化工进展 ›› 2023, Vol. 42 ›› Issue (S1): 400-410.DOI: 10.16085/j.issn.1000-6613.2023-0424
双子表面活性剂癸炔二醇的绿色合成
- 厦门大学化学化工学院,福建 厦门 361005
-
收稿日期:2023-03-21修回日期:2023-07-09出版日期:2023-10-25发布日期:2023-11-30 -
通讯作者:黎四芳 -
作者简介:王正坤(1993—),男,硕士研究生,研究方向为精细化工。E-mail:zkwang@stu.xmu.edu.cn。
Green synthesis of gemini surfactant decyne diol
- College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China
-
Received:2023-03-21Revised:2023-07-09Online:2023-10-25Published:2023-11-30 -
Contact:LI Sifang
摘要:
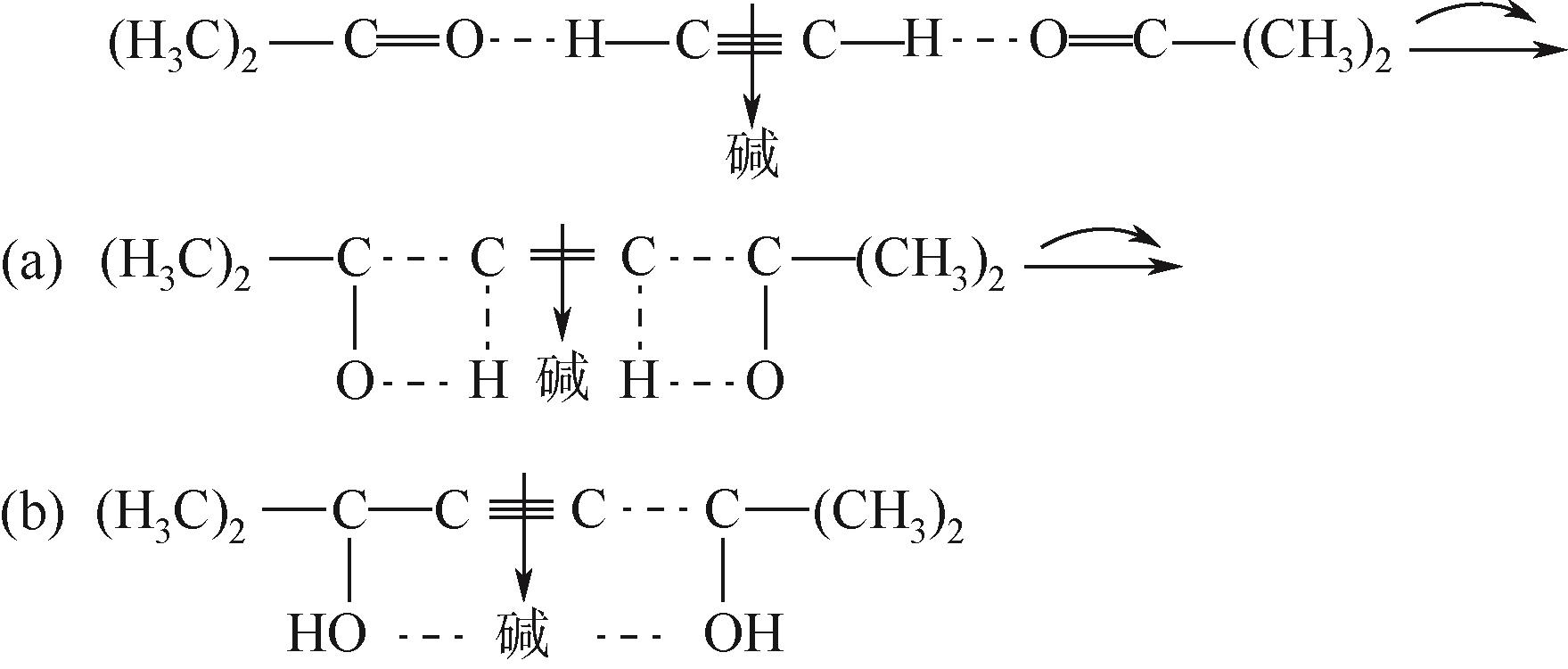
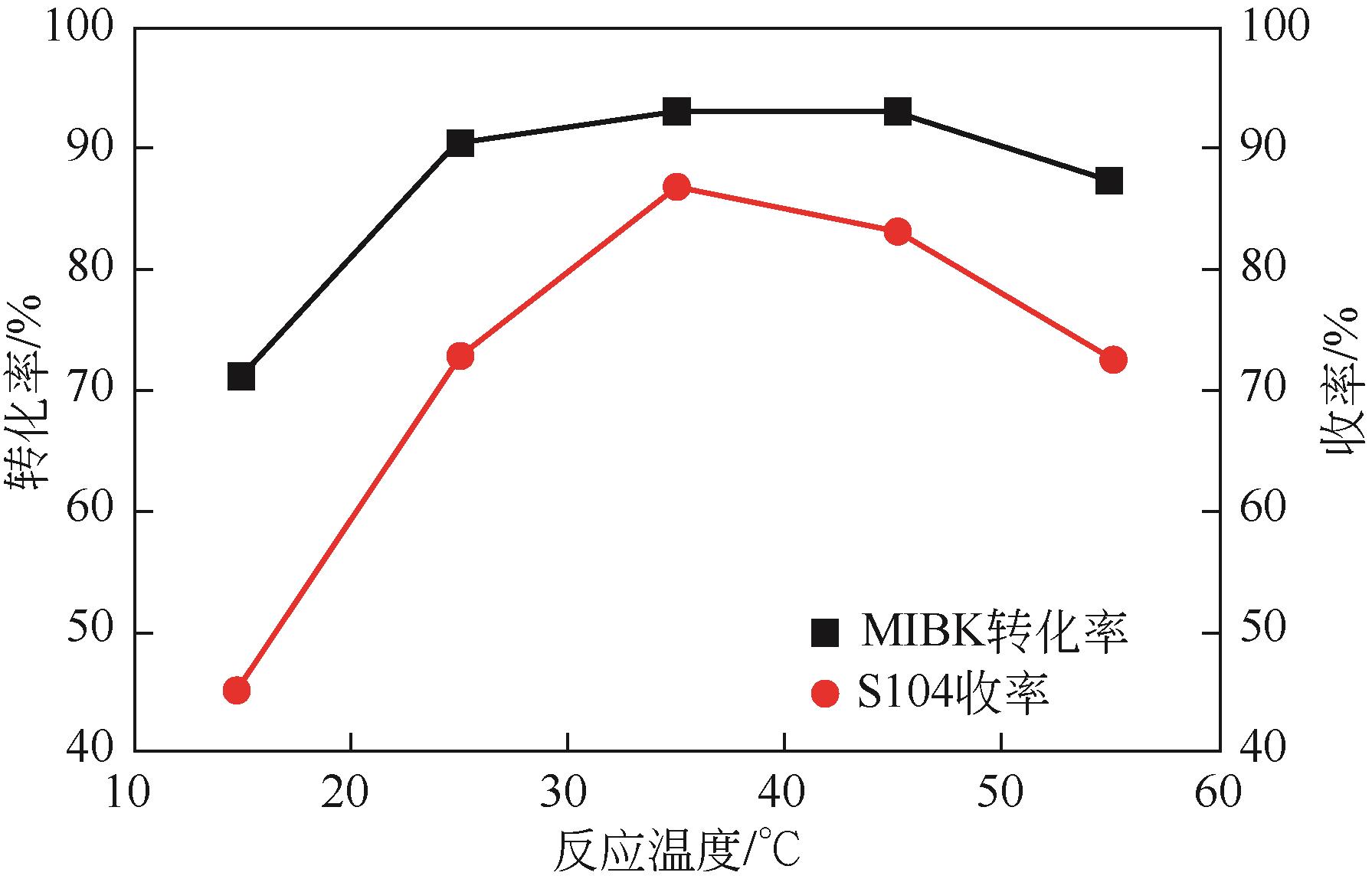
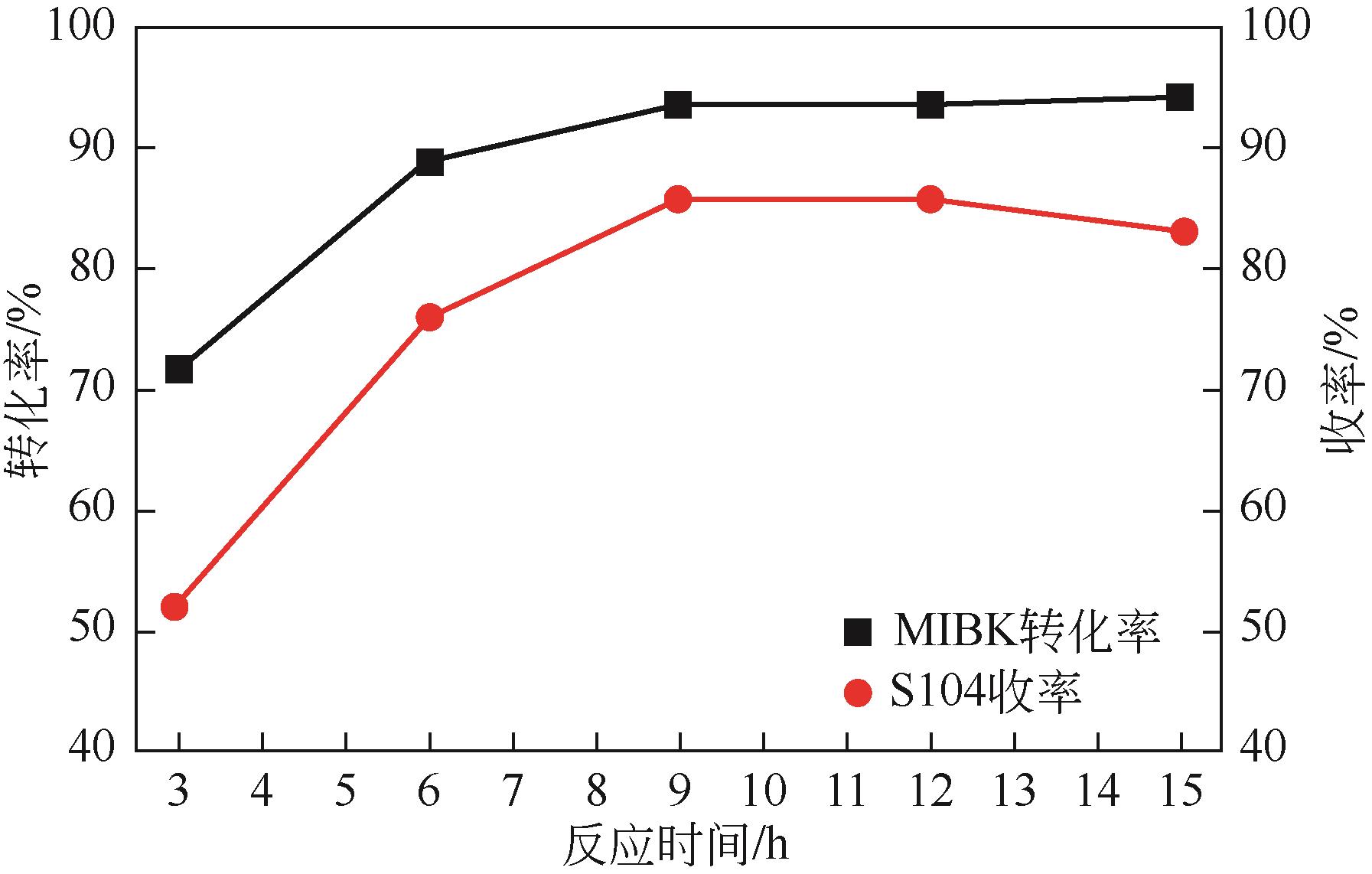
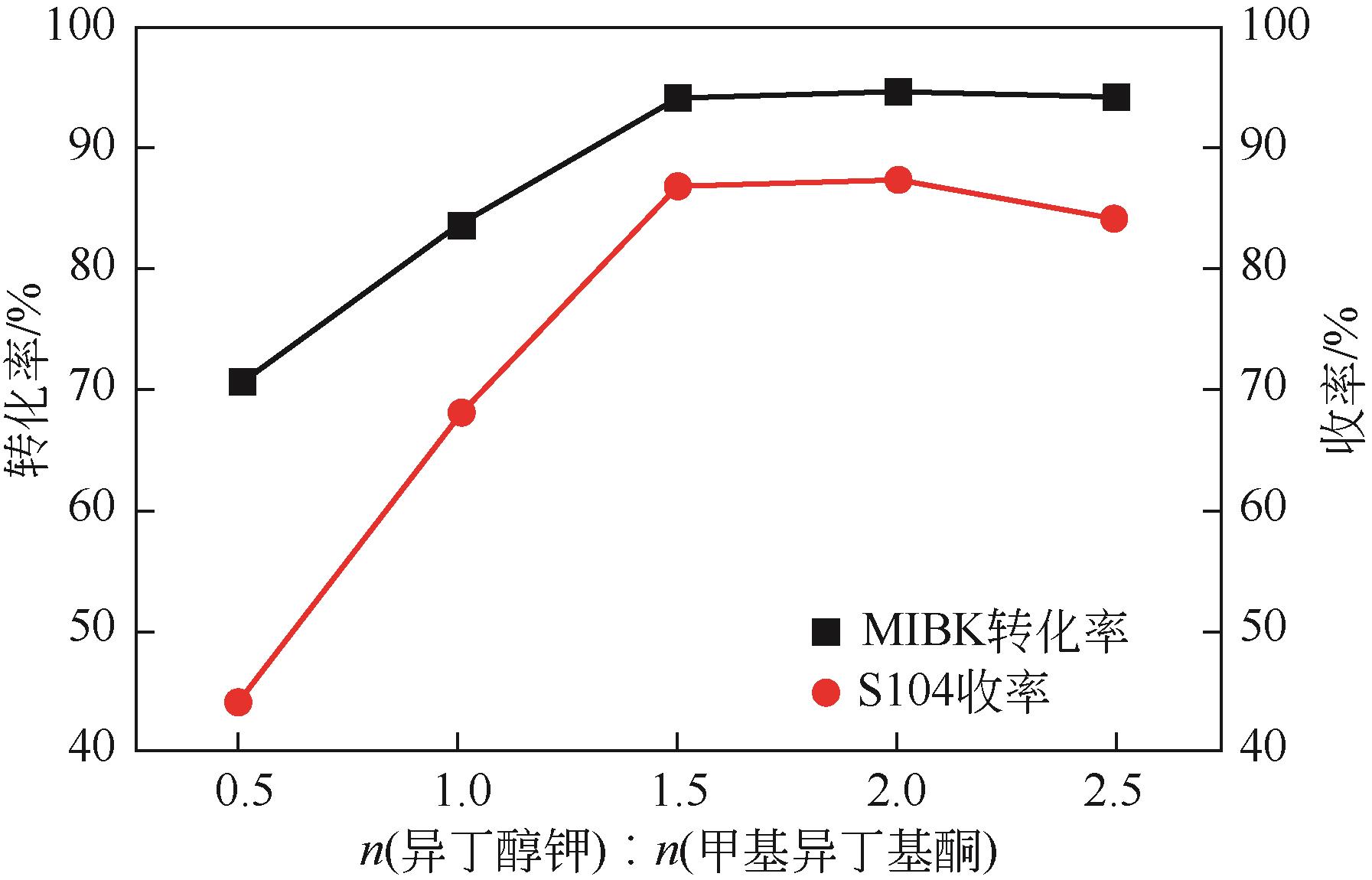
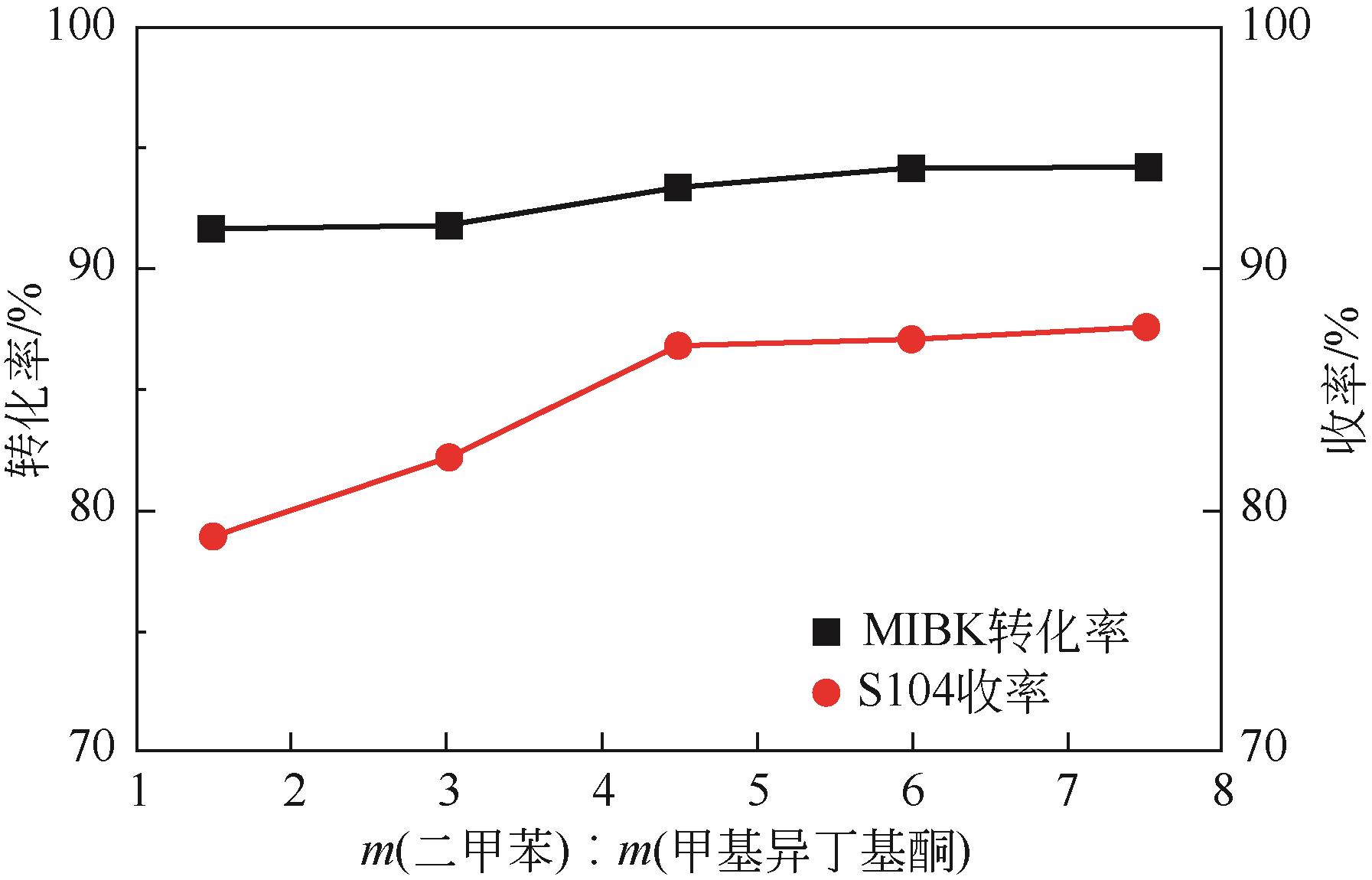
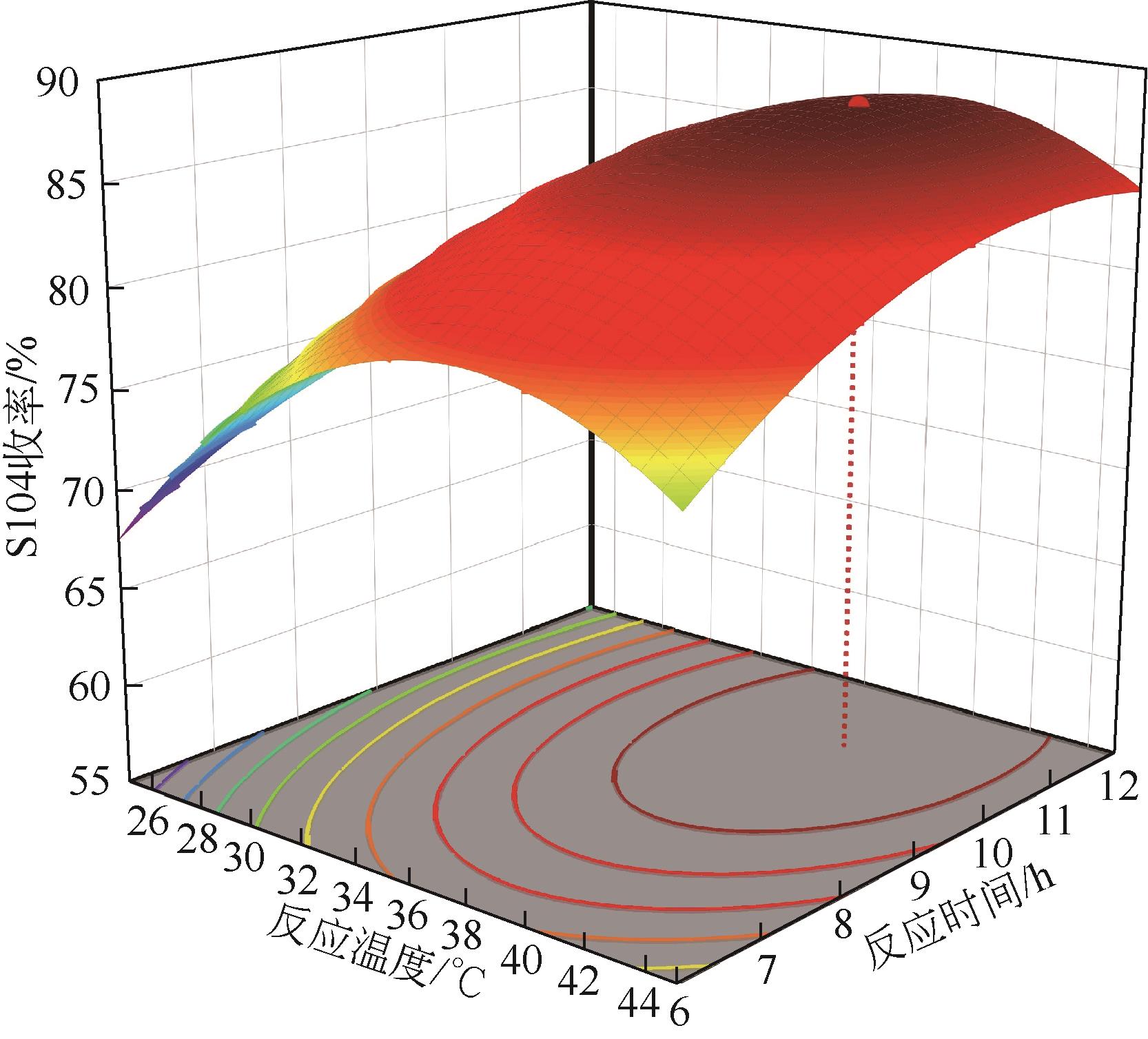
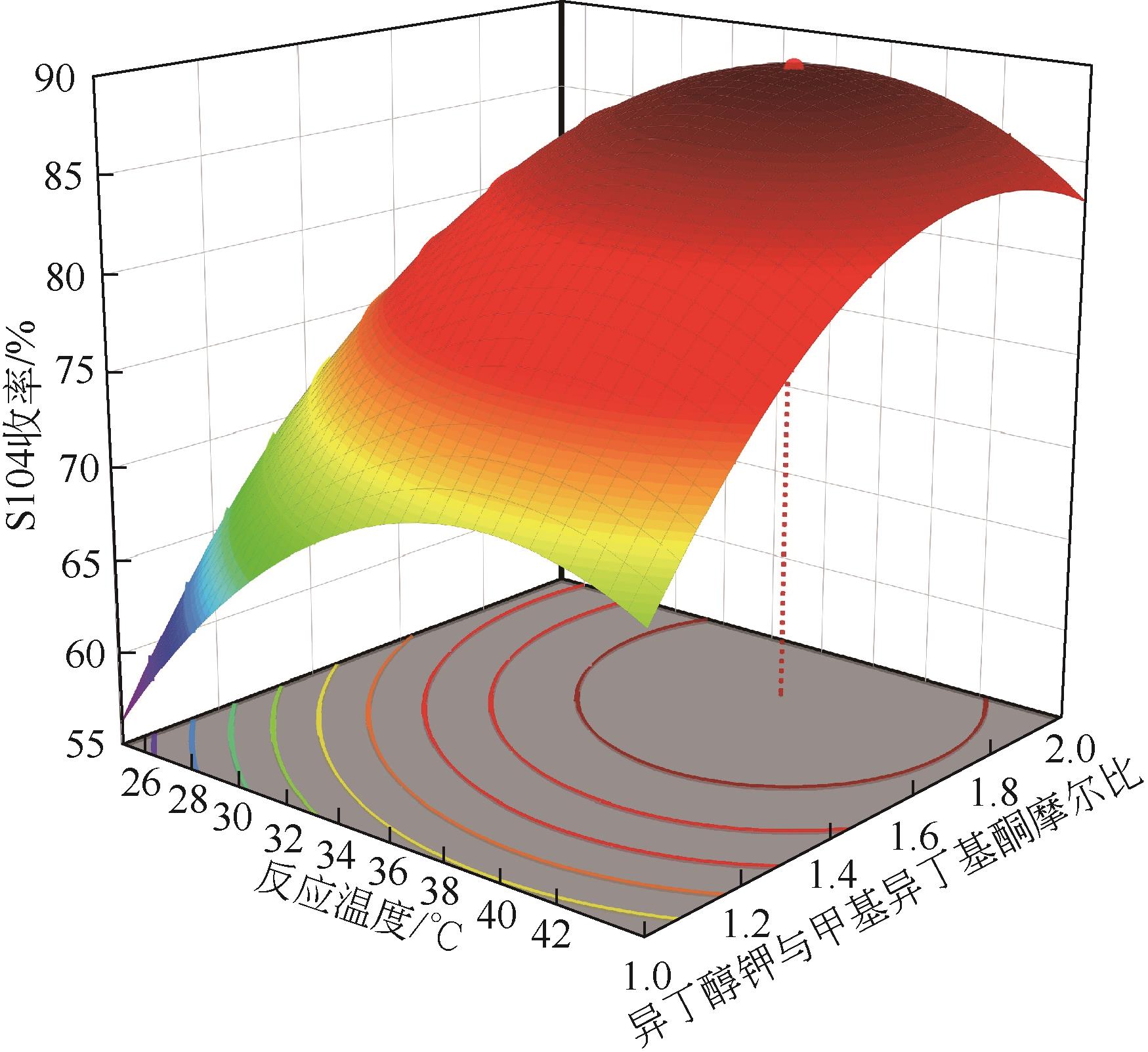
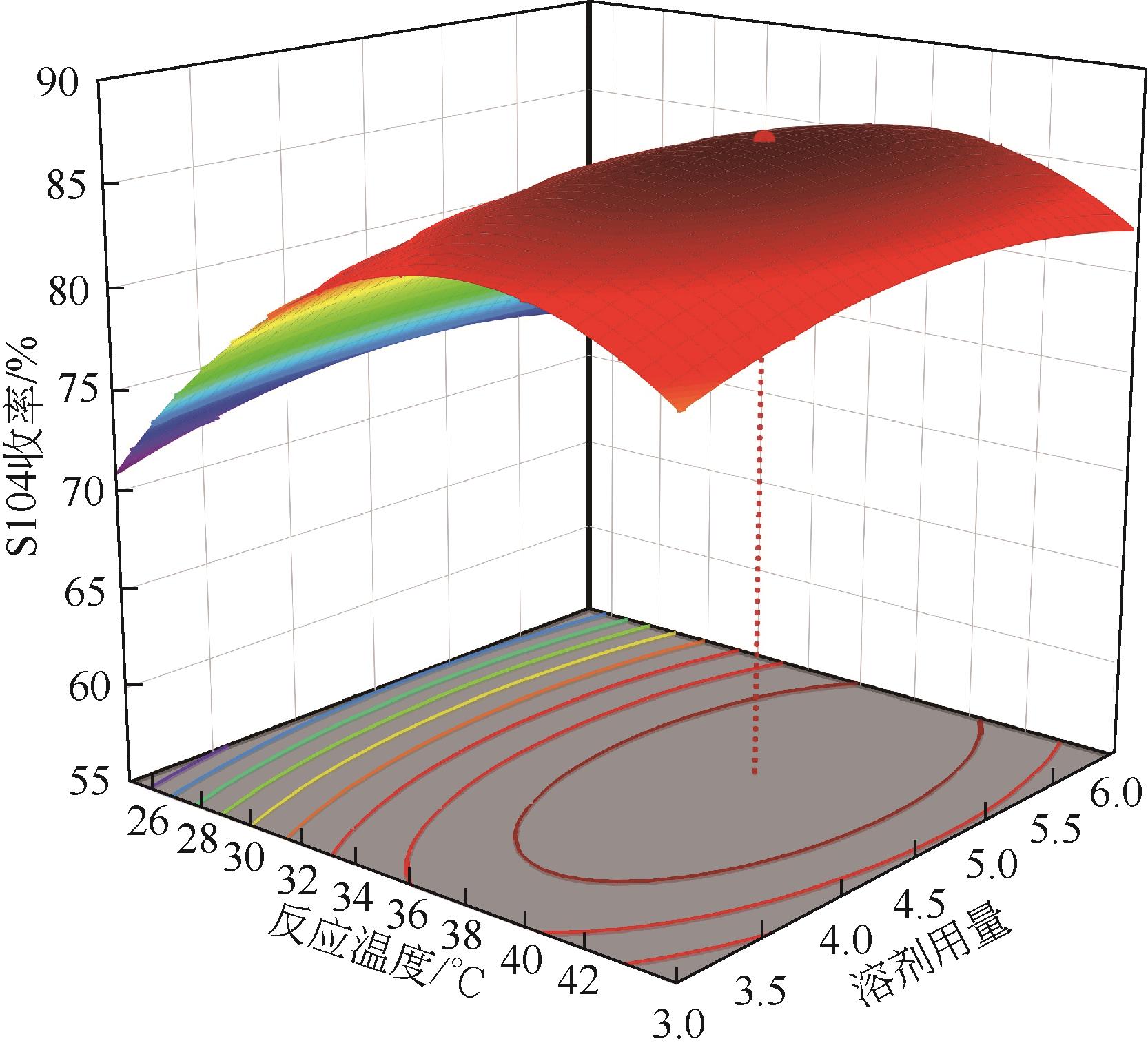
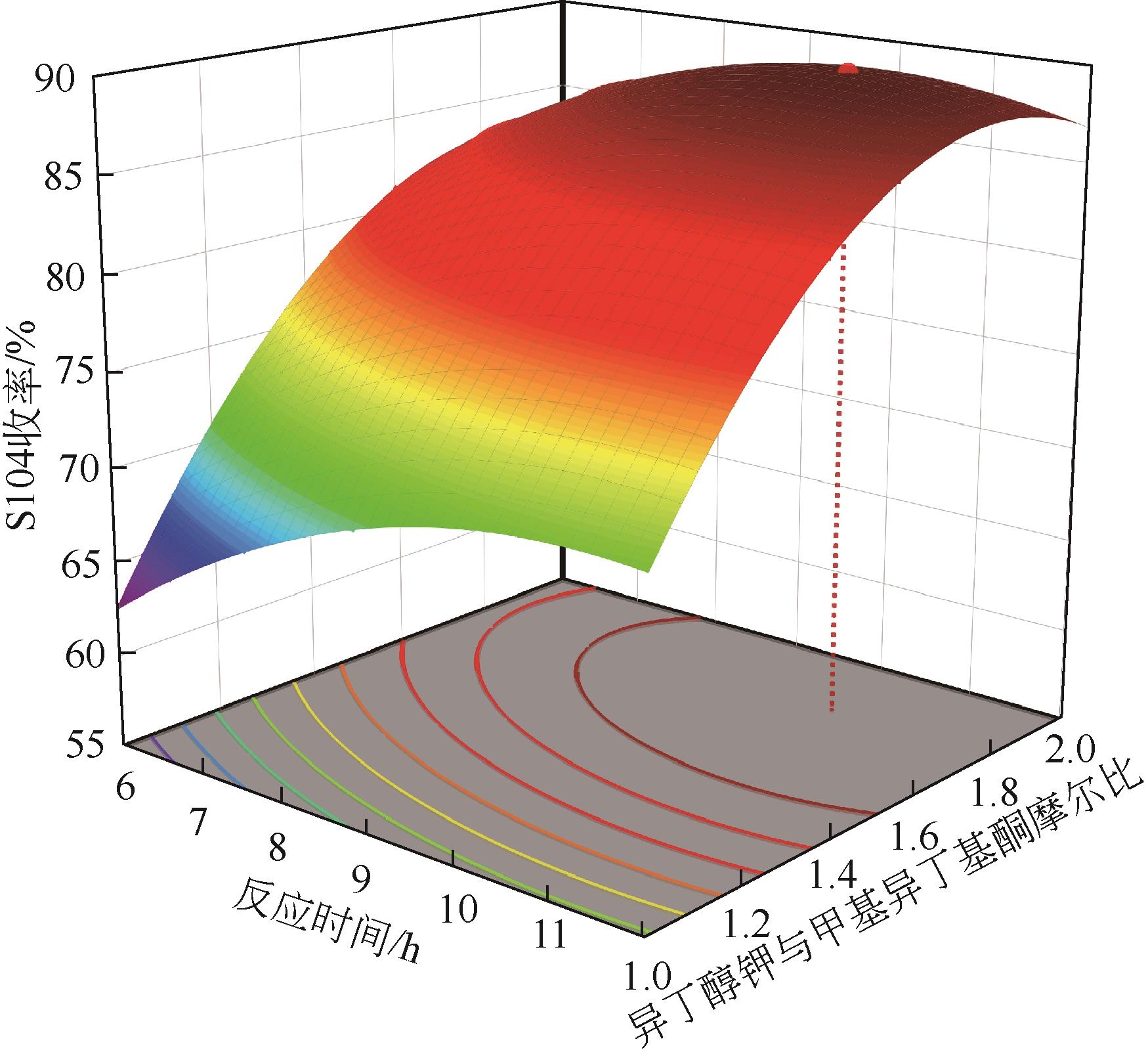
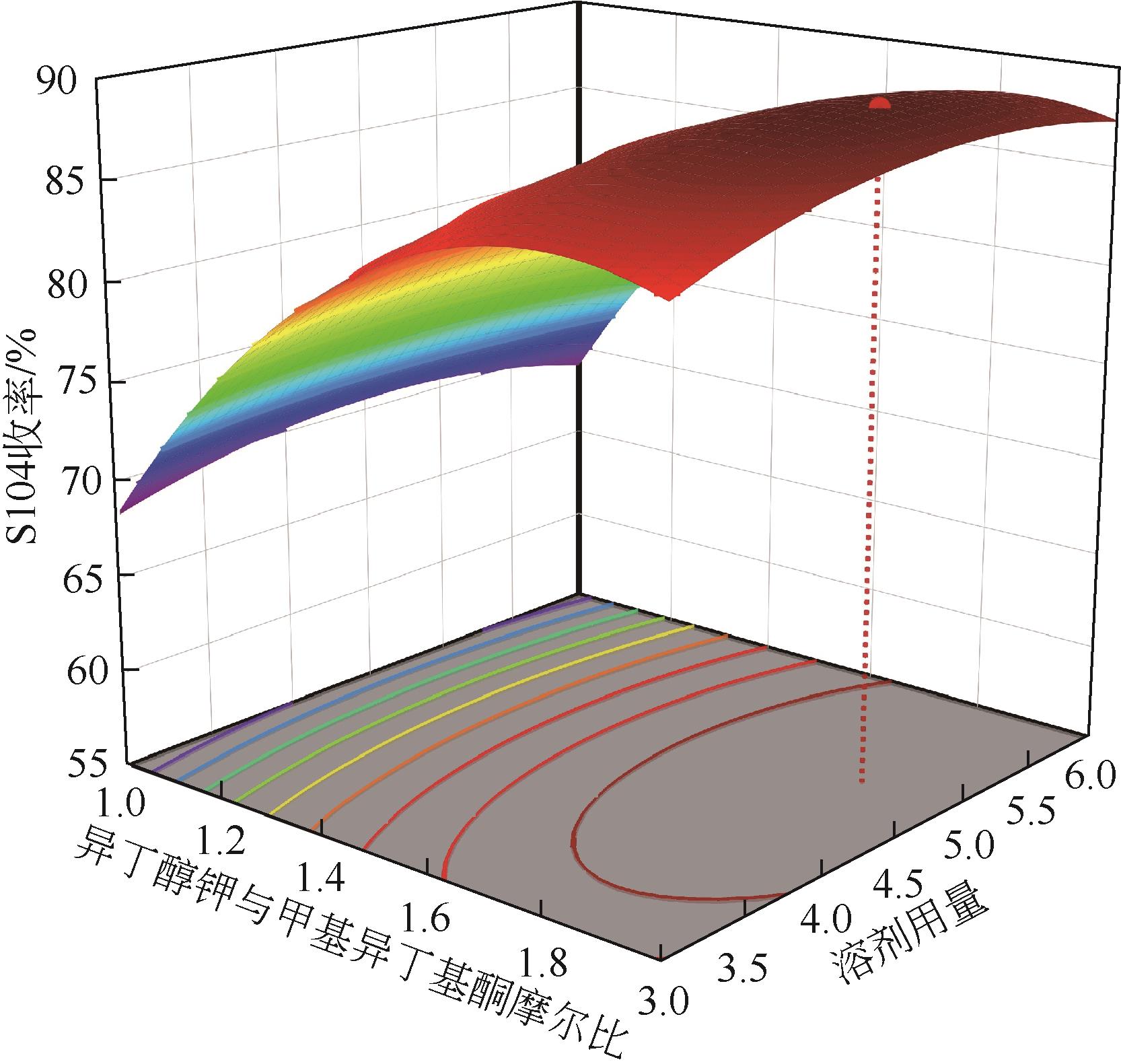
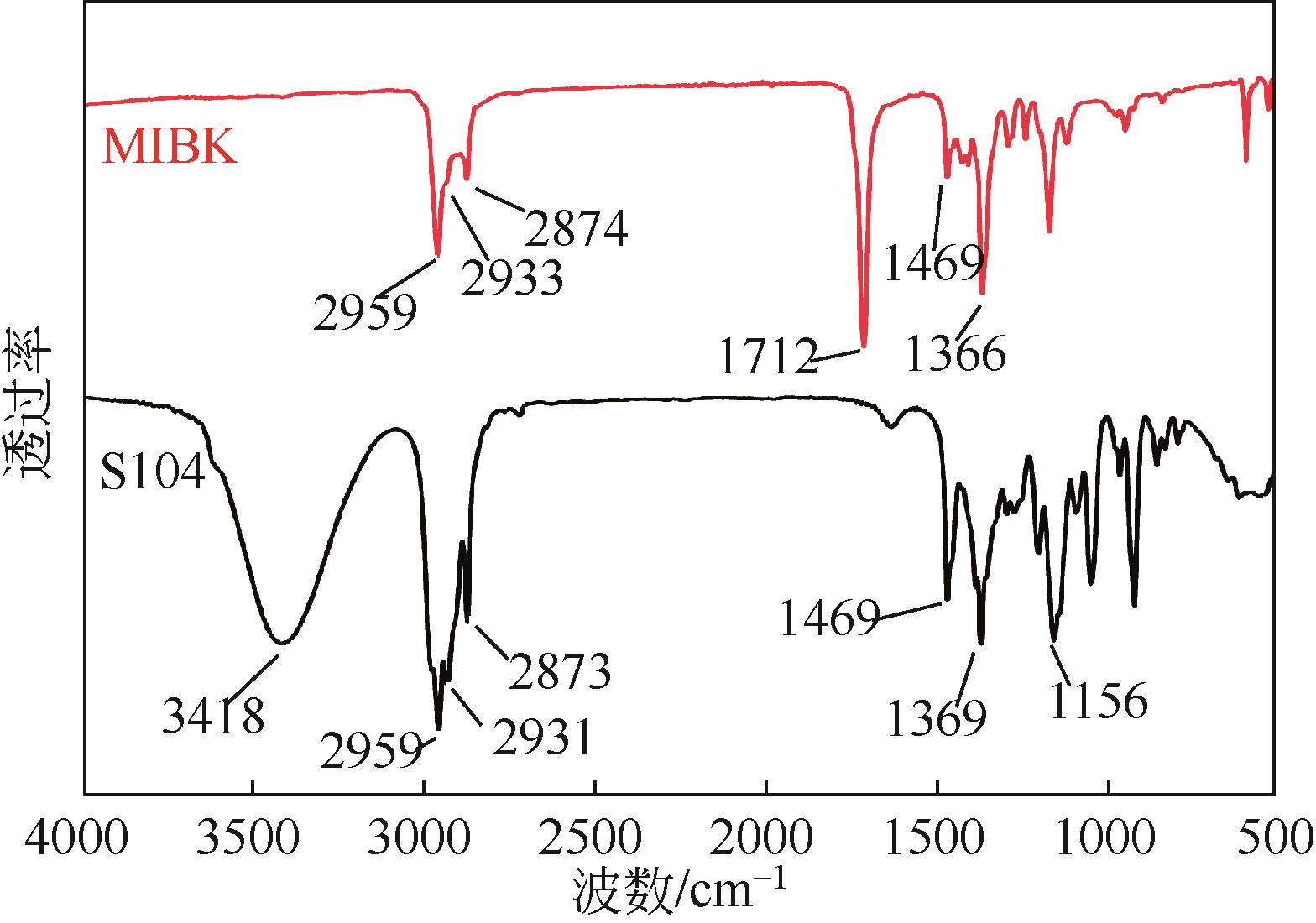
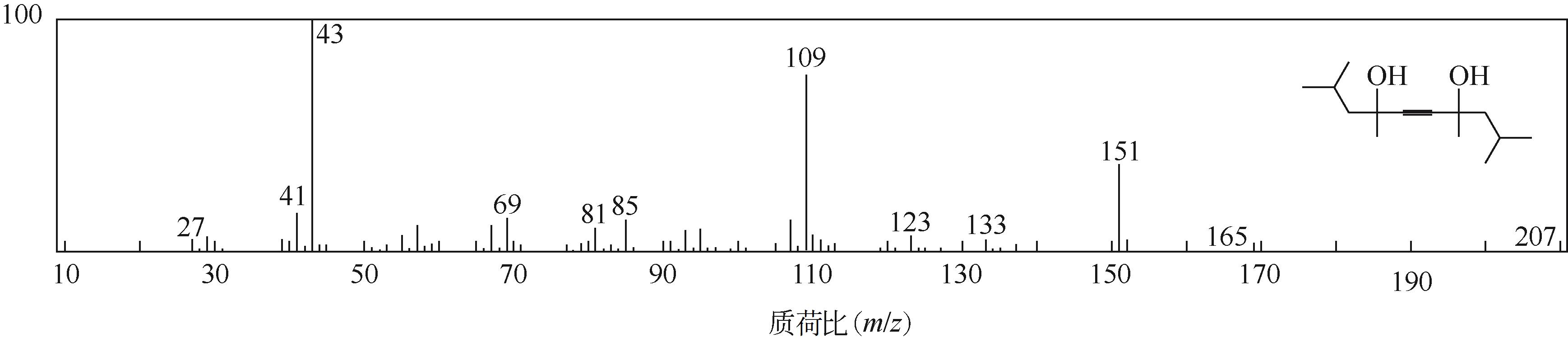
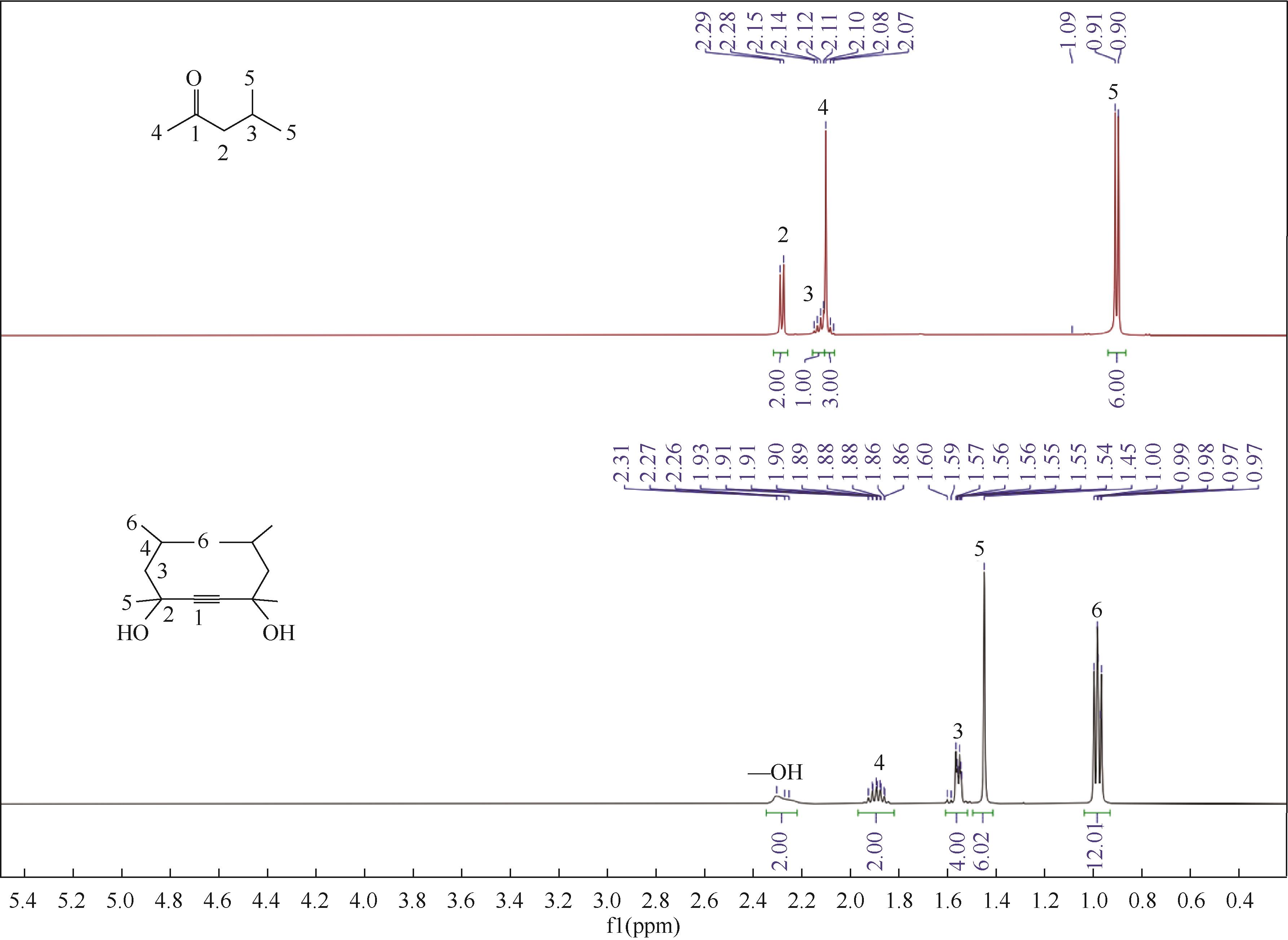
双子表面活性剂癸炔二醇(S104),即2,4,7,9-四甲基-5-癸炔-4,7-二醇,是一种用途广泛的炔二醇类表面活性剂。S104由甲基异丁基酮和乙炔在碱催化下进行炔化反应合成,目前的合成工艺有两种:固体KOH催化炔化法和液氨-KOH催化炔化法,但都存在严重的环保问题等缺点。为此本研究以悬浮于溶剂二甲苯中的固体异丁醇钾为催化剂催化乙炔和甲基异丁基酮的反应合成S104。采用单因素实验探究了各工艺条件对甲基异丁基酮转化率和S104收率的影响,并以此基础进行了响应面实验优化。在优选反应条件(反应温度35℃,反应时间9h,异丁醇钾与甲基异丁基酮摩尔比1.5∶1,溶剂二甲苯与甲基异丁基酮的质量比4.5∶1)下,S104的收率为86.52%±2.02%。将反应结束后水解得到的氢氧化钾溶液返回到异丁醇钾制备阶段循环使用,甲基异丁基酮转化率和S104收率无明显变化。该工艺在常压下进行,废碱液循环使用,避免了废水排放,安全环保,具有较高的绿色价值。
中图分类号:
引用本文
王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410.
WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410.
| 实验因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| 反应温度(A)/℃ | 25 | 35 | 45 |
| 反应时间(B)/h | 6 | 9 | 12 |
| 异丁醇钾与甲基异丁基酮摩尔比(C) | 1∶1 | 1.5∶1 | 2∶1 |
| 溶剂用量(D) | 3∶1 | 4.5∶1 | 6∶1 |
表1 响应面实验因素水平设计
| 实验因素 | 水平 | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| 反应温度(A)/℃ | 25 | 35 | 45 |
| 反应时间(B)/h | 6 | 9 | 12 |
| 异丁醇钾与甲基异丁基酮摩尔比(C) | 1∶1 | 1.5∶1 | 2∶1 |
| 溶剂用量(D) | 3∶1 | 4.5∶1 | 6∶1 |
| 序号 | 实验因素 | S104收率/% | |||
|---|---|---|---|---|---|
| A/℃ | B/h | C | D | ||
| 1 | 25 | 9 | 1 | 4.5 | 56.78 |
| 2 | 35 | 12 | 1 | 4.5 | 70.95 |
| 3 | 35 | 12 | 2 | 4.5 | 86.01 |
| 4 | 35 | 6 | 1.5 | 3 | 75.86 |
| 5 | 35 | 9 | 1.5 | 4.5 | 87.48 |
| 6 | 45 | 9 | 2 | 4.5 | 80.87 |
| 7 | 45 | 6 | 1.5 | 4.5 | 76.77 |
| 8 | 35 | 9 | 1.5 | 4.5 | 84.61 |
| 9 | 35 | 12 | 1.5 | 6 | 86.42 |
| 10 | 35 | 6 | 1.5 | 6 | 75.09 |
| 11 | 35 | 9 | 2 | 3 | 85.88 |
| 12 | 25 | 9 | 1.5 | 6 | 71.58 |
| 13 | 25 | 9 | 2 | 4.5 | 76.97 |
| 14 | 35 | 6 | 2 | 4.5 | 83.86 |
| 15 | 35 | 9 | 1.5 | 4.5 | 86.41 |
| 16 | 35 | 9 | 1.5 | 4.5 | 84.89 |
| 17 | 35 | 9 | 1 | 3 | 68.77 |
| 18 | 45 | 9 | 1.5 | 6 | 83.54 |
| 19 | 25 | 9 | 1.5 | 3 | 69.03 |
| 20 | 35 | 9 | 1.5 | 4.5 | 86.59 |
| 21 | 35 | 9 | 2 | 6 | 88.23 |
| 22 | 25 | 6 | 1.5 | 4.5 | 69.02 |
| 23 | 35 | 9 | 1 | 6 | 69.23 |
| 24 | 35 | 12 | 1.5 | 3 | 83.4 |
| 25 | 45 | 9 | 1.5 | 3 | 80.98 |
| 26 | 45 | 9 | 1 | 4.5 | 68.96 |
| 27 | 35 | 6 | 1 | 4.5 | 62.98 |
| 28 | 45 | 12 | 1.5 | 4.5 | 83.87 |
| 29 | 25 | 12 | 1.5 | 4.5 | 74.32 |
表2 响应面实验结果表
| 序号 | 实验因素 | S104收率/% | |||
|---|---|---|---|---|---|
| A/℃ | B/h | C | D | ||
| 1 | 25 | 9 | 1 | 4.5 | 56.78 |
| 2 | 35 | 12 | 1 | 4.5 | 70.95 |
| 3 | 35 | 12 | 2 | 4.5 | 86.01 |
| 4 | 35 | 6 | 1.5 | 3 | 75.86 |
| 5 | 35 | 9 | 1.5 | 4.5 | 87.48 |
| 6 | 45 | 9 | 2 | 4.5 | 80.87 |
| 7 | 45 | 6 | 1.5 | 4.5 | 76.77 |
| 8 | 35 | 9 | 1.5 | 4.5 | 84.61 |
| 9 | 35 | 12 | 1.5 | 6 | 86.42 |
| 10 | 35 | 6 | 1.5 | 6 | 75.09 |
| 11 | 35 | 9 | 2 | 3 | 85.88 |
| 12 | 25 | 9 | 1.5 | 6 | 71.58 |
| 13 | 25 | 9 | 2 | 4.5 | 76.97 |
| 14 | 35 | 6 | 2 | 4.5 | 83.86 |
| 15 | 35 | 9 | 1.5 | 4.5 | 86.41 |
| 16 | 35 | 9 | 1.5 | 4.5 | 84.89 |
| 17 | 35 | 9 | 1 | 3 | 68.77 |
| 18 | 45 | 9 | 1.5 | 6 | 83.54 |
| 19 | 25 | 9 | 1.5 | 3 | 69.03 |
| 20 | 35 | 9 | 1.5 | 4.5 | 86.59 |
| 21 | 35 | 9 | 2 | 6 | 88.23 |
| 22 | 25 | 6 | 1.5 | 4.5 | 69.02 |
| 23 | 35 | 9 | 1 | 6 | 69.23 |
| 24 | 35 | 12 | 1.5 | 3 | 83.4 |
| 25 | 45 | 9 | 1.5 | 3 | 80.98 |
| 26 | 45 | 9 | 1 | 4.5 | 68.96 |
| 27 | 35 | 6 | 1 | 4.5 | 62.98 |
| 28 | 45 | 12 | 1.5 | 4.5 | 83.87 |
| 29 | 25 | 12 | 1.5 | 4.5 | 74.32 |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 1936.259 | 14 | 138.3042 | 51.60691 | < 0.0001 | + + |
| 反应温度A | 273.512 | 1 | 273.512 | 102.0584 | < 0.0001 | + + |
| 反应时间B | 142.761 | 1 | 142.761 | 53.26993 | < 0.0001 | + + |
| 异丁醇钾与甲基异丁基酮摩尔比C | 903.9352 | 1 | 903.9352 | 337.2949 | < 0.0001 | + + |
| 溶剂用量D | 8.619075 | 1 | 8.619075 | 3.216127 | 0.0945 | |
| AB | 0.81 | 1 | 0.81 | 0.302244 | 0.5911 | |
| AC | 17.1396 | 1 | 17.1396 | 6.395481 | 0.0241 | + |
| AD | 2.5×10-5 | 1 | 2.5×10-5 | 9.33×10-6 | 0.9976 | |
| BC | 8.4681 | 1 | 8.4681 | 3.159792 | 0.0972 | |
| BD | 3.591025 | 1 | 3.591025 | 1.339957 | 0.2664 | |
| CD | 0.893025 | 1 | 0.893025 | 0.333224 | 0.5729 | |
| A2 | 378.1677 | 1 | 378.1677 | 141.1097 | < 0.0001 | + + |
| B2 | 64.48482 | 1 | 64.48482 | 24.0619 | 0.0002 | + + |
| C2 | 298.6574 | 1 | 298.6574 | 111.4412 | < 0.0001 | + + |
| D2 | 25.18619 | 1 | 25.18619 | 9.397988 | 0.0084 | + + |
| 残差 | 37.51937 | 14 | 2.679955 | |||
| 失拟项 | 31.64865 | 10 | 3.164865 | 2.156373 | 0.2388 | |
| 纯误差 | 5.87072 | 4 | 1.46768 | |||
| 总和 | 1973.778 | 28 |
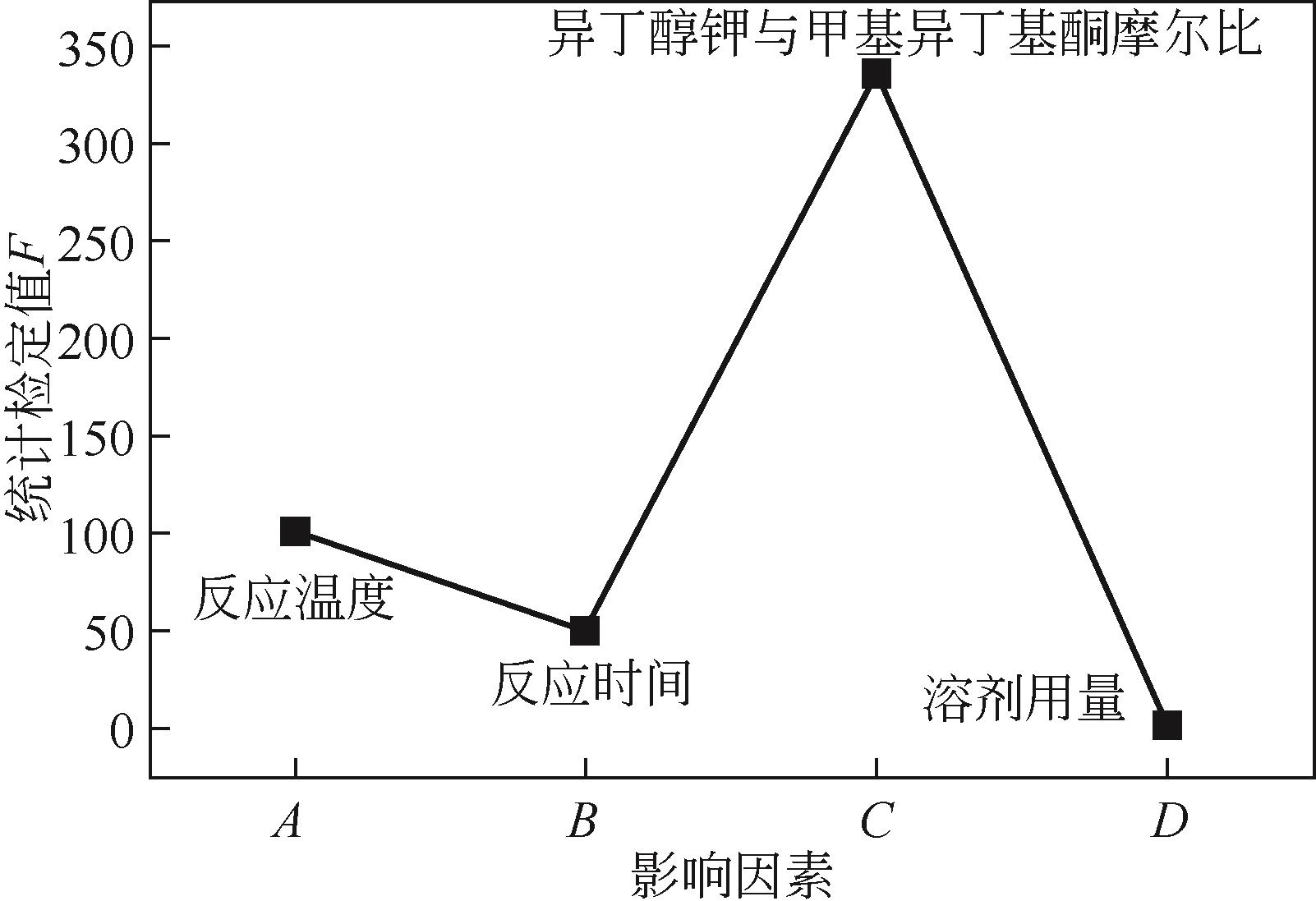
表3 方差分析表
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 回归方程 | 1936.259 | 14 | 138.3042 | 51.60691 | < 0.0001 | + + |
| 反应温度A | 273.512 | 1 | 273.512 | 102.0584 | < 0.0001 | + + |
| 反应时间B | 142.761 | 1 | 142.761 | 53.26993 | < 0.0001 | + + |
| 异丁醇钾与甲基异丁基酮摩尔比C | 903.9352 | 1 | 903.9352 | 337.2949 | < 0.0001 | + + |
| 溶剂用量D | 8.619075 | 1 | 8.619075 | 3.216127 | 0.0945 | |
| AB | 0.81 | 1 | 0.81 | 0.302244 | 0.5911 | |
| AC | 17.1396 | 1 | 17.1396 | 6.395481 | 0.0241 | + |
| AD | 2.5×10-5 | 1 | 2.5×10-5 | 9.33×10-6 | 0.9976 | |
| BC | 8.4681 | 1 | 8.4681 | 3.159792 | 0.0972 | |
| BD | 3.591025 | 1 | 3.591025 | 1.339957 | 0.2664 | |
| CD | 0.893025 | 1 | 0.893025 | 0.333224 | 0.5729 | |
| A2 | 378.1677 | 1 | 378.1677 | 141.1097 | < 0.0001 | + + |
| B2 | 64.48482 | 1 | 64.48482 | 24.0619 | 0.0002 | + + |
| C2 | 298.6574 | 1 | 298.6574 | 111.4412 | < 0.0001 | + + |
| D2 | 25.18619 | 1 | 25.18619 | 9.397988 | 0.0084 | + + |
| 残差 | 37.51937 | 14 | 2.679955 | |||
| 失拟项 | 31.64865 | 10 | 3.164865 | 2.156373 | 0.2388 | |
| 纯误差 | 5.87072 | 4 | 1.46768 | |||
| 总和 | 1973.778 | 28 |
| 循环次数 | 甲基异丁基酮转化率/% | S104收率/% |
|---|---|---|
| 新配氢氧化钾溶液 | 92.46 | 85.91 |
| 1 | 89.14 | 83.19 |
| 2 | 90.29 | 83.77 |
| 3 | 93.82 | 87.16 |
| 4 | 93.11 | 86.87 |
| 5 | 91.22 | 84.52 |
表4 氢氧化钾循环使用效果
| 循环次数 | 甲基异丁基酮转化率/% | S104收率/% |
|---|---|---|
| 新配氢氧化钾溶液 | 92.46 | 85.91 |
| 1 | 89.14 | 83.19 |
| 2 | 90.29 | 83.77 |
| 3 | 93.82 | 87.16 |
| 4 | 93.11 | 86.87 |
| 5 | 91.22 | 84.52 |
| 1 | GALGOCI E C, CHAN S Y, KHALIL Y. Innovative, gemini-type molecular defoamer technology for improved coating aesthetics[J]. JCT Research, 2006, 3(1): 77-85. |
| 2 | LEE F J. Acetylenic glycol-based surfactants for use in fountain solutions[J]. American Ink Maker, 1998,76(9): 30-36. |
| 3 | ZHANG P, KING D M, KARWACKI E J. Evaluating the impact of an acetylenic diol-type surfactant on DUV lithography performance[J]. Micro, 2002,20(6): 51-55. |
| 4 | JIAO Qingbin, ZHU Chunlin, TAN Xin, et al. The effect of ultrasonic vibration and surfactant additive on fabrication of 53.5gr/mm silicon echelle grating with low surface roughness in alkaline KOH solution[J]. Ultrasonics Sonochemistry, 2018, 40: 937-943. |
| 5 | TEDESCHI R J. Acetylene-based chemicals from coal and other natural resources[M]. New York: M. Dekker, 1982. |
| 6 | KELLAND M A, DIRDAL E G. Powerful synergy of acetylenic diol surfactants with kinetic hydrate inhibitor polymers—Choosing the correct synergist aqueous solubility[J]. Energy & Fuels, 2021, 35(19): 15721-15727. |
| 7 | CHRISTINE Louis. New additives for waterbased coatings: A new class of defoamers is born! [J]. Pitture e Vernici, European Coatings, 2003,79(20): 7-19. |
| 8 | DOUGLAS Bohn. The global waterborne coatings market keeps growing[J]. European Coatings Journal, 2021, 10: 16-17. |
| 9 | TROFIMOV B A, SCHMIDT E Y E. Reactions of acetylenes in superbasic media. recent advances[J]. Russian Chemical Reviews, 2014, 83(7): 600-619. |
| 10 | TROFIMOV B A, NOSYREVA V V, MAL'KINA A G. The role of potassium cation in the favorskii ethynylation of acetone: Effect of dibenzo-18-crown-6[J]. Russian Journal of Organic Chemistry, 2005, 41(9): 1254-1259. |
| 11 | DANILKINA N, VASIL'EVA A A, BALOVA I. A.E.Favorskii’s scientific legacy in modern organic chemistry: Prototropic acetylene-allene isomerization and the acetylene zipper reaction[J]. Russian Chemical Reviews, 2020, 89: 125-171. |
| 12 | TEDESCHI R J, WILSON M F, SCANLON J, et al. The reaction of alkali metal hydroxides with tertiary acetylenic carbinols and glycols[J]. The Journal of Organic Chemistry, 1963, 28(9): 2480-2483. |
| 13 | TEDESCHI R J. The mechanism of base-catalyzed ethynylation in donor solvents[J]. The Journal of Organic Chemistry, 1965, 30(9): 3045-3049. |
| 14 | 周飞, 陶川东, 李杰灵, 等. 游离碱对合成2,5-二甲基己炔二醇的影响[J]. 华东科技: 综合, 2018, 1(12): 399-401. |
| ZHOU Fei, TAO Chuandong, LI Jieling, et al. Effect of free base on the synthesis of 2,5 -dimethylhexynediol[J]. East China Technology: Comprehensive, 2018, 1(12): 399-401. | |
| 15 | 方善伦, 王丽蓉, 张征林. 叔炔醇的合成及用途[J]. 天然气化工(Cl化学与化工), 2003, 28(6): 43-47. |
| FANG Shanlun, WANG Lirong, ZHANG Zhenglin. Synthesis and application of tertiary alkynols[J]. Natural Gas Chemical Industry (C1 Chemistry and Technology), 2003, 28(6): 43-47. | |
| 16 | SHACHAT N, BAGNELL J J. Catalytic ethynylation of Ketones[J]. The Journal of Organic Chemistry, 1962, 27(5): 1498-1504. |
| 17 | GERHARD Thelen. Process for the manufacture of alkynediols by reaction of ketones with acetylene: US4960959[P]. 1990-10-02. |
| 18 | 齐建国, 周全凯, 金凤龙. 一种合成癸炔二醇的工艺: CN102964216A[P]. 2013-03-13. |
| QI Jianguo, ZHOU Quankai, JIN Fenglong. Decyne glycol synthesizing technology: CN102964216A[P]. 2013-03-13. | |
| 19 | 王立峰, 杨智, 孙健. 一种炔二醇系列产品的清洁生产工艺: CN103304376A[P]. 2013-09-18. |
| WANG Lifeng, YANG Zhi, SUN Jian. Clean production process of alkyne diol serial products: CN103304376A[P]. 2013-09-18. | |
| 20 | 程霜莉, 张一甫, 黄波. 一种炔二醇系列产品的生产方法: CN103242140A[P]. 2013-08-14. |
| CHENG Shuangli, ZHANG Yifu, HUANG Bo. Production method of acetylenic diol series products: CN103242140A[P]. 2013-08-14. | |
| 21 | 钟传蓉, 卢艾. 炔醇的应用与生产[J]. 油田化学, 2000, 17(3): 285-288. |
| ZHONG Chuanrong, LU Ai. The uses and production of alkynols: A review[J]. Oilfield Chemistry, 2000, 17(3): 285-288. | |
| 22 | LI Sifang. Manufacture of fine chemicals from acetylene[M]. Berlin: De Gruyter, 2021. |
| 23 | 芦艾, 饶增科. 醇钾催化合成甲基戊炔醇[J]. 化学工程师, 1998, 12(5): 5-7. |
| LU Ai, RAO Zengke. Synthesis of methyl pentynol with potassium alcoholate used as catalyst[J]. Chemical Engineer, 1998, 12(5): 5-7. | |
| 24 | KINDLER Alois, BRUNNER Melanie, TRAGUT Christian, et al. Method for producing alkyne diols: US6297407[P]. 2001-10-02. |
| 25 | 刘星玉. 2,5-二甲基己炔二醇的反应工程开发[J]. 天然气化工(C1化学与化工), 1996, 21(6): 36-40. |
| LIU Xingyu. Reaction engineering development of 2,5 dimethyl hexynediol[J]. Natural Gas Chemical Industry(C1 Chemistry and Technology), 1996, 21(6): 36-40. | |
| 26 | BADISCHE Anilin, SODA Fabric, AKTIENGESELL Schaft. Production of tertiary acetylenic glycols by reaction of acetylene with ketones: GB1329815A[P]. 1973-09-12. |
| 27 | BADISCHE Anilin, SODA Fabric, AKTIENGESELL Schaft. Production of acetylene glycols: GB1354011A[P]. 1974-5-22. |
| 28 | 刘栋昌, 李世模. 酮的乙炔化反应的化学平衡[J]. 成都科技大学学报, 1993, 25(6): 31-39. |
| LIU Dongchang, LI Shimo. Chemical equilibrium of the ethynation of ketones[J]. Journal of Chengdu University of Science and Technology, 1993, 25(6): 31-39. | |
| 29 | 方聪, 刘怡雪, 黎四芳. 爱德万甜中间体3-羟基-4-甲氧基肉桂醛的合成[J]. 化工进展, 2022, 41(11): 6053-6060. |
| FANG Cong, LIU Yixue, LI Sifang. Synthesis of 3-hydroxy-4-methoxycinnamaldehyde, an intermediate of advantame[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6053-6060. | |
| 30 | HORT E V, TAYLOR P. Kirk-Othmer encyclopedia of chemical technology, Vol. 1[M]. 5th ed. New Jersey: John Wiley and Sons, 2003. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [5] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [6] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [7] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [8] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [9] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [10] | 赵巍, 赵德银, 李世瀚, 刘洪达, 孙进, 郭艳秋. 三嗪型天然气管道缓蚀型减阻剂合成与应用[J]. 化工进展, 2023, 42(S1): 391-399. |
| [11] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [12] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [13] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [14] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [15] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||