化工进展 ›› 2024, Vol. 43 ›› Issue (4): 2126-2134.DOI: 10.16085/j.issn.1000-6613.2023-0514
• 资源与环境化工 • 上一篇
镁渣基多孔材料的制备及其对废水中Pb2+的吸附性能
- 山西大学资源与环境工程研究所,国家环境保护煤炭废弃物资源化高效利用技术重点实验室,山西 太原 030006
-
收稿日期:2023-04-04修回日期:2023-05-04出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:马志斌 -
作者简介:路广军(1983—),男,博士,副教授,研究方向为固废资源化利用。E-mail:lgj275@sxu.edu.cn。 -
基金资助:国家自然科学基金面上项目(22078181)
Preparation of magnesium slag-based porous materials and their performance for Pb2+ adsorption in wastewater
LU Guangjun( ), HAN Jingang, CHEN Ying, MA Zhibin(
), HAN Jingang, CHEN Ying, MA Zhibin( )
)
- Institute of Resources and Environmental Engineering of Shanxi University, State Environmental Protection Key Laboratory on Efficient Resource-utilization Techniques of Coal Waste, Taiyuan 030006, Shanxi, China
-
Received:2023-04-04Revised:2023-05-04Online:2024-04-15Published:2024-05-13 -
Contact:MA Zhibin
摘要:
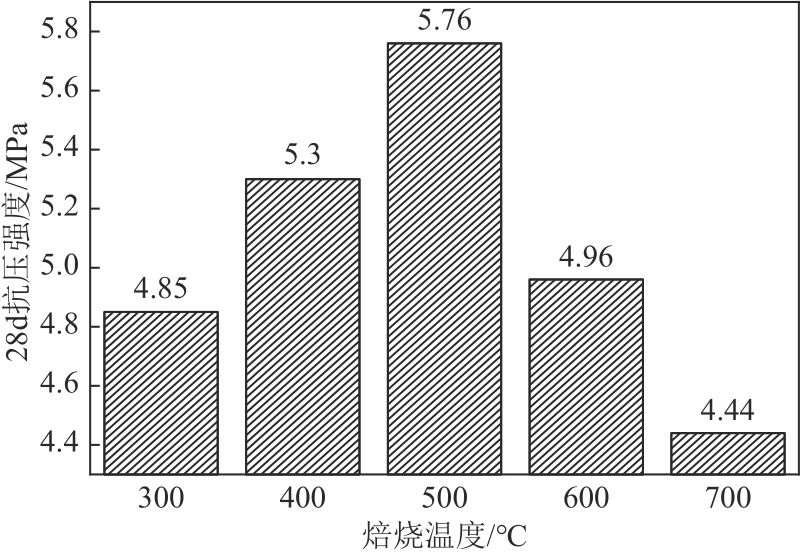
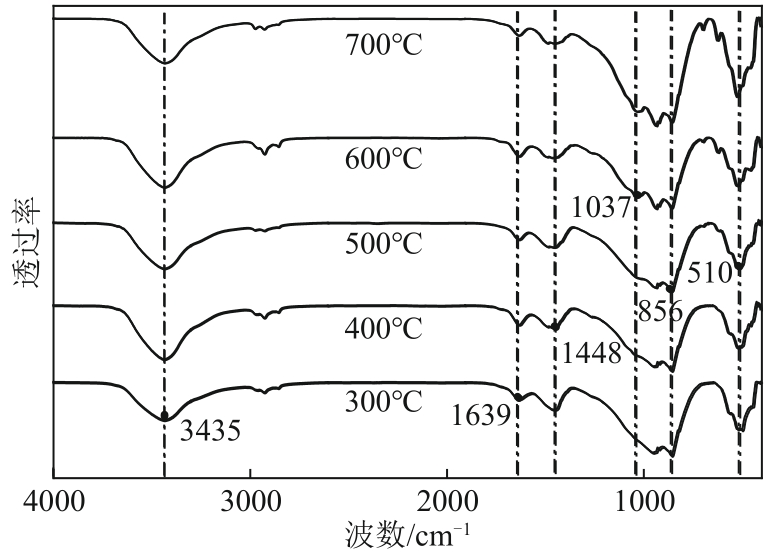
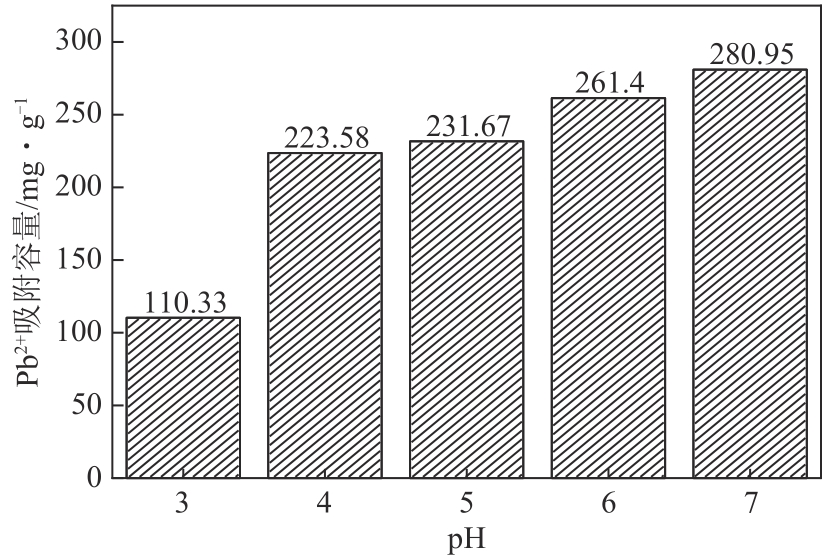
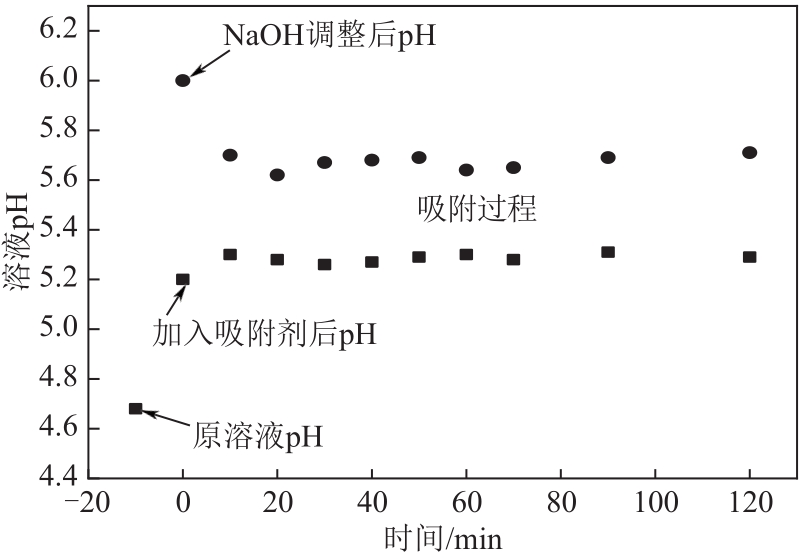
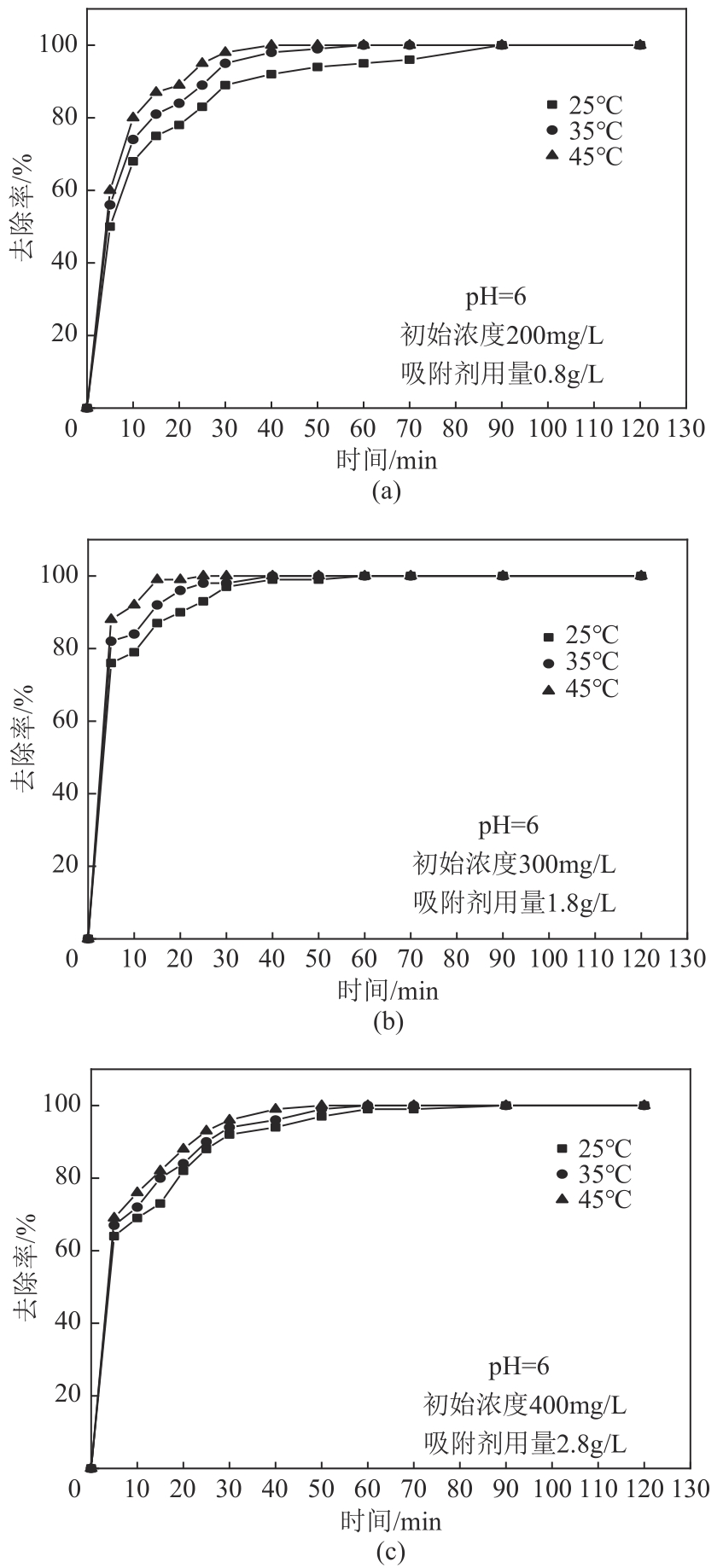
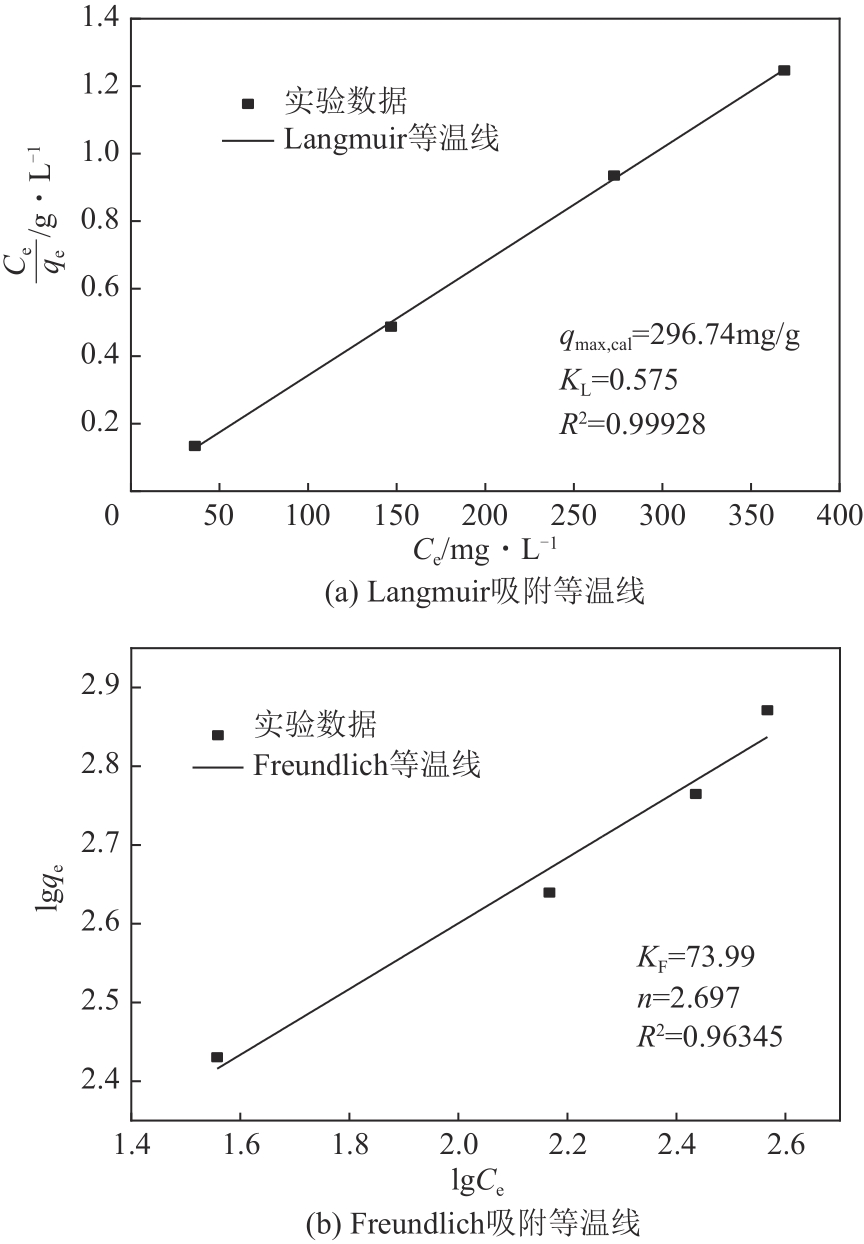
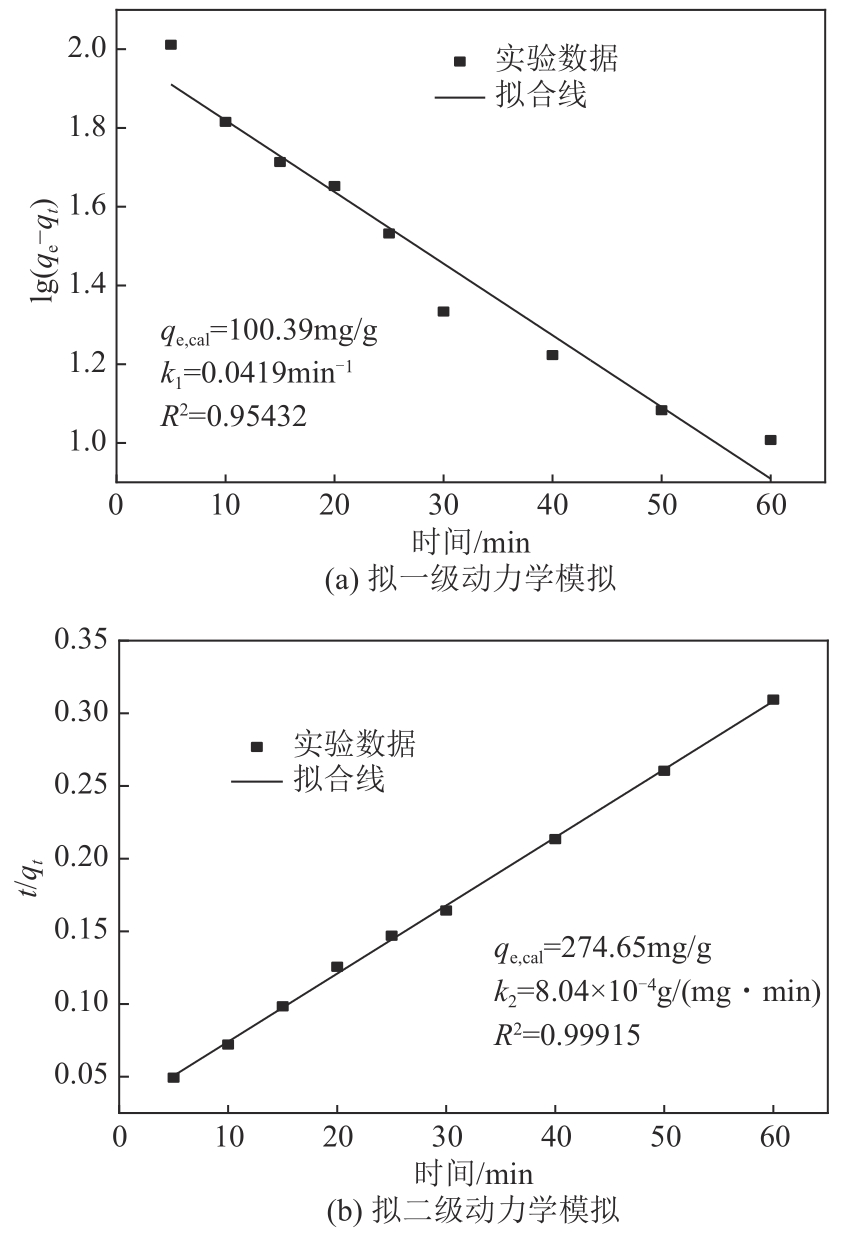
以镁渣为原料,通过添加复合激发剂与发泡剂制备镁渣基多孔材料(MSBPM)。首先,研究了水玻璃模数、碱掺量、发泡剂掺量和焙烧温度对MSBPM力学性能及吸附性能的影响,利用扫描电子显微镜对MSBPM的表观形貌和孔隙率进行了观察和分析。其次,研究了MSBPM对Pb2+的吸附性能,探究吸附剂投加量、溶液初始浓度、pH、吸附时间以及温度等参数对吸附性能的影响。结果表明:当发泡剂掺量为8%、碱掺量为10%、模数为1.3时,MSBPM对Pb2+的吸附性能最佳,饱和吸附量达到303.95mg/g,相较于原镁渣提高了27%,此时抗压强度为2.84MPa。当Pb2+初始浓度为500mg/L时,每升废水仅需3.4g吸附剂即可使Pb2+的去除率达到99%以上,吸附过程为单层化学吸附,符合拟二级动力学模型。
中图分类号:
引用本文
路广军, 韩晋钢, 陈英, 马志斌. 镁渣基多孔材料的制备及其对废水中Pb2+的吸附性能[J]. 化工进展, 2024, 43(4): 2126-2134.
LU Guangjun, HAN Jingang, CHEN Ying, MA Zhibin. Preparation of magnesium slag-based porous materials and their performance for Pb2+ adsorption in wastewater[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2126-2134.
| 配比 | 温度/℃ | 镁渣/g | 水玻璃/g | NaOH/g | 外加水/g | 发泡剂/g |
|---|---|---|---|---|---|---|
| 3%-10%-1.3 | 300 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 400 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 500 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 600 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 700 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
表1 MSBPM的制备条件
| 配比 | 温度/℃ | 镁渣/g | 水玻璃/g | NaOH/g | 外加水/g | 发泡剂/g |
|---|---|---|---|---|---|---|
| 3%-10%-1.3 | 300 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 400 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 500 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 600 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 3%-10%-1.3 | 700 | 150 | 72.9 | 11.7 | 26.9 | 4.5 |
| 考察条件 | pH | 初始浓度/mg·L-1 | 投加量/g·L-1 | 吸附时间/min | 吸附温度/℃ |
|---|---|---|---|---|---|
| 发泡剂掺量/碱掺量/模数 | 6 | 300 | 0.4 | 120 | 25 |
| pH | 3, 4, 5, 6, 7 | 500 | 0.4 | 120 | 25 |
| 初始浓度/投加量 | 6 | 200, 300, 400, 500 | 0.2, 0.4, 0.8, 1.2, 1.8, 2.0, 2.4, 2.8, 3.4, 4.0 | 120 | 25 |
| 吸附时间 | 6 | 200, 300, 400 | 0.8, 1.8, 2.8 | 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 90, 120 | 25, 35, 45 |
| 吸附等温曲线 | 6 | 200, 300, 400, 500 | 0.4 | 60 | 25 |
| 动力学 | 6 | 200 | 0.4 | 5, 10, 15, 20, 25,30, 40, 50, 60 | 25 |
表2 MSBPM对Pb2+吸附实验条件
| 考察条件 | pH | 初始浓度/mg·L-1 | 投加量/g·L-1 | 吸附时间/min | 吸附温度/℃ |
|---|---|---|---|---|---|
| 发泡剂掺量/碱掺量/模数 | 6 | 300 | 0.4 | 120 | 25 |
| pH | 3, 4, 5, 6, 7 | 500 | 0.4 | 120 | 25 |
| 初始浓度/投加量 | 6 | 200, 300, 400, 500 | 0.2, 0.4, 0.8, 1.2, 1.8, 2.0, 2.4, 2.8, 3.4, 4.0 | 120 | 25 |
| 吸附时间 | 6 | 200, 300, 400 | 0.8, 1.8, 2.8 | 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 90, 120 | 25, 35, 45 |
| 吸附等温曲线 | 6 | 200, 300, 400, 500 | 0.4 | 60 | 25 |
| 动力学 | 6 | 200 | 0.4 | 5, 10, 15, 20, 25,30, 40, 50, 60 | 25 |
| 焙烧温度/℃ | 质量分数/% | |||
|---|---|---|---|---|
| γ-C2S | β-C2S | MgO | 无定形组分 | |
| 300 | 43.7 | 9.2 | 2.4 | 44.7 |
| 400 | 41.5 | 8.5 | 2.5 | 47.5 |
| 500 | 38.5 | 7.7 | 1.8 | 51.9 |
| 600 | 33.8 | 18.8 | 1.7 | 45.7 |
| 700 | 27.8 | 15.4 | 2.7 | 54.2 |
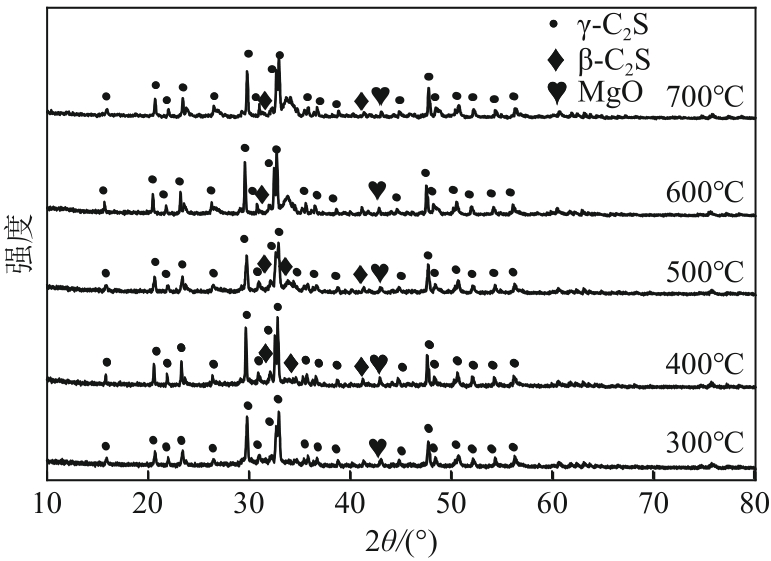
表3 不同焙烧温度下MSBPM的Rietveld定量分析结果
| 焙烧温度/℃ | 质量分数/% | |||
|---|---|---|---|---|
| γ-C2S | β-C2S | MgO | 无定形组分 | |
| 300 | 43.7 | 9.2 | 2.4 | 44.7 |
| 400 | 41.5 | 8.5 | 2.5 | 47.5 |
| 500 | 38.5 | 7.7 | 1.8 | 51.9 |
| 600 | 33.8 | 18.8 | 1.7 | 45.7 |
| 700 | 27.8 | 15.4 | 2.7 | 54.2 |
| 配比 | 镁渣/g | 水玻璃/g | NaOH/g | 外加水/g | 玉米芯粉末/g | 抗压 强度/MPa |
|---|---|---|---|---|---|---|
| 1%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 1.5 | 4.06 |
| 3%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 4.5 | 3.17 |
| 5%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 7.5 | 3.4 |
| 8%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 12 | 1.96 |
| 10%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 15 | 0.76 |
| 1%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 1.5 | 3.66 |
| 3%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 4.5 | 3.38 |
| 5%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 7.5 | 3.58 |
| 8%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 12 | 2.28 |
| 10%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 15 | 1.62 |
| 1%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 1.5 | 3.74 |
| 3%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 4.5 | 3.98 |
| 5%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 7.5 | 2.67 |
| 8%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 12 | 2.88 |
| 10%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 15 | 3.08 |
| 1%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 1.5 | 4.54 |
| 3%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 4.5 | 3.98 |
| 5%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 7.5 | 3.61 |
| 8%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 12 | 2.84 |
| 10%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 15 | 2.38 |
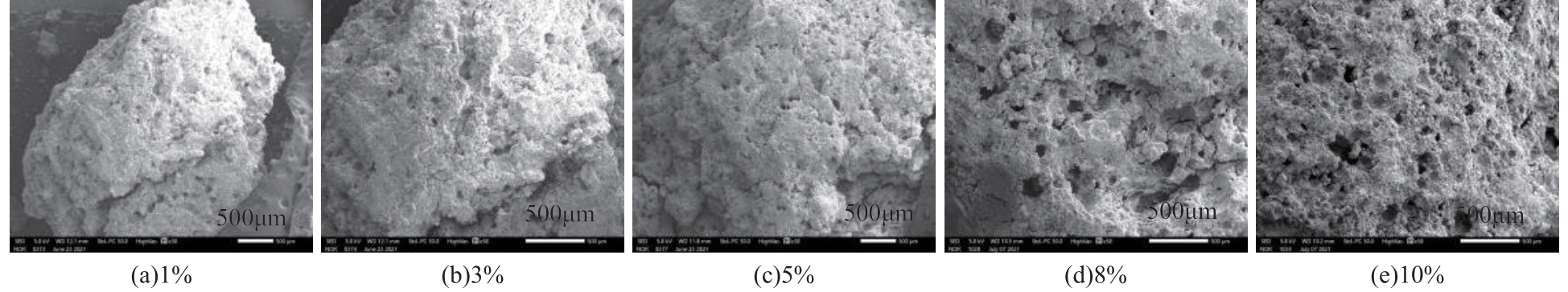
表4 MSBPM制备配比及试件28d抗压强度
| 配比 | 镁渣/g | 水玻璃/g | NaOH/g | 外加水/g | 玉米芯粉末/g | 抗压 强度/MPa |
|---|---|---|---|---|---|---|
| 1%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 1.5 | 4.06 |
| 3%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 4.5 | 3.17 |
| 5%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 7.5 | 3.4 |
| 8%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 12 | 1.96 |
| 10%-8%-1.0 | 150 | 44.8 | 10.8 | 45.4 | 15 | 0.76 |
| 1%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 1.5 | 3.66 |
| 3%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 4.5 | 3.38 |
| 5%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 7.5 | 3.58 |
| 8%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 12 | 2.28 |
| 10%-8%-1.3 | 150 | 58.3 | 9.4 | 36.5 | 15 | 1.62 |
| 1%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 1.5 | 3.74 |
| 3%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 4.5 | 3.98 |
| 5%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 7.5 | 2.67 |
| 8%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 12 | 2.88 |
| 10%-10%-1.0 | 150 | 56 | 13.5 | 38.04 | 15 | 3.08 |
| 1%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 1.5 | 4.54 |
| 3%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 4.5 | 3.98 |
| 5%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 7.5 | 3.61 |
| 8%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 12 | 2.84 |
| 10%-10%-1.3 | 150 | 72.9 | 11.7 | 26.9 | 15 | 2.38 |
| 发泡类型 | 配比 | 抗压强度/MPa | 吸附量/mg·g-1 |
|---|---|---|---|
| 发泡剂发泡 | 原镁渣 | 0.2 | 239.1 |
| 1%-8%-1.0 | 4.06 | 28.13 | |
| 3%-8%-1.0 | 3.17 | 49.22 | |
| 5%-8%-1.0 | 3.4 | 66.91 | |
| 8%-8%-1.0 | 1.96 | 89.15 | |
| 10%-8%-1.0 | 0.76 | 104.66 | |
| 1%-8%-1.3 | 3.66 | 34.79 | |
| 3%-8%-1.3 | 3.38 | 45.87 | |
| 5%-8%-1.3 | 3.58 | 33.8 | |
| 8%-8%-1.3 | 2.28 | 21.53 | |
| 10%-8%-1.3 | 1.62 | 24.42 | |
| 1%-10%-1.0 | 3.74 | 229.82 | |
| 3%-10%-1.0 | 3.98 | 122.06 | |
| 5%-10%-1.0 | 2.67 | 115.88 | |
| 8%-10%-1.0 | 2.88 | 137 | |
| 10%-10%-1.0 | 3.08 | 87.68 | |
| 1%-10%-1.3 | 4.54 | 208.21 | |
| 3%-10%-1.3 | 3.98 | 278.77 | |
| 5%-10%-1.3 | 3.61 | 298.65 | |
| 8%-10%-1.3 | 2.84 | 303.95 | |
| 10%-10%-1.3 | 2.38 | 236.36 |
表5 MSBPM对Pb2+的吸附性能
| 发泡类型 | 配比 | 抗压强度/MPa | 吸附量/mg·g-1 |
|---|---|---|---|
| 发泡剂发泡 | 原镁渣 | 0.2 | 239.1 |
| 1%-8%-1.0 | 4.06 | 28.13 | |
| 3%-8%-1.0 | 3.17 | 49.22 | |
| 5%-8%-1.0 | 3.4 | 66.91 | |
| 8%-8%-1.0 | 1.96 | 89.15 | |
| 10%-8%-1.0 | 0.76 | 104.66 | |
| 1%-8%-1.3 | 3.66 | 34.79 | |
| 3%-8%-1.3 | 3.38 | 45.87 | |
| 5%-8%-1.3 | 3.58 | 33.8 | |
| 8%-8%-1.3 | 2.28 | 21.53 | |
| 10%-8%-1.3 | 1.62 | 24.42 | |
| 1%-10%-1.0 | 3.74 | 229.82 | |
| 3%-10%-1.0 | 3.98 | 122.06 | |
| 5%-10%-1.0 | 2.67 | 115.88 | |
| 8%-10%-1.0 | 2.88 | 137 | |
| 10%-10%-1.0 | 3.08 | 87.68 | |
| 1%-10%-1.3 | 4.54 | 208.21 | |
| 3%-10%-1.3 | 3.98 | 278.77 | |
| 5%-10%-1.3 | 3.61 | 298.65 | |
| 8%-10%-1.3 | 2.84 | 303.95 | |
| 10%-10%-1.3 | 2.38 | 236.36 |
| 1 | 于栋, 罗庆, 苏伟, 等. 重金属废水电沉积处理技术研究及应用进展[J]. 化工进展, 2020, 39(5): 1938-1949. |
| YU Dong, LUO Qing, SU Wei, et al. A review on research and application of electrodeposition for heavy metal wastewater treatment[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 1938-1949. | |
| 2 | 周丽莎, 李若男, 卞雨洁, 等. TOCNF与磁性羧甲基壳聚糖纳米粒子复合物的制备及吸附Pb2+的特性[J]. 化工进展, 2022, 41(2): 901-910. |
| ZHOU Lisha, LI Ruonan, BIAN Yujie, et al. Preparation of TOCNF and magnetic carboxymethyl chitosan nanoparticles composite and adsorption properties of Pb2+ [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 901-910. | |
| 3 | YE Maoyou, LI Guojian, YAN Pingfang, et al. Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation[J]. Chemosphere, 2017, 185: 1189-1196. |
| 4 | WEN Zhipan, ZHANG Yalei, GUO Sheng, et al. Facile template-free fabrication of iron manganese bimetal oxides nanospheres with excellent capability for heavy metals removal[J]. Journal of Colloid and Interface Science, 2017, 486: 211-218. |
| 5 | ZHU Xianzheng, HUO Guangsheng, NI Jie, et al. Removal of tungsten and vanadium from molybdate solutions using ion exchange resin[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(12): 2727-2732. |
| 6 | FU Yunfeng, XIAO Qinggui, GAO Yiying, et al. Direct extraction of Mo(Ⅵ) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(8): 1660-1669. |
| 7 | GHERASIM Cristina-Veronica, Petr MIKULÁŠEK. Influence of operating variables on the removal of heavy metal ions from aqueous solutions by nanofiltration[J]. Desalination, 2014, 343: 67-74. |
| 8 | MALAMIS Simos, KATSOU Evina, TAKOPOULOS Konstantinos, et al. Assessment of metal removal, biomass activity and RO concentrate treatment in an MBR-RO system[J]. Journal of Hazardous Materials, 2012, 209/210: 1-8. |
| 9 | MEHTA Dhruv, MAZUMDAR Siddharth, SINGH S K. Magnetic adsorbents for the treatment of water/wastewater—a review[J]. Journal of Water Process Engineering, 2015, 7: 244-265. |
| 10 | XU Chenglong, FENG Yali, LI Haoran, et al. Adsorption of heavy metal ions by iron tailings: Behavior, mechanism, evaluation and new perspectives[J]. Journal of Cleaner Production, 2022, 344: 131065. |
| 11 | 李宪军, 张树元, 王芳芳. 镁渣废弃物再利用的研究综述[J]. 混凝土, 2011(8): 97-100, 124. |
| LI Xianjun, ZHANG Shuyuan, WANG Fangfang. Review on the recycle of magnesium slag wastes[J]. Concrete, 2011(8): 97-100, 124. | |
| 12 | 崔自治, 倪晓, 孟秀莉. 镁渣膨胀性机理试验研究[J]. 粉煤灰综合利用, 2006, 19(6): 8-11. |
| CUI Zizhi, NI Xiao, MENG Xiuli. Study on the expansibility of magnesium slag[J]. Fly Ash Comprehensive Utilization, 2006, 19(6): 8-11. | |
| 13 | 孙浩, 马志斌, 路广军, 等. 粉煤灰碱激发制备地质聚合物研究进展[J]. 洁净煤技术, 2023, 29(11): 140-153. |
| SUN Hao, MA Zhibin, LU Guangjun, et al. A review on geopolymer preparation by alkali activation of coal fly ash[J]. Clean Coal Technology, 2023, 29(11): 140-153. | |
| 14 | WEI Yufeng, WANG Jin, WANG Junxia, et al. Hydrothermal processing, characterization and leaching toxicity of Cr-added “fly ash-metakaolin” based geopolymer[J]. Construction and Building Materials, 2020, 251: 118931. |
| 15 | 韦尔娜. 不同地聚物沸石(NaA/NaX/SOD)微球的制备和转化机制及其对Pb2+吸附性能的研究[D]. 南宁: 广西大学, 2022. |
| WEI Erna. Preparation and transformation mechanism of different geopolymer zeolite (NaA/NaX/SOD) microspheres and their adsorption properties for Pb2+ [D]. Nanning: Guangxi University, 2022. | |
| 16 | KHALID Hammad R, LEE N K, PARK S M, et al. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential[J]. Journal of Hazardous Materials, 2018, 353: 522-533. |
| 17 | 苏俏俏. 地聚物基吸附剂与催化剂载体微球回收利用重金属的研究[D]. 南宁: 广西大学, 2020. |
| SU Qiaoqiao. Study on recovery and utilization of heavy metals by geopolymer-based adsorbent and catalyst carrier microspheres[D]. Nanning: Guangxi University, 2020. | |
| 18 | CHEN Fan, WANG Kaituo, SHAO Lin, et al. Synthesis of Fe2O3-modified porous geopolymer microspheres for highly selective adsorption and solidification of F- from waste-water[J]. Composites Part B: Engineering, 2019, 178: 107497. |
| 19 | Piotr ROŻEK, Magdalena KRÓL, Włodzimierz MOZGAWA. Lightweight geopolymer-expanded glass composites for removal of methylene blue from aqueous solutions[J]. Ceramics International, 2020, 46(12): 19785-19791. |
| 20 | 李灵. 发泡剂在泡沫混凝土中的应用研究[J]. 工程技术研究, 2022, 7(18): 74-76. |
| LI Ling. Study on application of foaming agent in foamed concrete[J]. Engineering and Technological Research, 2022, 7(18): 74-76. | |
| 21 | HAO Xixun, XU Feng, ZHANG Junhua. Effect of pretreatments on production of xylooligosaccharides and monosaccharides from corncob by a two-step hydrolysis[J]. Carbohydrate Polymers, 2022, 285: 119217. |
| 22 | 路广军, 韩晋钢, 马志斌. 碱激发镁渣胶凝特性实验设计[J]. 实验技术与管理, 2022, 39(9): 31-36, 47. |
| LU Guangjun, HAN Jingang, MA Zhibin. Experimental design of gelation characteristics of alkali-activated magnesium slag[J]. Experimental Technology and Management, 2022, 39(9): 31-36, 47. | |
| 23 | 赵耀. 秸秆灰-水泥基复合材料性能的研究[D]. 济南: 济南大学, 2021. |
| ZHAO Yao. Study on properties of straw ash-cement based composites[D]. Jinan: University of Jinan, 2021. | |
| 24 | 刘勇. 生物质灰对水泥基复合胶凝材料水化硬化性能的影响研究[D]. 泰安: 山东农业大学, 2017. |
| LIU Yong. Study on the influence of biomass ash on the hydration and hardening properties of cement-based composite cementitious materials[D]. Taian: Shandong Agricultural University, 2017. | |
| 25 | GE Qilong, MOEEN Muhammad, TIAN Qi, et al. Highly effective removal of Pb2+ in aqueous solution by Na-X zeolite derived from coal gangue[J]. Environmental Science and Pollution Research, 2020, 27(7): 7398-7408. |
| 26 | BU Naijing, LIU Xiaomeng, SONG Shaolei, et al. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution[J]. Advanced Powder Technology, 2020, 31(7): 2699-2710. |
| 27 | HE Xinping, YAO Bing, XIA Yang, et al. Coal fly ash derived zeolite for highly efficient removal of Ni2+ inwaste water[J]. Powder Technology, 2020, 367: 40-46. |
| 28 | LIU Yong, WANG Guodong, WANG Lu, et al. Zeolite P synthesis based on fly ash and its removal of Cu(II) and Ni(II) ions[J]. Chinese Journal of Chemical Engineering, 2019, 27(2): 341-348. |
| 29 | ZHOU Chunyu, GAO Qiang, LUO Wenjun, et al. Preparation, characterization and adsorption evaluation of spherical mesoporous Al-MCM-41 from coal fly ash[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 52: 147-157. |
| 30 | WANG Shaobin, SOUDI Mehdi, LI Li, et al. Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater[J]. Journal of Hazardous Materials, 2006, 133(1/2/3): 243-251. |
| 31 | 宋学锋, 陆伟宁. 转化方式对粉煤灰地聚物原位转化沸石及其Pb2+吸附性能的影响[J]. 材料导报, 2023, 37(6): 236-242. |
| SONG Xuefeng, LU Weining. Influence of conversion method on In-situ conversion of fly ash geopolymer to zeolite and its Pb2+ adsorption performance[J]. Materials Reports, 2023, 37(6): 236-242. | |
| 32 | HUANG Xunrong, ZHAO Hanghang, HU Xiongfei, et al. Optimization of preparation technology for modified coal fly ash and its adsorption properties for Cd2+ [J]. Journal of Hazardous Materials, 2020, 392: 122461. |
| 33 | FALAH Mahroo, MACKENZIE Kenneth J D. Synthesis and properties of novel photoactive composites of P25 titanium dioxide and copper (I) oxide with inorganic polymers[J]. Ceramics International, 2015, 41(10): 13702-13708. |
| [1] | 金彬浩, 朱小倩, 柯天, 张治国, 鲍宗必, 任其龙, 苏宝根, 杨启炜. 芳香烃/环烷烃吸附分离材料研究进展[J]. 化工进展, 2024, 43(4): 1863-1881. |
| [2] | 何兰, 高助威, 亓欣雨, 李成欣, 王世豪, 刘钟馨. 三聚氰胺海绵疏水改性及在油水分离领域的研究进展[J]. 化工进展, 2024, 43(2): 984-1000. |
| [3] | 陈森, 殷鹏远, 杨证禄, 莫一鸣, 崔希利, 锁显, 邢华斌. 功能固体材料智能合成研究进展[J]. 化工进展, 2023, 42(7): 3340-3348. |
| [4] | 任建鹏, 吴彩文, 刘慧君, 吴文娟. 木质素-聚苯胺复合材料的制备及对刚果红的吸附[J]. 化工进展, 2023, 42(6): 3087-3096. |
| [5] | 毛梦雷, 孟令玎, 高蕊, 孟子晖, 刘文芳. 多孔框架材料固定化酶研究进展[J]. 化工进展, 2023, 42(5): 2516-2535. |
| [6] | 孔祥如, 张肖阳, 孙鹏翔, 崔琳, 董勇. 直接空气捕碳固体多孔材料的研究进展[J]. 化工进展, 2023, 42(3): 1471-1483. |
| [7] | 金鑫, 李玉姗, 解青青, 王梦雨, 夏星帆, 杨朝合. 多孔材料催化丙酮缩甘油合成研究进展[J]. 化工进展, 2023, 42(2): 731-743. |
| [8] | 郭丽珍, 林详宇, 董阜豪, 王倬敏, 刘鹤. 多孔高硫聚合物的制备及其在汞吸附中的应用[J]. 化工进展, 2023, 42(11): 5764-5775. |
| [9] | 王书燕, 张新波, 彭安萍, 刘阳, NGO HUU HAO, 郭文珊, 温海涛. 生物炭回收水中氮磷营养物质的研究进展与挑战[J]. 化工进展, 2023, 42(10): 5459-5469. |
| [10] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [11] | 刘战剑, 杨金月, 景境, 张曦光, 汪怀远. 三维超浸润多孔材料在油水分离中的研究进展[J]. 化工进展, 2023, 42(1): 310-320. |
| [12] | 孔祥宇, 谢亮, 王延民, 翟尚鹏, 王建国. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| [13] | 梁格, 黄翔峰, 刘婉琪, 熊永娇, 彭开铭. 超疏水三维多孔材料在乳化液油水分离中的应用研究进展[J]. 化工进展, 2022, 41(12): 6557-6572. |
| [14] | 王文霞, 刘小丰, 陈浠, 许艳虹, 蒙振邦, 郑俊霞, 安太成. 多孔g-C3N4基光催化材料的制备及应用研究进展[J]. 化工进展, 2022, 41(1): 300-309. |
| [15] | 陈琦, 王文涛, 张志鹏, 晏太红. 共价有机框架材料对放射性核素吸附的研究进展[J]. 化工进展, 2021, 40(S2): 241-255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||