化工进展 ›› 2023, Vol. 42 ›› Issue (6): 2944-2953.DOI: 10.16085/j.issn.1000-6613.2022-1480
单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展
- 中国石油大学(华东)重质油国家重点实验室,山东 青岛 266580
-
收稿日期:2022-08-10修回日期:2022-12-05出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:潘原 -
作者简介:张鹏(1994—),男,博士研究生,研究方向为电催化能源转化。E-mail:zpeng.sdu@foxmail.com。 -
基金资助:国家自然科学基金(22108306);泰山学者科研项目(tsqn201909065);山东省优秀青年科学基金(ZR2021YQ15);山东省自然科学基金(ZR2020QB174)
Progress of single atom catalysts in electrocatalytic oxygen reduction to hydrogen peroxide
- State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, Shandong, China
-
Received:2022-08-10Revised:2022-12-05Online:2023-06-25Published:2023-06-29 -
Contact:PAN Yuan
摘要:
电催化二电子氧还原过程产过氧化氢具有绿色、高效、安全、即时的特点,是一项被认为能够代替传统高污染,能耗密集型蒽醌生产的潜在技术,但仍具有挑战性。单原子催化剂具有原子利用率高、位点均一、催化活性高的优点,在电催化氧还原产过氧化氢中展现了广阔的应用前景。本文重点综述了贵金属单原子催化剂和过渡金属单原子催化剂在氧还原二电子产过氧化氢中的研究进展,着重于通过实验和理论计算结合的方式揭示电催化氧还原二电子催化活性和结构的联系。总结提出了提升单原子催化剂在二电子氧还原反应性能的调控策略,包括金属活性中心调控、配位结构调控、局域微环境调控,旨在为设计高活性、高选择性电催化氧还原二电子产过氧化氢催化剂提供借鉴和设计思路。文章对单原子催化剂在电催化氧还原产过氧化氢应用中的机遇和挑战进行了展望。单原子催化剂在活性位点表征、稳定性、制备方法等方面亟需改善,以促进其在电催化氧还原制过氧化氢中的发展。
中图分类号:
引用本文
张鹏, 潘原. 单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展[J]. 化工进展, 2023, 42(6): 2944-2953.
ZHANG Peng, PAN Yuan. Progress of single atom catalysts in electrocatalytic oxygen reduction to hydrogen peroxide[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2944-2953.
| 1 | LU Zhiyi, CHEN Guangxu, SIAHROSTAMI Samira, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials[J]. Nature Catalysis, 2018, 1(2): 156-162. |
| 2 | KIM Hyo Won, ROSS Michael B, KORNIENKO Nikolay, et al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts[J]. Nature Catalysis, 2018, 1(4): 282-290. |
| 3 | LEWIS Richard J, UEURA Kenji, LIU Xi, et al. Highly efficient catalytic production of oximes from ketones using in situ-generated H2O2 [J]. Science, 2022, 376(6593): 615-620. |
| 4 | XIA Chuan, XIA Yang, ZHU Peng, et al. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte[J]. Science, 2019, 366(6462): 226-231. |
| 5 | LIU Jingjing, GONG Zhichao, YAN Minmin, et al. Electronic structure regulation of single-atom catalysts for electrochemical oxygen reduction to H2O2 [J]. Small, 2022, 18(3): 2103824. |
| 6 | WANG Shiyi, ZHU Enbo, HUANG Yu, et al. Direct correlation of oxygen adsorption on platinum-electrolyte interfaces with the activity in the oxygen reduction reaction[J]. Science Advances, 2021, 7(24): eabb1435. |
| 7 | ZHAO Xue, LI Xue, BI Zenghui, et al. Boron modulating electronic structure of FeN4C to initiate high-efficiency oxygen reduction reaction and high-performance zinc-air battery[J]. Journal of Energy Chemistry, 2022, 66: 514-524. |
| 8 | CAI Huizhu, ZHANG Guanghui, ZHANG Xiao, et al. Engineering the local coordination environment and density of FeN4 sites by Mn cooperation for electrocatalytic oxygen reduction[J]. Small, 2022, 18(18): 2200911. |
| 9 | SHAO Chunfeng, WU Lingmin, ZHANG Haocheng, et al. A versatile approach to boost oxygen reduction of Fe-N4 sites by controllably incorporating sulfur functionality[J]. Advanced Functional Materials, 2021, 31(25): 2100833. |
| 10 | SIAHROSTAMI Samira, Arnau VERDAGUER-CASADEVALL, KARAMAD Mohammadreza, et al. Enabling direct H2O2 production through rational electrocatalyst design[J]. Nature Materials, 2013, 12(12): 1137-1143. |
| 11 | Arnau VERDAGUER-CASADEVALL, DEIANA Davide, KARAMAD Mohammadreza, et al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering[J]. Nano Letters, 2014, 14(3): 1603-1608. |
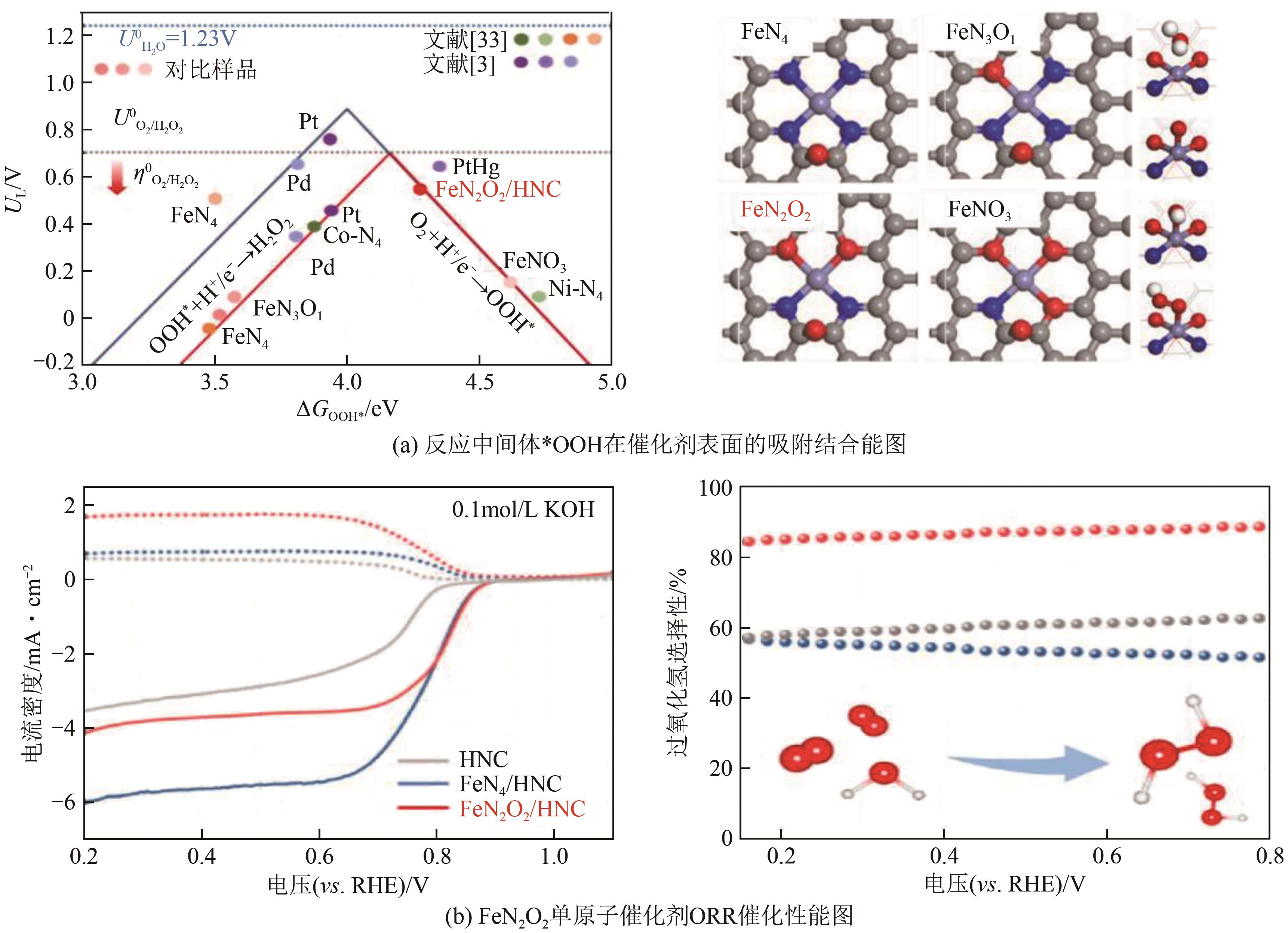
| 12 | QIAO Botao, WANG Aiqin, YANG Xiaofeng, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x [J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 13 | GUO Xiangyu, LIN Shiru, GU Jinxing, et al. Simultaneously achieving high activity and selectivity toward two- electron O2 electroreduction: the power of single-atom catalysts[J]. ACS Catalysis, 2019, 9(12): 11042-11054. |
| 14 | XIAO Fei, WANG Qi, XU Guiliang, et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells[J]. Nature Catalysis, 2022, 5(6): 503-512. |
| 15 | GARA Matthew, LABORDA Eduardo, HOLDWAY Philip, et al. Oxygen reduction at sparse arrays of platinum nanoparticles in aqueous acid: hydrogen peroxide as a liberated two electron intermediate[J]. Physical Chemistry Chemical Physics, 2013, 15(44): 19487-19495. |
| 16 | VON WEBER Alexander, BAXTER Eric T, WHITE Henry S, et al. Cluster size controls branching between water and hydrogen peroxide production in electrochemical oxygen reduction at Pt n /ITO[J]. The Journal of Physical Chemistry C, 2015, 119(20): 11160-11170. |
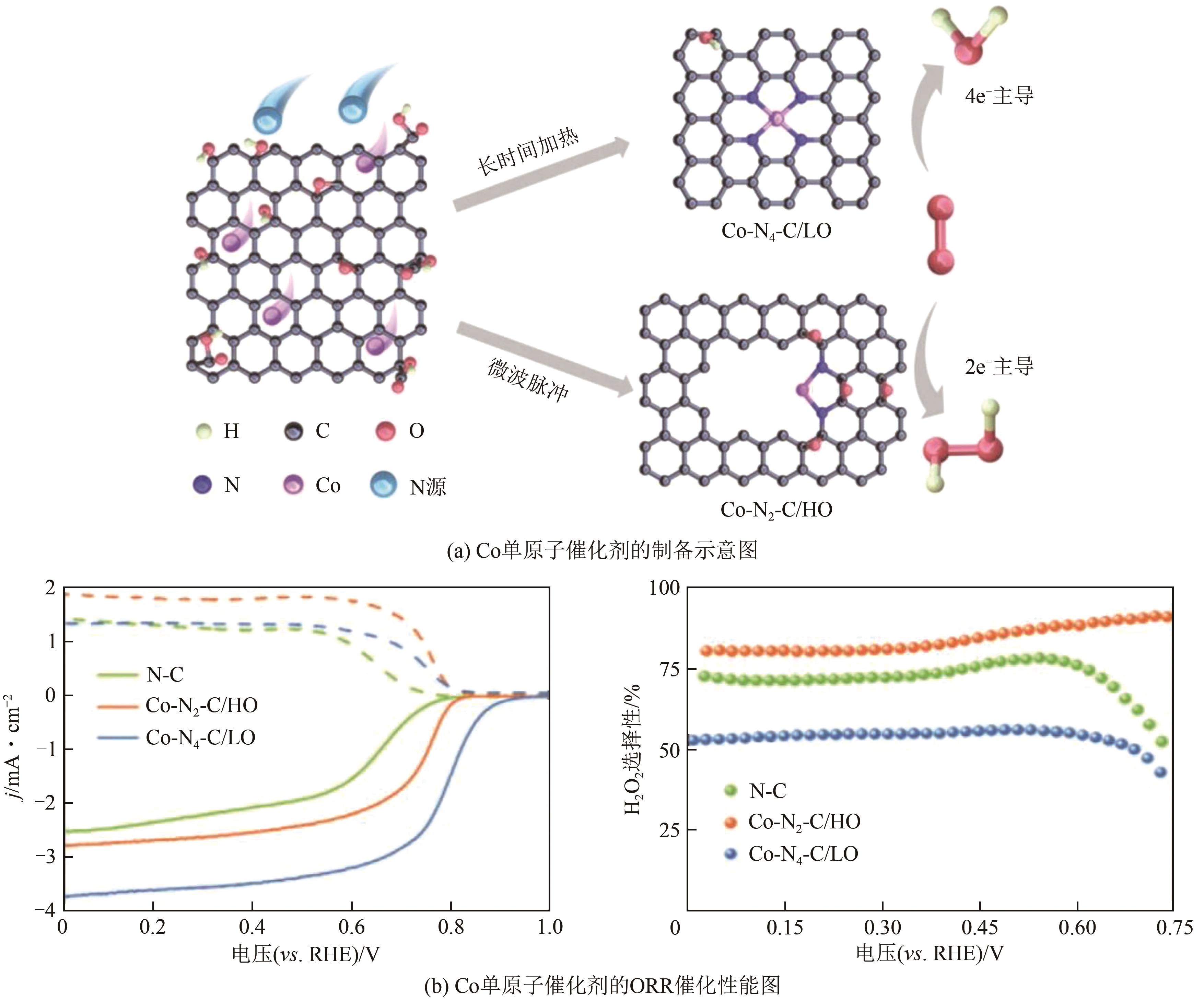
| 17 | SONG Xiaozhe, LI Ning, ZHANG Huan, et al. Promotion of hydrogen peroxide production on graphene-supported atomically dispersed platinum: effects of size on oxygen reduction reaction pathway[J]. Journal of Power Sources, 2019, 435: 226771. |
| 18 | YIN Yunchao, SHI Yi, SUN Yibai, et al. Selective electrochemical generation of hydrogen peroxide from oxygen reduction on atomically dispersed platinum[J]. ACS Applied Energy Materials, 2021, 4(10): 10843-10848. |
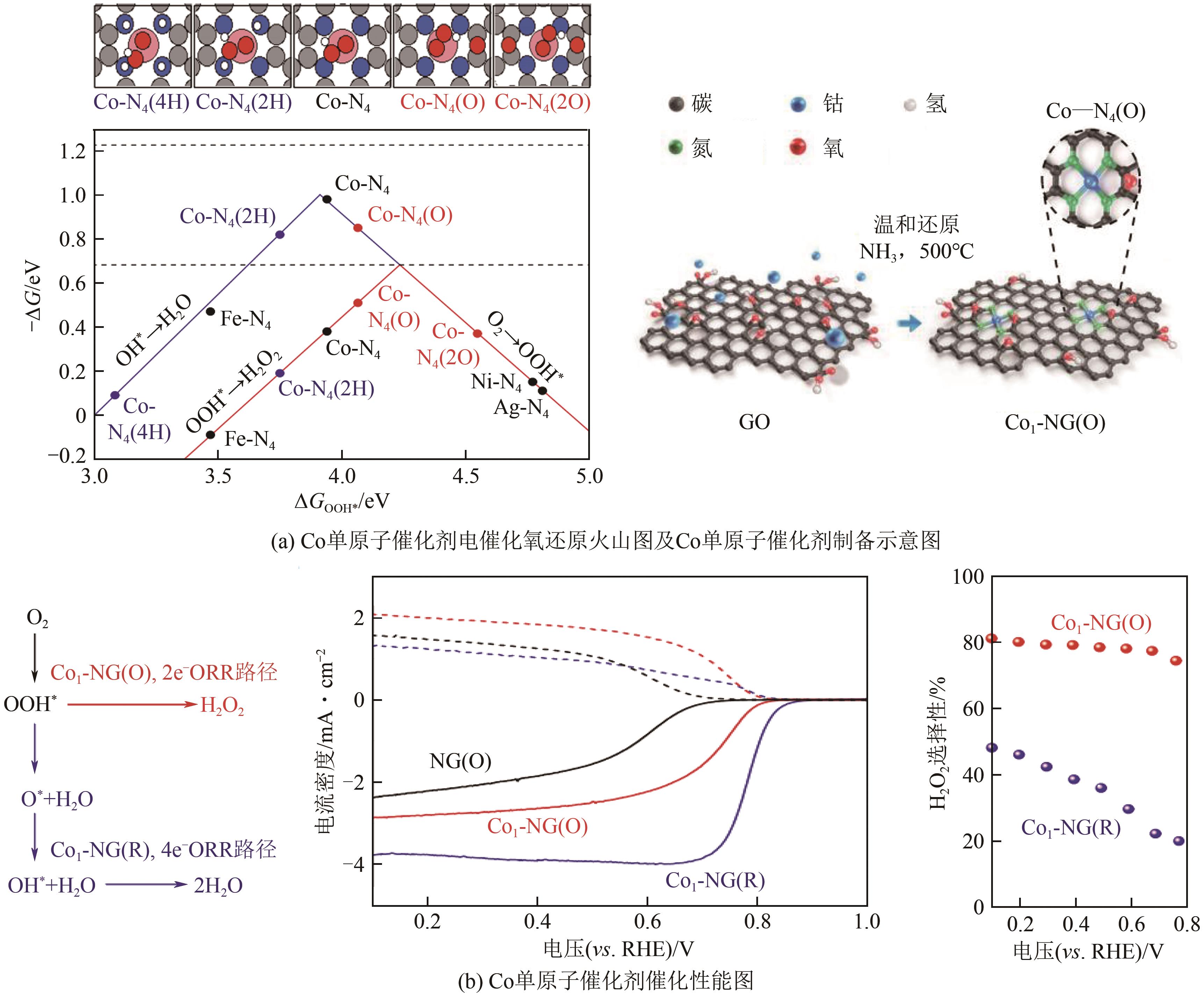
| 19 | ZHAO Jiajun, FU Cehuang, YE Ke, et al. Manipulating the oxygen reduction reaction pathway on Pt-coordinated motifs[J]. Nature Communications, 2022, 13: 685. |
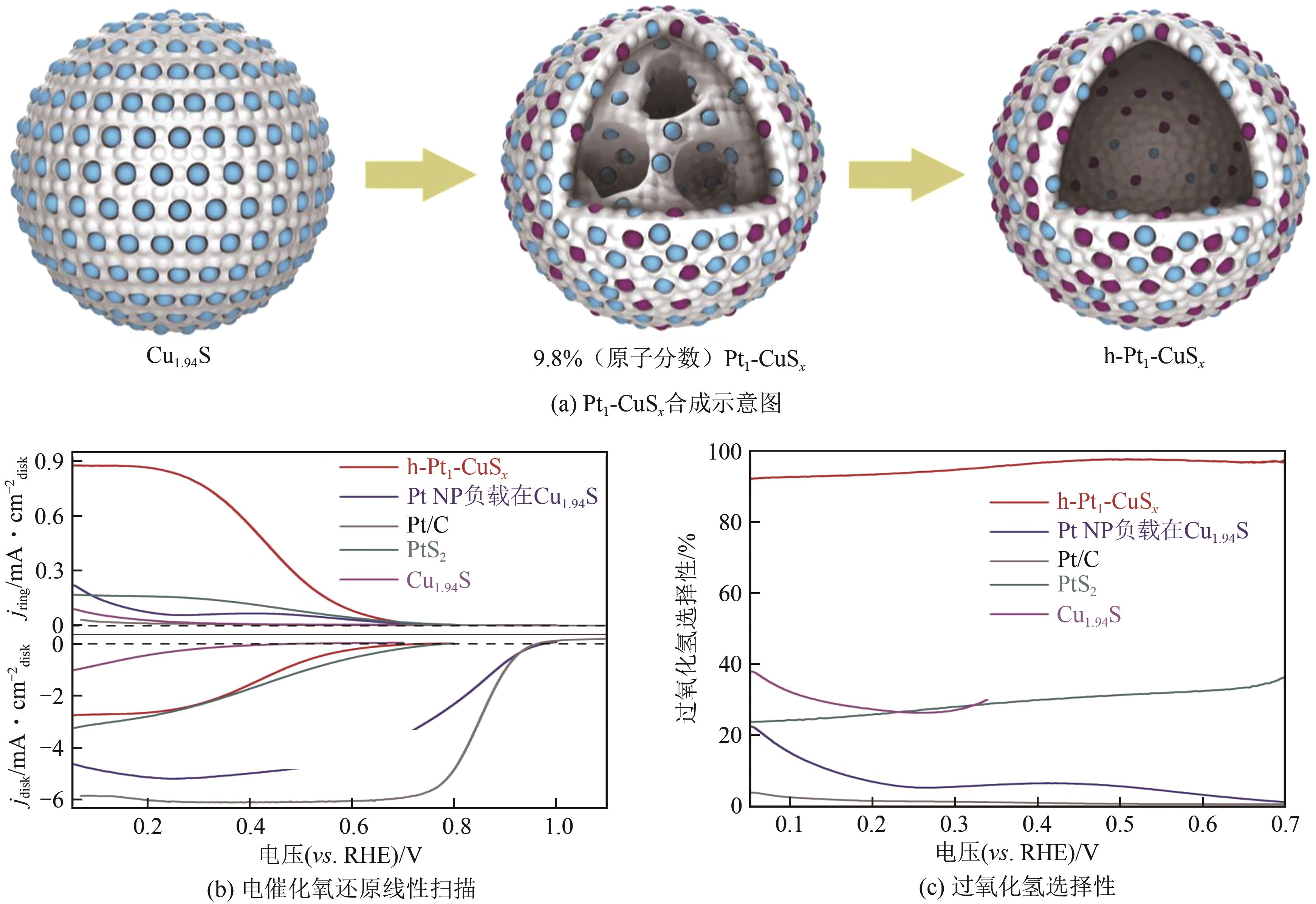
| 20 | SHEN Rongan, CHEN Wenxing, PENG Qing, et al. High-concentration single atomic Pt sites on hollow CuS x for selective O2 reduction to H2O2 in acid solution[J]. Chem, 2019, 5(8): 2099-2110. |
| 21 | CHEN Jinxing, MA Qian, ZHENG Xiliang, et al. Kinetically restrained oxygen reduction to hydrogen peroxide with nearly 100% selectivity[J]. Nature Communications, 2022, 13(1): 2808. |
| 22 | XUE Qi, XU Guangrui, MAO Rundong, et al. Polyethyleneimine modified AuPd@PdAu alloy nanocrystals as advanced electrocatalysts towards the oxygen reduction reaction[J]. Journal of Energy Chemistry, 2017, 26(6): 1153-1159. |
| 23 | WANG Chenyu, CHEN Dennis P, SANG Xiahan, et al. Size-dependent disorder-order transformation in the synthesis of monodisperse intermetallic PdCu nanocatalysts[J]. ACS Nano, 2016, 10(6): 6345-6353. |
| 24 | WEI Zengxi, DENG Bowen, CHEN Peng, et al. Palladium-based single atom catalysts for high-performance electrochemical production of hydrogen peroxide[J]. Chemical Engineering Journal, 2022, 428: 131112. |
| 25 | CHANG Qiaowan, ZHANG Pu, MOSTAGHIMI Amir Hassan Bagherzadeh, et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon[J]. Nature Communications, 2020, 11: 2178. |
| 26 | WANG Nan, ZHAO Xunhua, ZHANG Rui, et al. Highly selective oxygen reduction to hydrogen peroxide on a carbon-supported single-atom Pd electrocatalyst[J]. ACS Catalysis, 2022, 12(7): 4156-4164. |
| 27 | ZHANG Erhuan, TAO Lei, AN Jingkun, et al. Engineering the local atomic environments of indium single-atom catalysts for efficient electrochemical production of hydrogen peroxide[J]. Angewandte Chemie International Edition, 2022, 61(12): 1-10. |
| 28 | ZAGAL José H, KOPER Marc T M. Reaktivitätsdeskriptoren für die Aktivität von molekularen MN4-katalysatoren zur sauerstoffreduktion[J]. Angewandte Chemie International Edition, 2016, 128(47): 14726-14738. |
| 29 | JIANG Kun, BACK Seoin, AKEY Austin J, et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination[J]. Nature Communications, 2019, 10(1): 3997. |
| 30 | WU Yuhan, DING Yifan, HAN Xiao, et al. Modulating coordination environment of Fe single atoms for high-efficiency all-pH-tolerated H2O2 electrochemical production[J]. Applied Catalysis B: Environmental, 2022, 315: 121578. |
| 31 | SUN Yanyan, SILVIOLI Luca, SAHRAIE Nastaran Ranjbar, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts[J]. Journal of the American Chemical Society, 2019, 141(31): 12372-12381. |
| 32 | KULKARNI Ambarish, SIAHROSTAMI Samira, PATEL Anjli, et al. Understanding catalytic activity trends in the oxygen reduction reaction[J]. Chemical Reviews, 2018, 118(5): 2302-2312. |
| 33 | PEGIS Michael L, WISE Catherine F, MARTIN Daniel J, et al. Oxygen reduction by homogeneous molecular catalysts and electrocatalysts[J]. Chemical Reviews, 2018, 118(5): 2340-2391. |
| 34 | HE Yanghua, HWANG Sooyeon, CULLEN David A, et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy[J]. Energy & Environmental Science, 2019, 12(1): 250-260. |
| 35 | JUNG Euiyeon, SHIN Heejong, LEE Byoung Hoon, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials, 2020, 19(4): 436-442. |
| 36 | DING Jie, HUANG Jian, ZHANG Qiao, et al. A hierarchical monolithic cobalt-single-atom electrode for efficient hydrogen peroxide production in acid[J]. Catalysis Science & Technology, 2022, 12(8): 2416-2419. |
| 37 | ZHANG Binwei, ZHENG Tao, WANG Yunxiao, et al. Highly efficient and selective electrocatalytic hydrogen peroxide production on Co-O-C active centers on graphene oxide[J]. Communications Chemistry, 2022, 5(1): 1-7. |
| 38 | LIN Zeheng, ZHANG Qingran, PAN Jian, et al. Atomic Co decorated free-standing graphene electrode assembly for efficient hydrogen peroxide production in acid[J]. Energy & Environmental Science, 2022, 15(3): 1172-1182. |
| 39 | CHEN Shanyong, LUO Tao, LI Xiaoqing, et al. Identification of the highly active Co-N4 coordination motif for selective oxygen reduction to hydrogen peroxide[J]. Journal of the American Chemical Society, 2022, 144(32): 14505-14516. |
| 40 | PAN Jinkong, FANG Qiaojun, XIA Qian, et al. Dual effect of the coordination field and sulphuric acid on the properties of a single-atom catalyst in the electrosynthesis of H2O2 [J]. Physical Chemistry Chemical Physics, 2021, 23(37): 21338-21349. |
| 41 | WU Huixiang, HE Taihe, DAN Meng, et al. Activated Ni-based metal-organic framework catalyst with well-defined structure for electrosynthesis of hydrogen peroxide[J]. Chemical Engineering Journal, 2022, 435: 134863. |
| 42 | SONG Xiaozhe, LI Ning, ZHANG Huan, et al. Graphene-supported single nickel atom catalyst for highly selective and efficient hydrogen peroxide production[J]. ACS Applied Materials & Interfaces, 2020, 12(15): 17519-17527. |
| 43 | XIAO Chuqian, CHENG Ling, ZHU Yihua, et al. Super-coordinated nickel N4Ni1O2 site single-atom catalyst for selective H2O2 electrosynthesis at high current densities[J]. Angewandte Chemie International Edition, 2022, 61(38): e202206544. |
| 44 | ZHAO Qinglan, WANG Yian, LAI Werihong, et al. Approaching a high-rate and sustainable production of hydrogen peroxide: oxygen reduction on Co-N-C single-atom electrocatalysts in simulated seawater[J]. Energy & environmental science, 2021, 14(10): 5444-5456. |
| 45 | GONG Haisheng, WEI Zengxi, GONG Zhichao, et al. Low-coordinated Co-N-C on oxygenated graphene for efficient electrocatalytic H2O2 production[J]. Advanced Functional Materials, 2022, 32(5): 2106886. |
| 46 | LI Boquan, ZHAO Changxin, LIU Jianing, et al. Electrosynthesis of hydrogen peroxide synergistically catalyzed by atomic Co-N x -C sites and oxygen functional groups in noble-metal-free electrocatalysts[J]. Advanced Materials, 2019, 31(35): 1808173. |
| 47 | ZHANG Qingran, TAN Xin, BEDFORD Nicholas M, et al. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production[J]. Nature Communications, 2020, 11: 4181. |
| 48 | WU Yuhan, SUN Jianhui, DOU Shixue, et al. Non-precious metal electrocatalysts for two-electron oxygen electrochemistry: mechanisms, progress, and outlooks[J]. Journal of Energy Chemistry, 2022, 69: 54-69. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [6] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [10] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [11] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [12] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [13] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [14] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [15] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||