化工进展 ›› 2023, Vol. 42 ›› Issue (11): 5764-5775.DOI: 10.16085/j.issn.1000-6613.2022-2267
多孔高硫聚合物的制备及其在汞吸附中的应用
- 中国林业科学研究院林产化学工业研究所,生物质化学利用国家工程实验室,江苏省生物质能源与材料重点实验室,江苏 南京 210042
-
收稿日期:2022-12-06修回日期:2023-01-20出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:刘鹤 -
作者简介:郭丽珍(1994—),女,硕士,实习研究员,研究方向为林业生物质资源的化学利用。E-mail:guolizhen2014@163.com。 -
基金资助:国家自然科学基金(32071708)
Preparation of the porous high sulfur polymers and its application in mercury adsorption
GUO Lizhen( ), LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He(
), LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He( )
)
- National Engineering Laboratory for Biomass Chemical Utilization, Key Laboratory of Biomass Energy and Materials, Institute of Chemical Industry of Forest Products CAF, Nanjing 210042, Jiangsu, China
-
Received:2022-12-06Revised:2023-01-20Online:2023-11-20Published:2023-12-15 -
Contact:LIU He
摘要:
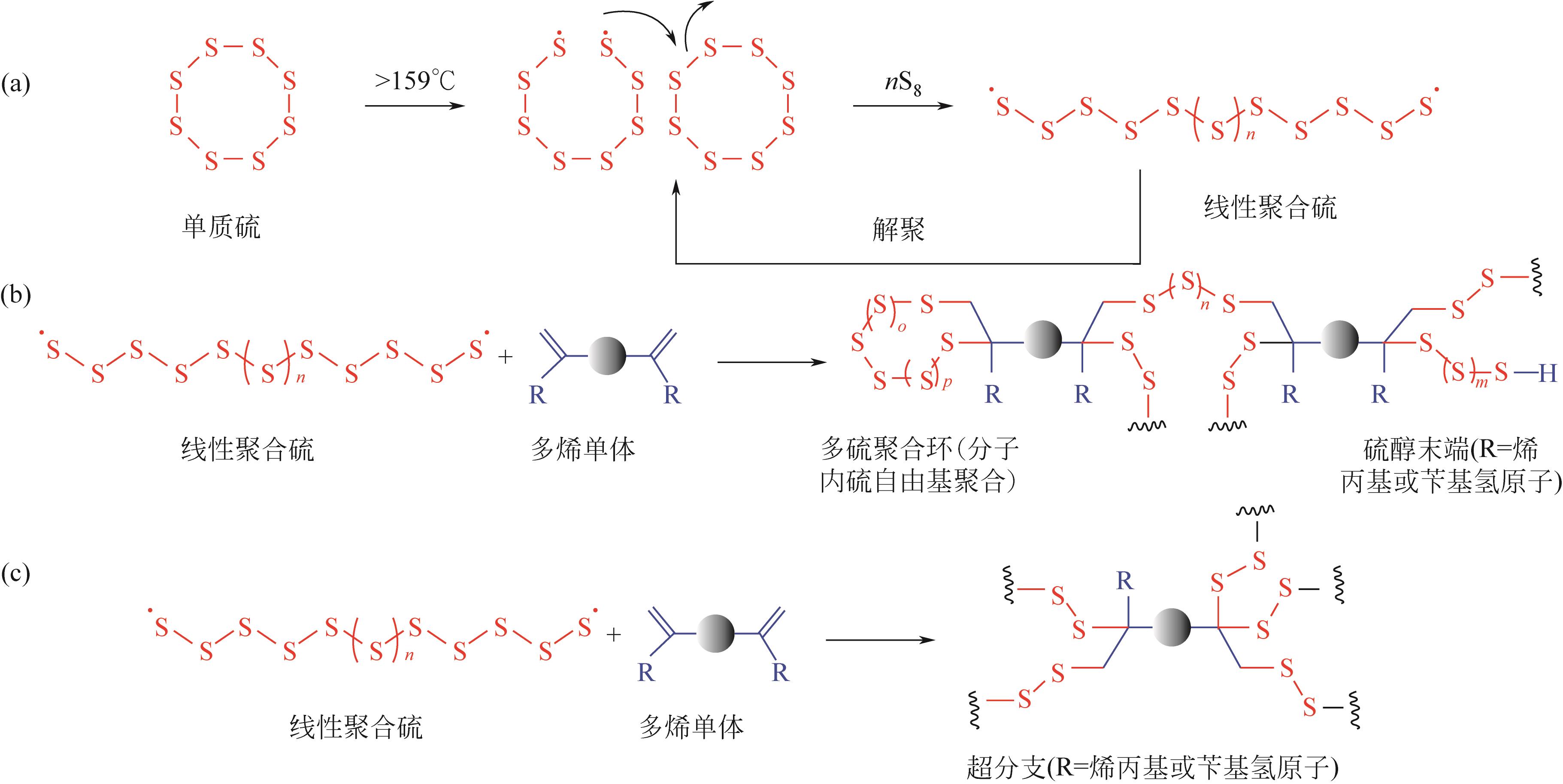
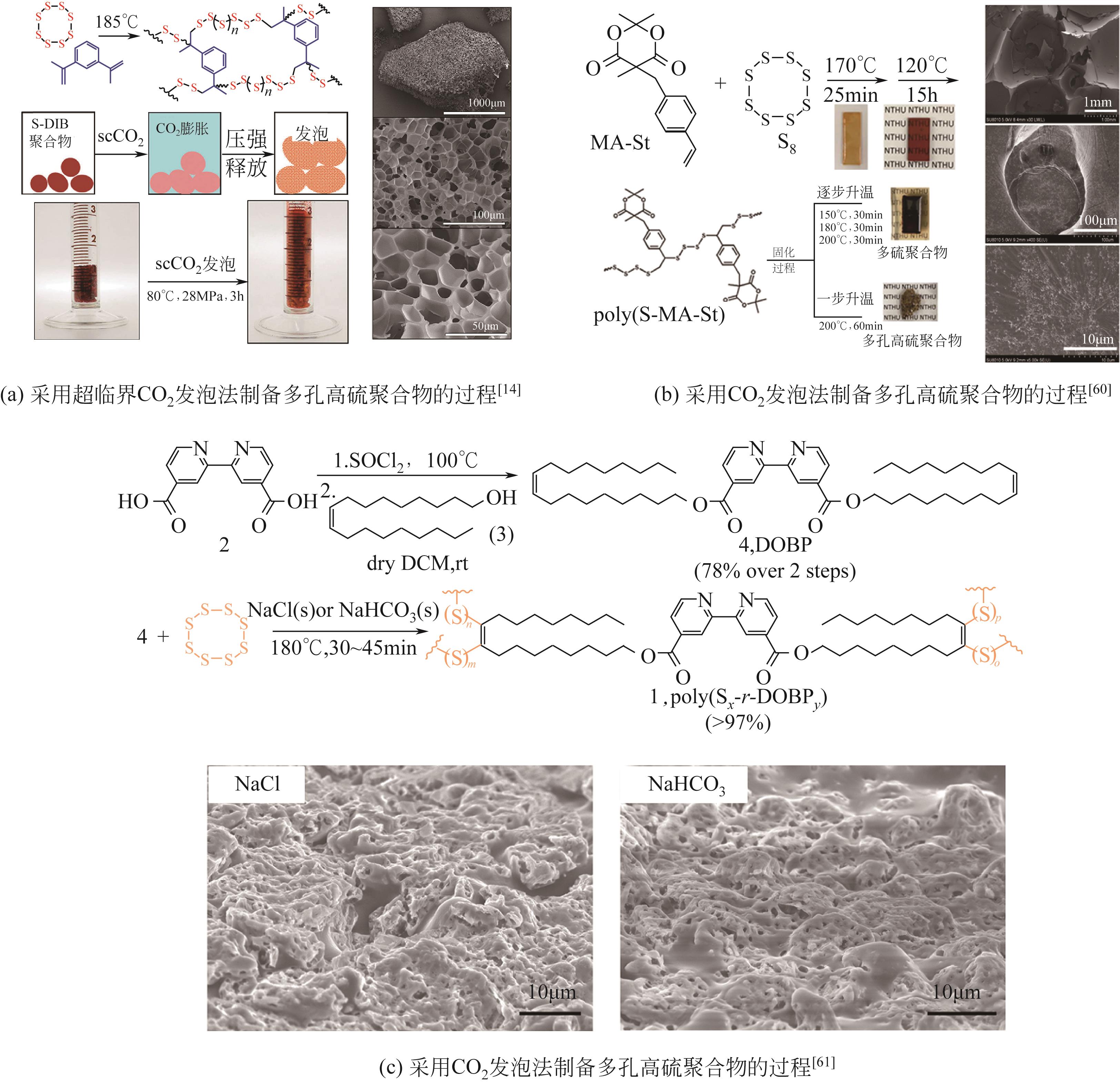
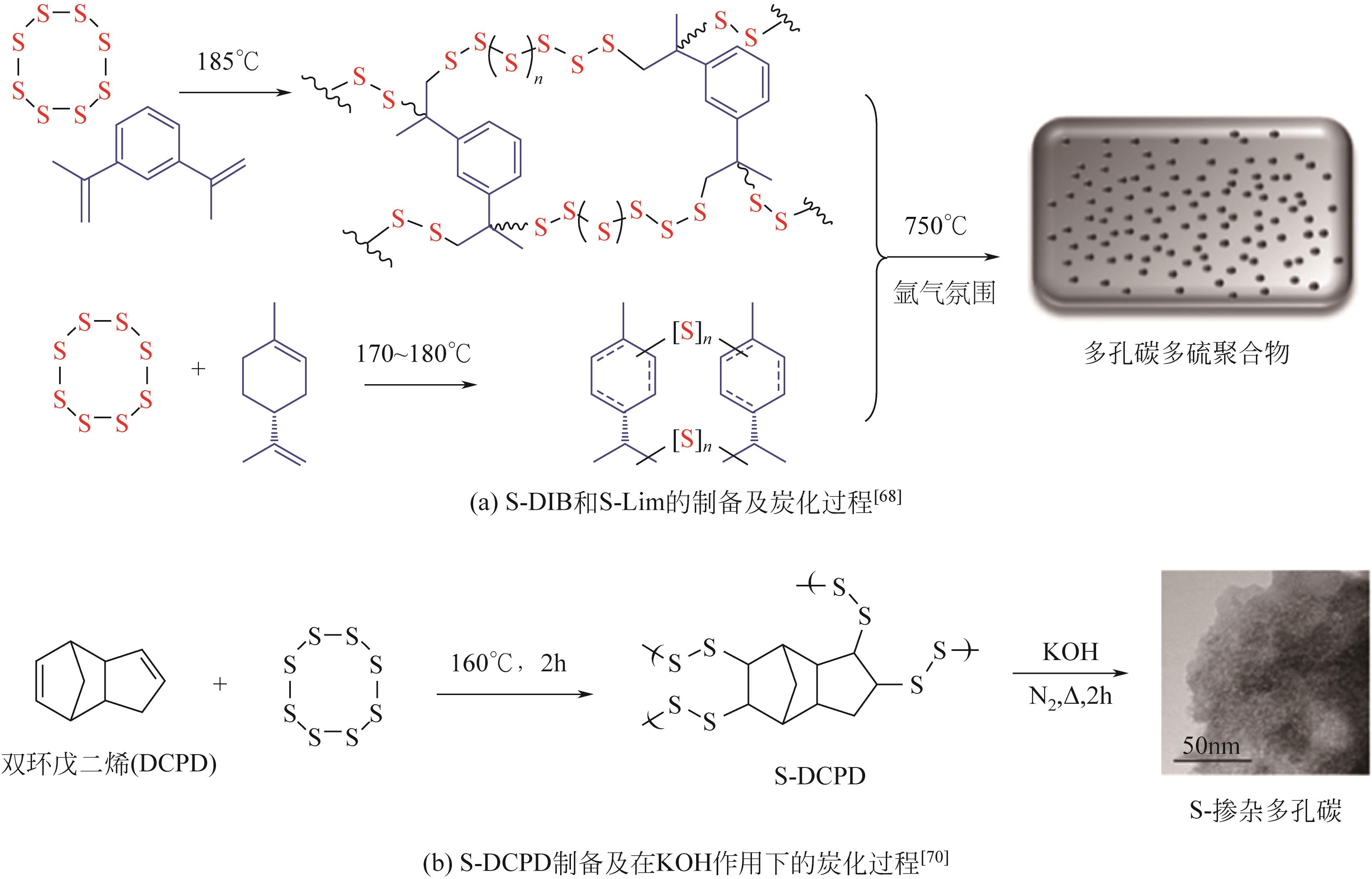
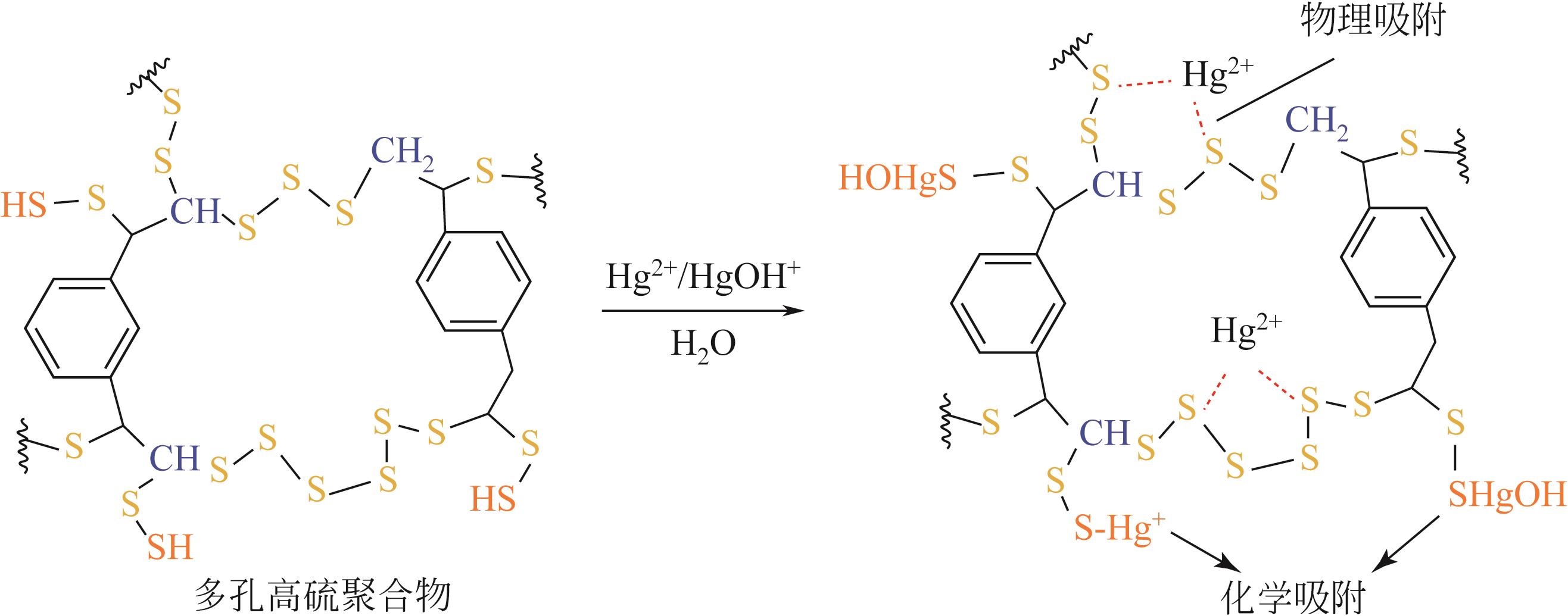
随着人类社会工业的快速发展,汞排放对生态环境及人类健康带来了巨大的隐患。汞处理常用方法包括化学沉淀法、离子交换法、吸附法等方法。其中,化学沉淀法虽可以有效去除废水中大部分的汞,但硫化物用量难以控制,同时易引起水质硬化和二次污染;离子交换法处理的水质高、设备简单、汞吸收速度快且无二次污染,但因受交换剂种类、用量和成本等限制而尚未被广泛推广使用;吸附法通常不会向需处理的水体中引入新污染物,并且具有较高的汞去除率,同时易操作、选择性好,因此其相关技术的研究开发更具潜力。由逆硫化法制备的聚合物——硫系有机/无机聚合物(CHIPs)是一种新型高效的汞吸附剂,为解决汞污染提供了新思路。本文介绍了逆硫化法的基本原理,概括了利用致孔剂、二氧化碳发泡、静电纺丝、碳化等制备多孔CHIPs的不同方法,归纳了多孔CHIPs的汞吸附机理以及在汞吸附领域的应用研究进展,并展望了多孔CHIPs的发展前景,指出具有生物相容性和可降解性的多孔CHIPs将成为下一步研究重点,同时多孔CHIPs的毒性探究、在大气和土壤中的汞吸附应用也将成为重要的研究方向之一,从而为多孔CHIPs在汞吸附领域的应用拓宽新思路。
中图分类号:
引用本文
郭丽珍, 林详宇, 董阜豪, 王倬敏, 刘鹤. 多孔高硫聚合物的制备及其在汞吸附中的应用[J]. 化工进展, 2023, 42(11): 5764-5775.
GUO Lizhen, LIN Xiangyu, DONG Fuhao, WANG Zhuomin, LIU He. Preparation of the porous high sulfur polymers and its application in mercury adsorption[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5764-5775.
制备 方法 | 孔径 | 比表面积 | 吸附 等温线 | 金属吸附量 | 参考 文献 |
|---|---|---|---|---|---|
| 致孔剂 | 100~200μm | — | Ⅰ型 | 119~470μg/g | [ |
二氧化碳 发泡 | 0.2~20μm | — | — | (52±1.8)mg/g | [ |
| 静电纺丝 | <1μm | 2.35m2/g | Ⅱ型 | — | [ |
| 炭化 | <2nm和 2~50nm | >110m2/g | Ⅰ和Ⅱ型 | 1.1~187mg/g | [ |
表1 不同方法制备的多孔CHIPs的性能
制备 方法 | 孔径 | 比表面积 | 吸附 等温线 | 金属吸附量 | 参考 文献 |
|---|---|---|---|---|---|
| 致孔剂 | 100~200μm | — | Ⅰ型 | 119~470μg/g | [ |
二氧化碳 发泡 | 0.2~20μm | — | — | (52±1.8)mg/g | [ |
| 静电纺丝 | <1μm | 2.35m2/g | Ⅱ型 | — | [ |
| 炭化 | <2nm和 2~50nm | >110m2/g | Ⅰ和Ⅱ型 | 1.1~187mg/g | [ |
| 1 | WU Y, WANG S X, STREETS D G, et al. Trends in anthropogenic mercury emissions in China from 1995 to 2003[J]. Environmental Science & Technology, 2006, 40(17): 5312-5318. |
| 2 | CLARKSON T W, MAGOS L. The toxicology of mercury and its chemical compounds[J]. Critical Reviews in Toxicology, 2006, 36(8): 609-662. |
| 3 | ULLRICH S M, TANTON T W, ABDRASHITOVA S A. Mercury in the aquatic environment: A review of factors affecting methylation[J]. Critical Reviews in Environmental Science and Technology, 2001, 31(3): 241-293. |
| 4 | ZAHIR F, RIZWI S J, HAQ S K, et al. Low dose mercury toxicity and human health[J]. Environmental Toxicology and Pharmacology, 2005, 20(2): 351-360. |
| 5 | BECKERS Felix, RINKLEBE Joerg. Cycling of mercury in the environment: Sources, fate, and human health implications: A review[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(9): 693-794. |
| 6 | O'CONNOR D, HOU D, OK Y S, et al. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review[J]. Environment International, 2019, 126: 747-761. |
| 7 | ARIYA P A, AMYOT M, DASTOOR A, et al. Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: A review and future directions[J]. Chemical Reviews, 2015, 115(10): 3760-3802. |
| 8 | SELIN N E. Global change and mercury cycling: Challenges for implementing a global mercury treaty[J]. Environmental Toxicology and Chemistry, 2014, 33(6): 1202-1210. |
| 9 | LIN C J, PEHKONEN S O. The chemistry of atmospheric mercury: A review[J]. Atmospheric Environment, 1999, 33(13): 2067-2079. |
| 10 | WU C Y, MOURI H, CHEN S S, et al. Removal of trace-amount mercury from wastewater by forward osmosis[J]. Journal of Water Process Engineering, 2016, 14: 108-116. |
| 11 | CAI Chong, WANG Ran, LIU Shufeng, et al. Synthesis of self-assembled phytic acid-MXene nanocomposites via a facile hydrothermal approach with elevated dye adsorption capacities[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 589: 124468. |
| 12 | FENG Wenguo, KWON Seokjoon, FENG Xue, et al. Sulfur impregnation on activated carbon fibers through H2S oxidation for vapor phase mercury removal[J]. Journal of Environmental Engineering, 2006, 132(3): 292-300. |
| 13 | HSI H C, ROOD M J, ROSTAM-ABADI M, et al. Effects of sulfur impregnation temperature on the properties and mercury adsorption capacities of activated carbon fibers (ACFs)[J]. Environmental Science & Technology, 2001, 35(13): 2785-2791. |
| 14 | HASELL T, PARKER D J, JONES H A, et al. Porous inverse vulcanised polymers for mercury capture[J]. Chemical Communications, 2016, 52(31): 5383-5386. |
| 15 | BEAR J C, PEVELER W J, MCNAUGHTER P D, et al. Nanoparticle-sulphur “inverse vulcanisation” polymer composites[J]. Chemical Communications, 2015, 51(52): 10467-10470. |
| 16 | HOEFLING A, NGUYEN D T, LEE Y J, et al. A sulfur-eugenol allyl ether copolymer: A material synthesized via inverse vulcanization from renewable resources and its application in Li-S batteries[J]. Materials Chemistry Frontiers, 2017, 1(9): 1818-1822. |
| 17 | HOEFLING A, LEE Y J, THEATO P. Sulfur-based polymer composites from vegetable oils and elemental sulfur: A sustainable active material for Li-S batteries[J]. Macromolecular Chemistry and Physics, 2017, 218(1): 1600303. |
| 18 | MANN M, KRUGER J E, ANDARI F, et al. Sulfur polymer composites as controlled-release fertilisers[J]. Organic & Biomolecular Chemistry, 2019, 17(7): 1929-1936. |
| 19 | DIRLAM P T, SIMMONDS A G, KLEINE T S, et al. Inverse vulcanization of elemental sulfur with 1,4-diphenylbutadiyne for cathode materials in Li-S batteries[J]. RSC Advances, 2015, 5(31): 24718-24722. |
| 20 | SMITH J A, WU X F, BERRY N G, et al. High sulfur content polymers: The effect of crosslinker structure on inverse vulcanization[J]. Journal of Polymer Science A: Polymer Chemistry, 2018, 56(16): 1777-1781. |
| 21 | WESTERMAN C R, JENKINS C L. Dynamic sulfur bonds initiate polymerization of vinyl and allyl ethers at mild temperatures[J]. Macromolecules, 2018, 51(18): 7233-7238. |
| 22 | KWON Minho, LEE Hongchan, LEE Seohui, et al. Dynamic covalent polymerization of chalcogenide hybrid inorganic/organic polymer resins with norbornenyl comonomers[J]. Macromolecular Research, 2020, 28(11): 1003-1009. |
| 23 | LIU Yanxia, CHEN Yidan, ZHANG Yagang, et al. Density-adjustable bio-based polysulfide composite prepared by inverse vulcanization and bio-based fillers[J]. Polymers, 2020, 12(9): 2127. |
| 24 | SMITH J A, GREEN S J, PETCHER S, et al. Crosslinker copolymerization for property control in inverse vulcanization[J]. Chemistry: A European Journal, 2019, 25(44): 10433-10440. |
| 25 | ZENG Shuaibo, LI Ligui, XIE Lihong, et al. Conducting polymers crosslinked with sulfur as cathode materials for high-rate, ultralong-life lithium-sulfur batteries[J]. ChemSusChem, 2017, 10(17): 3378-3386. |
| 26 | PARKER D J, JONES H A, PETCHER S, et al. Low cost and renewable sulfur-polymers by inverse vulcanisation, and their potential for mercury capture[J]. Journal of Materials Chemistry A, 2017, 5(23): 11682-11692. |
| 27 | NGUYEN T B. Elemental sulfur and molecular iodine as efficient tools for carbon-nitrogen bond formation through redox reactions[J]. Asian Journal of Organic Chemistry, 2017, 6(5): 477-491. |
| 28 | WRECZYCKI J, BIELIŃSKI D M, ANYSZKA R. Sulfur/organic copolymers as curing agents for rubber[J]. Polymers, 2018, 10(8): 870. |
| 29 | KUTNEY Gerald. Sulfur: History, technology, applications & industry[M]. Toronto: Chem. Tec. Publishing, 2007. |
| 30 | HU Jinghong, LEI Zhengdong, CHEN Zhangxin, et al. Effect of sulphur deposition on well performance in a sour gas reservoir[J]. The Canadian Journal of Chemical Engineering, 2018, 96(4): 886-894. |
| 31 | MILLER G W. Thermal analysis of polymers. VIII. Dilatometric and thermal optical behavior of sulfur[J]. Journal of Applied Polymer Science, 1971, 15(8): 1985-1994. |
| 32 | BOYD D A. Sulfur and its role in modern materials science[J]. Angewandte Chemie-International Edition, 2016, 55(50): 15486-15502. |
| 33 | NGUYEN T B. Recent advances in organic reactions involving elemental sulfur[J]. Advanced Synthesis & Catalysis, 2017, 359(7): 1066-1130. |
| 34 | GRIEBEL J J, GLASS R S, CHAR K, et al. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense[J]. Progress in Polymer Science, 2016, 58: 90-125. |
| 35 | WORTHINGTON M J, KUCERA R L, CHALKER J M. Green chemistry and polymers made from sulfur[J]. Green Chemistry, 2017, 19(12): 2748-2761. |
| 36 | DIEZ Sergei, HOEFLING Alexande, THEATO Patrick, et al. Mechanical and electrical properties of sulfur-containing polymeric materials prepared via inverse vulcanization[J]. Polymers, 2017, 9(2): 59. |
| 37 | CHUNG W J, GRIEBEL J J, KIM E T, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials[J]. Nature Chemistry, 2013, 5(6): 518-524. |
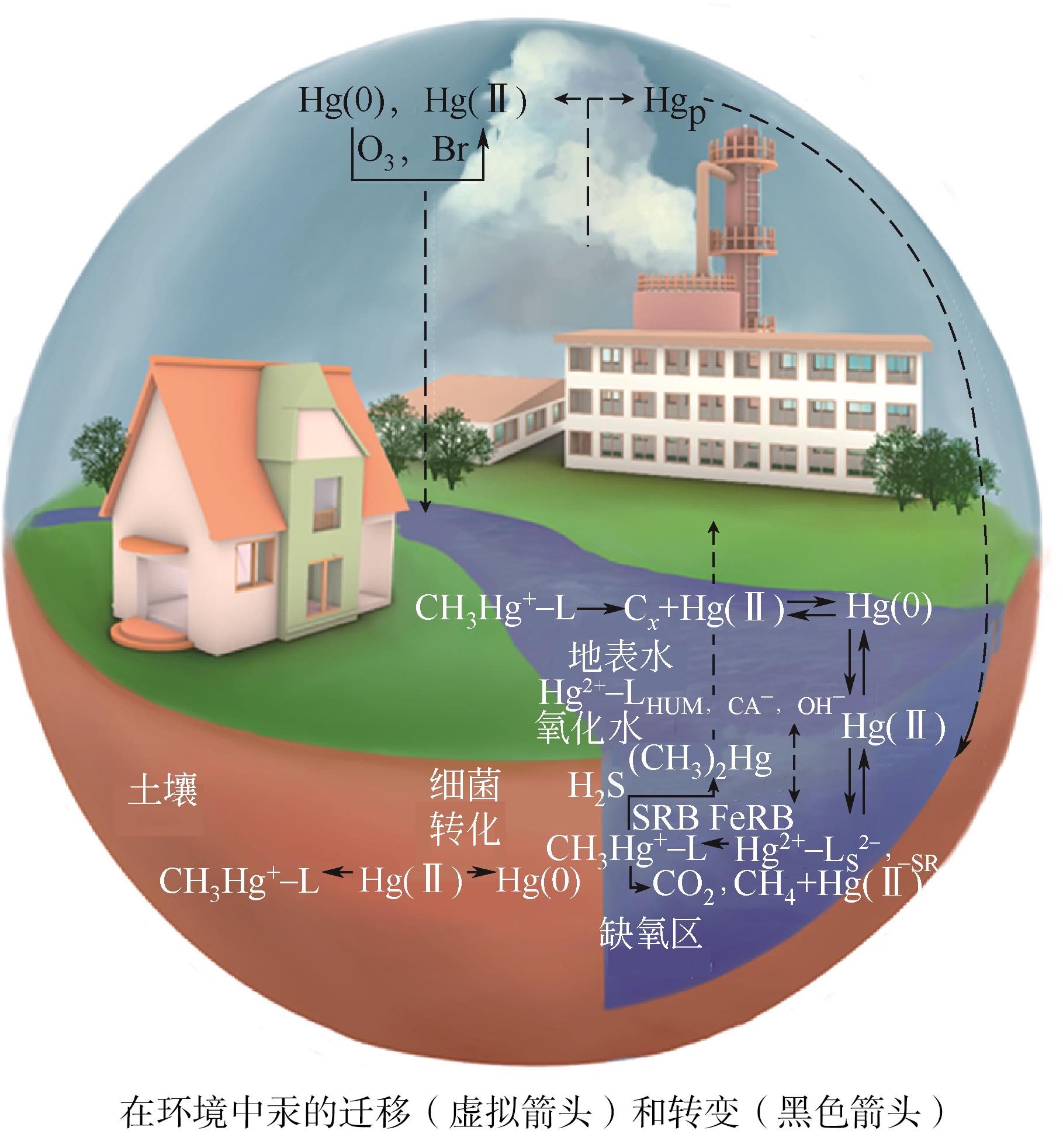
| 38 | BOYD D A, BAKER C C, MYERS J D, et al. ORMOCHALCs: Organically modified chalcogenide polymers for infrared optics[J]. Chemical Communications, 2016, 53(1): 259-262. |
| 39 | LUNDQUIST N A, TIKOALU A D, WORTHINGTON M J, et al. Reactive compression molding post-inverse vulcanization: A method to assemble, recycle, and repurpose sulfur polymers and composites[J]. Chemistry: A European Journal, 2020, 26(44): 10035-10044. |
| 40 | HERRERA C, YSINGA K J, JENKINS C L. Polysulfides synthesized from renewable garlic components and repurposed sulfur form environmentally friendly adhesives[J]. ACS Applied Materials & Interfaces, 2019, 11(38): 35312-35318. |
| 41 | PETCHER Samuel, ZHANG Bowen, HASELL Tom. Mesoporous knitted inverse vulcanised polymers[J]. Chemical Communications, 2021, 57(41): 5059-5062. |
| 42 | TIKOALU A D, LUNDQUIST N A, CHALKER J M. Mercury sorbents made by inverse vulcanization of sustainable triglycerides: The plant oil structure influences the rate of mercury removal from water[J]. Advanced Sustainable Systems, 2020, 4(3): 1900111. |
| 43 | GOMEZ I, LEONET O, BLAZQUEZ J A, et al. Inverse vulcanization of sulfur using natural dienes as sustainable materials for lithium-sulfur batteries[J]. ChemSusChem, 2016, 9(24): 3419-3425. |
| 44 | CHALKER J M, WORTHINGTON M J, LUNDQUIST N A, et al. Synthesis and applications of polymers made by inverse vulcanization[J]. Topics in Current Chemistry, 2019, 377(3): 16. |
| 45 | WU X F, SMITH J A, PETCHER S, et al. Catalytic inverse vulcanization[J]. Nature Communications, 2019, 10: 647. |
| 46 | KLEINE T S, NGUYEN N A, ANDERSON L E, et al. High refractive index copolymers with improved thermomechanical properties via the inverse vulcanization of sulfur and 1,3,5-triisopropenylbenzene[J]. ACS Macro Letters, 2016, 5(10): 1152-1156. |
| 47 | SING K S. Assessment of surface area by gas adsorption[M]. France: Adsorption by Powders and Porous Solids, 2013: 237-268. |
| 48 | TIEN Chi. Introduction to adsorption: Chapter 3-Adsorption equilibrium relationships, isotherm expressions, their determinations, and predictions[M]. USA: Elsevier, 2019: 23-85. |
| 49 | BARTON T J, BULL LUCY M, KLEMPERER W G, et al. Tailored porous materials[J]. Chemistry of Materials, 1999, 11(10): 2633-2656. |
| 50 | SING K S W, EVERETT D H, HAUL R A W, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 51 | WORTHINGTON M J, KUCERA R L, ALBUQUERQUE I S, et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils[J]. Chemistry—A European Journal, 2017, 23(64): 16219-16230. |
| 52 | LUNDQUIST N A, WORTHINGTON M J, ADAMSON N, et al. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water[J]. RSC Advances, 2018, 8(3): 1232-1236. |
| 53 | ABRAHAM A M, KUMAR S V, ALHASSAN S M. Porous sulphur copolymer for gas-phase mercury removal and thermal insulation[J]. Chemical Engineering Journal, 2018, 332: 1-7. |
| 54 | REN Zixuan, JIANG Xue, LIU Lingli, et al. Modification of high-sulfur polymer using a mixture porogen and its application as advanced adsorbents for Au( Ⅲ ) from wastewater[J]. Journal of Molecular Liquids, 2021, 328: 115437. |
| 55 | KOEBEL M, STRUTZ E O. Thermal and hydrolytic decomposition of urea for automotive selective catalytic reduction systems: thermochemical and practical aspects[J]. Industrial & Engineering Chemistry Research, 2003, 42(10): 2093-2100. |
| 56 | JACOBS L J, KEMMERE M F, KEURENTJES J T. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications[J]. Green Chemistry, 2008, 10(7): 731-738. |
| 57 | COOPER A I. Polymer synthesis and processing using supercritical carbon dioxide[J]. Journal of Materials Chemistry, 2000, 10(2): 207-234. |
| 58 | NALAWADE S P, PICCHIONI F, JANSSEN L P. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications[J]. Progress in Polymer Science, 2006, 31(1): 19-43. |
| 59 | PICCHIONI Francesco. Supercritical carbon dioxide and polymers: An interplay of science and technology[J]. Polymer International, 2014, 63(8): 1394-1399. |
| 60 | LIN H K, LAI Y S, LIU Y L. Cross-linkable and self-foaming polysulfide materials for repairable and mercury capture applications[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(4): 4515-4522. |
| 61 | KO L A, HUANG Y S, LIN Y A. Bipyridine-containing polysulfide materials for broad-spectrum removal of heavy metals from water[J]. ACS Applied Polymer Materials, 2021, 3(7): 3363-3372. |
| 62 | XUE Jiajia, WU Tong, DAI Yunqian, et al. Electrospinning and electrospun nanofibers: Methods, materials, and applications[J]. Chemical Reviews, 2019, 119(8): 5298-5415. |
| 63 | HUANG Yunpeng, MIAO Yue'e, LIU Tianxi. Electrospun fibrous membranes for efficient heavy metal removal[J]. Journal of Applied Polymer Science, 2014, 131(19): 40864. |
| 64 | WANJALE Santosh, BIRAJDAR Mallinath, Jyoti JOG, et al. Surface tailored PS/TiO2 composite nanofiber membrane for copper removal from water[J]. Journal of Colloid and Interface Science, 2016, 469: 31-37. |
| 65 | SANG Yimin, LI Fasheng, GU Qingbao, et al. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane[J]. Desalination, 2008, 223(1/2/3): 349-360. |
| 66 | THIELKE M W, BULTEMA L A, BRAUER D D, et al. Rapid mercury(Ⅱ) removal by electrospun sulfur copolymers[J]. Polymers, 2016, 8(7): 266. |
| 67 | LIMJUCO L A, NISOLA G M, PAROHINOG K J, et al. Water-insoluble hydrophilic polysulfides as microfibrous composites towards highly effective and practical Hg2+ capture[J]. Chemical Engineering Journal, 2019, 378: 122216. |
| 68 | BEAR J C, MCGETTRICK J D, PARKIN I P, et al. Porous carbons from inverse vulcanised polymers[J]. Microporous and Mesoporous Materials, 2016, 232: 189-195. |
| 69 | MANN M, LUO X, TIKOALU A D, et al. Carbonisation of a polymer made from sulfur and canola oil[J]. Chemical Communications, 2021, 57(51): 6296-6299. |
| 70 | LEE J M, PARKER D J, COOPER A I, et al. High surface area sulfur-doped microporous carbons from inverse vulcanised polymers[J]. Journal of Materials Chemistry A, 2017, 5(35): 18603-18609. |
| 71 | ZHANG Bowen, PETCHER Samuel, GAO Hui, et al. Magnetic sulfur-doped carbons for mercury adsorption[J]. Journal of Colloid and Interface Science, 2021, 603: 728-737. |
| 72 | 冯新斌, 陈玖斌, 付学吾, 等. 汞的环境地球化学研究进展[J]. 矿物岩石地球化学通报, 2013, 32(5): 503-530. |
| FENG Xinbin, CHEN Jiubin, FU Xuewu, et al. Progresses on environmental geochemistry of mercury[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2013, 32(5): 503-530. | |
| 73 | 孙阳昭, 陈扬, 蓝虹, 等. 中国汞污染的来源、成因及控制技术路径分析[J]. 环境化学, 2013, 32(6): 937-942. |
| SUN Yangzhao, CHEN Yang, LAN Hong, et al. Study on pollution sources, cause of mercury pollution and its control technical roadmap in China[J]. Environmental Chemistry, 2013, 32(6): 937-942. | |
| 74 | 张霞忠, 袁烈梅. 汞吸附研究进展[J]. 环境污染与防治, 2017, 39(5): 563-568. |
| ZHANG Xiazhong, YUAN Liemei. Research progress of mercury adsorption[J]. Environmental Pollution & Control, 2017, 39(5): 563-568. | |
| 75 | AKAY Sema, KAYAN Berkant, KALDERIS Dimitrios, et al. Poly(benzoxazine-co-sulfur): An efficient sorbent for mercury removal from aqueous solution[J]. Journal of Applied Polymer Science, 2017, 134(38): 45306. |
| 76 | RICE K M, WALKER E M, WU M Z, et al. Environmental mercury and its toxic effects[J]. Journal of Preventive Medicine and Public Health, 2014, 47(2): 74-83. |
| 77 | WADI V S, MITTAL H, FOSSO-KANKEU E, et al. Mercury removal by porous sulfur copolymers: Adsorption isotherm and kinetics studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 606: 125333. |
| 78 | WADI V K, JENA K K, KHAWAJA S Z, et al. NMR and EPR structural analysis and stability study of inverse vulcanized sulfur copolymers[J]. ACS Omega, 2018, 3(3): 3330-3339. |
| 79 | XU Yang, WANG Tianqi, HE Zidong, et al. A polymerization-cutting strategy: Self-protection synthesis of thiol-based nanoporous adsorbents for efficient mercury removal[J]. Chemistry: A European Journal, 2018, 24(54): 14436-14441. |
| 80 | WANG Qingfeng, LIU Yue, YANG Zhenmei, et al. Study of mercury re-emission in a simulated WFGD solution containing thiocyanate and sulfide ions[J]. Fuel, 2014, 134: 588-594. |
| 81 | DIRINGER S E, FEINGOLD B J, ORTIZ E J, et al. River transport of mercury from artisanal and small-scale gold mining and risks for dietary mercury exposure in Madre de Dios, Peru[J]. Environmental Science Processes & Impacts, 2015, 17(2): 478-487. |
| 82 | TCHOUNWOU P B, AYENSU W K, NINASHVILI N, et al. Environmental exposure to mercury and its toxicopathologic implications for public health[J]. Environmental Toxicology, 2003, 18(3): 149-175. |
| 83 | MASON R P, ROLFHUS K R, FITZGERALD W F. Methylated and elemental mercury cycling in surface and deep ocean waters of the North Atlantic[J]. Water, Air, and Soil Pollution, 1995, 80(1): 665-677. |
| 84 | CROCKETT M P, EVANS A M, WORTHINGTON M J, et al. Sulfur-limonene polysulfide: A material synthesized entirely from industrial by-products and its use in removing toxic metals from water and soil[J]. Angewandte Chemie-International Edition, 2016, 55(5): 1714-1718. |
| [1] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [2] | 张婷婷, 潘大伟, 巨晓洁, 刘壮, 谢锐, 汪伟, 褚良银. Hg2+响应型智能凝胶检测光栅的构建与性能[J]. 化工进展, 2023, 42(8): 4143-4152. |
| [3] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [4] | 陈森, 殷鹏远, 杨证禄, 莫一鸣, 崔希利, 锁显, 邢华斌. 功能固体材料智能合成研究进展[J]. 化工进展, 2023, 42(7): 3340-3348. |
| [5] | 毛梦雷, 孟令玎, 高蕊, 孟子晖, 刘文芳. 多孔框架材料固定化酶研究进展[J]. 化工进展, 2023, 42(5): 2516-2535. |
| [6] | 范昀培, 金晶, 刘敦禹, 王静杰, 刘秋祺, 许开龙. CaSO4载氧体在煤气化化学链燃烧中的脱汞[J]. 化工进展, 2023, 42(3): 1638-1648. |
| [7] | 孔祥如, 张肖阳, 孙鹏翔, 崔琳, 董勇. 直接空气捕碳固体多孔材料的研究进展[J]. 化工进展, 2023, 42(3): 1471-1483. |
| [8] | 金鑫, 李玉姗, 解青青, 王梦雨, 夏星帆, 杨朝合. 多孔材料催化丙酮缩甘油合成研究进展[J]. 化工进展, 2023, 42(2): 731-743. |
| [9] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [10] | 刘战剑, 杨金月, 景境, 张曦光, 汪怀远. 三维超浸润多孔材料在油水分离中的研究进展[J]. 化工进展, 2023, 42(1): 310-320. |
| [11] | 曾军建, 赵基钢. 乙炔氢氯化金基无汞催化剂的研究进展[J]. 化工进展, 2022, 41(7): 3589-3596. |
| [12] | 狄冠丞, 周强, 陶信, 尚瑜, 宋涛, 卢平, 徐贵玲. 掺硫介孔炭的制备及其汞脱除特性[J]. 化工进展, 2022, 41(5): 2761-2769. |
| [13] | 孔祥宇, 谢亮, 王延民, 翟尚鹏, 王建国. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| [14] | 高天, 张伊黎, 熊卓, 赵永椿, 张军营. 改性氧化钛光催化氧化单质汞性能及其影响因素研究进展[J]. 化工进展, 2022, 41(2): 690-700. |
| [15] | 梁格, 黄翔峰, 刘婉琪, 熊永娇, 彭开铭. 超疏水三维多孔材料在乳化液油水分离中的应用研究进展[J]. 化工进展, 2022, 41(12): 6557-6572. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||