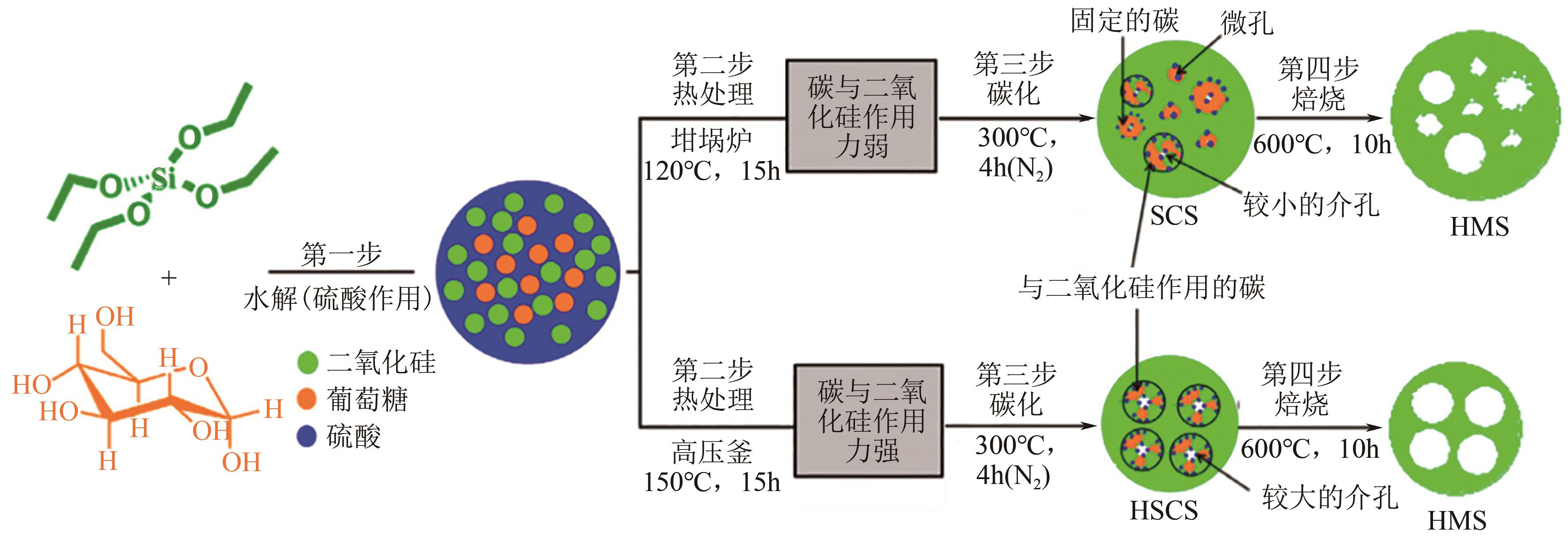
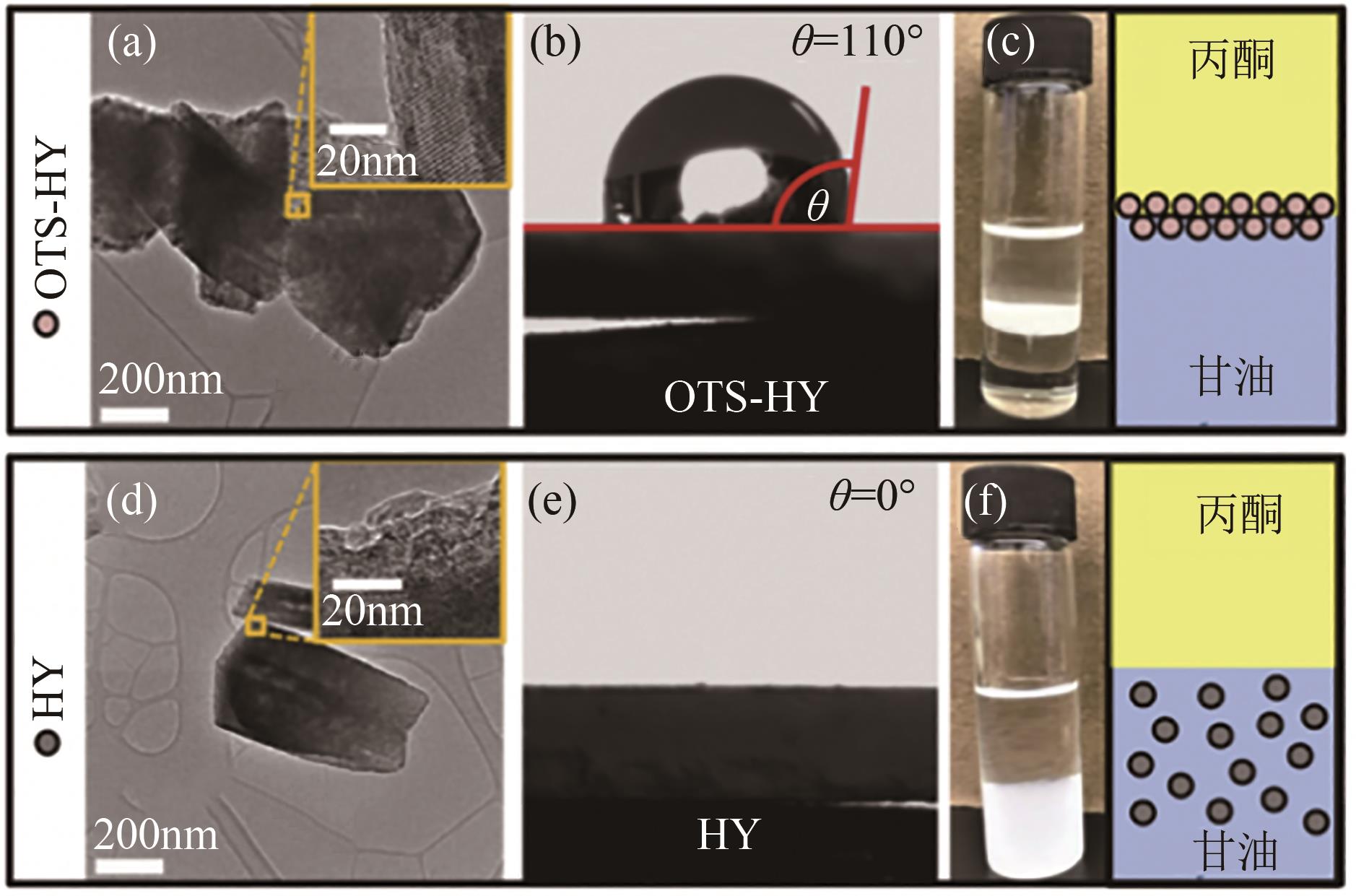
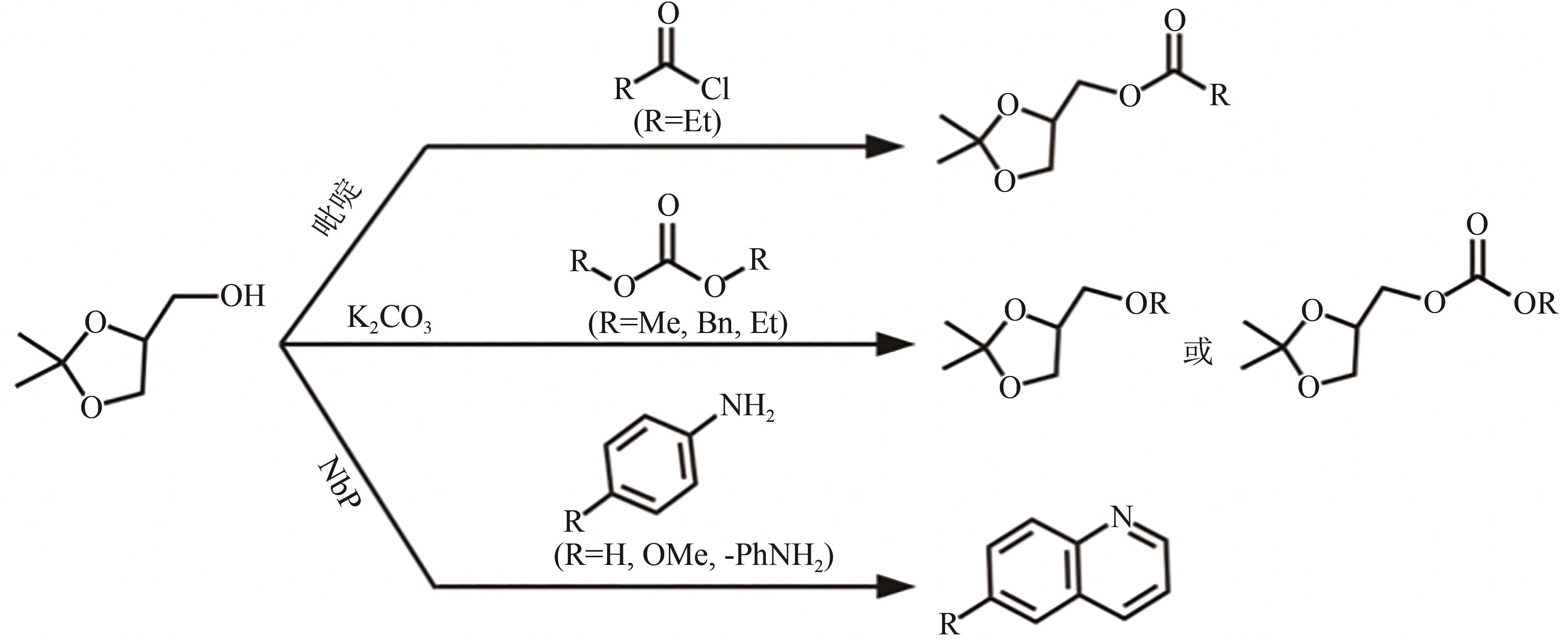
化工进展 ›› 2023, Vol. 42 ›› Issue (2): 731-743.DOI: 10.16085/j.issn.1000-6613.2022-0759
多孔材料催化丙酮缩甘油合成研究进展
金鑫1( ), 李玉姗1, 解青青1, 王梦雨1, 夏星帆2, 杨朝合1
), 李玉姗1, 解青青1, 王梦雨1, 夏星帆2, 杨朝合1
- 1.中国石油大学(华东)化学化工学院,山东 青岛 266580
2.中国石油大学(华东)石油工业训练中心,山东 青岛 266580
-
收稿日期:2022-04-26修回日期:2022-06-10出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:金鑫 -
作者简介:金鑫(1984—),男,教授,研究方向为生物基高端化学品的合成机理以及催化剂设计和相关工艺技术的开发。E-mail:jamesjinxin@upc.edu.cn。 -
基金资助:国家自然科学基金(22078365)
Progress on solketal synthesis catalyzed by porous materials
JIN Xin1( ), LI Yushan1, XIE Qingqing1, WANG Mengyu1, XIA Xingfan2, YANG Chaohe1
), LI Yushan1, XIE Qingqing1, WANG Mengyu1, XIA Xingfan2, YANG Chaohe1
- 1.College of Chemistry and Chemical Engineering, China University of Petroleum, Qingdao 266580, Shandong, China
2.Petroleum Engineering Experimental Teaching Center, China University of Petroleum, Qingdao 266580, Shandong, China
-
Received:2022-04-26Revised:2022-06-10Online:2023-02-25Published:2023-03-13 -
Contact:JIN Xin
摘要:
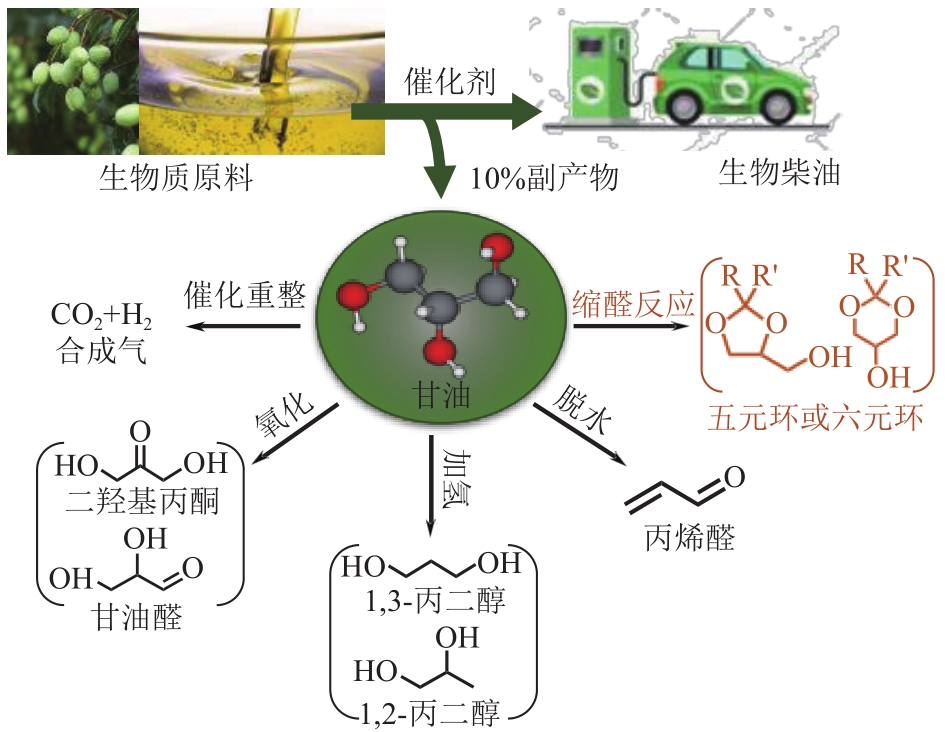
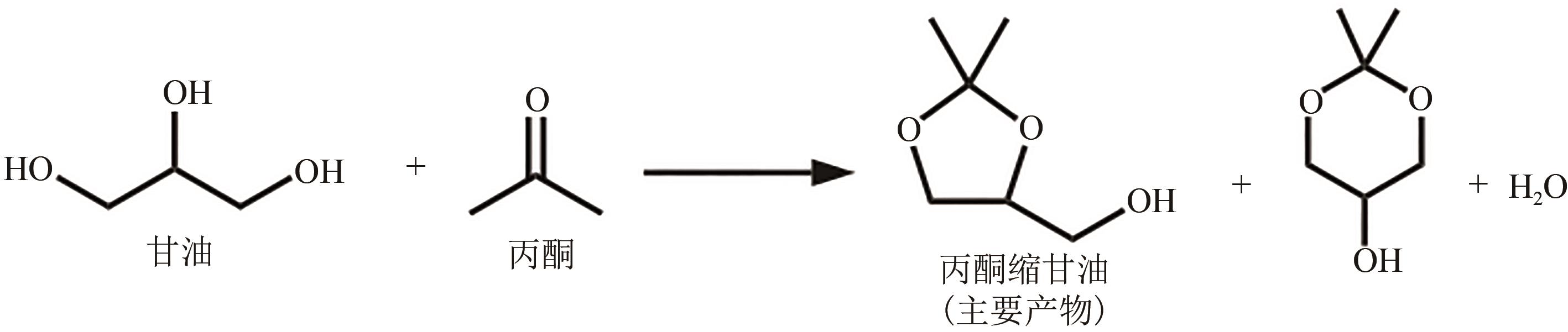
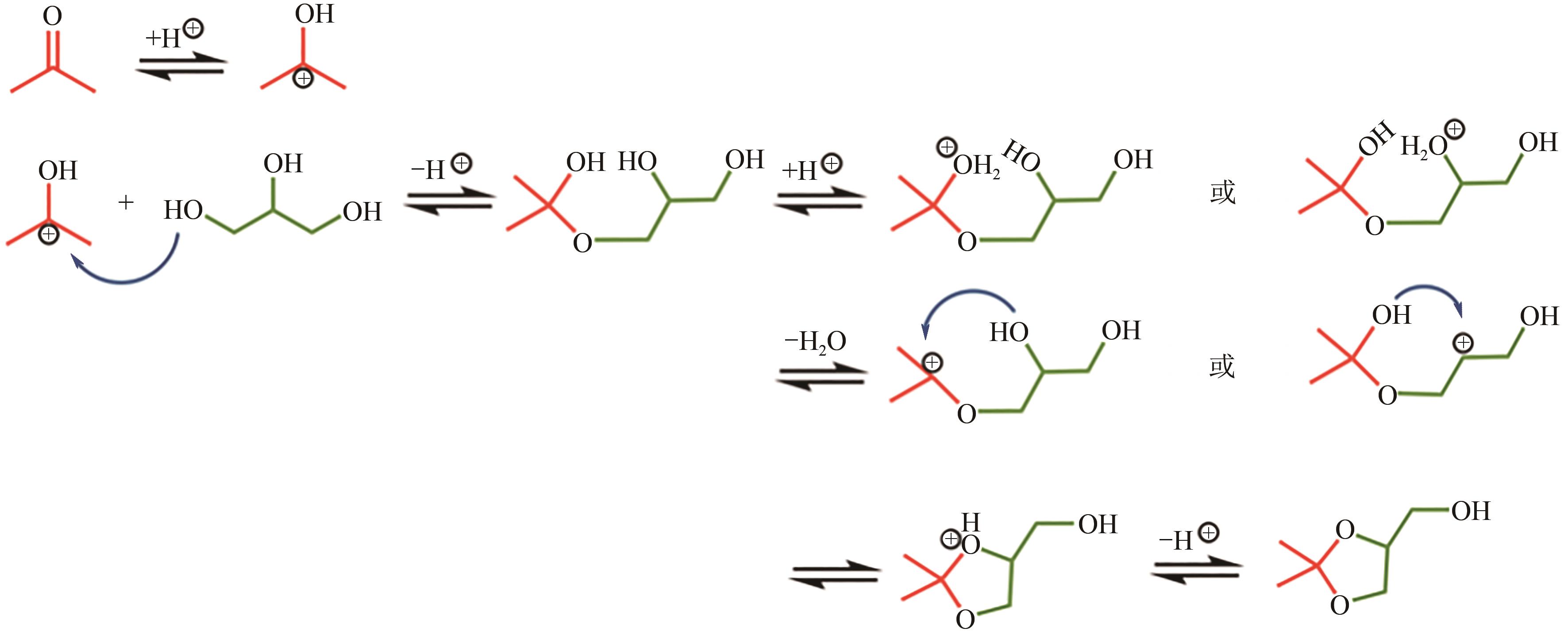
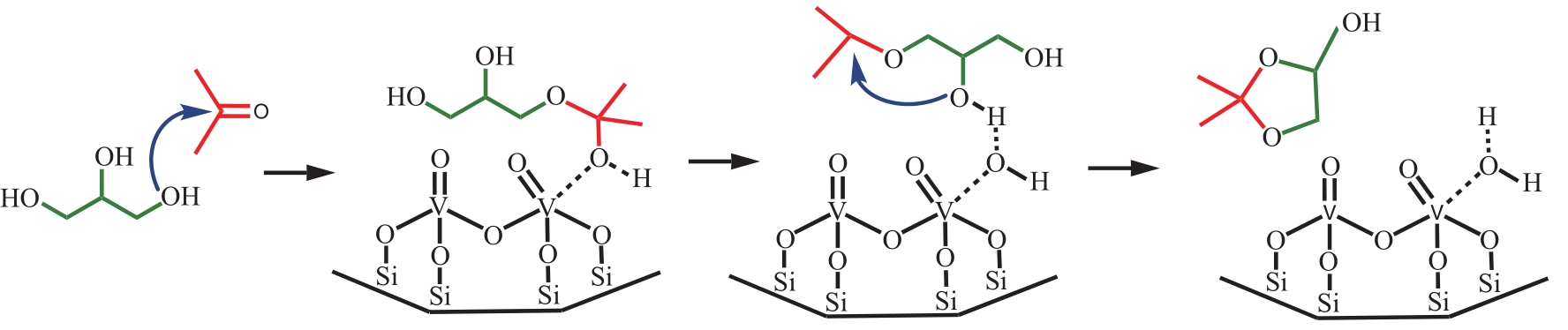
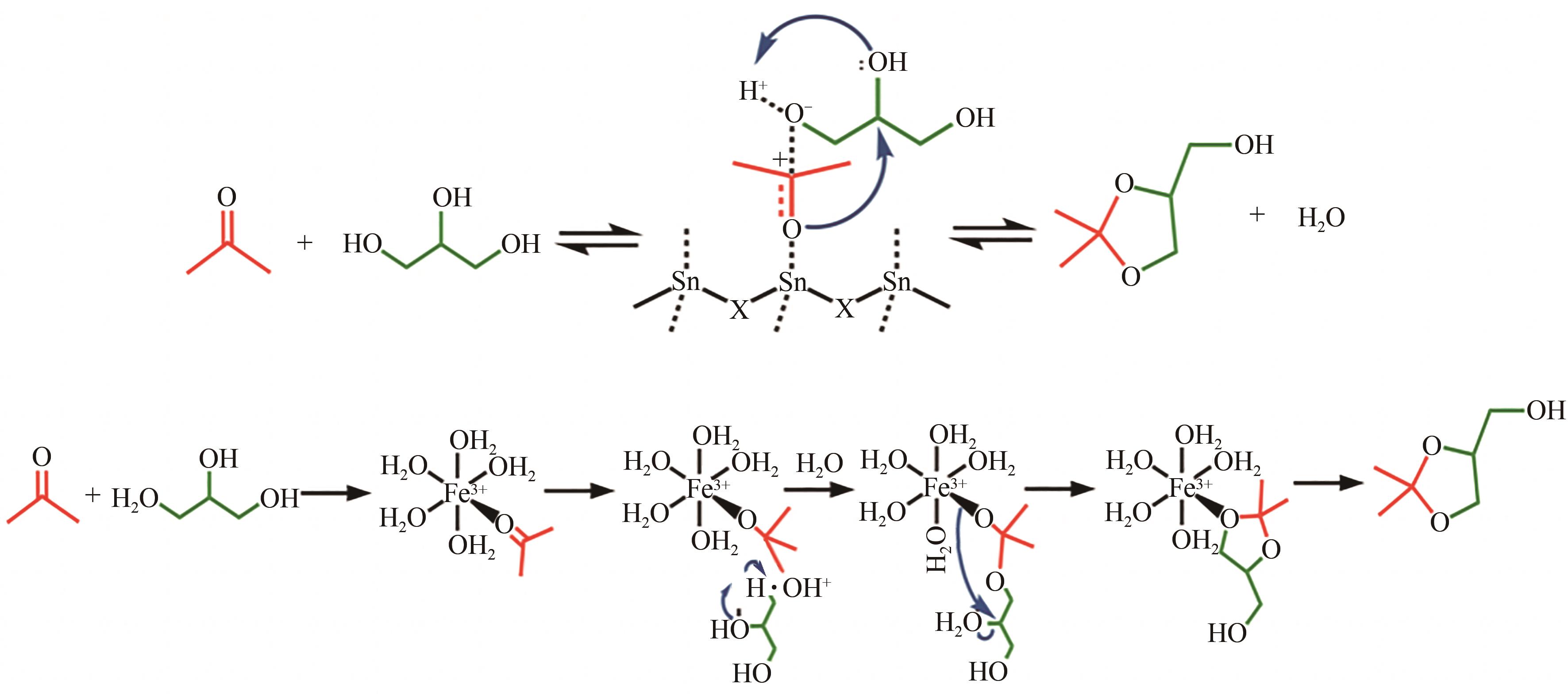
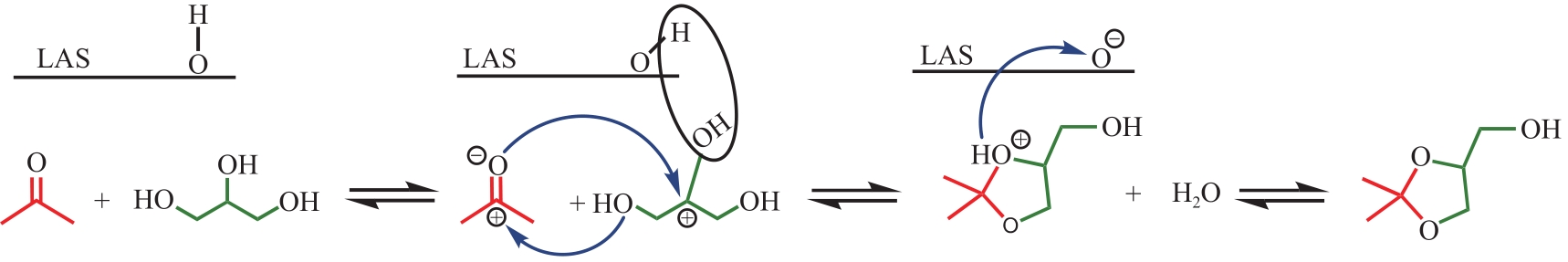
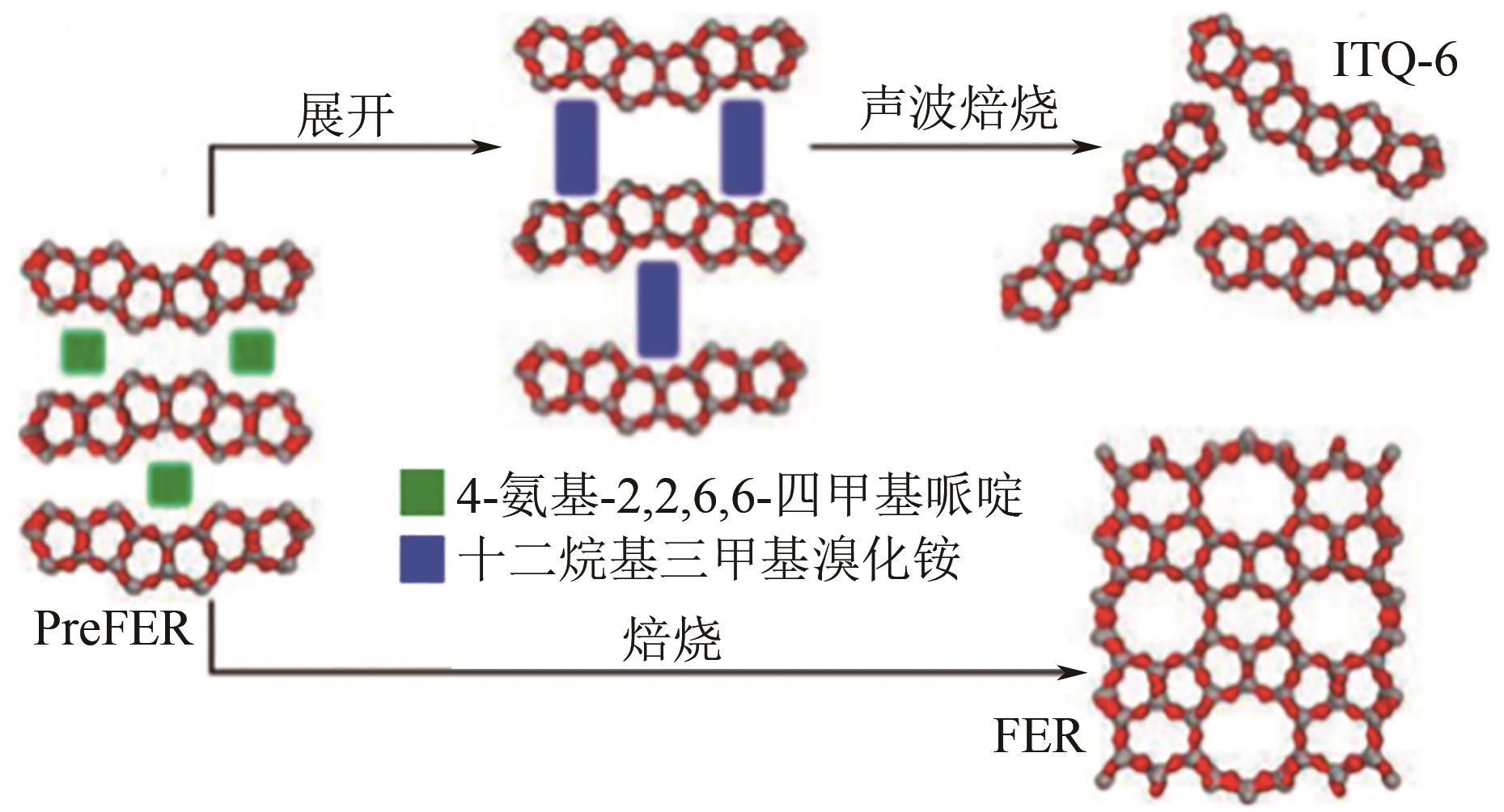
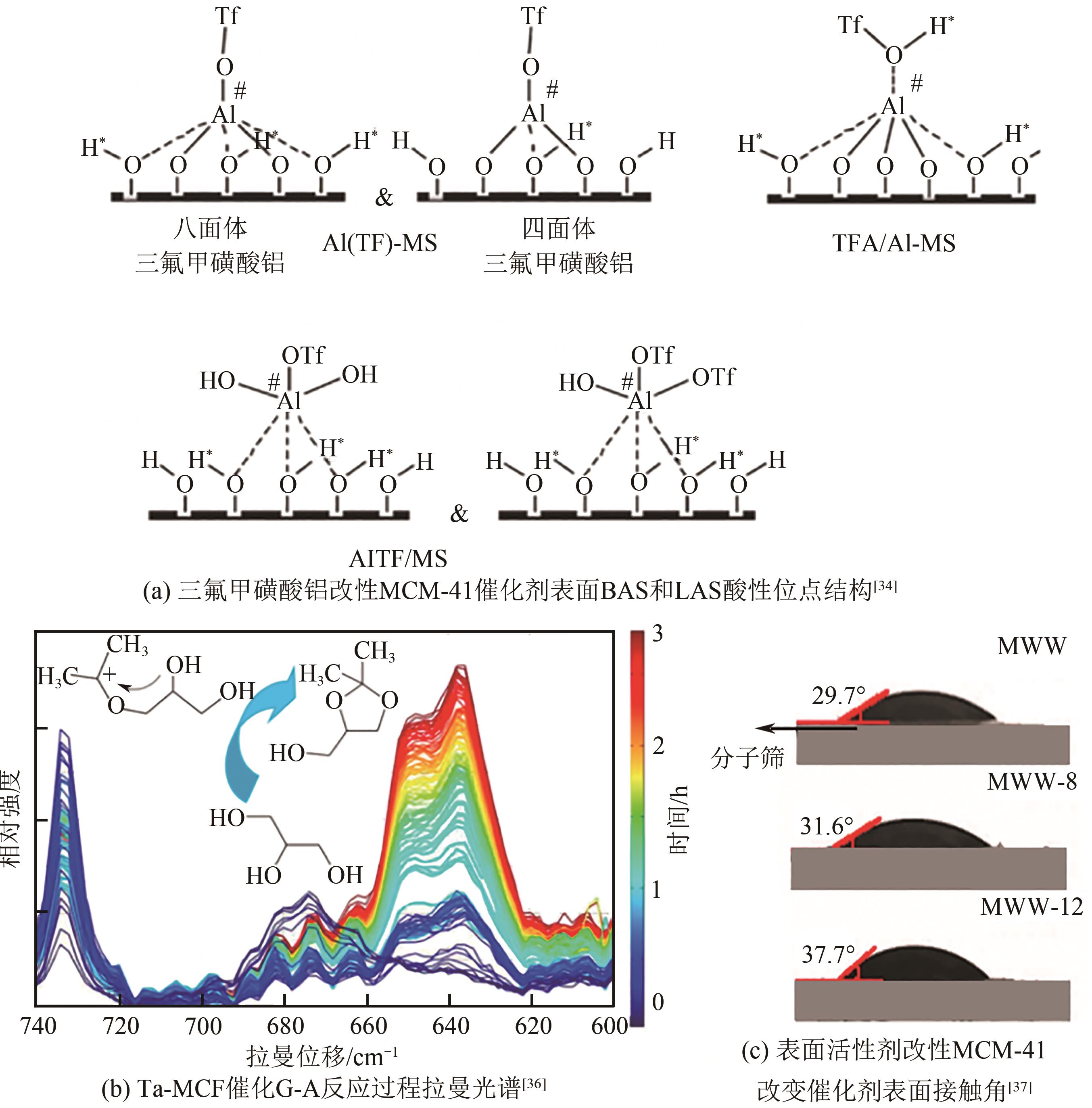
丙酮缩甘油是生物柴油行业新兴的高附加值衍生物,可以用作高性能增塑剂和汽柴油增强剂,具有低毒和可降解等诸多优点。如何高效实现丙酮缩甘油的高效和绿色合成一直是本行业需要解决的重大关键难题。本文从反应合成机理和多孔材料催化剂表面理化性质调控两个角度,详细分析了丙酮缩甘油反应过程机理和催化剂构效关系研究进展,生成丙酮缩甘油的反应机理主要包括BAS、LAS、BAS-LAS协同和酸碱协同催化反应机理,催化剂性能调控方法主要为负载金属氧化物、酸/盐改性和表面活性剂处理。未来该领域仍须在抑制催化剂水合极化失活方面进一步研究,提高催化剂抗盐抗杂性能,为未来丙酮缩甘油及其衍生物的低碳绿色合成工艺开发提供思路。
中图分类号:
引用本文
金鑫, 李玉姗, 解青青, 王梦雨, 夏星帆, 杨朝合. 多孔材料催化丙酮缩甘油合成研究进展[J]. 化工进展, 2023, 42(2): 731-743.
JIN Xin, LI Yushan, XIE Qingqing, WANG Mengyu, XIA Xingfan, YANG Chaohe. Progress on solketal synthesis catalyzed by porous materials[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 731-743.
| 反应温度(Si/Al=90)/℃ | 初始反应速率/Con1/2·min-1 | 反应级数 | Si/Al比(25℃下) | 初始反应速率/Con1/2·min-1 | 反应级数 |
|---|---|---|---|---|---|
| 25 | 0.7415 | 1/2 | 30 | 0.6320 | 1/2 |
| 50 | 4.436 | -2.5 | 90 | 0.7415 | 1/2 |
| 60 | 1.3256 | -1.3 | 160 | 0.6500 | 1/2 |
表1 温度和Si/Al影响动力学参数[17]
| 反应温度(Si/Al=90)/℃ | 初始反应速率/Con1/2·min-1 | 反应级数 | Si/Al比(25℃下) | 初始反应速率/Con1/2·min-1 | 反应级数 |
|---|---|---|---|---|---|
| 25 | 0.7415 | 1/2 | 30 | 0.6320 | 1/2 |
| 50 | 4.436 | -2.5 | 90 | 0.7415 | 1/2 |
| 60 | 1.3256 | -1.3 | 160 | 0.6500 | 1/2 |
| 分子筛 | 初始反应速率/Con1/2·min-1 | 反应级数 |
|---|---|---|
| MCM-41 | 0.5766 | 0.1 |
| 磺化MCM-41 | 3.9 | -1 |
表2 磺化处理MCM-41影响动力学参数[17]
| 分子筛 | 初始反应速率/Con1/2·min-1 | 反应级数 |
|---|---|---|
| MCM-41 | 0.5766 | 0.1 |
| 磺化MCM-41 | 3.9 | -1 |
| PL速率方程 | ER速率方程 | LHHW速率方程 |
|---|---|---|
| PL1 | ER1 | LHHW1 |
| PL2 | ER2 | LHHW2 |
| PL3 | ER3 | LHHW3 |
| PL4 | ER4 | LHHW4 |
表3 动力学模型一览表[28]
| PL速率方程 | ER速率方程 | LHHW速率方程 |
|---|---|---|
| PL1 | ER1 | LHHW1 |
| PL2 | ER2 | LHHW2 |
| PL3 | ER3 | LHHW3 |
| PL4 | ER4 | LHHW4 |
| 催化剂 | 温度/℃ | 溶剂 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|
| OTS-HY | 30 | — | 89 | 95 | [ |
| HUSY-W20 | 40 | — | 100 | 97.87 | [ |
| Sil-F | 70 | — | 65.8 | 63.9 | [ |
| 5%V-Si-ITQ-6 | 60 | — | 100 | 99 | [ |
| HUSY-Nb5 | 40 | — | 66 | 98 | [ |
| MIL-100-V | 70 | 5mL乙腈 | 85.4 | 97.7 | [ |
| MIL-100-Al | 70 | 5mL乙腈 | 44.2 | 94.3 | [ |
| MIL-100-Fe | 70 | 5mL乙腈 | 19.5 | 86.1 | [ |
| MIL-100-Cr | 70 | 5mL乙腈 | 4.2 | 77.5 | [ |
| Cu-Mor | RT | — | 95 | 98 | [ |
| Zn-Mor | RT | — | 90 | 81 | [ |
| Ni-Mor | RT | — | 93 | 88 | [ |
| Co-Mor | RT | — | 87 | 73 | [ |
| Fe-Mor | RT | — | 88 | 84 | [ |
| MFI-CA | 70 | — | 81 | 99 | [ |
| BEA-CA | 70 | — | 82 | 99 | [ |
| Mor-CA | 70 | — | 79 | 98 | [ |
| PDSA-BEA | 30 | — | 80 | 100 | [ |
| pTSA-BEA | 30 | — | 82 | 90 | [ |
| H-Beta | RT | — | 86 | 98.5 | [ |
| Sn-TUD-1 | 80 | 10mmol叔丁醇 | 44 | 99 | [ |
| Zr-TUD-1 | 80 | 10mmol叔丁醇 | 46 | 99 | [ |
| Hf-TUD-1 | 80 | 10mmol叔丁醇 | 52 | 99 | [ |
| Ga-MCM-41 | 25 | 15mL乙腈 | 75 | 95 | [ |
| XS-Ga-MCM-41 | 80 | 1.8g叔丁醇 | 28 | 99 | [ |
| TFAl-MCM-41 | 25 | 15mL乙腈 | 97 | 97 | [ |
| OS-MCM-22 | 40 | — | 81 | 95 | [ |
| Nb-SBA-16 | 50 | — | 86 | 96 | [ |
| ZrMo-KIT-6 | 50 | — | 85.8 | 97.8 | [ |
| Nb-MCF | 40 | — | 48 | 99 | [ |
| Ta-MCF | 40 | — | 74 | 99 | [ |
| Mo-MCF | 40 | — | 48 | 99 | [ |
| MP-MCF | 40 | — | 83 | 99 | [ |
| CS-MCF | 40 | — | 87 | 99 | [ |
| TFAl-MCM-41 | 30 | — | 92 | [ | |
| Sn-TUD-1 | 80 | 10mmol叔丁醇 | 44 | 99 | [ |
| Zr-TUD-1 | 80 | 10mmol叔丁醇 | 46 | 99 | [ |
| Hf-TUD-1 | 80 | 10mmol叔丁醇 | 52 | 99 | [ |
| MoPO-SBA-15 | RT | — | 100 | 98 | [ |
| Pr-SBA-15 | 70 | — | 79 | — | [ |
| Ar-SBA-15 | 70 | — | 82 | — | [ |
| 1M-MM | 50 | — | 57 | 97.4 | [ |
| HSCS | 70 | — | 80 | 98 | [ |
| SO3H-Cel | — | 80 | 95 | [ | |
| C-HSO3 | 50 | — | 80 | 99.5 | [ |
| 5%Ni–1%Zr/AC | 45 | — | 82 | 74 | [ |
表4 改性分子筛和介孔材料催化剂研究结果汇总表
| 催化剂 | 温度/℃ | 溶剂 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|
| OTS-HY | 30 | — | 89 | 95 | [ |
| HUSY-W20 | 40 | — | 100 | 97.87 | [ |
| Sil-F | 70 | — | 65.8 | 63.9 | [ |
| 5%V-Si-ITQ-6 | 60 | — | 100 | 99 | [ |
| HUSY-Nb5 | 40 | — | 66 | 98 | [ |
| MIL-100-V | 70 | 5mL乙腈 | 85.4 | 97.7 | [ |
| MIL-100-Al | 70 | 5mL乙腈 | 44.2 | 94.3 | [ |
| MIL-100-Fe | 70 | 5mL乙腈 | 19.5 | 86.1 | [ |
| MIL-100-Cr | 70 | 5mL乙腈 | 4.2 | 77.5 | [ |
| Cu-Mor | RT | — | 95 | 98 | [ |
| Zn-Mor | RT | — | 90 | 81 | [ |
| Ni-Mor | RT | — | 93 | 88 | [ |
| Co-Mor | RT | — | 87 | 73 | [ |
| Fe-Mor | RT | — | 88 | 84 | [ |
| MFI-CA | 70 | — | 81 | 99 | [ |
| BEA-CA | 70 | — | 82 | 99 | [ |
| Mor-CA | 70 | — | 79 | 98 | [ |
| PDSA-BEA | 30 | — | 80 | 100 | [ |
| pTSA-BEA | 30 | — | 82 | 90 | [ |
| H-Beta | RT | — | 86 | 98.5 | [ |
| Sn-TUD-1 | 80 | 10mmol叔丁醇 | 44 | 99 | [ |
| Zr-TUD-1 | 80 | 10mmol叔丁醇 | 46 | 99 | [ |
| Hf-TUD-1 | 80 | 10mmol叔丁醇 | 52 | 99 | [ |
| Ga-MCM-41 | 25 | 15mL乙腈 | 75 | 95 | [ |
| XS-Ga-MCM-41 | 80 | 1.8g叔丁醇 | 28 | 99 | [ |
| TFAl-MCM-41 | 25 | 15mL乙腈 | 97 | 97 | [ |
| OS-MCM-22 | 40 | — | 81 | 95 | [ |
| Nb-SBA-16 | 50 | — | 86 | 96 | [ |
| ZrMo-KIT-6 | 50 | — | 85.8 | 97.8 | [ |
| Nb-MCF | 40 | — | 48 | 99 | [ |
| Ta-MCF | 40 | — | 74 | 99 | [ |
| Mo-MCF | 40 | — | 48 | 99 | [ |
| MP-MCF | 40 | — | 83 | 99 | [ |
| CS-MCF | 40 | — | 87 | 99 | [ |
| TFAl-MCM-41 | 30 | — | 92 | [ | |
| Sn-TUD-1 | 80 | 10mmol叔丁醇 | 44 | 99 | [ |
| Zr-TUD-1 | 80 | 10mmol叔丁醇 | 46 | 99 | [ |
| Hf-TUD-1 | 80 | 10mmol叔丁醇 | 52 | 99 | [ |
| MoPO-SBA-15 | RT | — | 100 | 98 | [ |
| Pr-SBA-15 | 70 | — | 79 | — | [ |
| Ar-SBA-15 | 70 | — | 82 | — | [ |
| 1M-MM | 50 | — | 57 | 97.4 | [ |
| HSCS | 70 | — | 80 | 98 | [ |
| SO3H-Cel | — | 80 | 95 | [ | |
| C-HSO3 | 50 | — | 80 | 99.5 | [ |
| 5%Ni–1%Zr/AC | 45 | — | 82 | 74 | [ |
| 1 | 李亮荣, 李秋平, 艾盛, 等. 传统化石与新型生物质能源重整制氢研究现状[J]. 化学与生物工程, 2021, 38(11): 1-6. |
| LI Liangrong, LI Qiuping, AI Sheng, et al. Research status of hydrogen production from reforming of fossil energy and new biomass energy[J]. Chemistry & Bioengineering, 2021, 38(11): 1-6. | |
| 2 | 洪浩. 生物质能源产业的比较优势[J]. 能源, 2018(8): 71-73. |
| HONG Hao. Comparative advantages of biomass energy industry[J]. Energy, 2018(8): 71-73. | |
| 3 | ANDRE T. Renewables 2020 global status report[M]. Renewables 2020 Global Status Report, 2020. |
| 4 | RODRIGUES Raphael, MANDELLI Dalmo, GONÇALVES Norberto S, et al. Acetalization of acetone with glycerol catalyzed by niobium-aluminum mixed oxides synthesized by a sol-gel process[J]. Journal of Molecular Catalysis A: Chemical, 2016, 422: 122-130. |
| 5 | 姜莉莉, 朱宝伟, 李昌丽, 等. 微生物转化粗甘油制备高附加值产品的研究进展[J]. 生物质化学工程, 2021, 55(5): 60-66. |
| JIANG Lili, ZHU Baowei, LI Changli, et al. Research progress in microbial conversion crude glycerol to high value-added products[J]. Biomass Chemical Engineering, 2021, 55(5): 60-66. | |
| 6 | 胡琦艳, 杨德育. (R)-甘油醛缩丙酮的合成及Wittig反应选择性[J]. 化学研究, 2006, 17(2): 12-16. |
| HU Qiyan, YANG Deyu. Synthesis of (R)-glyceraldehyde acetonide and stereoselectivity of its Wittig reaction[J]. Chemical Research, 2006, 17(2): 12-16. | |
| 7 | 赵军, 王亚东. (S)-普萘洛尔的立体选择性合成[J]. 浙江工业大学学报, 1997, 25(3): 252-255. |
| ZHAO Jun, WANG Yadong. Stereoselective synthesis of β-blocker receptor (S)-propranolol[J]. Journal of Zhejiang University of Technology, 1997, 25(3): 252-255. | |
| 8 | EIBL Hansjörg. An improved method for the preparation of 1,2-isopropylidene-SN-glycerol[J]. Chemistry and Physics of Lipids, 1981, 28(1): 1-5. |
| 9 | 尹显洪. 相转移催化D-甘露醇合成镇咳药左旋羟苯哌嗪[J]. 化学试剂, 2001, 23(6): 349-350. |
| YIN Xianhong. Phase transfer catalyzed synthesis of (-)-dropropizine with D-mannitol as starting material[J]. Chemical Reagents, 2001, 23(6): 349-350. | |
| 10 | 赵军, 王亚东, 杨世琰. 催化氧化法合成缩丙酮-(R)-和(S)-甘油酸[J]. 合成化学, 1996, 4(1): 88-90. |
| ZHAO Jun, WANG Yadong, YANG Shiyan. Synthesis of 2,3-O-isopropylidene (R) glyceric acid and its (S) isomer by oxidation of RuCl3-NaClO[J]. Chinese Journal of Synthetic Chemistry, 1996, 4(1): 88-90. | |
| 11 | JANISZEWSKA E, KOWALSKA-KUŚ J, GÓRA-MAREK K, et al. Modification of silicalite-1 with ammonium compounds aimed at preparation of acidic catalyst for acetalization of glycerol with acetone[J]. Applied Catalysis A: General, 2019, 581: 1-10. |
| 12 | LOPES N F, CAIADO M, CANHÃO P, et al. Synthesis of bio-fuel additives from glycerol over poly(vinyl alcohol) with sulfonic acid groups[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2015, 37(17): 1928-1936. |
| 13 | FERREIRA P, FONSECA I M, RAMOS A M, et al. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids[J]. Applied Catalysis B: Environmental, 2010, 98(1/2): 94-99. |
| 14 | MENEZES Fernanda D L, GUIMARAES Matheus D O, SILVA Márcio J DA. Highly selective SnCl2-catalyzed solketal synthesis at room temperature[J]. Industrial & Engineering Chemistry Research, 2013, 52(47): 16709-16713. |
| 15 | MANJUNATHAN Pandian, MARADUR Sanjeev P, HALGERI A B, et al. Room temperature synthesis of solketal from acetalization of glycerol with acetone: Effect of crystallite size and the role of acidity of beta zeolite[J]. Journal of Molecular Catalysis A: Chemical, 2015, 396: 47-54. |
| 16 | TIMOFEEVA M N, PANCHENKO V N, KHAN N A, et al. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone[J]. Applied Catalysis a-General, 2017, 529: 167-174. |
| 17 | ALSAWALHA Murad. Catalytic activity and kinetic modeling of various modules HZMS-5 and treated MCM-41 catalysts, for the liquid-phase ketalization of glycerol with acetone[J]. Frontiers in Chemistry, 2019, 7: 799. |
| 18 | LI Zhenbin, MIAO Zhichao, WANG Xuan, et al. One-pot synthesis of ZrMo-KIT-6 solid acid catalyst for solvent-free conversion of glycerol to solketal[J]. Fuel, 2018, 233: 377-387. |
| 19 | Amin TALEBIAN-KIAKALAIEH, TARIGHI Sara. Hierarchical faujasite zeolite-supported heteropoly acid catalyst for acetalization of crude-glycerol to fuel additives[J]. Journal of Industrial and Engineering Chemistry, 2019, 79: 452-464. |
| 20 | DE CARVALHO Davi C, OLIVEIRA Alcemira C, FERREIRA Odair P, et al. Titanate nanotubes as acid catalysts for acetalization of glycerol with acetone: Influence of the synthesis time and the role of structure on the catalytic performance[J]. Chemical Engineering Journal, 2017, 313: 1454-1467. |
| 21 | VIEIRA Luiz H, POSSATO Luiz G, CHAVES Thiago F, et al. Studies on dispersion and reactivity of vanadium oxides deposited on lamellar ferrierite zeolites for condensation of glycerol into bulky products[J]. Molecular Catalysis, 2018, 458: 161-170. |
| 22 | LI Li, KORáNYI Tamás I, SELS Bert F, et al. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts[J]. Green Chemistry, 2012, 14(6): 1611-1619. |
| 23 | PRIYA Samudrala Shanthi, SELVAKANNAN P R, CHARY Komandur V R, et al. Solvent-free microwave-assisted synthesis of solketal from glycerol using transition metal ions promoted mordenite solid acid catalysts[J]. Molecular Catalysis, 2017, 434: 184-193. |
| 24 | SILVA Márcio José DA, DE ÁVILA RODRIGUES Fabio, JÚLIO Armanda Aparecida. SnF2-catalyzed glycerol ketalization: A friendly environmentally process to synthesize solketal at room temperature over on solid and reusable Lewis acid[J]. Chemical Engineering Journal, 2017, 307: 828-835. |
| 25 | ESPOSITO Roberto, RAUCCI Umberto, CUCCIOLITO Maria E, et al. Iron(Ⅲ) complexes for highly efficient and sustainable ketalization of glycerol: A combined experimental and theoretical study[J]. ACS Omega, 2019, 4(1): 688-698. |
| 26 | VISWANADHAM Nagabhatla, DEBNATH Suman, SREENIVASULU Peta, et al. Nano porous hydroxyapatite as a bi-functional catalyst for bio-fuel production[J]. RSC Advances, 2015, 5(83): 67380-67383. |
| 27 | NANDA Malaya R, YUAN Zhongshun, QIN Wensheng, et al. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive[J]. Fuel, 2014, 117: 470-477. |
| 28 | ESTEBAN Jesús, LADERO Miguel, Félix GARCÍA-OCHOA. Kinetic modelling of the solventless synthesis of solketal with a sulphonic ion exchange resin[J]. Chemical Engineering Journal, 2015, 269: 194-202. |
| 29 | JOLANTA Kowalska-Kus, AGNIESZKA Held, KRYSTYNA Nowinska. Enhancement of the catalytic activity of H-ZSM-5 zeolites for glycerol acetalization by mechanical grinding[J]. Reaction Kinetics, Mechanisms and Catalysis, 2016, 117(1): 341-352. |
| 30 | RAHAMAN Mohammad Shahinur, PHUNG Thanh Khoa, HOSSAIN Md Anwar, et al. Hydrophobic functionalization of HY zeolites for efficient conversion of glycerol to solketal[J]. Applied Catalysis A: General, 2020, 592: 117369. |
| 31 | FERREIRA C, ARAUJO A, CALVINO-CASILDA V, et al. Y zeolite-supported niobium pentoxide catalysts for the glycerol acetalization reaction[J]. Microporous and Mesoporous Materials, 2018, 271: 243-251. |
| 32 | Agnieszka FELICZAK-GUZIK, NOWAK Izabela. Application of glycerol to synthesis of solvo-surfactants by using mesoporous materials containing niobium[J]. Microporous and Mesoporous Materials, 2019, 277: 301-308. |
| 33 | COLLARD Xavier, LI Li, LUEANGCHAICHAWENG Warunee, et al. Ga-MCM-41 nanoparticles: Synthesis and application of versatile heterogeneous catalysts[J]. Catalysis Today, 2014, 235: 184-192. |
| 34 | TAYADE Kamlesh N, MISHRA Manish, Munusamy K, et al. Synthesis of aluminium triflate-grafted MCM-41 as a water-tolerant acid catalyst for the ketalization of glycerol with acetone[J]. Catalysis Science & Technology, 2015, 5(4): 2427-2440. |
| 35 | KHAYOON M S, HAMEED B H. Solventless acetalization of glycerol with acetone to fuel oxygenates over Ni-Zr supported on mesoporous activated carbon catalyst[J]. Applied Catalysis A: General, 2013, 464/465: 191-199. |
| 36 | CALVINO-CASILDA V, STAWICKA K, TREJDA M, et al. Real-time Raman monitoring and control of the catalytic acetalization of glycerol with acetone over modified mesoporous cellular foams[J]. The Journal of Physical Chemistry C, 2014, 118(20): 10780-10791. |
| 37 | RODRIGUES Mariana, OKOLIE Chukwuemeka, SIEVERS Carsten, et al. Organosilane-assisted synthesis of hierarchical MCM-22 zeolites for condensation of glycerol into bulky products[J]. Crystal Growth & Design, 2019, 19(1): 231-241. |
| 38 | LI Li, CANI Damiano, PESCARMONA Paolo P. Metal-containing TUD-1 mesoporous silicates as versatile solid acid catalysts for the conversion of bio-based compounds into valuable chemicals[J]. Inorganica Chimica Acta, 2015, 431: 289-296. |
| 39 | Katarzyna Stawicka, DÍAZ ÁLVAREZ ALBA E, Calvino Casilda Vanesa, et al. The role of Brønsted and Lewis acid sites in acetalization of glycerol over modified mesoporous cellular foams[J]. The Journal of Physical Chemistry C, 2016, 120(30): 16699-16711. |
| 40 | KOWALSKA-KUS J, HELD A, FRANKOWSKI M, et al. Solketal formation from glycerol and acetone over hierarchical zeolites of different structure as catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2017, 426: 205-212. |
| 41 | VENKATESHA N J, BHAT Y S, PRAKASH B S JAI. Dealuminated BEA zeolite for selective synthesis of five-membered cyclic acetal from glycerol under ambient conditions[J]. RSC Advances, 2016, 6(23): 18824-18833. |
| 42 | VICENTE Gemma, MELERO Juan A, MORALES Gabriel, et al. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas[J]. Green Chemistry, 2010, 12(5): 899-907. |
| 43 | NANDAN Devaki, SREENIVASULU Peta, SIVAKUMAR KONATHALA L N, et al. Acid functionalized carbon-silica composite and its application for solketal production[J]. Microporous and Mesoporous Materials, 2013, 179: 182-190. |
| 44 | FERNÁNDEZ P, FRAILE J M, GARCÍA-BORDEJÉ E, et al. Sulfonated hydrothermal carbons from cellulose and glucose as catalysts for glycerol ketalization[J]. Catalysts, 2019, 9(10): 804. |
| 45 | KONWAR Lakhya Jyoti, SAMIKANNU Ajaikumar, Päivi MÄKI-ARVELA, et al. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives[J]. Applied Catalysis B: Environmental, 2018, 220: 314-323. |
| 46 | Maraísa GONÇALVES, RODRIGUES Raphael, GALHARDO Thalita S, et al. Highly selective acetalization of glycerol with acetone to solketal over acidic carbon-based catalysts from biodiesel waste[J]. Fuel, 2016, 181: 46-54. |
| 47 | TIMOFEEVA Maria N, PANCHENKO Valentina N, KRUPSKAYA Victoria V, et al. Effect of nitric acid modification of montmorillonite clay on synthesis of solketal from glycerol and acetone[J]. Catalysis Communications, 2017, 90: 65-69. |
| 48 | RODRIGUES Raphael, Maraisa GONçALVES, MANDELLI Dalmo, et al. Solvent-free conversion of glycerol to solketal catalysed by activated carbons functionalised with acid groups[J]. Catalysis Science & Technology, 2014, 4(8): 2293-2301. |
| 49 | GADAMSETTI Sailaja, Pethan RAJAN N, RAO G Srinivasa, et al. Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2015, 410: 49-57. |
| 50 | KITTICHAI Chaiseeda, LADAWAN Chantharadet, WARINTHORN Chavasiri. Utilization of hexabromoacetone for protection of alcohols and aldehydes and deprotection of acetals, ketals, and oximes under UV irradiation[J]. Research on Chemical Intermediates, 2018, 44(2): 1305-1323. |
| 51 | SELVA Maurizio, BENEDET Vanni, FABRIS Massimo. Selective catalytic etherification of glycerol formal and solketal with dialkyl carbonates and K2CO3 [J]. Green Chemistry, 2012, 14(1): 188-200. |
| 52 | JIN Jing, GUIDI Sandro, ABADA Zahra, et al. Continuous niobium phosphate catalysed Skraup reaction for quinoline synthesis from solketal[J]. Green Chemistry, 2017, 19(10): 2439-2447. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||