化工进展 ›› 2022, Vol. 41 ›› Issue (2): 666-681.DOI: 10.16085/j.issn.1000-6613.2021-0658
单原子位点催化剂及其电催化应用研究进展
竹涛1( ), 韩一伟1, 刘帅1, 谢蔚1, 苑博1, 宋慧平2, 程芳琴2
), 韩一伟1, 刘帅1, 谢蔚1, 苑博1, 宋慧平2, 程芳琴2
- 1.中国矿业大学(北京)大气环境管理与污染控制研究所,北京 100083
2.山西大学资源与环境工程研究所,山西 太原 030006
-
收稿日期:2021-03-30修回日期:2021-06-14出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:竹涛 -
作者简介:竹涛(1979—),男,博士,教授,博士生导师,研究方向为大气环境管理与污染控制。E-mail:bamboozt@cumtb.edu.cn 。 -
基金资助:山西省揭榜招标项目(20191101007);国家重点研发计划(2019YFC1805505)
Progress in electrocatalysis by single-atom site catalysts
ZHU Tao1( ), HAN Yiwei1, LIU Shuai1, XIE Wei1, YUAN Bo1, SONG Huiping2, CHEGN Fangqin2
), HAN Yiwei1, LIU Shuai1, XIE Wei1, YUAN Bo1, SONG Huiping2, CHEGN Fangqin2
- 1.Institute of Atmospheric Environmental Management and Pollution Control, China University of Mining and Technology-Beijing, Beijing 100083, China
2.Institute of Resources and Environment Engineering of Shanxi University, Taiyuan 030006, Shanxi, China
-
Received:2021-03-30Revised:2021-06-14Online:2022-02-05Published:2022-02-23 -
Contact:ZHU Tao
摘要:
单原子位点催化剂作为新兴类别,由于具有接近100%的高效原子利用率,出色的活性、选择性和稳定性等优异特性,受到广泛的关注和研究。本文综述了单原子位点催化剂的最新研究成果及在电催化领域的应用。详细介绍了单原子位点催化剂的制备方法,包括“自下而上”合成策略中的共沉淀法、电化学沉淀法、原子层沉积法、光化学法和原子约束法等,“自上而下”合成策略中的高温原子迁移捕获法、高温热解法和悬挂键捕获法。分析了用于表征单原子位点催化剂的高角环形暗场透射扫描显微镜和X射线吸收光谱表征技术,进行单原子位点催化剂理论计算的密度泛函理论(DFT)和第一性原理。在电催化领域的应用主要包括氧还原反应(ORR)、氮还原反应(NRR)、CO2还原反应(CO2RR)、氢析出反应(HER)和氧析出反应(OER)。最后指出目前单原子位点催化剂存在无法大规模生产和催化机制不清晰等问题并给出相关建议,展望了单原子位点催化剂的发展前景,指出创新制备方法以实现稳定型单原子位点催化剂的大规模制备及工业应用,利用先进表征技术进一步明确单原子位点催化剂催化机制是未来发展的方向。
中图分类号:
引用本文
竹涛, 韩一伟, 刘帅, 谢蔚, 苑博, 宋慧平, 程芳琴. 单原子位点催化剂及其电催化应用研究进展[J]. 化工进展, 2022, 41(2): 666-681.
ZHU Tao, HAN Yiwei, LIU Shuai, XIE Wei, YUAN Bo, SONG Huiping, CHEGN Fangqin. Progress in electrocatalysis by single-atom site catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 666-681.
| 制备方法 | 催化剂 | 金属负载量(质量分数) | 应用 | 文献 |
|---|---|---|---|---|
| 共沉淀法 | Ni1/HAP-Ce | 0.5%~8.5%(ICP-AES) | DRM | [ |
| 电化学沉淀法 | Ir1/Co(OH)2 | 2.0%(ICP-AES) | 氢析出反应 | [ |
| Ni1/GD和Fe1/GD | 0.278%和0.680%(ICP-MS) | 氢析出反应 | [ | |
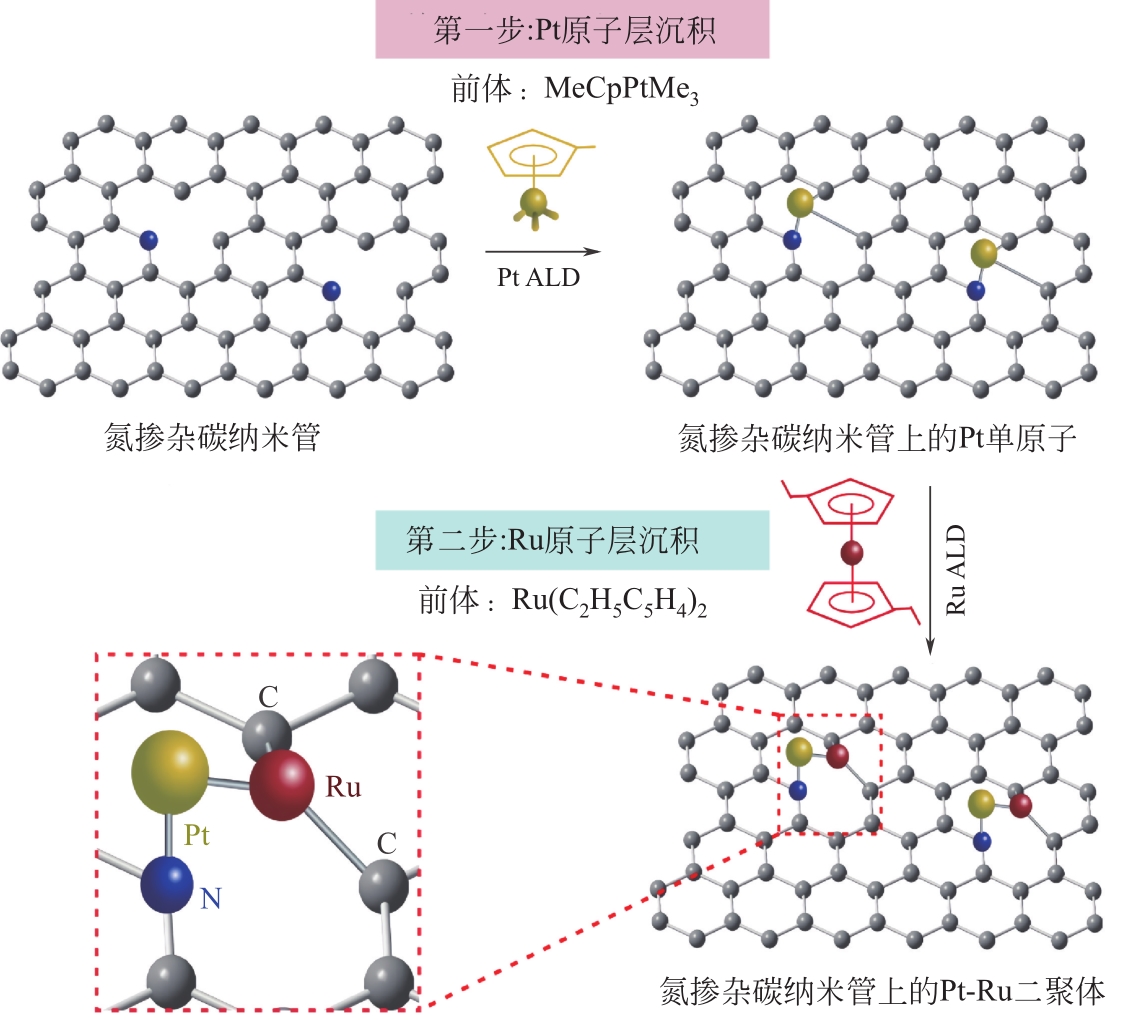
| 原子层沉积法 | Pt-Ru/NCNT | 0.9% Pt/0.31% Ru(ICP-OES) | 氢析出反应 | [ |
| Pt1/GNS | 10.5%(ICP-OES) | 甲醇氧化反应(MOR) | [ | |
| 光化学法 | Pd1/TiO2 | 1.5%(ICP-MS) | C | [ |
| Pt1/CoSe2-x | 2.25%(ICP-AES) | 氧析出反应 | [ | |
| Pt1/NPC | 3.8%(ICP-OES) | 燃料电池和制氢 | [ | |
| 原子约束法 | Er1/CN-NT | 2.5%(ICP-AES) | 光催化CO2还原反应 | [ |
| M/meso_S-C(M=Ru,Rh,Pd,Ir,Pt) | 10%(ICP-AES) | 甲酸氧化和喹啉加氢 | [ | |
| 配体法 | M-SASCs(M=Ni,Mn,Fe,Co,Cr,Cu,Zn,Ru,Pt及其组合) | 2.5%(ICP-OES) | CO2电化学还原为CO | [ |
| Fe1/N-C | 1.5%(ICP-OES) | 氧还原反应 | [ | |
| Pt1/MNSs | 12%(ICP-OES) | 光催化水制氢 | [ | |
| 模板辅助法 | Fe1/NSC | 0.87%(ICP-MS) | 氧还原反应 | [ |
| Co1/HNCS | 2.2%(ICP-OES) | 氧还原反应 | [ | |
| 高温原子迁移捕集法 | Cu1/NC | 0.45%(ICP-AES) | 氧还原反应 | [ |
| Pt1/Fe2O3 | 1.9%(ICP-AES) | 甲烷催化燃烧 | [ | |
| 高温热解 | Ru1/MAFO | 2.09%(ICP-OES) | N2O分解 | [ |
| 悬挂键捕获法 | Fe1/GO | 6.7%(ICP-AES) | 氧还原反应 | [ |
表1 单原子位点催化剂常见制备方法汇总
| 制备方法 | 催化剂 | 金属负载量(质量分数) | 应用 | 文献 |
|---|---|---|---|---|
| 共沉淀法 | Ni1/HAP-Ce | 0.5%~8.5%(ICP-AES) | DRM | [ |
| 电化学沉淀法 | Ir1/Co(OH)2 | 2.0%(ICP-AES) | 氢析出反应 | [ |
| Ni1/GD和Fe1/GD | 0.278%和0.680%(ICP-MS) | 氢析出反应 | [ | |
| 原子层沉积法 | Pt-Ru/NCNT | 0.9% Pt/0.31% Ru(ICP-OES) | 氢析出反应 | [ |
| Pt1/GNS | 10.5%(ICP-OES) | 甲醇氧化反应(MOR) | [ | |
| 光化学法 | Pd1/TiO2 | 1.5%(ICP-MS) | C | [ |
| Pt1/CoSe2-x | 2.25%(ICP-AES) | 氧析出反应 | [ | |
| Pt1/NPC | 3.8%(ICP-OES) | 燃料电池和制氢 | [ | |
| 原子约束法 | Er1/CN-NT | 2.5%(ICP-AES) | 光催化CO2还原反应 | [ |
| M/meso_S-C(M=Ru,Rh,Pd,Ir,Pt) | 10%(ICP-AES) | 甲酸氧化和喹啉加氢 | [ | |
| 配体法 | M-SASCs(M=Ni,Mn,Fe,Co,Cr,Cu,Zn,Ru,Pt及其组合) | 2.5%(ICP-OES) | CO2电化学还原为CO | [ |
| Fe1/N-C | 1.5%(ICP-OES) | 氧还原反应 | [ | |
| Pt1/MNSs | 12%(ICP-OES) | 光催化水制氢 | [ | |
| 模板辅助法 | Fe1/NSC | 0.87%(ICP-MS) | 氧还原反应 | [ |
| Co1/HNCS | 2.2%(ICP-OES) | 氧还原反应 | [ | |
| 高温原子迁移捕集法 | Cu1/NC | 0.45%(ICP-AES) | 氧还原反应 | [ |
| Pt1/Fe2O3 | 1.9%(ICP-AES) | 甲烷催化燃烧 | [ | |
| 高温热解 | Ru1/MAFO | 2.09%(ICP-OES) | N2O分解 | [ |
| 悬挂键捕获法 | Fe1/GO | 6.7%(ICP-AES) | 氧还原反应 | [ |

图5 HAADF-STEM图像和结构模型[58](a)Pt4Au96核壳结构的STEM及EDX图谱;(b)Pt7Au93核壳结构的STEM及EDX图谱;(c)Pt78Au22核壳结构的STEM及EDX图谱;(d)单原子覆盖的纳米颗粒模型;(e)PtAu表面的单原子Pt位点;(f)PtAu表面的少量Pt团簇;(g)PtAu表面的Pt壳层
| 1 | SAMANTARAY Manoja K, Valerio D’ELIA, PUMP Eva, et al. The comparison between single atom catalysis and surface organometallic catalysis[J]. Chemical Reviews, 2020, 120(2): 734-813. |
| 2 | DING Shipeng, HÜLSEY Max J, Javier PÉREZ-RAMÍREZ, et al. Transforming energy with single-atom catalysts[J]. Joule, 2019, 3(12): 2897-2929. |
| 3 | QIAO Botao, WANG Aiqin, YANG Xiaofeng, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 4 | QIN Ruixuan, LIU Pengxin, FU Gang, et al. Strategies for stabilizing atomically dispersed metal catalysts[J]. Small Methods, 2018, 2(1): 1700286. |
| 5 | LI Zhi, JI Shufang, LIU Yiwei, et al. Well-defined materials for heterogeneous catalysis: from nanoparticles to isolated single-atom sites[J]. Chemical Reviews, 2020, 120(2): 623-682. |
| 6 | JI Shufang, CHEN Yuanjun, WANG Xiaolu, et al. Chemical synthesis of single atomic site catalysts[J]. Chemical Reviews, 2020, 120(21): 11900-11955. |
| 7 | WANG Jing, LI Zhijun, WU Yuen, et al. Fabrication of single-atom catalysts with precise structure and high metal loading[J]. Advanced Materials, 2018, 30(48): 1801649. |
| 8 | LIU Jingyue. Catalysis by supported single metal atoms[J]. ACS Catalysis, 2017, 7(1): 34-59. |
| 9 | JAMES Trevor E, HEMMINGSON Stephanie L, CAMPBELL Charles T. Energy of supported metal catalysts: from single atoms to large metal nanoparticles[J]. ACS Catalysis, 2015, 5(10): 5673-5678. |
| 10 | LIU Lichen, CORMA Avelino. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 11 | WANG Aiqin, LI Jun, ZHANG Tao. Heterogeneous single-atom catalysis[J]. Nature Reviews Chemistry, 2018, 2(6): 65-81. |
| 12 | BABUCCI Melike, OZTUNA F Eylul SARAC, DEBEFVE Louise M, et al. Atomically dispersed reduced graphene aerogel-supported iridium catalyst with an iridium loading of 14.8%[J]. ACS Catalysis, 2019, 9(11): 9905-9913. |
| 13 | WANG Qi, HUANG Xiang, ZHAO Zhi Liang, et al. Ultrahigh-loading of Ir single atoms on NiO matrix to dramatically enhance oxygen evolution reaction[J]. Journal of the American Chemical Society, 2020, 142(16): 7425-7433. |
| 14 | DING Shipeng, GUO Yalin, HÜLSEY Max J, et al. Electrostatic stabilization of single-atom catalysts by ionic liquids[J]. Chem, 2019, 5(12): 3207-3219. |
| 15 | LI Pengsong, WANG Maoyu, DUAN Xinxuan, et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides[J]. Nature Communications, 2019, 10: 1711. |
| 16 | KAISER Selina K, FAKO Edvin, MANZOCCHI Gabriele, et al. Nanostructuring unlocks high performance of platinum single-atom catalysts for stable vinyl chloride production[J]. Nature Catalysis, 2020, 3(4): 376-385. |
| 17 | GIANNAKAKIS Georgios, Maria FLYTZANI-STEPHANOPOULOS, SYKES E Charles H. Single-atom alloys as a reductionist approach to the rational design of heterogeneous catalysts[J]. Accounts of Chemical Research, 2019, 52(1): 237-247. |
| 18 | ZHANG Leilei, ZHOU Maoxiang, WANG Aiqin, et al. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 19 | LOU Yang, ZHENG Yongping, LI Xu, et al. Pocketlike active site of Rh1/MoS2 single-atom catalyst for selective crotonaldehyde hydrogenation[J]. Journal of the American Chemical Society, 2019, 141(49): 19289-19295. |
| 20 | CUI Xinjiang, JUNGE Kathrin, DAI Xingchao, et al. Synthesis of single atom based heterogeneous platinum catalysts: high selectivity and activity for hydrosilylation reactions[J]. ACS Central Science, 2017, 3(6): 580-585. |
| 21 | RONG Xin, WANG Hong Juan, LU Xiu Li, et al. Controlled synthesis of a vacancy-defect single-atom catalyst for boosting CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 59(5): 1961-1965. |
| 22 | LOU Yang, CAI Yafeng, HU Wende, et al. Identification of active area as active center for CO oxidation over single Au atom catalyst[J]. ACS Catalysis, 2020, 10(11): 6094-6101. |
| 23 | ZHAO Shu, CHEN Fang, DUAN Sibin, et al. Remarkable active-site dependent H2O promoting effect in CO oxidation[J]. Nature Communications, 2019, 10(1): 3824. |
| 24 | ZHANG Jian, WANG Ziyun, CHEN Wenxing, et al. Tuning polarity of Cu-O bond in heterogeneous Cu catalyst to promote additive-free hydroboration of alkynes[J]. Chem, 2020, 6(3): 725-737. |
| 25 | XIONG Yu, DONG Juncai, HUANG Zheng-Qing, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation[J]. Nature Nanotechnology, 2020, 15(5): 390-397. |
| 26 | YAN D X, CHEN J, JIA H P. Temperature-induced structure reconstruction to prepare a thermally stable single-atom platinum catalyst[J]. Angewandte Chemie International Edition, 2020, 59(32): 13562-13567. |
| 27 | AKRI Mohcin, ZHAO Shu, LI Xiaoyu, et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming[J]. Nature Communications, 2019, 10: 5181. |
| 28 | ZHANG Zhirong, FENG Chen, LIU Chunxiao, et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts[J]. Nature Communications, 2020, 11: 1215. |
| 29 | XUE Yurui, HUANG Bolong, YI Yuanping, et al. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution[J]. Nature Communications, 2018, 9: 1460. |
| 30 | ZHANG Lei, SI Rutong, LIU Hanshuo, et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction[J]. Nature Communications, 2019, 10: 4936. |
| 31 | SUN Shuhui, ZHANG Gaixia, GAUQUELIN Nicolas, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports, 2013, 3: 1775. |
| 32 | LIU Pengxin, ZHAO Yun, QIN Ruixuan, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts[J]. Science, 2016, 352(6287): 797-801. |
| 33 | ZHUANG Linzhou, JIA Yi, LIU Hongli, et al. Defect-induced Pt-Co-Se coordinated sites with highly asymmetrical electronic distribution for boosting oxygen-involving electrocatalysis[J]. Advanced Materials, 2019, 31(4): 1805581. |
| 34 | LI Tuanfeng, LIU Jingjun, SONG Ye, et al. Photochemical solid-phase synthesis of platinum single atoms on nitrogen-doped carbon with high loading as bifunctional catalysts for hydrogen evolution and oxygen reduction reactions[J]. ACS Catalysis, 2018, 8(9): 8450-8458. |
| 35 | JI Shufang, QU Yang, WANG Tao, et al. Rare-earth single erbium atoms for enhanced photocatalytic CO2 reduction[J]. Angewandte Chemie, 2020, 132(26): 10738-10744. |
| 36 | WANG Lei, CHEN Ming-Xi, YAN Qiang-Qiang, et al. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts[J]. Science Advances, 2019, 5(10): eaax6322. |
| 37 | YANG Hongzhou, SHANG Lu, ZHANG Qinghua, et al. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts[J]. Nature Communications, 2019, 10: 4585. |
| 38 | LIN Yichao, LIU Pingying, VELASCO Ever, et al. Fabricating single-atom catalysts from chelating metal in open frameworks[J]. Advanced Materials, 2019, 31(18): 1808193. |
| 39 | ZUO Quan, LIU Tingting, CHEN Chuanshuang, et al. Ultrathin metal-organic framework nanosheets with ultrahigh loading of single Pt atoms for efficient visible-light-driven photocatalytic H2 evolution[J]. Angewandte Chemie International Edition, 2019, 58(30): 10198-10203. |
| 40 | ZHANG Jinqiang, ZHAO Yufei, CHEN Chen, et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions[J]. Journal of the American Chemical Society, 2019, 141(51): 20118-20126. |
| 41 | HAN Yunhu, WANG Yang-Gang, CHEN Wenxing, et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2017, 139(48): 17269-17272. |
| 42 | YANG Zhengkun, CHEN Bingxu, CHEN Wenxing, et al. Directly transforming copper (Ⅰ) oxide bulk into isolated single-atom copper sites catalyst through gas-transport approach[J]. Nature Communications, 2019, 10: 3734. |
| 43 | LANG Rui, XI Wei, LIU Jin-Cheng, et al. Non defect-stabilized thermally stable single-atom catalyst[J]. Nature Communications, 2019, 10: 234. |
| 44 | LIU Kaipeng, ZHAO Xintian, REN Guoqing, et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts[J]. Nature Communications, 2020, 11: 1263. |
| 45 | QU Y T, WANG L G, LI Z J, et al. Ambient synthesis of single-atom catalysts from bulk metal via trapping of atoms by surface dangling bonds[J]. Advanced Materials, 2019, 31(44): 1904496. |
| 46 | LI Zhijun, WANG Dehua, WU Yuen, et al. Recent advances in the precise control of isolated single-site catalysts by chemical methods[J]. National Science Review, 2018, 5(5): 673-689. |
| 47 | AHONEN M, PESSA M, SUNTOLA T. A study of ZnTe films grown on glass substrates using an atomic layer evaporation method[J]. Thin Solid Films, 1980, 65(3): 301-307. |
| 48 | GEORGE S M. Atomic layer deposition: an overview[J]. Chemical Reviews, 2010, 110(1): 111-131. |
| 49 | ZHANG Xiaoyan, ZHANG Shan, YANG Yong, et al. A general method for transition metal single atoms anchored on honeycomb-like nitrogen-doped carbon nanosheets[J]. Advanced Materials, 2020, 32(10): 1906905. |
| 50 | FENG Jiaqi, GAO Hongshuai, ZHENG Lirong, et al. A Mn-N3 single-atom catalyst embedded in graphitic carbon nitride for efficient CO2 electroreduction[J]. Nature Communications, 2020, 11: 4341. |
| 51 | 李丽, 余愿, 孙东峰, 等. Ru单原子负载g-C3N4催化剂的制备及其光催化固氮性能研究[J]. 中国材料进展, 2021, 40(3): 234-240. |
| LI Li, YU Yuan, SUN Dongfeng, et al. Ruthenium single atoms supported on graphitic carbon nitride for nitrogen photofixation[J]. Materials China, 2021, 40(3): 234-240. | |
| 52 | 徐劼, 王柯晴, 田丹, 等. 单原子Co-C-N催化过一硫酸盐降解金橙Ⅱ[J]. 中国环境科学, 2021, 41(1): 151-160. |
| XU Jie, WANG Keqing, TIAN Dan, et al. Degradation of AO7 with peroxymonosulfate catalyzed by Co-C-N single atom[J]. China Environmental Science, 2021, 41(1): 151-160. | |
| 53 | ZHANG Jinqiang, ZHAO Yufei, CHEN Chen, et al. Tuning the coordination environment in single-atom catalysts to achieve highly efficient oxygen reduction reactions[J]. Journal of the American Chemical Society, 2019, 141(51): 20118-20126. |
| 54 | YANG Y, PERRY I B, LU G, et al. Copper-catalyzed asymmetric addition of olefin-derived nucleophiles to ketones[J]. Science, 2016, 353(6295): 144-150. |
| 55 | YAO Yonggang, HUANG Zhennan, XIE Pengfei, et al. High temperature shockwave stabilized single atoms[J]. Nature Nanotechnology, 2019, 14(9): 851-857. |
| 56 | LIU Jingyue. Aberration-corrected scanning transmission electron microscopy in single-atom catalysis: Probing the catalytically active centers[J]. Chinese Journal of Catalysis, 2017, 38(9): 1460-1472. |
| 57 | WAN Xin, LIU Xiaofang, LI Yongcheng, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells[J]. Nature Catalysis, 2019, 2(3): 259-268. |
| 58 | DUCHESNE P N, LI Z Y, DEMING C P, et al. Golden single-atomic-site platinum electrocatalysts[J]. Nature Materials, 2018, 17(11): 1033-1039. |
| 59 | YANG Jian, QIU Zongyang, ZHAO Changming, et al. In situ thermal atomization to convert supported nickel nanoparticles into surface-bound nickel single-atom catalysts[J]. Angewandte Chemie International Edition, 2018, 57(43): 14095-14100. |
| 60 | WANG Maoyu, Líney ÁRNADÓTTIR, XU Zhichuan, et al. In situ X-ray absorption spectroscopy studies of nanoscale electrocatalysts[J]. Nano-Micro Letters, 2019, 11(1): 1-18. |
| 61 | LU Yubing, WANG Jiamin, YU Liang, et al. Identification of the active complex for CO oxidation over single-atom Ir-on-MgAl2O4 catalysts[J]. Nature Catalysis, 2019, 2(2): 149-156. |
| 62 | LIU Wengang, ZHANG Leilei, YAN Wensheng, et al. Single-atom dispersed Co-N-C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes[J]. Chemical Science, 2016, 7(9): 5758-5764. |
| 63 | ZHAO Yongqiang, LING Tao, CHEN Shuangming, et al. Non-metal single-iodine-atom electrocatalysts for the hydrogen evolution reaction[J]. Angewandte Chemie International Edition, 2019, 58(35): 12252-12257. |
| 64 | HÜLSEY M J, ZHANG B, MA Z R, et al. In situ spectroscopy-guided engineering of rhodium single-atom catalysts for CO oxidation[J]. Nature Communications, 2019, 10: 1330. |
| 65 | ZHOU Guangmin, ZHAO Shiyong, WANG Tianshuai, et al. Theoretical calculation guided design of single-atom catalysts toward fast kinetic and long-life Li–S batteries[J]. Nano Letters, 2020, 20(2): 1252-1261. |
| 66 | CHENG Mu-Jeng, CLARK Ezra L, PHAM Hieu H, et al. Quantum mechanical screening of single-atom bimetallic alloys for the selective reduction of CO2 to C1 hydrocarbons[J]. ACS Catalysis, 2016, 6(11): 7769-7777. |
| 67 | LIU Xin, JIAO Yan, ZHENG Yao, et al. Building up a picture of the electrocatalytic nitrogen reduction activity of transition metal single-atom catalysts[J]. Journal of the American Chemical Society, 2019, 141(24): 9664-9672. |
| 68 | LI Fengyu, LI Yafei, ZENG Xiao Cheng, et al. Exploration of high-performance single-atom catalysts on support M1/FeOx for CO oxidation via computational study[J]. ACS Catalysis, 2015, 5(2): 544-552. |
| 69 | SONG Weiyu, HENSEN Emiel J M. Structure sensitivity in CO oxidation by a single Au atom supported on ceria[J]. The Journal of Physical Chemistry C, 2013, 117(15): 7721-7726. |
| 70 | 肖香珍, 蔡颖莹, 张建伟. N2在单原子催化剂Ir/MoS2表面上吸附的第一性原理研究[J]. 原子与分子物理学报, 2020, 37(3): 317-322. |
| XIAO Xiangzhen, CAI Yingying, ZHANG Jianwei. First-principles study of N2 adsorption on Ir/MoS2 surface of single-atom catalysts[J]. Journal of Atomic and Molecular Physics, 2020, 37(3): 317-322. | |
| 71 | LIANG Jin Xia, LIN Jian, LIU Jingyue, et al. Dual metal active sites in an Ir1/FeOx single-atom catalyst: a redox mechanism for the water-gas shift reaction[J]. Angewandte Chemie International Edition, 2020, 59(31): 12868-12875. |
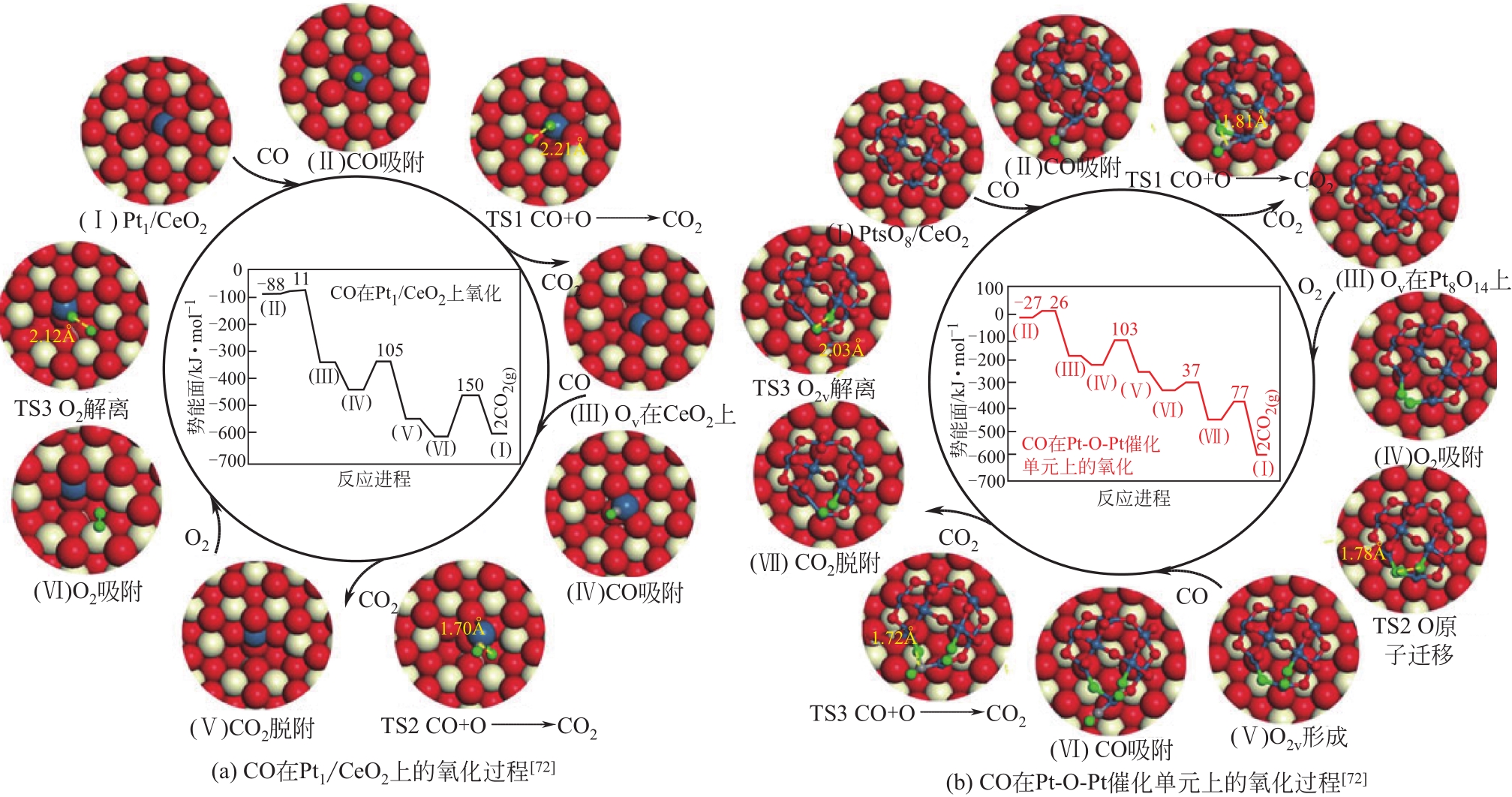
| 72 | WANG Hui, LIU Jin-Xun, ALLARD Lawrence F, et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms[J]. Nature Communications, 2019, 10: 3808. |
| 73 | KONG Ningning, FAN Xing, LIU Fangfang, et al. Single vanadium atoms anchored on graphitic carbon nitride as a high-performance catalyst for non-oxidative propane dehydrogenation[J]. ACS Nano, 2020, 14(5): 5772-5779. |
| 74 | WANG Yu, TANG Yu-Jia, ZHOU Kun, et al. Self-adjusting activity induced by intrinsic reaction intermediate in Fe-N-C single-atom catalysts[J]. Journal of the American Chemical Society, 2019, 141(36): 14115-14119. |
| 75 | YI Ding, LU Fei, ZHANG Fengchu, et al. Regulating charge transfer of lattice oxygen in single-atom-doped titania for hydrogen evolution[J]. Angewandte Chemie International Edition, 2020, 59(37): 15855-15859. |
| 76 | ZHANG Qiaoqiao, GUAN Jingqi. Single-atom catalysts for electrocatalytic applications[J]. Advanced Functional Materials, 2020, 30(31): 2000768. |
| 77 | LIU S W, WANG M Y, YANG X X, et al. Chemical vapor deposition for atomically dispersed and nitrogen coordinated single metal site catalysts[J]. Angewandte Chemie, 2020, 132(48): 21882-21889. |
| 78 | HE Yanghua, SHI Qiurong, SHAN Weitao, et al. Dynamically unveiling metal-nitrogen coordination during thermal activation to design high-efficient atomically dispersed CoN4 active sites[J]. Angewandte Chemie International Edition, 2021, 60(17): 9516-9526. |
| 79 | XIE Xiaohong, HE Cheng, LI Boyang, et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells[J]. Nature Catalysis, 2020, 3(12): 1044-1054. |
| 80 | LUO E G, ZHANG H, WANG X, et al. Single-atom Cr-N4 sites designed for durable oxygen reduction catalysis in acid media[J]. Angewandte Chemie, 2019, 131(36): 12599-12605. |
| 81 | GONG L, ZHANG H, WANG Y, et al. Bridge bonded oxygen ligands between approximated FeN4 sites confer catalysts with high ORR performance[J]. Angewandte Chemie International Edition, 2020, 59(33): 13923-13928. |
| 82 | XIAO Meiling, GAO Liqin, WANG Ying, et al. Engineering energy level of metal center: Ru single-atom site for efficient and durable oxygen reduction catalysis[J]. Journal of the American Chemical Society, 2019, 141(50): 19800-19806. |
| 83 | HAN Aijuan, CHEN Wenxing, ZHANG Shaolong, et al. A polymer encapsulation strategy to synthesize porous nitrogen-doped carbon-nanosphere-supported metal isolated-single-atomic-site catalysts[J]. Advanced Materials, 2018, 30(15): 1706508. |
| 84 | HAN Lili, LIU Xijun, CHEN Jinping, et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation[J]. Angewandte Chemie International Edition, 2019, 58(8): 2321-2325. |
| 85 | YU Bing, LI Hao, WHITE Jai, et al. Tuning the catalytic preference of ruthenium catalysts for nitrogen reduction by atomic dispersion[J]. Advanced Functional Materials, 2020, 30(6): 1905665. |
| 86 | FENG Jiaqi, GAO Hongshuai, ZHENG Lirong, et al. A Mn-N3 single-atom catalyst embedded in graphitic carbon nitride for efficient CO2 electroreduction[J]. Nature Communications, 2020, 11: 4341. |
| 87 | ZHANG Erhuan, WANG Tao, YU Ke, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction[J]. Journal of the American Chemical Society, 2019, 141(42): 16569-16573. |
| 88 | ZHAO Shiyong, CHEN Guangxu, ZHOU Guangmin, et al. A universal seeding strategy to synthesize single atom catalysts on 2D materials for electrocatalytic applications[J]. Advanced Functional Materials, 2020, 30(6): 1906157. |
| 89 | HE Qun, TIAN Dong, JIANG Hongliang, et al. Achieving efficient alkaline hydrogen evolution reaction over a Ni5P4 catalyst incorporating single-atomic Ru sites[J]. Advanced Materials, 2020, 32(11): 1906972. |
| 90 | PATTENGALE Brian, HUANG Yichao, YAN Xingxu, et al. Dynamic evolution and reversibility of single-atom Ni(Ⅱ) active site in 1T-MoS2 electrocatalysts for hydrogen evolution[J]. Nature Communications, 2020, 11: 4114. |
| 91 | BAI Lichen, HSU Chia-Shuo, ALEXANDER Duncan T L, et al. A cobalt-iron double-atom catalyst for the oxygen evolution reaction[J]. Journal of the American Chemical Society, 2019, 141(36): 14190-14199. |
| 92 | ZHU Siyuan, GE Junjie, LIU Changpeng, et al. Atomic-level dispersed catalysts for PEMFCs: progress and future prospects[J]. EnergyChem, 2019, 1(3): 100018. |
| 93 | LI Wei, WANG Dongdong, ZHANG Yiqiong, et al. Defect engineering for fuel-cell electrocatalysts[J]. Advanced Materials, 2020, 32(19): 1907879. |
| 94 | LI Mufan, DUANMU Kaining, WAN Chengzhang, et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis[J]. Nature Catalysis, 2019, 2(6): 495-503. |
| 95 | YANG Sungeun, KIM Jiwhan, Young Joo TAK, et al. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions[J]. Angewandte Chemie, 2016, 128(6): 2098-2102. |
| 96 | MAO Junjie, HE Chun-Ting, PEI Jiajing, et al. Isolated Ni atoms dispersed on Ru nanosheets: high-performance electrocatalysts toward hydrogen oxidation reaction[J]. Nano Letters, 2020, 20(5): 3442-3448. |
| 97 | AN Lulu, ZHAO Xu, ZHAO Tonghui, et al. Atomic-level insight into reasonable design of metal-based catalysts for hydrogen oxidation in alkaline electrolytes[J]. Energy & Environmental Science, 2021, 14(5): 2620-2638. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [4] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [5] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [6] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [7] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [8] | 吴正浩, 周天航, 蓝兴英, 徐春明. 人工智能驱动化学品创新设计的实践与展望[J]. 化工进展, 2023, 42(8): 3910-3916. |
| [9] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| [10] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [11] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [12] | 李润蕾, 王子彦, 王志苗, 李芳, 薛伟, 赵新强, 王延吉. CuO-CeO2/TiO 2 高效催化CO低温氧化反应性能[J]. 化工进展, 2023, 42(8): 4264-4274. |
| [13] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [14] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [15] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||