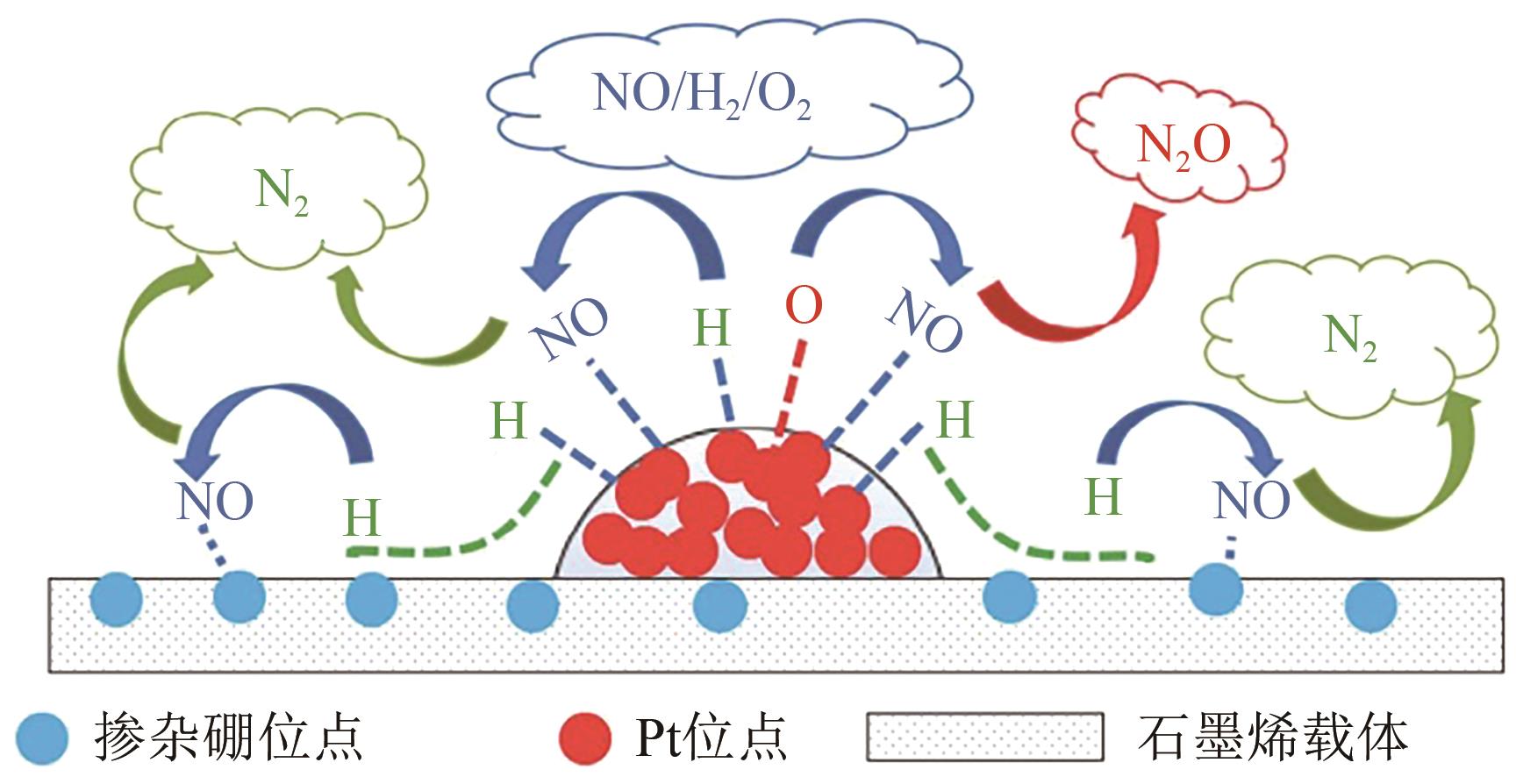
| 41 | HU Maocong, YAO Zhenhua, LI Lili, et al. Boron-doped graphene nanosheet-supported Pt: a highly active and selective catalyst for low temperature H2-SCR[J]. Nanoscale, 2018, 10 (21): 10203-10212. |
| 42 | SHIBATA J, HASHIMOTO M, SHIMIZU K, et al. Factors controlling activity and selectivity for SCR of NO by hydrogen over supported platinum catalysts[J]. The Journal of Physical Chemistry B, 2004, 108(47): 18327-18335. |
| 43 | JONG K G, HUN S J, BIN K S, et al. The role of Pt valence state and La doping on titanium supported Pt-La/TiO2 catalyst for selective catalytic reduction with H2 [J]. Applied Surface Science, 2023, 608. |
| 44 | PARK S M, JANG H G, KIM E S, et al. Incorporation of zirconia onto silica for improved Pt/SiO2 catalysts for the selective reduction of NO by H2 [J]. Applied Catalysis A: General, 2012, 427-428: 155-64. |
| 45 | SCHOTT F J P, BALLE P, ADLER J, et al. Reduction of NO x by H2 on Pt/WO3/ZrO2 catalysts in oxygen-rich exhaust[J]. Applied Catalysis B: Environmental, 2009, 87(1/2): 18-29. |
| 46 | LU Jianyi, ZHOU Zhiyong, ZHANG Hanzhi, et al. Influenced factors study and evaluation for SO2/SO3 conversion rate in SCR process[J]. Fuel, 2019, 245: 528-533. |
| 47 | OLSSON L, KARLSSON H. The beneficial effect of SO2 on platinum migration and NO oxidation over Pt containing monolith catalysts[J]. Catalysis Today, 2009, 147: S290-S294. |
| 48 | CORRO G, RAMON M, CARLOS V L. Promoting and inhibiting effect of SO2 on propane oxidation over Pt/Al2O3 [J]. Catalysis Communications, 2002, 3(11): 533-539. |
| 49 | DAWODY J, SKOGLUNDH M, OLSSON L, et al. Sulfur deactivation of Pt/SiO2, Pt/BaO/Al2O3, and BaO/Al2O3 NO x storage catalysts: influence of SO2 exposure conditions[J]. Journal of Catalysis, 2005, 234 (1): 206-218. |
| 50 | HAMZEHLOUYAN T, SAMPARA C S, LI J, et al. Kinetic study of adsorption and desorption of SO2 over γ-Al2O3 and Pt/γ-Al2O3 [J]. Applied Catalysis B: Environmental, 2016, 181: 587-598. |
| 51 | WAKITA H, KANI Y, UKAI K, et al. Effect of SO2 and H2O on CO preferential oxidation in H2-rich gas over Ru/Al2O3 and Pt/Al2O3 catalysts[J]. Applied Catalysis A: General, 2005, 283 (1-2): 53-61. |
| 52 | WILBURN M S, EPLING W S. SO2 adsorption and desorption characteristics of bimetallic Pd-Pt catalysts: Pd∶Pt ratio dependency[J]. Catalysis Today, 2019, 320: 11-19. |
| 53 | LANA I D, KARGE H G, GEORGE Z M. Dissociative adsorption of sulfur dioxide on γ-alumina investigated by TPD and mass spectrometry[J]. The Journal of Physical Chemistry, 1993, 97(30): 8005-8011. |
| 54 | DATTA A, CAVELL R G, TOWER R W, et al. Claus catalysis. 1. Adsorption of SO2 on the alumina catalyst studied by FTIR and EPR spectroscopy[J]. Journal of Physical Chemistry, 1985, 89(3): 443-449. |
| 55 | MIKHAIL Yu Smirnov, Kalinkin ALEXANDER V, Pashis ANDREI V, et al. Interaction of Al2O3 and CeO2 surfaces with SO2 and SO2 + O2 studied by X-ray photoelectron spectroscopy[J]. The Journal of Physical Chemistry B, 2005, 109(23): 11712–11719. |
| 56 | LIU Y, TURSUN M, Yu H, et al. Surface property and activity of Pt/Nb2O5-ZrO2 for selective catalytic reduction of NO by H2 [J]. Molecular Catalysis, 2019, 464: 22-28. |
| 57 | YOSHIDA H, YAZAWA Y, HATTORA T. Effects of support and additive on oxidation state and activity of Pt catalyst in propane combustion[J]. Catalysis Today, 2003, 87 (1/2/3/4): 19-28. |
| 58 | YOSHITERU Y N T, HISAO Y, SHIN K, et al. The support effect on propane combustion over platinum catalyst: control of the oxidation-resistance of platinum by the acid strength of support materials[J]. Applied Catalysis A: General, 2002, 233 (1/2): 103-112. |
| [1] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [2] | 于志庆, 黄文斌, 王晓晗, 邓开鑫, 魏强, 周亚松, 姜鹏. B掺杂Al2O3@C负载CoMo型加氢脱硫催化剂性能[J]. 化工进展, 2023, 42(7): 3550-3560. |
| [3] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [4] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| [5] | 王嘉, 彭冲, 唐磊, 陆安慧. 渣油加氢催化剂活性相结构调控及对反应性能影响[J]. 化工进展, 2023, 42(4): 1811-1821. |
| [6] | 刘亮, 王朝曦, 李鑫龙, 张高山, 王守阳, 张林林, 陆畅, 卿梦霞. 钒钛系脱硝催化剂抗硫酸氢铵中毒改进措施研究进展[J]. 化工进展, 2023, 42(1): 215-225. |
| [7] | 汪兴, 赵子龙, 张小山, 王宏杰, 董文艺, 陈慧慧. 制备条件对生物炭载铁催化剂催化破络Ni-EDTA性能及活性组分浸出的影响[J]. 化工进展, 2022, 41(9): 4831-4839. |
| [8] | 曾军建, 赵基钢. 乙炔氢氯化金基无汞催化剂的研究进展[J]. 化工进展, 2022, 41(7): 3589-3596. |
| [9] | 唐金琼, 孔勇, 沈晓冬. 碳化物衍生碳的制备及其应用研究进展[J]. 化工进展, 2022, 41(2): 791-802. |
| [10] | 张永祥, 王德龙, 郭晓燕, 邵怀启. CrO x /Ti-Al2O3催化剂结构及其催化丙烷脱氢性能[J]. 化工进展, 2022, 41(11): 5879-5886. |
| [11] | 陈志强, 车春霞, 吴登峰, 温翯, 韩伟, 张峰, 许昊翔, 程道建. 乙炔选择性加氢催化剂研究进展[J]. 化工进展, 2022, 41(10): 5390-5405. |
| [12] | 代校军, 成艳, 王晓晗, 黄文斌, 魏强, 周亚松. 小粒径SAPO-11分子筛合成的研究进展[J]. 化工进展, 2021, 40(S1): 191-203. |
| [13] | 张雯惠, 华睿, 齐随涛. 低温费托合成蜡油加氢裂化精制技术的研究进展[J]. 化工进展, 2021, 40(S1): 81-87. |
| [14] | 丁鑫, 张栋铭, 焦纬洲, 刘有智. 直接甲醇燃料电池阳极催化剂研究进展[J]. 化工进展, 2021, 40(9): 4918-4930. |
| [15] | 曾茂株, 佘煜琪, 胡玉彬, 吴林军, 袁慢景, 漆毅, 王欢, 林绪亮, 秦延林. 木质素多孔炭的制备及应用研究进展[J]. 化工进展, 2021, 40(8): 4573-4586. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||