化工进展 ›› 2023, Vol. 42 ›› Issue (9): 4706-4715.DOI: 10.16085/j.issn.1000-6613.2022-1924
醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚
王伟涛1( ), 鲍婷玉1, 姜旭禄1, 何珍红1, 王宽1, 杨阳1, 刘昭铁1,2(
), 鲍婷玉1, 姜旭禄1, 何珍红1, 王宽1, 杨阳1, 刘昭铁1,2( )
)
- 1.陕西科技大学化学与化工学院,陕西 西安 710021
2.陕西师范大学化学与化工学院, 陕西 西安 710119
-
收稿日期:2022-10-17修回日期:2023-03-15出版日期:2023-09-15发布日期:2023-09-28 -
通讯作者:刘昭铁 -
作者简介:王伟涛(1985—),男,副教授,硕士生导师,研究方向为绿色催化反应。E-mail:wangweitao@sust.edu.cn。 -
基金资助:陕西省自然科学基础研究计划(2019JM-080)
Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst
WANG Weitao1( ), BAO Tingyu1, JIANG Xulu1, HE Zhenhong1, WANG Kuan1, YANG Yang1, LIU Zhaotie1,2(
), BAO Tingyu1, JIANG Xulu1, HE Zhenhong1, WANG Kuan1, YANG Yang1, LIU Zhaotie1,2( )
)
- 1.College of Chemistry & Chemical Engineering, Shaanxi University of Science & Technology, Xi’an 710021, Shaanxi, China
2.School of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi’an 710119, Shaanxi, China
-
Received:2022-10-17Revised:2023-03-15Online:2023-09-15Published:2023-09-28 -
Contact:LIU Zhaotie
摘要:
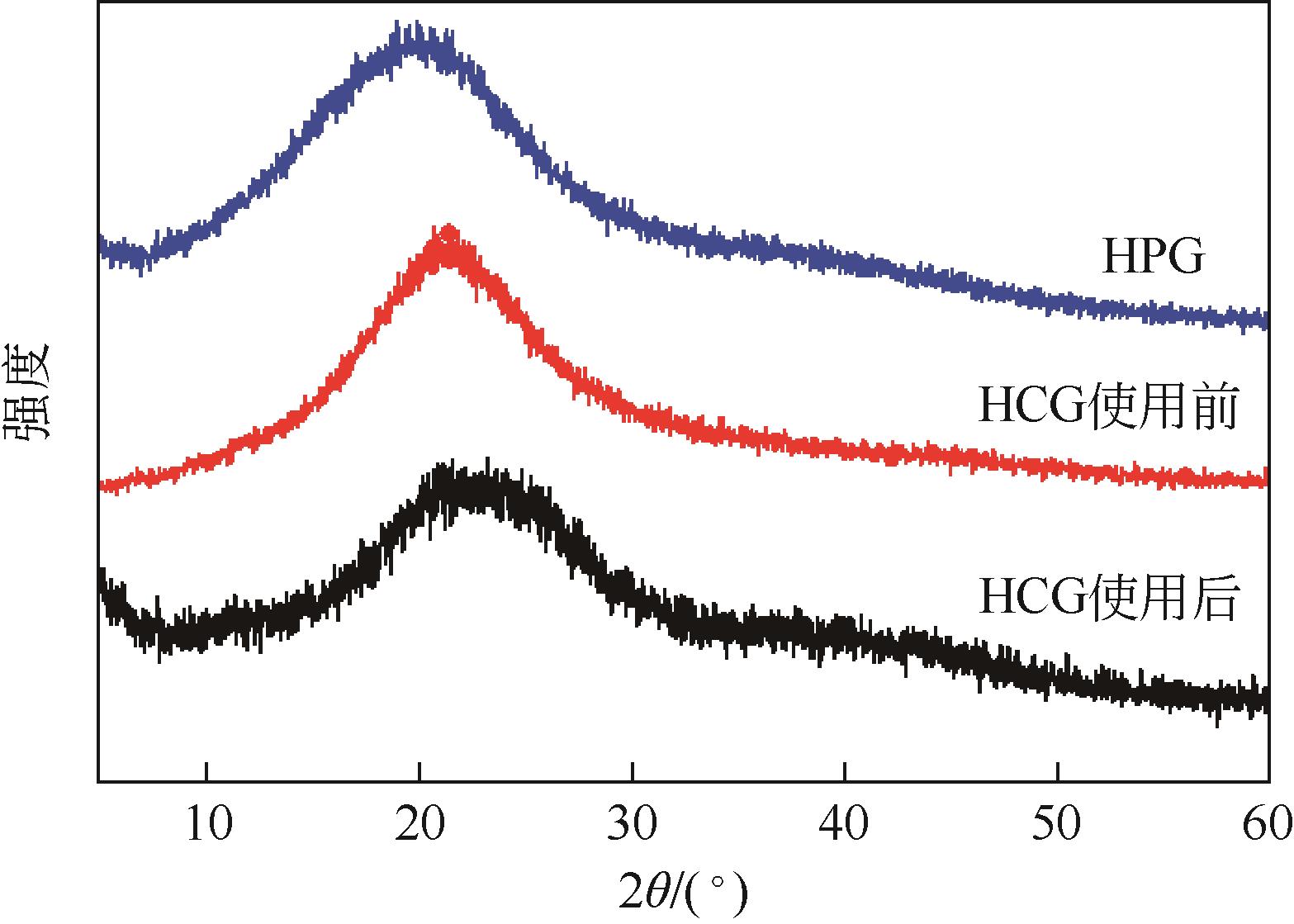
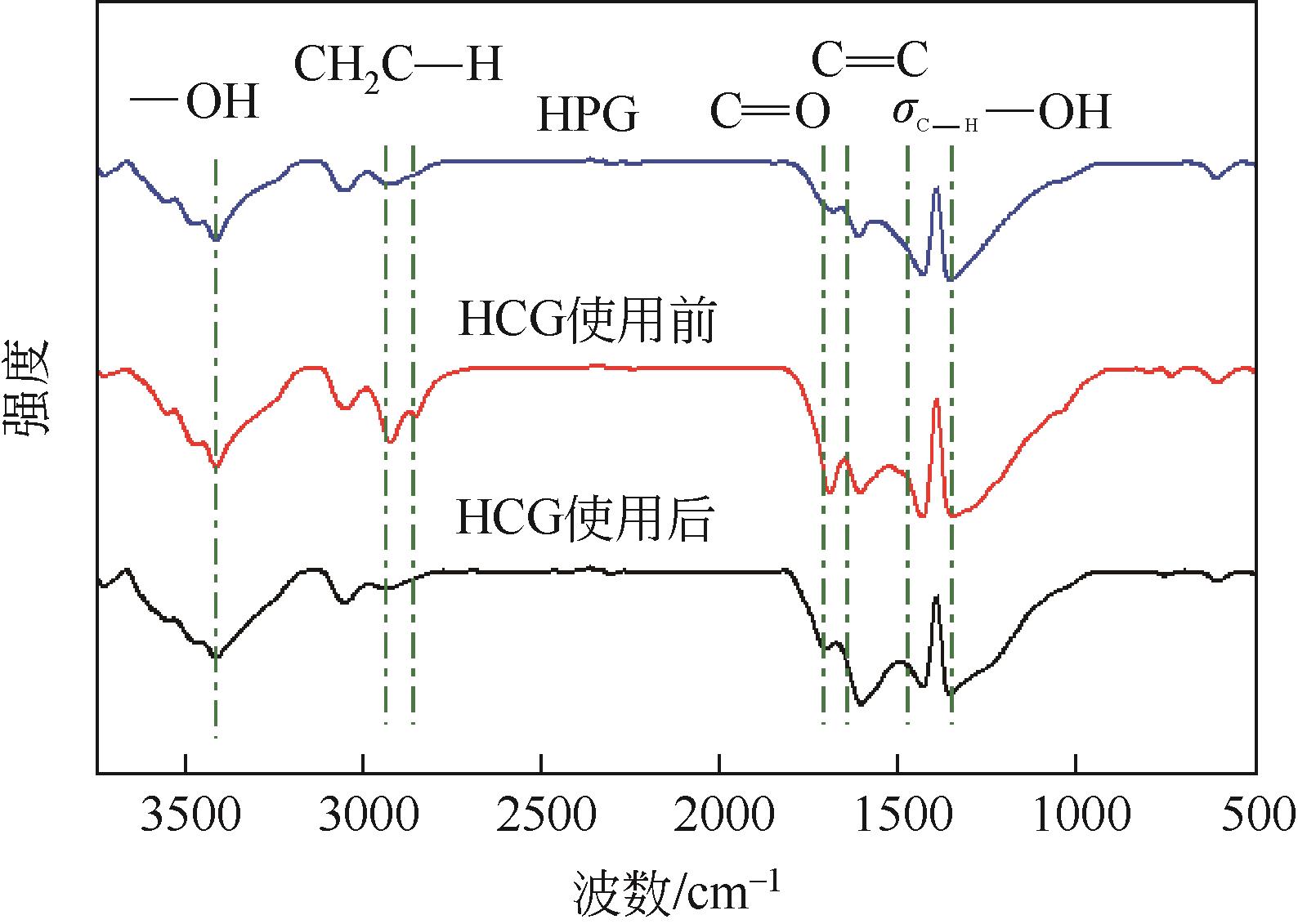
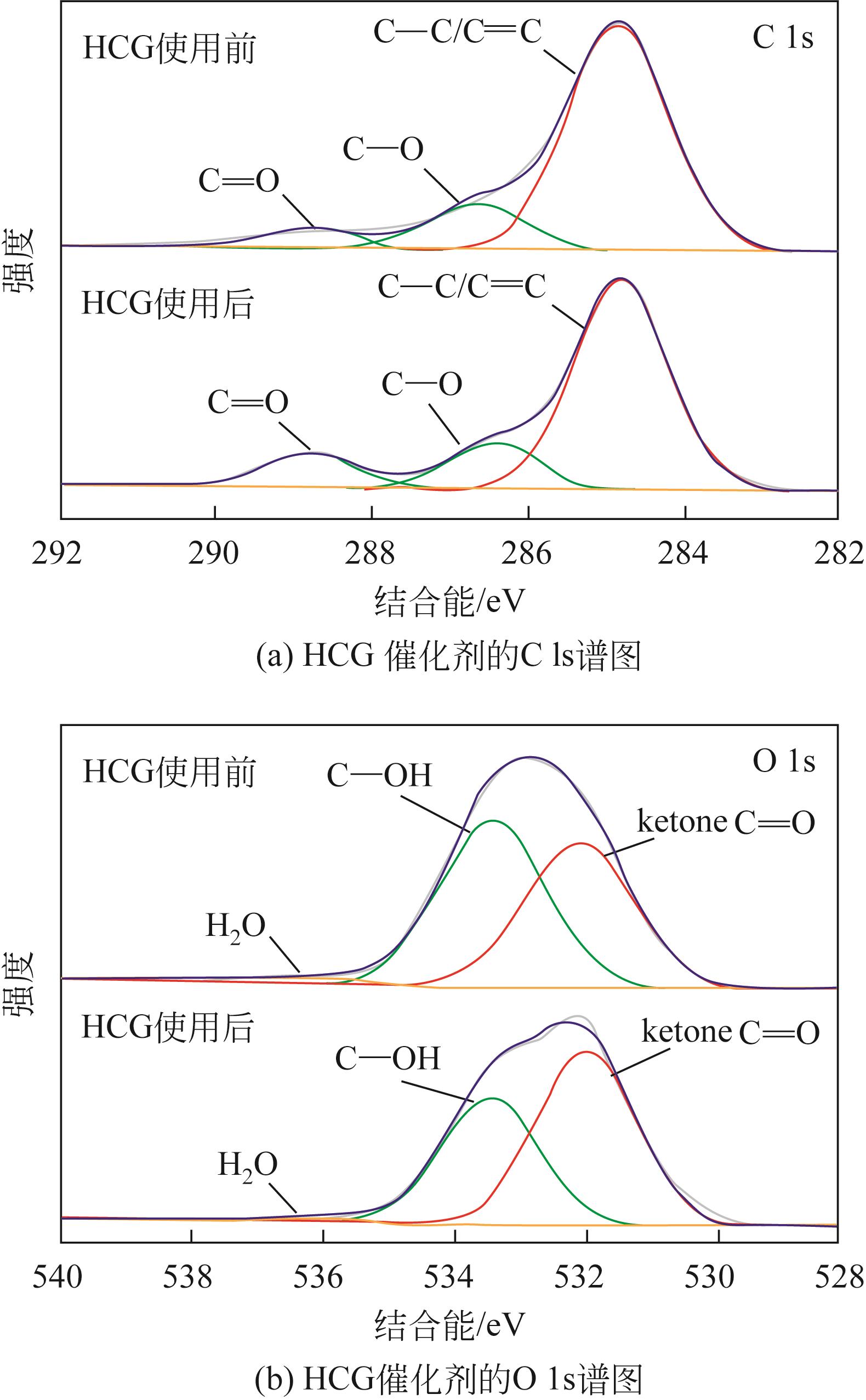
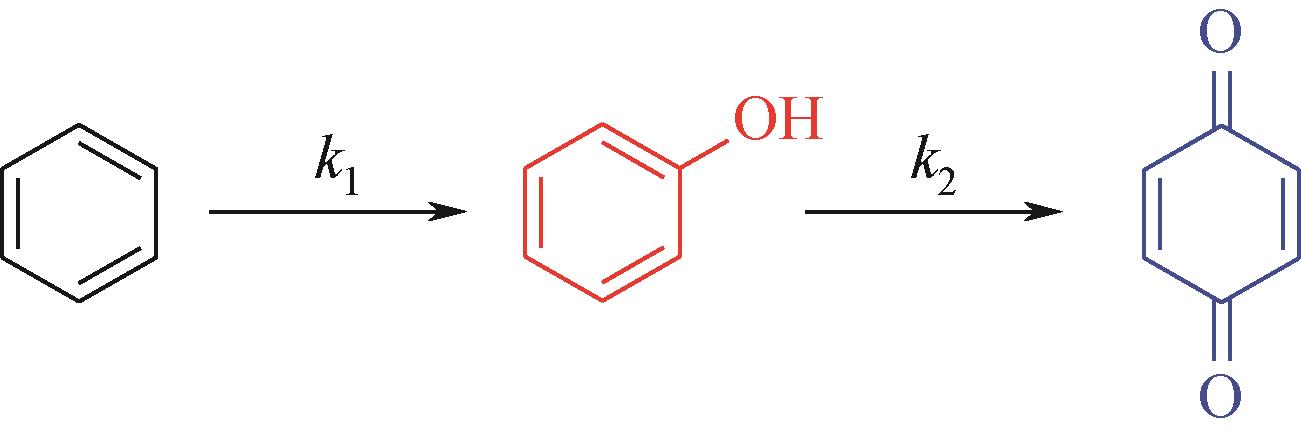
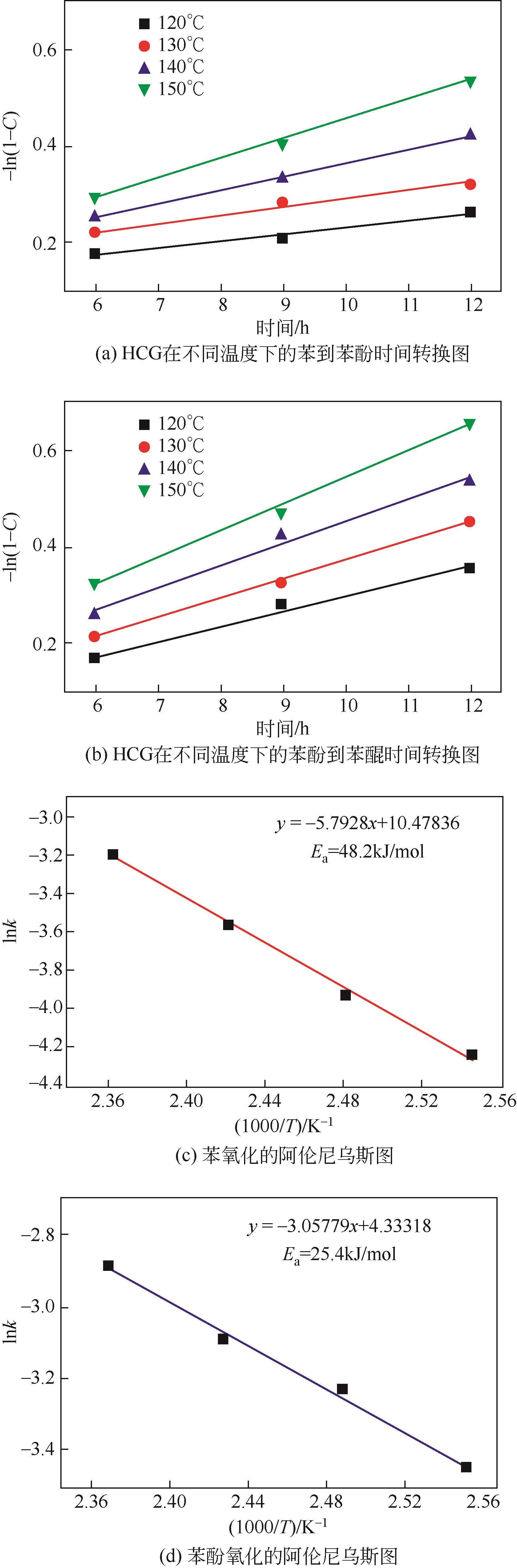
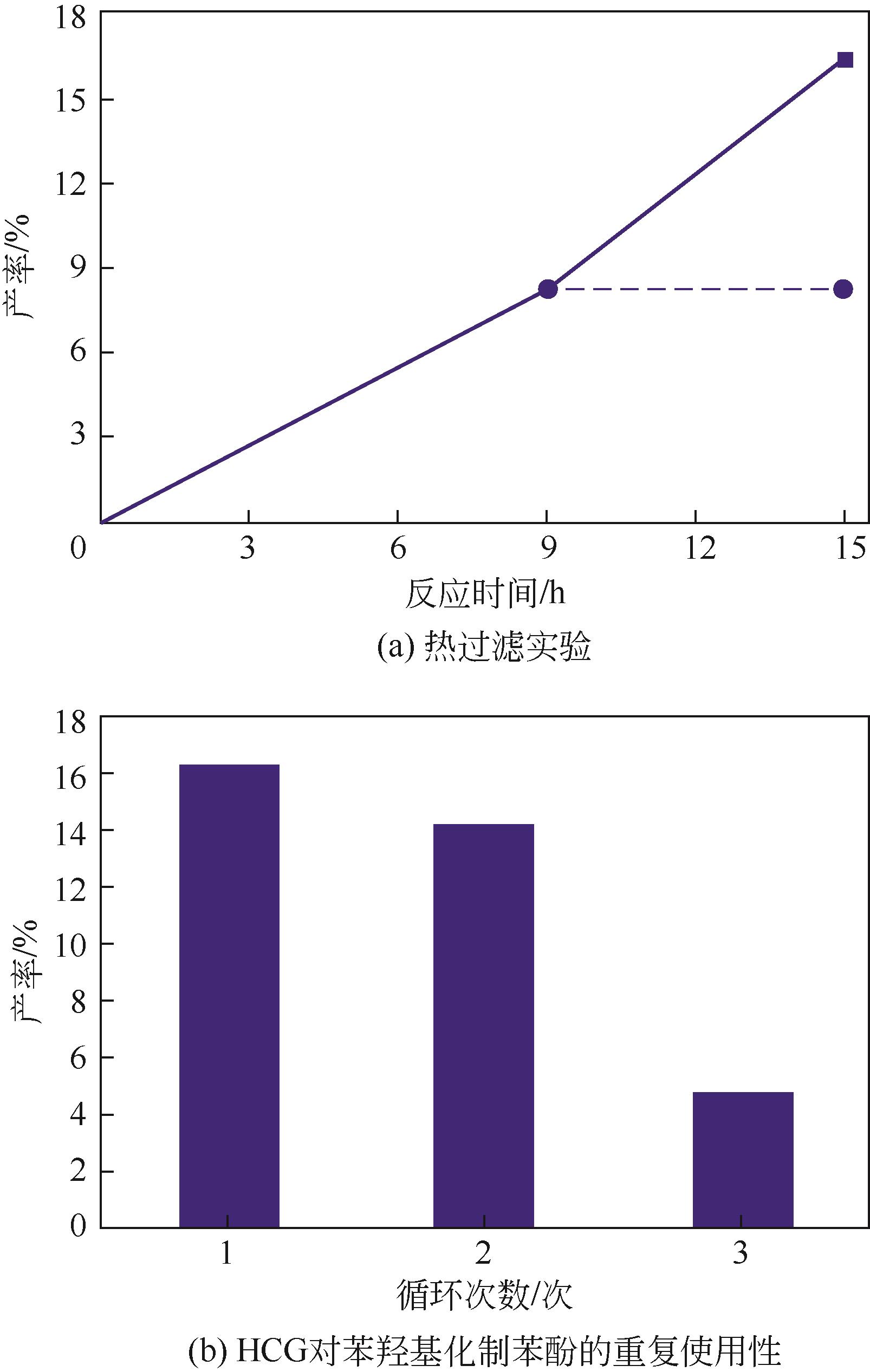
苯酚是一种具有广泛应用价值的化工原料,氧气直接氧化苯制备苯酚是一条绿色的路径。本文设计并制备了一种新型醛酮树脂基非金属催化剂用于氧气直接氧化苯制备苯酚。通过X射线衍射仪、傅里叶变换红外光谱仪、扫描电镜、X射线光电子能谱和N2吸附-脱附等技术对催化剂的形貌、结构进行了表征。结果表明,制备的催化剂是具有大量羟基和羰基官能团的无定形碳材料,不同的酮对催化剂官能团的含量有影响。考察了所制备的催化剂催化氧气直接氧化苯制备苯酚反应的性能,优化了反应条件。在最佳反应条件下,苯酚的产率达到16.3%,其催化性能可与金属催化剂相媲美。此外,通过动力学研究表明该反应为一级反应并计算了各步骤的反应速率常数。结合表征、动力学实验和对照实验得出催化剂表面丰富的酮羰基是反应的活性位点,并据此提出了反应机理。
中图分类号:
引用本文
王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715.
WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715.
| 催化剂 | 比表面积①/m2·g-1 | 孔体积②/cm3·g-1 | 孔径③/nm |
|---|---|---|---|
| HPG | 7.4 | 0.0086 | 4.7 |
| HCG | 5.5 | 0.0077 | 5.5 |
| 使用后HCG | 9.1 | 0.0148 | 6.6 |
表1 HPG、HCG和使用后HCG催化剂的结构特性
| 催化剂 | 比表面积①/m2·g-1 | 孔体积②/cm3·g-1 | 孔径③/nm |
|---|---|---|---|
| HPG | 7.4 | 0.0086 | 4.7 |
| HCG | 5.5 | 0.0077 | 5.5 |
| 使用后HCG | 9.1 | 0.0148 | 6.6 |
| 序号 | 催化剂 | 苯酚产率/% |
|---|---|---|
| 1 | — | 0.4 |
| 2② | HCG | 0.0 |
| 3③ | HCG | 4.8 |
| 4 | HCG | 12.5 |
| 5 | HPG | 11.9 |
表2 不同催化剂对苯羟基化制苯酚的催化性能①
| 序号 | 催化剂 | 苯酚产率/% |
|---|---|---|
| 1 | — | 0.4 |
| 2② | HCG | 0.0 |
| 3③ | HCG | 4.8 |
| 4 | HCG | 12.5 |
| 5 | HPG | 11.9 |
| 序号 | 条件 | 苯酚产率/% |
|---|---|---|
| 1 | 环己酮(100mg) | 13.3 |
| 2 | 乙二醛(100mg) | 0.8 |
| 3 | 环己醇(100mg) | 0.9 |
| 4 | TBA(2mmol) | 5.1 |
| 5 | BHT(2mmol) | 5.8 |
| 6 | TBA(5mmol) | 3.7 |
| 7 | BHT(5mmol) | 1.6 |
| 8② | 无氧气 | — |
| 9③ | H2O2 | 6.3 |
| 10④ | LiCl | 1.7 |
| 11⑤ | NaOAc | 12.5 |
| 12 | 标准条件 | 16.3 |
表3 苯羟基化制苯酚的对照实验①
| 序号 | 条件 | 苯酚产率/% |
|---|---|---|
| 1 | 环己酮(100mg) | 13.3 |
| 2 | 乙二醛(100mg) | 0.8 |
| 3 | 环己醇(100mg) | 0.9 |
| 4 | TBA(2mmol) | 5.1 |
| 5 | BHT(2mmol) | 5.8 |
| 6 | TBA(5mmol) | 3.7 |
| 7 | BHT(5mmol) | 1.6 |
| 8② | 无氧气 | — |
| 9③ | H2O2 | 6.3 |
| 10④ | LiCl | 1.7 |
| 11⑤ | NaOAc | 12.5 |
| 12 | 标准条件 | 16.3 |
| 1 | WU Yuzhou, ZHANG Xubin, WANG Fumin, et al. Synergistic effect between Fe and Cu species on mesoporous silica for hydroxylation of benzene to phenol[J]. Industrial & Engineering Chemistry Research, 2021, 60(23): 8386-8395. |
| 2 | GU Yaqi, LI Qi, ZANG Dejin, et al. Light-induced efficient hydroxylation of benzene to phenol by quinolinium and polyoxovanadate-based supramolecular catalysts[J]. Angewandte Chemie International Edition, 2021, 60(24): 13310-13316. |
| 3 | HIROSE Kensaku, OHKUBO Kei, FUKUZUMI Shunichi. Catalytic hydroxylation of benzene to phenol by dioxygen with an NADH analogue[J]. Chemistry: A European Journal, 2016, 22(36): 12904-12909. |
| 4 | YAMADA Mihoko, KARLIN Kenneth D, FUKUZUMI Shunichi. One-step selective hydroxylation of benzene to phenol with hydrogen peroxide catalysed by copper complexes incorporated into mesoporous silica-alumina[J]. Chemical Science, 2016, 7(4): 2856-2863. |
| 5 | MISHRA Subhashree, Rajaram BAL, DEY R K. Heterogeneous recyclable copper oxide supported on activated red mud as an efficient and stable catalyst for the one pot hydroxylation of benzene to phenol[J]. Molecular Catalysis, 2021, 499: 111310. |
| 6 | OKEMOTO Atsushi, UEYAMA Kohei, TANIYA Keita, et al. Direct oxidation of benzene with molecular oxygen in liquid phase catalysed by heterogeneous copper complexes encapsulated in Y-type zeolite[J]. Catalysis Communications, 2017, 100: 29-32. |
| 7 | FARAHMAND Shohreh, GHIACI Mehran, VATANPARAST Morteza, et al. One-step hydroxylation of benzene to phenol over Schiff base complexes incorporated onto mesoporous organosilica in the presence of different axial ligands[J]. New Journal of Chemistry, 2020, 44(18): 7517-7527. |
| 8 | 王伟涛, 姚敏, 马养民, 等. 氧气直接氧化苯制备苯酚[J]. 化学进展, 2014, 26(10): 1665-1672. |
| WANG Weitao, YAO Min, MA Yangmin, et al. Direct oxidation of liquid benzene to phenol with molecular oxygen[J]. Progress in Chemistry, 2014, 26(10): 1665-1672. | |
| 9 | LONG Zhouyang, LIU Yangqing, ZHAO Pingping, et al. Aerobic oxidation of benzene to phenol over polyoxometalate-paired PdⅡ-coordinated hybrid: Reductant-free heterogeneous catalysis[J]. Catalysis Communications, 2015, 59: 1-4. |
| 10 | LONG Zhouyang, ZHOU Yu, GE Weilin, et al. Ionic-liquid-functionalized polyoxometalates for heterogeneously catalyzing the aerobic oxidation of benzene to phenol: Raising efficacy through specific design[J]. ChemPlusChem, 2014, 79(11): 1590-1596. |
| 11 | CHEN Qiang, PENG Qingyu, ZHAO Xu, et al. Grafting carbon nanotubes densely on carbon fibers by poly(propylene imine) for interfacial enhancement of carbon fiber composites[J]. Carbon, 2020, 158: 704-710. |
| 12 | ZOU Rongge, QIAN Moriko, WANG Chenxi, et al. Biochar: From by-products of agro-industrial lignocellulosic waste to tailored carbon-based catalysts for biomass thermochemical conversions[J]. Chemical Engineering Journal, 2022, 441: 135972. |
| 13 | LIU Xiaohong, CHEN Xiejie, ZHANG Qiang, et al. Effect of N, P co-doped activated carbon supported Cu-based catalyst for acetylene hydration[J]. Molecular Catalysis, 2022, 522: 112223. |
| 14 | SANGSIRI Pimpajee, LAOSIRIPOJANA Navadol, DAORATTANACHAI Pornlada. Synthesis of sulfonated carbon-based catalysts from organosolv lignin and methanesulfonic acid: Its activity toward esterification of stearic acid[J]. Renewable Energy, 2022, 193: 113-127. |
| 15 | SHAN Wanjian, LI Shuai, CAI Xiaochun, et al. Carbon catalyzed hydroxylation of benzene with dioxygen to phenol over surface carbonyl groups[J]. ChemCatChem, 2019, 11(3): 1076-1085. |
| 16 | CHEN Tao, YE Tingting, ZHU Jie, et al. Small-sized biomass-derived hydrothermal carbon with enriched oxygen groups quickens benzene hydroxylation to phenol with dioxygen[J]. Applied Catalysis A: General, 2021, 626: 118356. |
| 17 | ZHU Jie, LI Guoqing, WANG Qian, et al. Engineering surface groups of commercially activated carbon for benzene hydroxylation to phenol with dioxygen[J]. Industrial & Engineering Chemistry Research, 2019, 58(44): 20226-20235. |
| 18 | WANG Weitao, SHI Leilei, LI Na, et al. V x O y @C catalyst prepared from biomass for hydroxylation of benzene to phenol with molecular oxygen[J]. RSC Advances, 2017, 7(21): 12738-12744. |
| 19 | WANG Weitao, TANG Hao, JIANG Xulu, et al. Quinone-amine polymers as metal-free and reductant-free catalysts for hydroxylation of benzene to phenol with molecular oxygen[J]. Chemical Communications, 2019, 55(54): 7772-7775. |
| 20 | WANG Weitao, LI Na, SHI Leilei, et al. Vanadium-zirconium catalyst on different support for hydroxylation of benzene to phenol with O2 as the oxidant[J]. Applied Catalysis A: General, 2018, 553: 117-125. |
| 21 | GONG Yutong, XIE Lei, LI Haoran, et al. Sustainable and scalable production of monodisperse and highly uniform colloidal carbonaceous spheres using sodium polyacrylate as the dispersant[J]. Chemical Communications, 2014, 50(84): 12633-12636. |
| 22 | WANG Qisong, YE Chao, ZHAO Yuan, et al. Preparation of polydopamine-derived carbon-based nano-Fe catalysts and its catalytic conversion of toluene for hydrogen production[J]. Fuel, 2022, 324: 124692. |
| 23 | SHENG Yeliang, PENG Jinfei, MA Lei, et al. Nickel nanoparticles embedded in porous carbon-coated honeycomb ceramics: A potential monolithic catalyst for continuous hydrogenation reaction[J]. Carbon, 2022, 197: 171-182. |
| 24 | 李鹏, 张一甫, 林晓丹, 等. 苯乙酮-环己酮-甲醛共缩聚树脂的合成研究[J]. 化学与黏合, 2006, 28(5): 295-298. |
| LI Peng, ZHANG Yifu, LIN Xiaodan, et al. Study on synthesis of acetophenone-cyclohexanone-formaldehyde resin[J]. Chemistry and Adhesion, 2006, 28(5): 295-298. | |
| 25 | YANG Guo, JIANG Junqing, ZHANG Yanwu. Synthesis of cyclohexanone-formaldehyde resin catalyzed by rehydrated Mg-Al hydrotalcite[J]. Progress in Organic Coatings, 2015, 78: 55-58. |
| 26 | LASKAR Ikbal B, RAJKUMARI Kalyani, GUPTA Rajat, et al. Acid-functionalized mesoporous polymer-catalyzed acetalization of glycerol to solketal, a potential fuel additive under solvent-free conditions[J]. Energy & Fuels, 2018, 32(12): 12567-12576. |
| 27 | ZHANG Kejing, MIN Xiaoye, ZHANG Tingzheng, et al. Selenium and nitrogen co-doped biochar as a new metal-free catalyst for adsorption of phenol and activation of peroxymonosulfate: Elucidating the enhanced catalytic performance and stability[J]. Journal of Hazardous Materials, 2021, 413: 125294. |
| 28 | PAN Fuping, CAO Zhongyue, ZHAO Qiuping, et al. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction[J]. Journal of Power Sources, 2014, 272: 8-15. |
| 29 | YANG Xu, ZHANG Rongyu, CHEN Nan, et al. Assembly of SnSe nanoparticles confined in graphene for enhanced sodium-ion storage performance[J]. Chemistry: A European Journal, 2016, 22(4): 1445-1451. |
| 30 | KUBO Shiori, TAN Irene, WHITE Robin J, et al. Template synthesis of carbonaceous tubular nanostructures with tunable surface properties[J]. Chemistry of Materials, 2010, 22(24): 6590-6597. |
| 31 | ZHAO Xiaochen, ZHANG Qiang, ZHANG Bingsen, et al. Dual-heteroatom-modified ordered mesoporous carbon: Hydrothermal functionalization, structure, and its electrochemical performance[J]. Journal of Materials Chemistry, 2012, 22(11): 4963-4969. |
| 32 | CHEN Yandan, AI Xiaolin, HUANG Biao, et al. Consecutive preparation of hydrochar catalyst functionalized in situ with sulfonic groups for efficient cellulose hydrolysis[J]. Cellulose, 2017, 24(7): 2743-2752. |
| 33 | YAN Pengqiang, ZHANG Bingsen, WU Kuang-Hsu, et al. Surface chemistry of nanocarbon: Characterization strategies from the viewpoint of catalysis and energy conversion[J]. Carbon, 2019, 143: 915-936. |
| 34 | WEI Qinhong, FAN Huailin, QIN Fangfang, et al. Metal-free honeycomb-like porous carbon as catalyst for direct oxidation of benzene to phenol[J]. Carbon, 2018, 133: 6-13. |
| 35 | YANG Jinghe, SUN Geng, GAO Yongjun, et al. Direct catalytic oxidation of benzene to phenol over metal-free graphene-based catalyst[J]. Energy & Environmental Science, 2013, 6(3): 793-798. |
| 36 | HEATH Aubrey A, EHRENHAUSER Franz S, VALSARAJ Kalliat T. Effects of temperature, oxygen level, ionic strength, and pH on the reaction of benzene with hydroxyl radicals in aqueous atmospheric systems[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 822-830. |
| 37 | SUN Wei, GAO Lingfeng, ZHENG Gengxiu. A radical capture mechanism for immediate Csp2-H bond hydroxylation via a heterogeneous Cu-graphene catalyst[J]. Chemical Communications, 2019, 55(61): 8915-8918. |
| 38 | 张进, 罗茜, 唐英, 等. 钒钼磷杂多酸催化苯直接羟化为苯酚的动力学研究[J]. 化学研究与应用, 2005, 17(5): 603-606. |
| ZHANG Jin, LUO Qian, TANG Ying, et al. Kinetics studies of the direct hydroxylation of benzene to phenol catalysed by vanadium substituted heteropolymolybdates[J]. Chemical Research and Application, 2005, 17(5): 603-606. | |
| 39 | QIN Qin, LIU Yangqing, SHAN Wanjian, et al. Synergistic catalysis of Fe2O3 nanoparticles on mesoporous poly(ionic liquid)-derived carbon for benzene hydroxylation with dioxygen[J]. Industrial & Engineering Chemistry Research, 2017, 56(43): 12289-12296. |
| 40 | WANG Weitao, WEI Yaoyao, JIANG Xulu, et al. Rational designed polymer as a metal-free catalyst for hydroxylation of benzene to phenol with dioxygen[J]. Catalysis Letters, 2021, 151(5): 1330-1335. |
| 41 | MUSTATA FANICA, BICU IOAN. Cyclohexanone-aniline-formaldehyde resins—Synthesis and characterization[J]. Polimery, 2002, 47(11/12): 817-821. |
| 42 | SHIMIZU Atsushi, TANAKA Katsutoshi, OGAWA Hiroo, et al. An industrial process for adipic acid production by the liquid-phase oxidation of cyclohexanone with molecular oxygen[J]. Bulletin of the Chemical Society of Japan, 2003, 76(10): 1993-2001. |
| 43 | WILSON R J, BEEZER A E, MITCHELL J C. A kinetic study of the oxidation of L-ascorbic acid (vitamin C) in solution using an isothermal microcalorimeter[J]. Thermochimica Acta, 1995, 264: 27-40. |
| 44 | GAO Jinhao, WANG Huan, CAO Xiaomei, et al. Nitrogen doped carbon solid acid for improving its catalytic transformation of xylose and agricultural biomass residues to furfural[J]. Molecular Catalysis, 2023, 535: 112890. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [10] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [11] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [14] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [15] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||