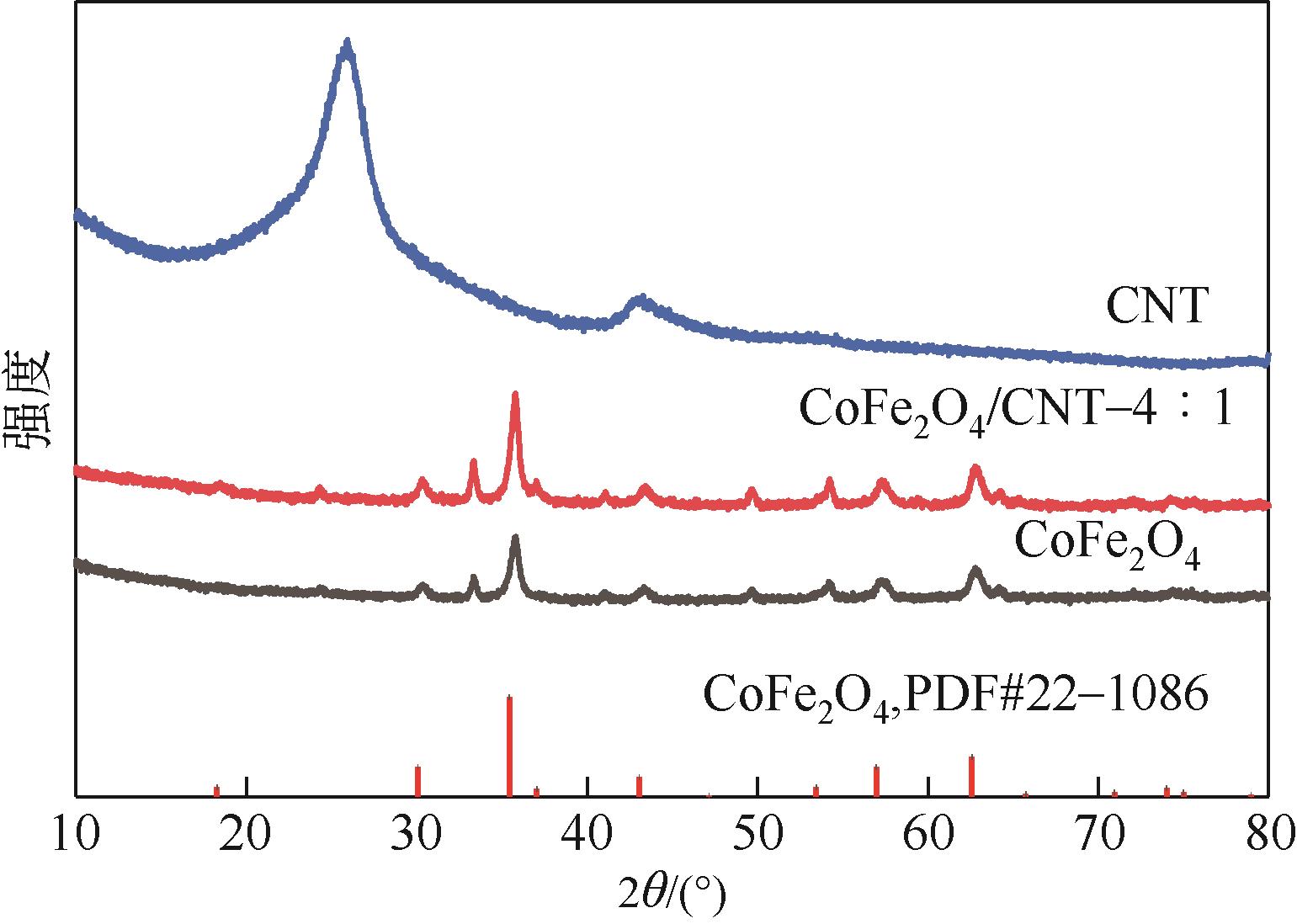
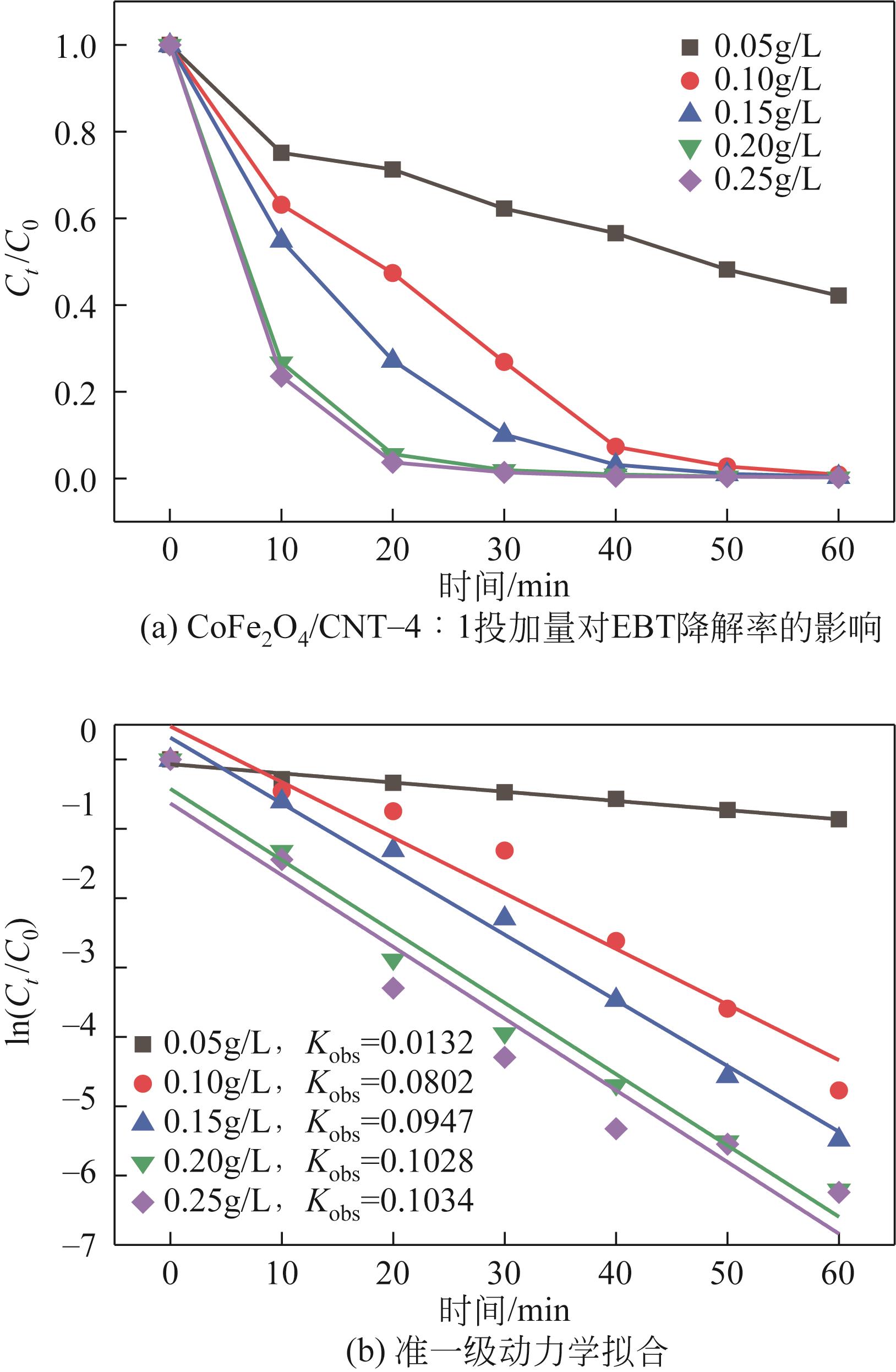
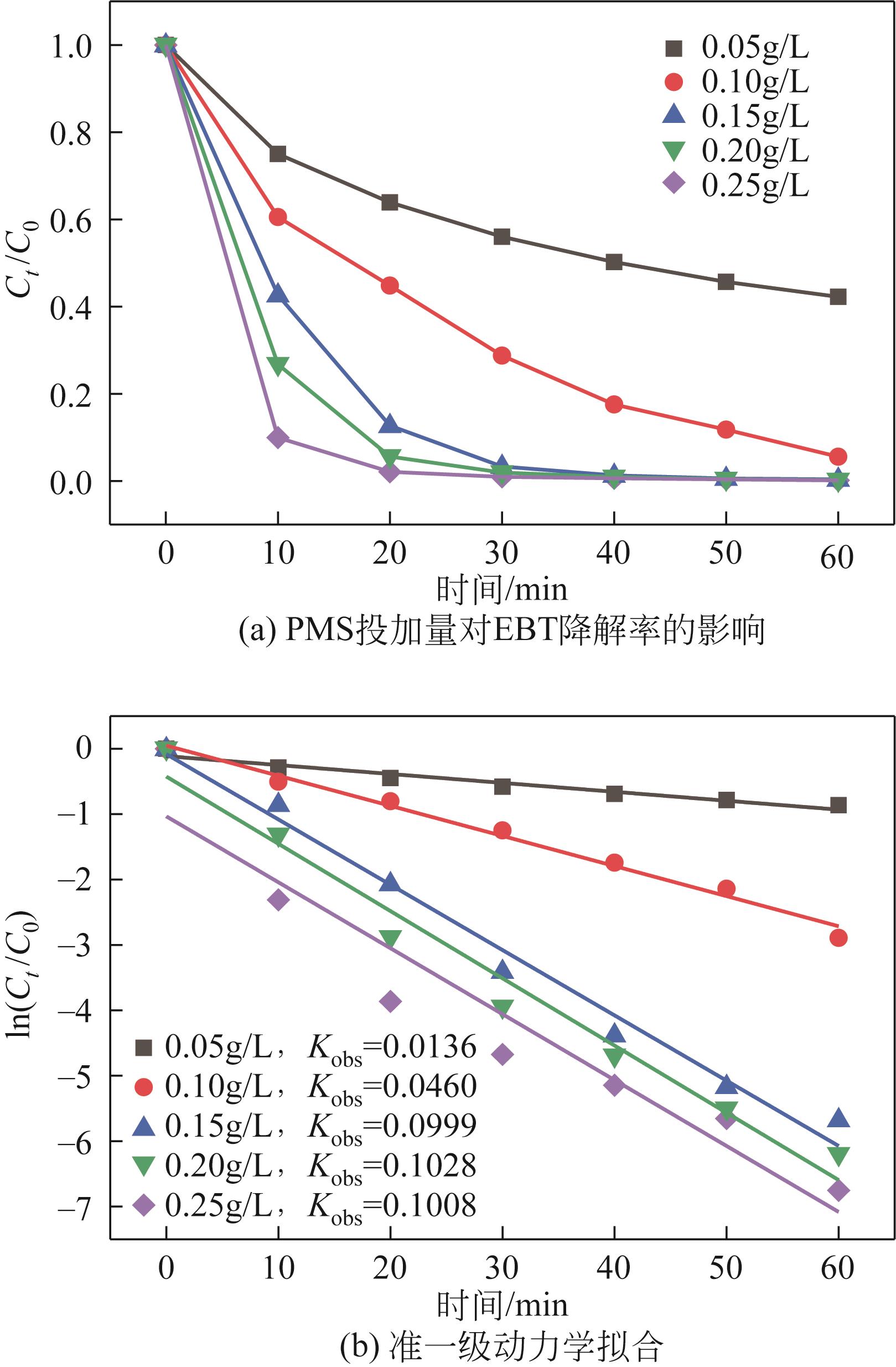
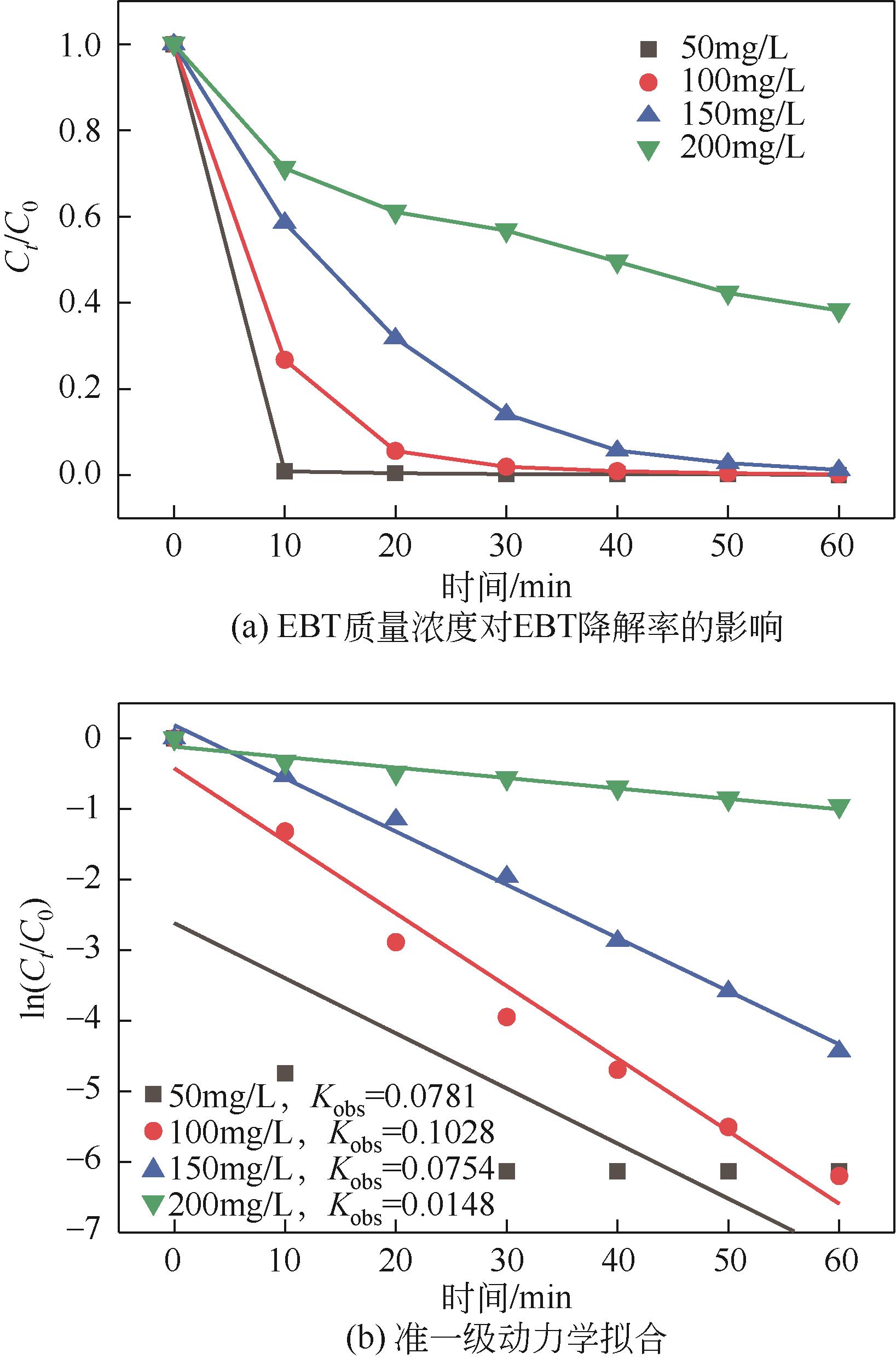
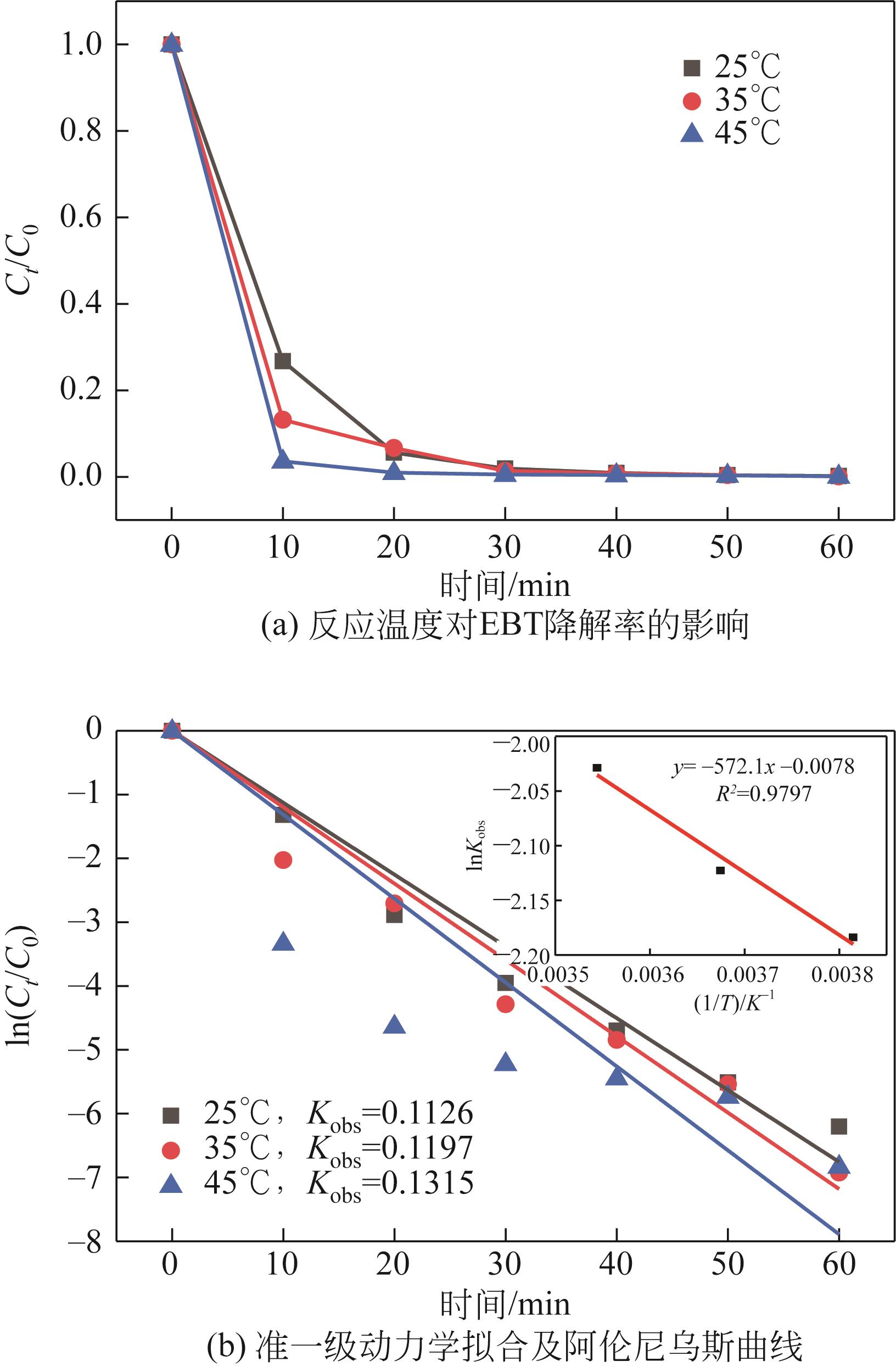
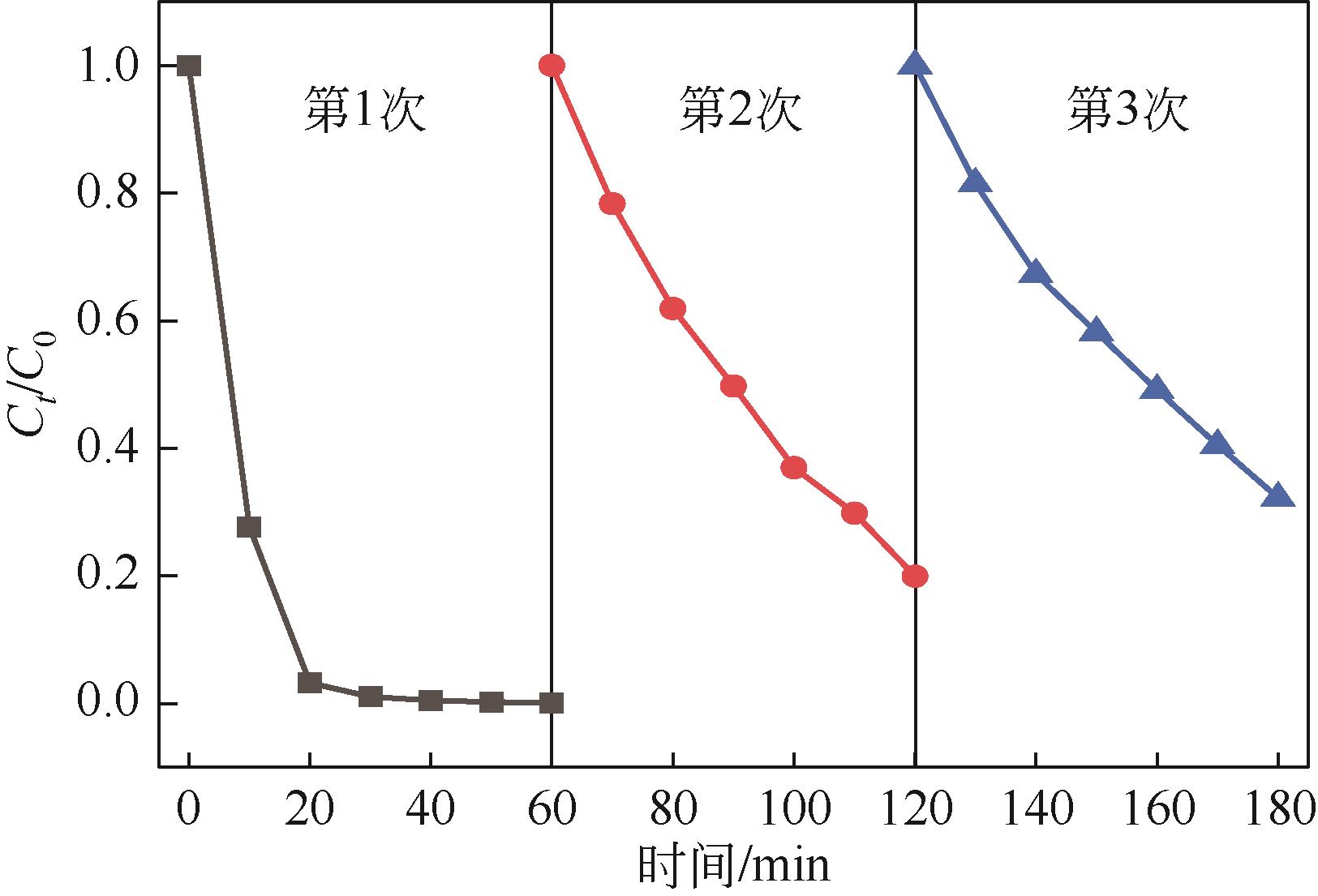
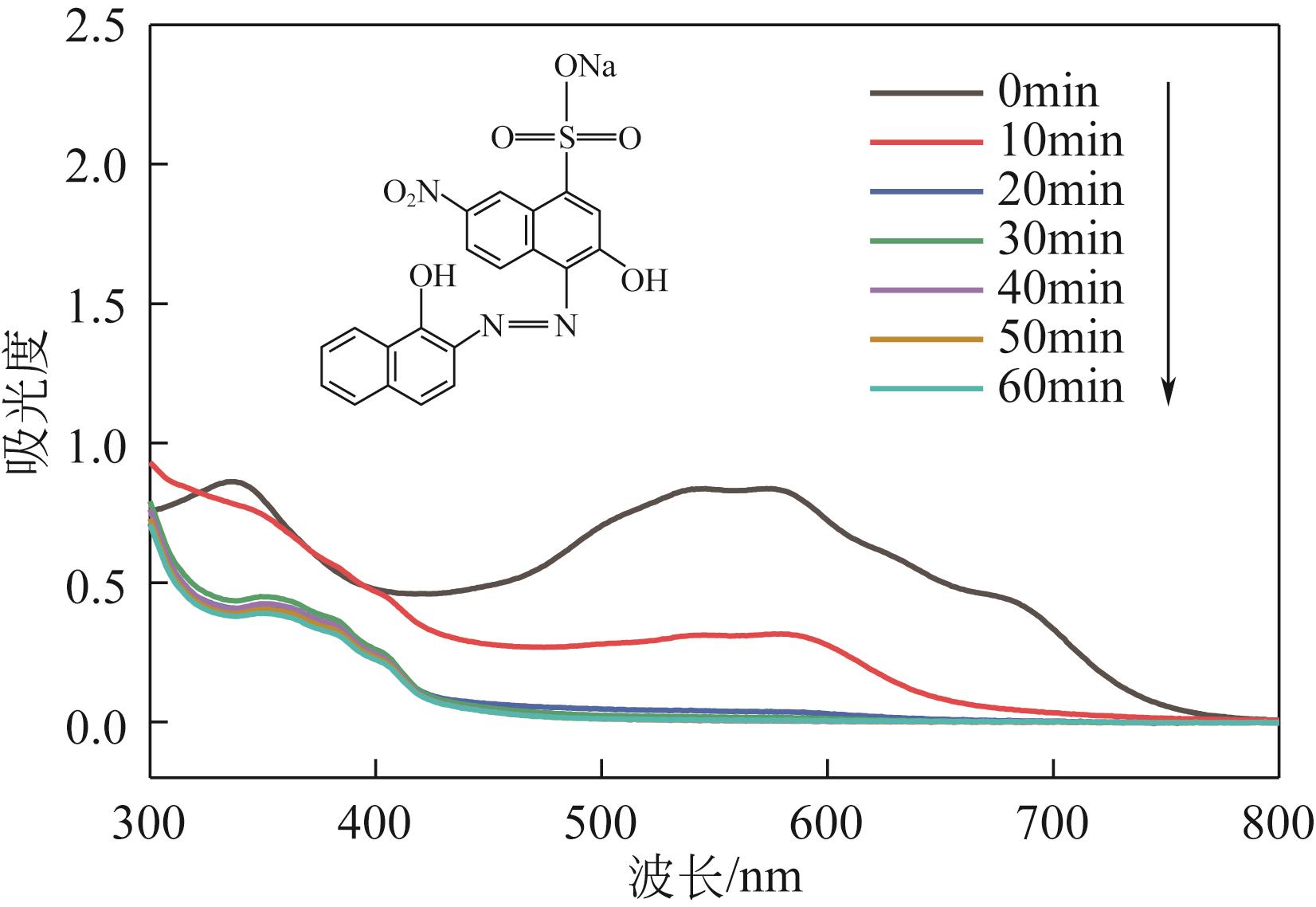
| 7 |
XIAO Pengfei, AN Lu, HAN Shuang. Research advances on applying carbon materials to activate persulfate in advanced oxidation technology[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3293-3306.
|
| 8 |
MA Qiuling, NENGZI Lichao, ZHANG Xinyi, et al. Enhanced activation of persulfate by AC@CoFe2O4 nanocomposites for effective removal of lomefloxacin[J]. Separation and Purification Technology, 2020, 233: 115978.
|
| 9 |
CHEN Liwei, DING Dahu, LIU Chao, et al. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration[J]. Chemical Engineering Journal, 2018, 334: 273-284.
|
| 10 |
CHEN Shaohua, MA Liying, DU Yaguang, et al. Highly efficient degradation of rhodamine B by carbon nanotubes-activated persulfate[J]. Separation and Purification Technology, 2021, 256: 117788.
|
| 11 |
WU Lin, WU Ting, LIU Zhifeng, et al. Carbon nanotube-based materials for persulfate activation to degrade organic contaminants: Properties, mechanisms and modification insights[J]. Journal of Hazardous Materials, 2022, 431: 128536.
|
| 12 |
LIU Desheng, LI Minna, LI Xiaochun, et al. Core-shell Zn/Co MOFs derived Co3O4/CNTs as an efficient magnetic heterogeneous catalyst for persulfate activation and oxytetracycline degradation[J]. Chemical Engineering Journal, 2020, 387: 124008.
|
| 13 |
ZHU Fang, JI Qiuyue, LEI Yu, et al. Efficient degradation of orange Ⅱ by core shell CoFe2O4-CeO2 nanocomposite with the synergistic effect from sodium persulfate[J]. Chemosphere, 2022, 291(2): 132765.
|
| 14 |
HUANG Quanlong, CHEN Congjin, ZHAO Xilian, et al. Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: Efficiency, stability and mechanism[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105800.
|
| 15 |
LIANG Yu, LI Lihua, YANG Chunmeng, et al. Bimetallic zeolitic imidazolate framework-derived nitrogen-doped porous carbon-coated CoFe2O4 core-shell composite with high catalytic performance for peroxymonosulfate activation in Rhodamine B degradation[J]. Journal of Alloys and Compounds, 2022, 907: 164504.
|
| 16 |
LIU Dongdong, CHEN Dengqian, HAO Zhengkai, et al. Efficient degradation of Rhodamine B in water by CoFe2O4/H2O2 and CoFe2O4/PMS systems: A comparative study[J]. Chemosphere, 2022, 307(2): 135935.
|
| 17 |
ZHI Zejian, WU Di, MENG Fanyue, et al. Facile synthesis of CoFe2O4@BC activated peroxymonosulfate for p-nitrochlorobenzene degradation: Matrix effect and toxicity evaluation[J]. Science of the Total Environment, 2022, 828: 154275.
|
| 18 |
LONG Xinxin, YANG Shengjiong, QIU Xiaojie, et al. Heterogeneous activation of peroxymonosulfate for bisphenol A degradation using CoFe2O4 derived by hybrid cobalt-ion hexacyanoferrate nanoparticles[J]. Chemical Engineering Journal, 2021, 404: 127052.
|
| 19 |
YANG Zhiquan, LI Ying, ZHANG Xinyi, et al. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate[J]. Chemical Engineering Journal, 2020, 384: 123319.
|
| 20 |
ZHANG Qianyu, SUN Xiaoqin, DANG Yuan, et al. A novel electrochemically enhanced homogeneous PMS-heterogeneous CoFe2O4 synergistic catalysis for the efficient removal of levofloxacin[J]. Journal of Hazardous Materials, 2022, 424: 127651.
|
| 21 |
FAN Yan, LIU Yanru, HU Xiang, et al. Preparation of metal organic framework derived materials CoFe2O4@NC and its application for degradation of norfloxacin from aqueous solutions by activated peroxymonosulfate[J]. Chemosphere, 2021, 275: 130059.
|
| 22 |
GUAN Yinghong, MA Jun, REN Yueming, et al. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals[J]. Water Research, 2013, 47(14): 5431-5438.
|
| 23 |
李英豪, 郑向前, 高晓亚, 等. CoFe2O4的制备及其活化过一硫酸盐降解磺胺甲𫫇唑[J]. 精细化工, 2022, 39(5): 1020-1027.
|
|
LI Yinghao, ZHENG Xiangqian, GAO Xiaoya, et al. Preparation of CoFe2O4 and its peroxymonosulfate activation for degradation of sulfamethoxazole[J]. Fine Chemicals, 2022, 39(5): 1020-1027.
|
| 24 |
XU Siyu, WEN Liangtao, YU Chen, et al. Activation of peroxymonosulfate by MnFe2O4@BC composite for bisphenol A Degradation: The coexisting of free-radical and non-radical pathways[J]. Chemical Engineering Journal, 2022, 442: 136250.
|
| 25 |
PENG Xiaoming, YANG Zhanhong, HU Fengping, et al. Mechanistic investigation of rapid catalytic degradation of tetracycline using CoFe2O4@MoS2 by activation of peroxymonosulfate[J]. Separation and Purification Technology, 2022, 287: 120525.
|
| 26 |
XU Mengjuan, LI Jun, YAN Yan, et al. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles[J]. Chemical Engineering Journal, 2019, 369: 403-413.
|
| 27 |
ZHAO Yan, SONG Min, CAO Qi, et al. The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil[J]. Journal of Hazardous Materials, 2020, 400: 122887.
|
| 28 |
TAN Ye, LI Chunquan, SUN Zhiming, et al. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate[J]. Chemical Engineering Journal, 2020, 388: 124386.
|
| 29 |
TAN Chaoqun, GAO Naiyun, FU Dafang, et al. Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate[J]. Separation and Purification Technology, 2017, 175: 47-57.
|
| 30 |
FU Haichao, MA Shuanglong, ZHAO Peng, et al. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: Structure dependence and mechanism[J]. Chemical Engineering Journal, 2019, 360: 157-170.
|
| 1 |
ZHANG Chengji, CHEN Hong, XUE Gang, et al. A critical review of the aniline transformation fate in azo dye wastewater treatment[J]. Journal of Cleaner Production, 2021, 321: 128971.
|
| 2 |
田婷婷, 李朝阳, 王召东, 等. 过渡金属活化过硫酸盐降解有机废水技术研究进展[J]. 化工进展, 2021, 40(6): 3480-3488.
|
|
TIAN Tingting, LI Chaoyang, WANG Shaodong, et al. Research progress of transition metal activated persulfate to degrade organic wastewater[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3480-3488.
|
| 3 |
WANG Jianlong, WANG Shizong. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517.
|
| 4 |
REN Yueming, LIN Lingqiang, MA Jun, et al. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Applied Catalysis B: Environmental, 2015, 165: 572-578.
|
| 5 |
SONG Qingyun, FENG Yiping, WANG Zhu, et al. Degradation of triphenyl phosphate (TPhP) by CoFe2O4-activated peroxymonosulfate oxidation process: Kinetics, pathways, and mechanisms[J]. Science of the Total Environment, 2019, 681: 331-338.
|
| 6 |
MENGELIZADEH Nezamaddin, MOHSENI Esmail, DEHGHANI Mohammad Hossein. Heterogeneous activation of peroxymonosulfate by GO-CoFe2O4 for degradation of reactive black 5 from aqueous solutions: Optimization, mechanism, degradation intermediates and toxicity[J]. Journal of Molecular Liquids, 2021, 327: 114838.
|
| 7 |
肖鹏飞, 安璐, 韩爽. 炭质材料在活化过硫酸盐高级氧化技术中的应用进展[J]. 化工进展, 2020, 39(8): 3293-3306.
|
| 31 |
LI Yi, MA Shuanglong, XU Shengjun, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4 [J]. Chemical Engineering Journal, 2020, 387: 124094.
|
| 32 |
Maryam KARIMI-SHAMSABADI, BEHPOUR Mohsen, BABAHEIDARI Ali Kazemi, et al. Efficiently enhancing photocatalytic activity of NiO-ZnO doped onto nanozeoliteX by synergistic effects of p-n heterojunction, supporting and zeolite nanoparticles in photo-degradation of Eriochrome Black T and Methyl Orange[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 346: 133-143.
|
 ), 严群1(
), 严群1( ), 陈锦富1,2, 温俊宇1,2, 苏晓洁1,2
), 陈锦富1,2, 温俊宇1,2, 苏晓洁1,2
 ), YAN Qun1(
), YAN Qun1( ), CHEN Jinfu1,2, WEN Junyu1,2, SU Xiaojie1,2
), CHEN Jinfu1,2, WEN Junyu1,2, SU Xiaojie1,2