化工进展 ›› 2023, Vol. 42 ›› Issue (8): 4043-4057.DOI: 10.16085/j.issn.1000-6613.2023-0397
铜基催化剂电还原二氧化碳选择性的调控策略
王耀刚1,2,3,4( ), 韩子姗1,2,3,4, 高嘉辰1,2,3,4, 王新宇1,2,3,4, 李思琪1,2,3,4, 杨全红1,2,3,4, 翁哲1,2,3,4(
), 韩子姗1,2,3,4, 高嘉辰1,2,3,4, 王新宇1,2,3,4, 李思琪1,2,3,4, 杨全红1,2,3,4, 翁哲1,2,3,4( )
)
- 1.天津市先进碳与电化学储能重点实验室,天津 300072
2.国家储能技术产教融合创新平台,天津 300072
3.物质绿色创造与制造海河实验室,天津 300192
4.天津化学化工协同创新中心,天津 300072
-
收稿日期:2023-03-15修回日期:2023-07-27出版日期:2023-08-15发布日期:2023-09-19 -
通讯作者:翁哲 -
作者简介:王耀刚(1996—),男,硕士研究生,研究方向为电催化二氧化碳还原催化剂的设计。E-mail:e158263980@163.com。 -
基金资助:国家自然科学基金(51972223);天津市自然科学基金(20JCYBJC01550)
Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction
WANG Yaogang1,2,3,4( ), HAN Zishan1,2,3,4, GAO Jiachen1,2,3,4, WANG Xinyu1,2,3,4, LI Siqi1,2,3,4, YANG Quanhong1,2,3,4, WENG Zhe1,2,3,4(
), HAN Zishan1,2,3,4, GAO Jiachen1,2,3,4, WANG Xinyu1,2,3,4, LI Siqi1,2,3,4, YANG Quanhong1,2,3,4, WENG Zhe1,2,3,4( )
)
- 1.Nanoyang Group, Tianjin Key Laboratory of Advanced Carbon and Electrochemical Energy Storage, Tianjin 30072, China
2.National Industry-Education Integration Platform of Energy Storage, Tianjin 300072, China
3.Haihe Laboratory of Sustainable Chemical Transformations, Tianjin 300192, China
4.Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
-
Received:2023-03-15Revised:2023-07-27Online:2023-08-15Published:2023-09-19 -
Contact:WENG Zhe
摘要:
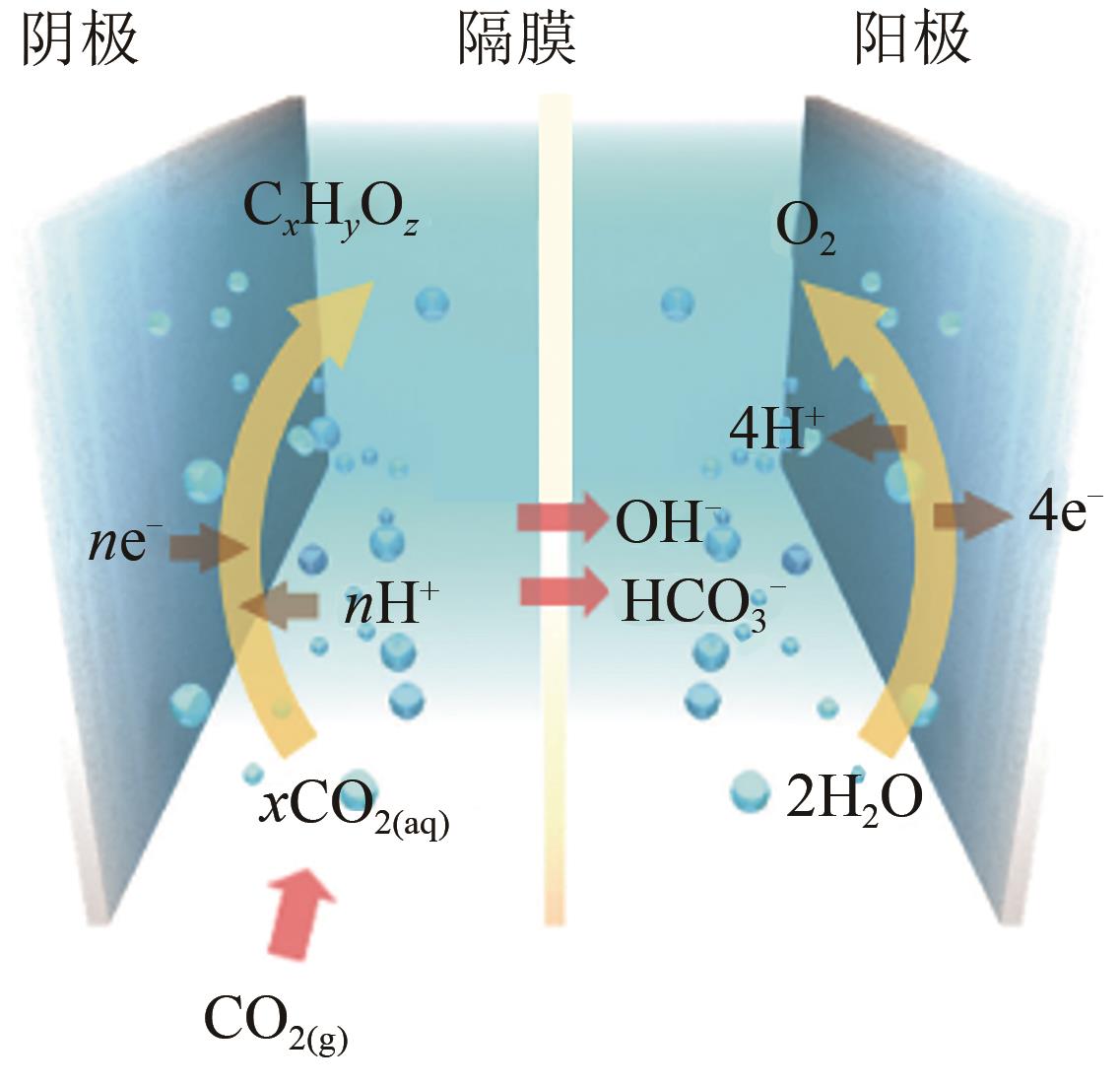
在水系电解液中利用电能直接将CO2还原成基础化学品为CO2资源化利用提供了一种绿色可行的策略。铜是唯一能够高效地将CO2还原成C2+产物的金属催化剂,然而其催化产物多达16种,产物的多样性严重增加了后期产物分离的成本并大幅降低了整个电催化二氧化碳还原(eCO2RR)系统的能量转换效率,是eCO2RR走向工业化生产的一个重要瓶颈,因此对铜基催化剂进行合理的调控,以提高其对单一产物的选择性一直是研究的热点问题。经过30余年的发展,铜基催化剂的研究已经取得了巨大的进展,回溯近期铜基催化剂电催化CO2还原领域的研究历程,本文综述了电催化二氧化碳还原的反应原理、反应路径和针对不同产物的调控策略,着重总结了提高铜基催化剂对单一产物选择性的设计策略和设计方法,最后展望了铜基催化剂面临的挑战和未来的发展方向。
中图分类号:
引用本文
王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057.
WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057.
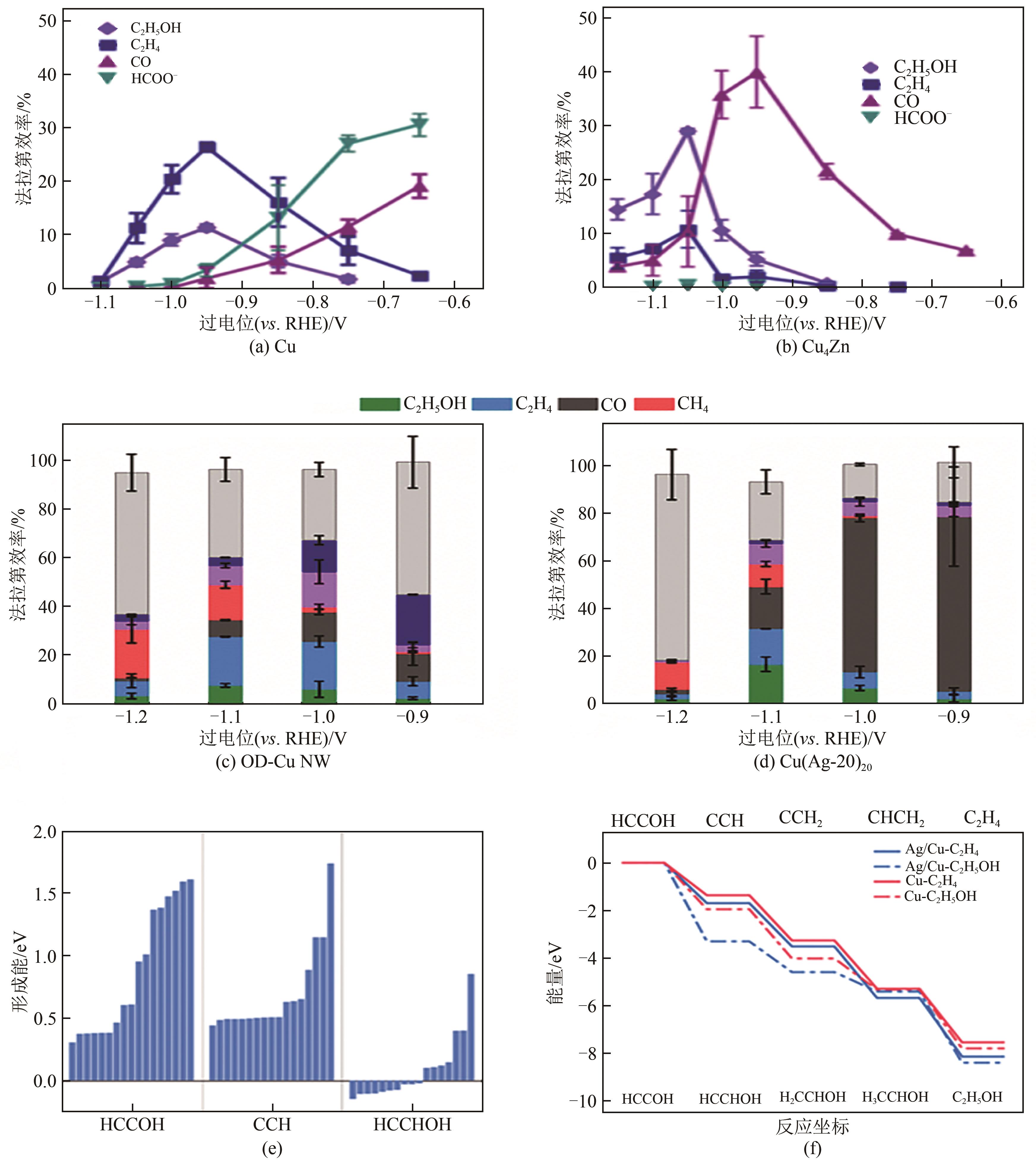
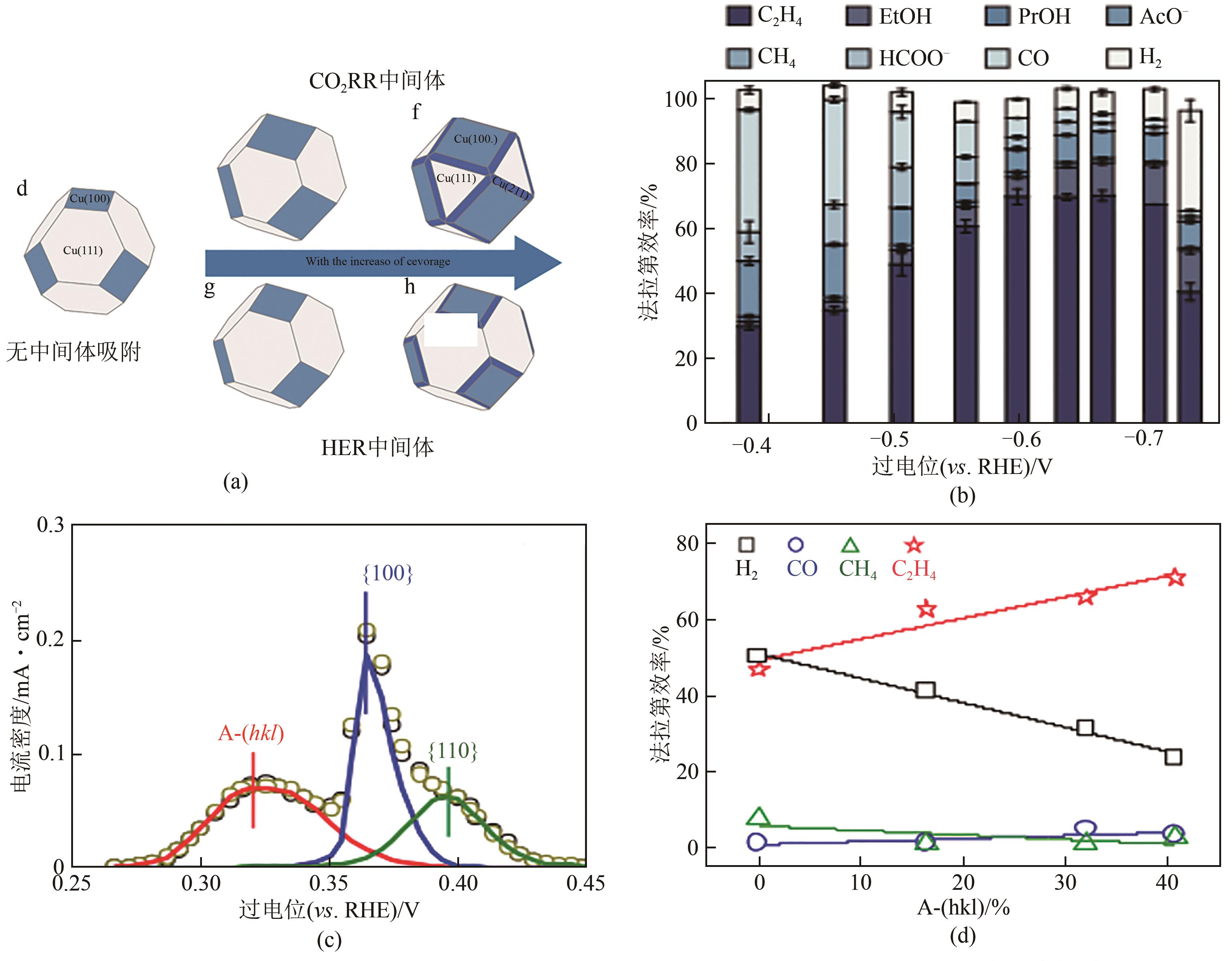
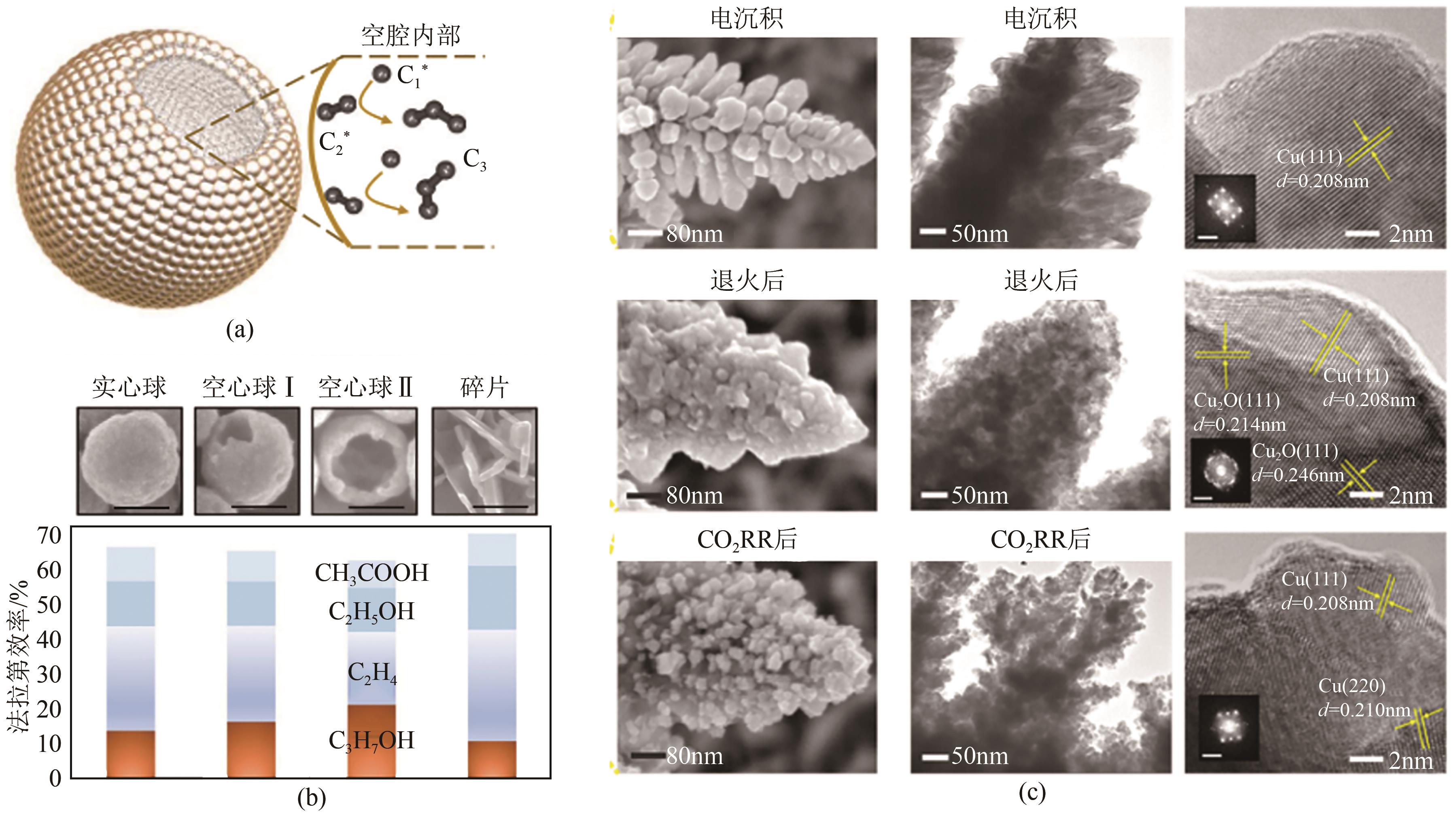
图9 Cu(a)、Cu4Zn(b)在不同电位下的产物选择性[56];OD-Cu NW(c)、Cu(Ag-20)20(d)在不同电位下的产物选择性[39];Ag/Cu上*HCCOH、*CCH(乙烯路径)和*HCCHOH(乙醇路径)的形成能(e) 在Cu和Ag/Cu催化剂上乙烯和乙醇路径的反应能垒计算(f)[57]
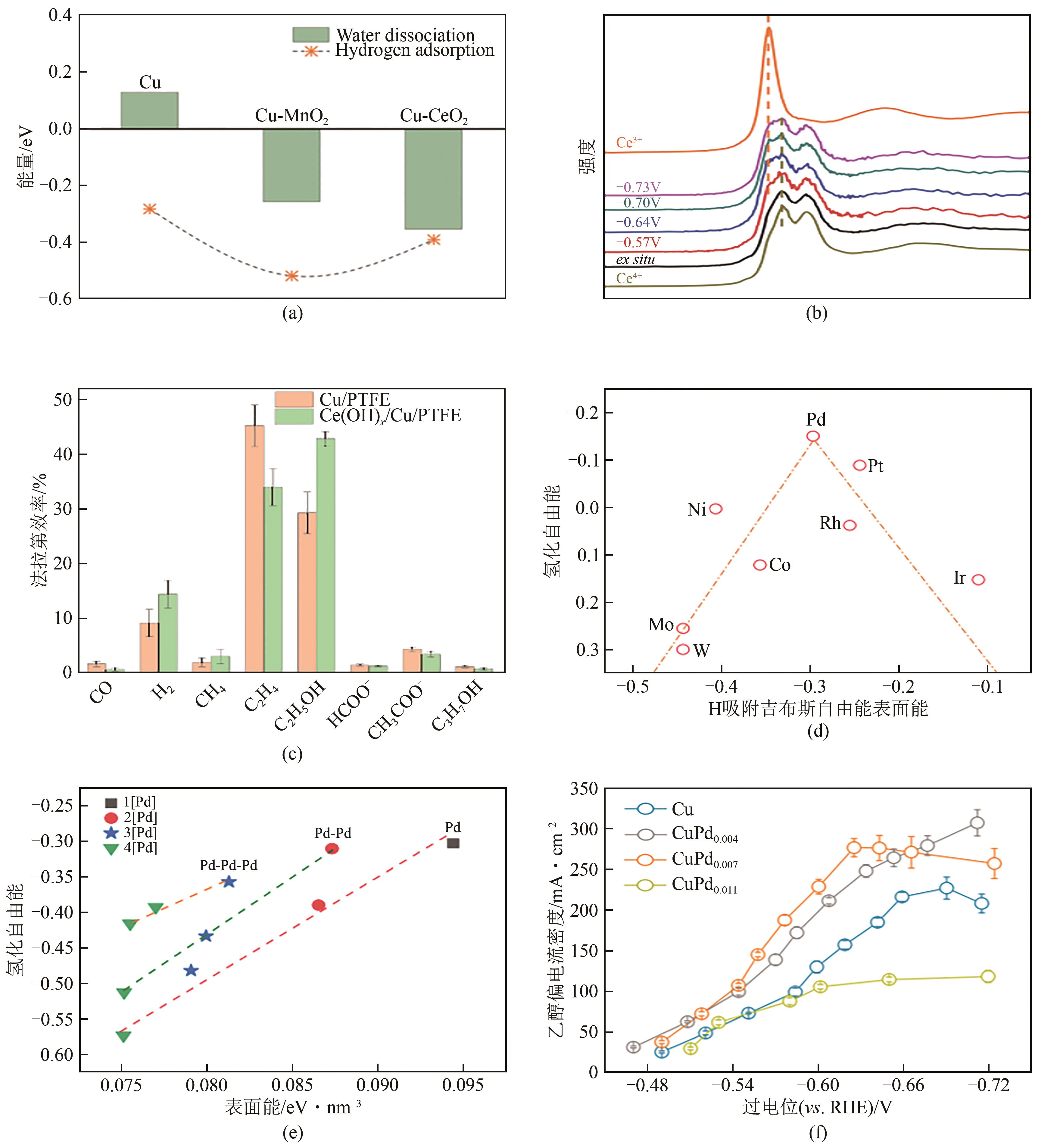
图10 Cu/Cu-MnO2/Cu-CeO2表面的水解离能和氢吸附能(a);Ce(OH) x /Cu/PTFE在不同电位下的原位XAS谱图(b);Ce(OH) x /Cu/PTFE和Cu在-0.7V(vs. RHE)下的产物分布(c);不同掺杂剂下HOCCH*自由能与H吸附自由能之间的关系图(d)[58];不同Pd掺杂构型下HOCCH*的氢化反应自由能,1[Pd]、2[Pd]、3[Pd]、4[Pd]是指表面Pd含量分别为1/16、1/8、3/16、1/4(e);不同电位下醇类产物的偏电流密度(f)[59]
| 1 | HORI Yoshio, MURATA Akira, TAKAHASHI Ryutaro, et al. Electroreduction of carbon monoxide to methane and ethylene at a copper electrode in aqueous solutions at ambient temperature and pressure[J]. Journal of the American Chemical Society, 1987, 109(16): 5022-5023. |
| 2 | XIE Shunji, MA Wenchao, WU Xuejiao, et al. Photocatalytic and electrocatalytic transformations of C1 molecules involving C—C coupling[J]. Energy & Environmental Science, 2021, 14(1): 37-89. |
| 3 | CHENG Tao, XIAO Hai, GODDARD William A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298K[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(8): 1795-1800. |
| 4 | PETERSON Andrew A, NØRSKOV Jens K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts[J]. The Journal of Physical Chemistry Letters, 2012, 3(2): 251-258. |
| 5 | KOPER Marc T M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis[J]. Journal of Electroanalytical Chemistry, 2011, 660(2): 254-260. |
| 6 | MOU Shiyong, WU Tongwei, XIE Junfeng, et al. Boron phosphide nanoparticles: A nonmetal catalyst for high-selectivity electrochemical reduction of CO2 to CH3OH[J]. Advanced Materials, 2019, 31(36): e1903499. |
| 7 | CHENG Wenhui, RICHTER Matthias H, SULLIVAN Ian, et al. CO2 reduction to CO with 19% efficiency in a solar-driven gas diffusion electrode flow cell under outdoor solar illumination[J]. ACS Energy Letters, 2020, 5(2): 470-476. |
| 8 | CHRISTENSEN Oliver, ZHAO Siqi, SUN Zhaozong, et al. Can the CO2 reduction reaction be improved on Cu: Selectivity and intrinsic activity of functionalized Cu surfaces[J]. ACS Catalysis, 2022, 12(24): 15737-15749. |
| 9 | LI Minhan, SONG Nan, LUO Wei, et al. Engineering surface oxophilicity of copper for electrochemical CO2 reduction to ethanol[J]. Advanced Science, 2023, 10(2): e2204579. |
| 10 | OKATENKO Valery, LOIUDICE Anna, NEWTON Mark A, et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction[J]. Journal of the American Chemical Society, 2023, 145(9): 5370-5383. |
| 11 | Nitopi Stephanie, Bertheussen Erlend, SCOTT Soren B, et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J]. Chemical Reviews, 2019, 119(12): 7610-7672. |
| 12 | SEONG Hoeun, Yongsung JO, EFREMOV Vladimir, et al. Transplanting gold active sites into non-precious-metal nanoclusters for efficient CO2-to-CO electroreduction[J]. Journal of the American Chemical Society, 2023, 145(4): 2152-2160. |
| 13 | JIANG Yunling, SHAN Jieqiong, WANG Pengtang, et al. Stabilizing oxidation state of SnO2 for highly selective CO2 electroreduction to formate at large current densities[J]. ACS Catalysis, 2023, 13(5): 3101-3108. |
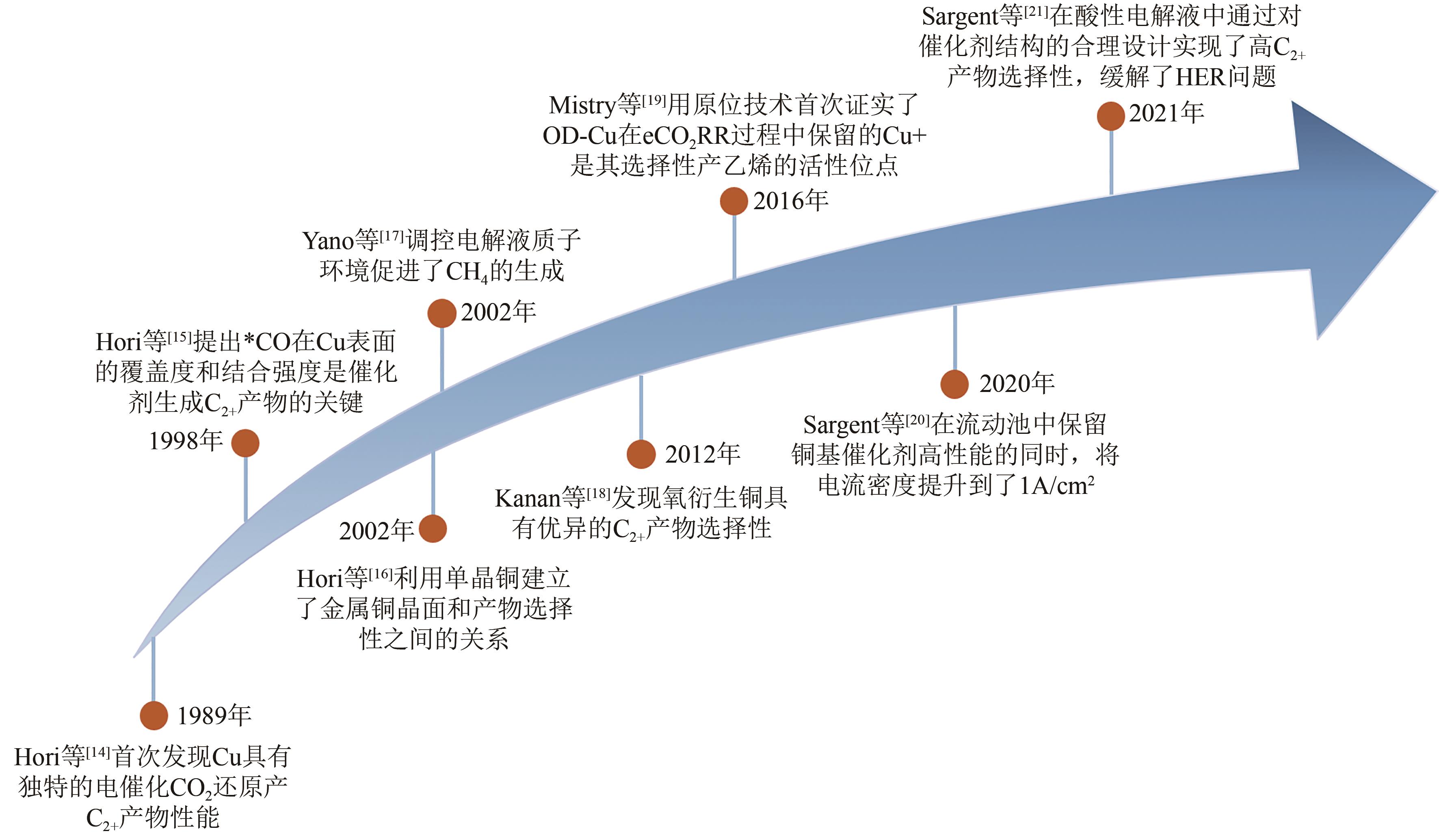
| 14 | HORI Yoshio, MURATA Akira, TAKAHASHI Ryutaro. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1989, 85(8): 2309. |
| 15 | KOGA Osamu, MATSUO Tadanori, YAMAZAKI Hiroki, et al. Infrared spectroscopic observation of intermediate species on Ni and Fe electrodes in the electrochemical reduction of CO2 and CO to hydrocarbons[J]. Bulletin of the Chemical Society of Japan, 1998, 71(2): 315-320. |
| 16 | HORI Y, TAKAHASHI I, KOGA O, et al. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes[J]. Journal of Molecular Catalysis A: Chemical, 2003, 199(1/2): 39-47. |
| 17 | YANO H, SHIRAI F, NAKAYAMA M, et al. Efficient electrochemical conversion of CO2 to CO, C2H4 and CH4 at a three-phase interface on a Cu net electrode in acidic solution[J]. Journal of Electroanalytical Chemistry, 2002, 519(1/2): 93-100. |
| 18 | LI Christina W, KANAN Matthew W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films[J]. Journal of the American Chemical Society, 2012, 134(17): 7231-7234. |
| 19 | MISTRY Hemma, VARELA Ana Sofia, BONIFACIO Cecile S, et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene[J]. Nature Communications, 2016, 7: 12123. |
| 20 | DE ARQUER F Pelayo García, Dinh Cao-Thang, Ozden Adnan, et al. CO2 electrolysis to multicarbon products at activities greater than 1 A·cm-2 [J]. Science, 2020, 367(6478): 661-666. |
| 21 | Erick Huang Jianan, LI Fengwang, Ozden Adnan, et al. CO2 electrolysis to multicarbon products in strong acid[J]. Science, 2021, 372(6546): 1074-1078. |
| 22 | MA Wenchao, HE Xiaoyang, WANG Wei, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts[J]. Chemical Society Reviews, 2021, 50(23): 12897-12914. |
| 23 | BIRDJA Yuvraj Y, Elena PÉREZ-GALLENT, FIGUEIREDO Marta C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nature Energy, 2019, 4(9): 732-745. |
| 24 | KUHL Kendra P, CAVE Etosha R, ABRAM David N, et al. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces[J]. Energy & Environmental Science, 2012, 5(5): 7050-7059. |
| 25 | HANSEN Heine A, VARLEY Joel B, PETERSON Andrew A, et al. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO[J]. The Journal of Physical Chemistry Letters, 2013, 4(3): 388-392. |
| 26 | SINGH Meenesh R, CLARK Ezra L, BELL Alexis T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide[J]. Physical Chemistry Chemical Physics, 2015, 17(29): 18924-18936. |
| 27 | TAN Xinyi, YU Chang, REN Yongwen, et al. Recent advances in innovative strategies for the CO2 electroreduction reaction[J]. Energy & Environmental Science, 2021, 14(2): 765-780. |
| 28 | WUTTIG Anna, YAGUCHI Momo, MOTOBAYASHI Kenta, et al. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(32): E4585-4593. |
| 29 | ZHANG Benjamin A, OZEL Tuncay, ELIAS Joseph S, et al. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes[J]. ACS Central Science, 2019, 5(6): 1097-1105. |
| 30 | DUNWELL Marco, LU Qi, HEYES Jeffrey M, et al. The central role of bicarbonate in the electrochemical reduction of carbon dioxide on gold[J]. Journal of the American Chemical Society, 2017, 139(10): 3774-3783. |
| 31 | BANERJEE Soumyodip, ZHANG Zhuoqun, HALL Anthony Shoji, et al. Surfactant perturbation of cation interactions at the electrode-electrolyte interface in carbon dioxide reduction[J]. ACS Catalysis, 2020, 10(17): 9907-9914. |
| 32 | GABARDO Christine M, O’BRIEN Colin P, EDWARDS Jonathan P, et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly[J]. Joule, 2019, 3(11): 2777-2791. |
| 33 | Young Jin SA, LEE Chan Woo, LEE Si Young, et al. Catalyst-electrolyte interface chemistry for electrochemical CO2 reduction[J]. Chemical Society Reviews, 2020, 49(18): 6632-6665. |
| 34 | GÖTTLE Adrien J, KOPER Marc T M. Determinant role of electrogenerated reactive nucleophilic species on selectivity during reduction of CO2 catalyzed by metalloporphyrins[J]. Journal of the American Chemical Society, 2018, 140(14): 4826-4834. |
| 35 | WANG Jialin, HUANG Yucheng, WANG Yiqing, et al. Atomically dispersed metal-nitrogen-carbon catalysts with d-orbital electronic configuration-dependent selectivity for electrochemical CO2-to-CO reduction[J]. ACS Catalysis, 2023, 13(4): 2374-2385. |
| 36 | ZHENG Min, WANG Pengtang, ZHI Xing, et al. Electrocatalytic CO2-to-C2+ with ampere-level current on heteroatom-engineered copper via tuning *CO intermediate coverage[J]. Journal of the American Chemical Society, 2022, 144(32): 14936-14944. |
| 37 | JEONG Hyung Mo, KWON Youngkook, WON Jong Ho, et al. CO2 reduction: Atomic-scale spacing between copper facets for the electrochemical reduction of carbon dioxide (adv. energy mater. 10/2020)[J]. Advanced Energy Materials, 2020, 10(10): 2070041. |
| 38 | LUO Wenjia, NIE Xiaowa, JANIK Michael J, et al. Facet dependence of CO2 reduction paths on Cu electrodes[J]. ACS Catalysis, 2016, 6(1): 219-229. |
| 39 | TING Louisa Rui Lin, Oriol PIQUÉ, Si Ying LIM, et al. Enhancing CO2 electroreduction to ethanol on copper-silver composites by opening an alternative catalytic pathway[J]. ACS Catalysis, 2020, 10(7): 4059-4069. |
| 40 | ZHUANG Taotao, PANG Yuanjie, LIANG Zhiqin, et al. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide[J]. Nature Catalysis, 2018, 1(12): 946-951. |
| 41 | WU Guoling, SONG Yaru, ZHENG Qiang, et al. Selective electroreduction of CO2 to n-propanol in two-step tandem catalytic system[J]. Advanced Energy Materials, 2022, 12(36): 2202054. |
| 42 | REN Dan, WONG Nian Tee, HANDOKO Albertus Denny, et al. Mechanistic insights into the enhanced activity and stability of agglomerated Cu nanocrystals for the electrochemical reduction of carbon dioxide to n-propanol[J]. The Journal of Physical Chemistry Letters, 2016, 7(1): 20-24. |
| 43 | ZHAO Runbo, DING Peng, WEI Peipei, et al. Recent progress in electrocatalytic methanation of CO2 at ambient conditions[J]. Advanced Functional Materials, 2021, 31(13): 2009449. |
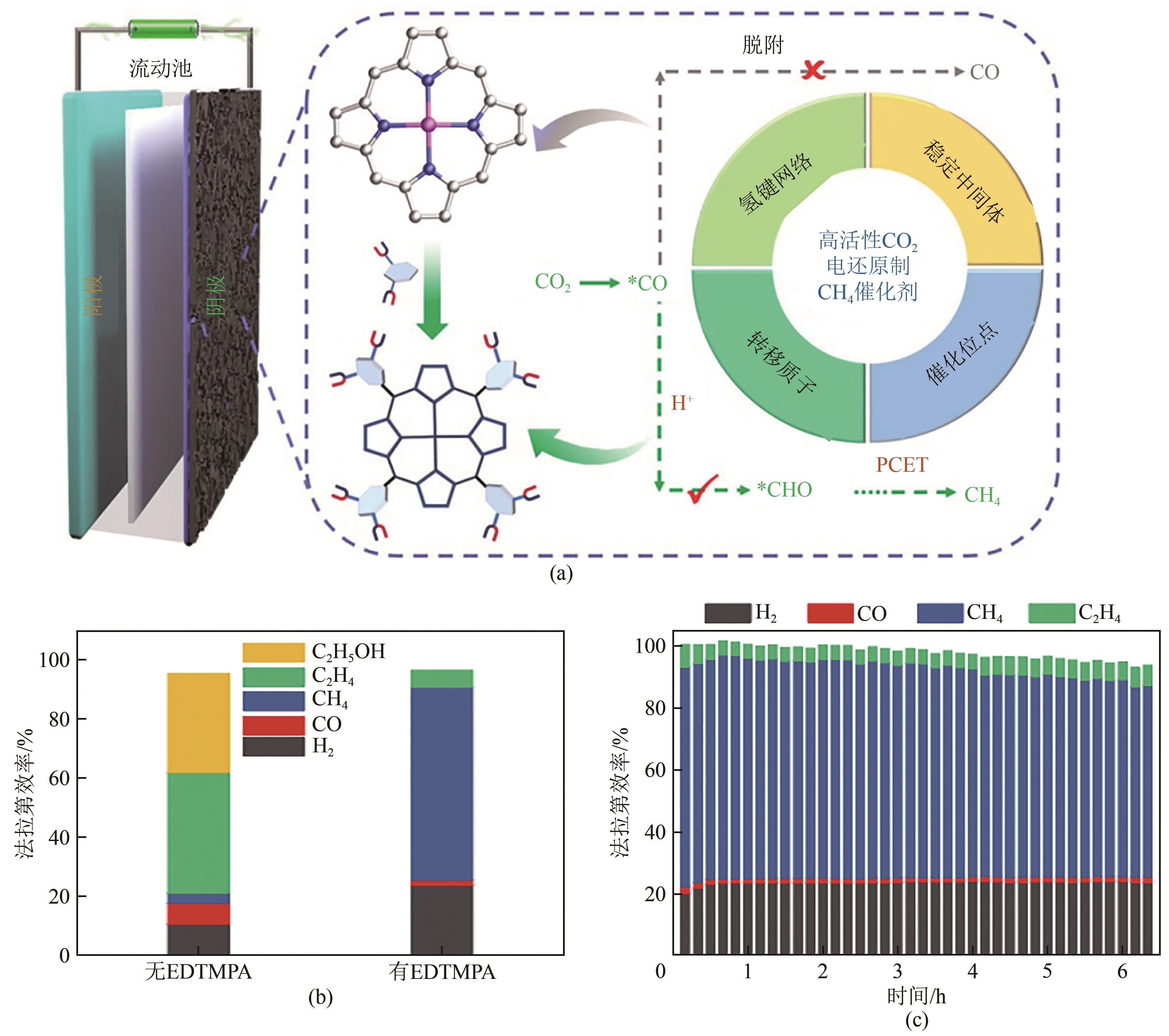
| 44 | WANG Yirong, LIU Ming, GAO Guangkuo, et al. Implanting numerous hydrogen-bonding networks in a Cu-porphyrin-based nanosheet to boost CH4 selectivity in neutral-media CO2 electroreduction[J]. Angewandte Chemie International Edition, 2021, 60(40): 21952-21958. |
| 45 | PAN Hanqing, BARILE Christopher J. Electrochemical CO2 reduction to methane with remarkably high Faradaic efficiency in the presence of a proton permeable membrane[J]. Energy & Environmental Science, 2020, 13(10): 3567-3578. |
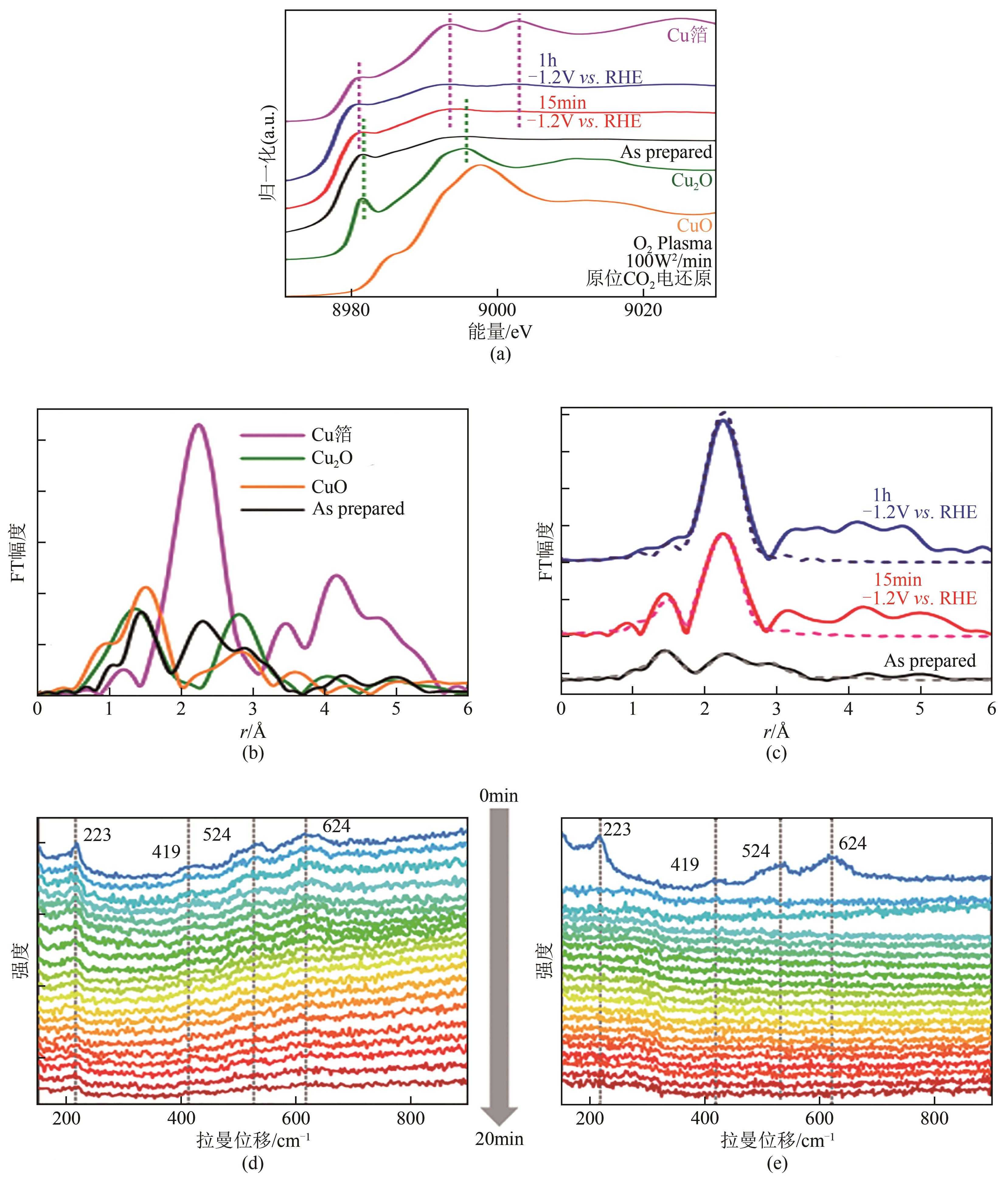
| 46 | HAN Zishan, HAN Daliang, CHEN Zhe, et al. Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction[J]. Nature Communications, 2022, 13: 3158. |
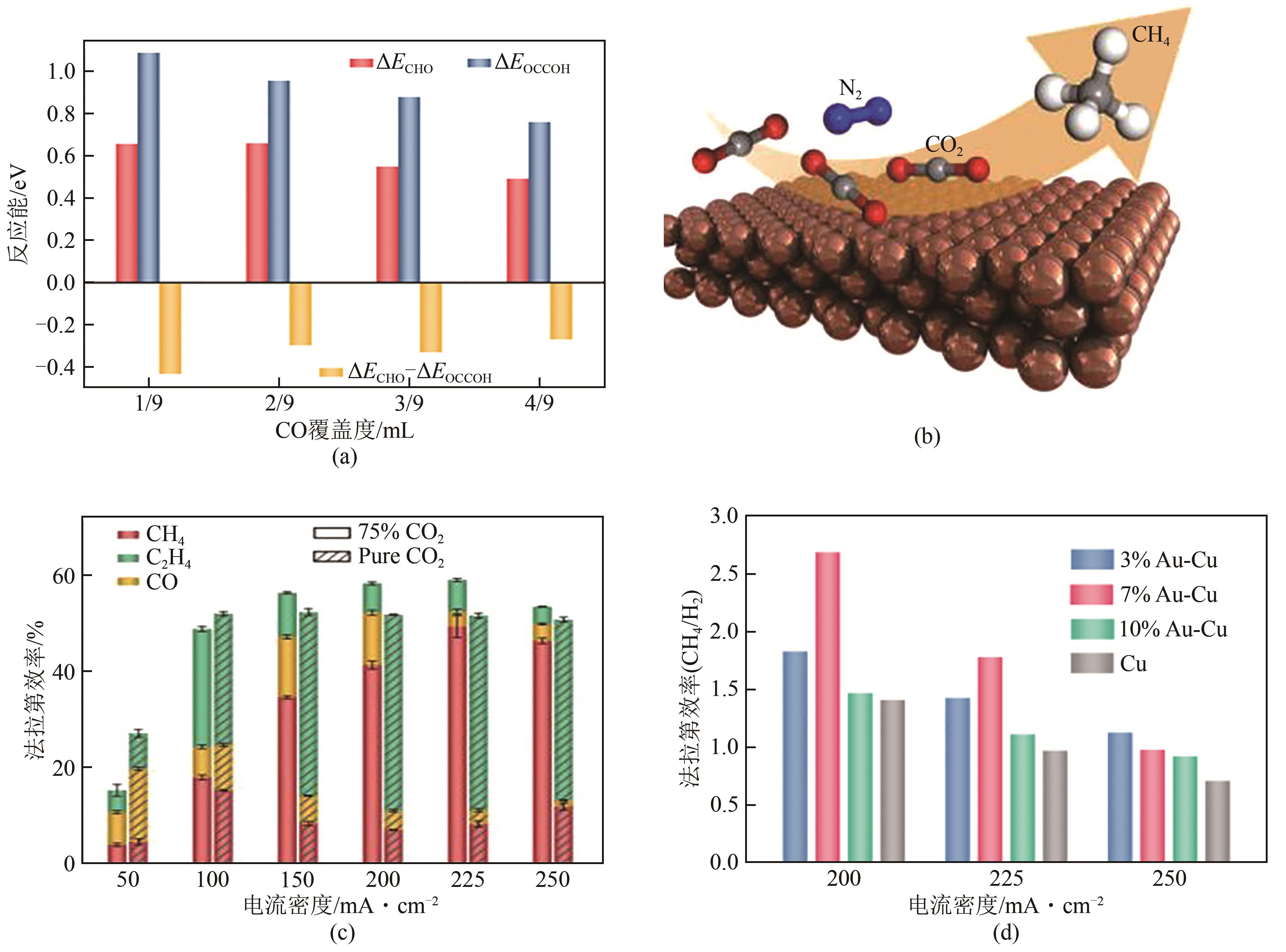
| 47 | WANG Xue, XU Aoni, LI Fengwang, et al. Efficient methane electrosynthesis enabled by tuning local CO2 availability[J]. Journal of the American Chemical Society, 2020, 142(7): 3525-3531. |
| 48 | WANG Xue, Pengfei OU, WICKS Joshua, et al. Gold-in-copper at low *CO coverage enables efficient electromethanation of CO2 [J]. Nature Communications, 2021, 12: 3387. |
| 49 | WANG Yuhang, WANG Ziyun, DINH Cao-Thang, et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis[J]. Nature Catalysis, 2020, 3(2): 98-106. |
| 50 | CHOI Chungseok, KWON Soonho, CHENG Tao, et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4 [J]. Nature Catalysis, 2020, 3(10): 804-812. |
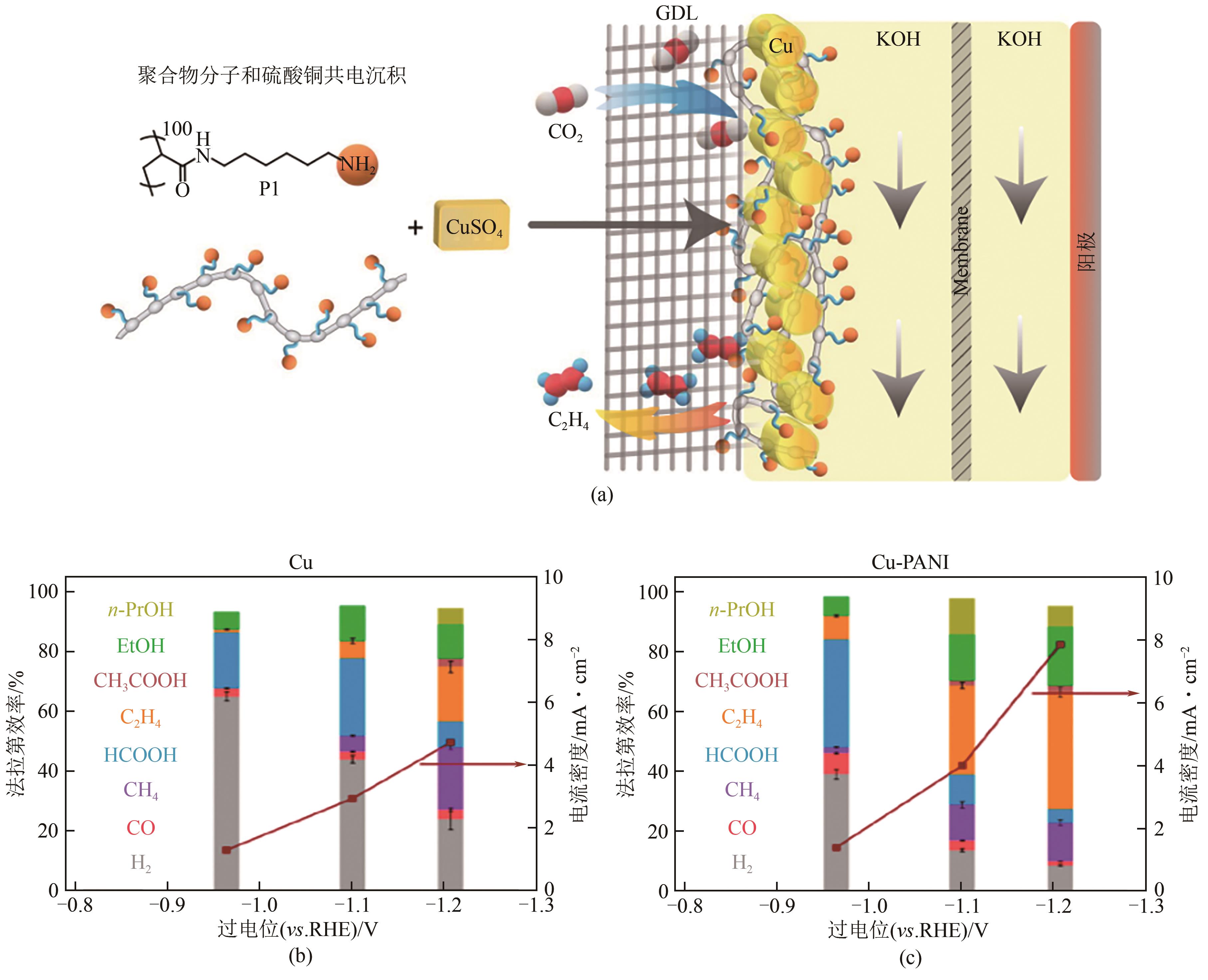
| 51 | CHEN Xinyi, CHEN Junfeng, ALGHORAIBI Nawal M, et al. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes[J]. Nature Catalysis, 2021, 4(1): 20-27. |
| 52 | WEI Xing, YIN Zhenglei, Kangjie LYU, et al. Highly selective reduction of CO2 to C2+ hydrocarbons at copper/polyaniline interfaces[J]. ACS Catalysis, 2020, 10(7): 4103-4111. |
| 53 | YANG Pengpeng, ZHANG Xiaolong, GAO Feiyue, et al. Protecting copper oxidation state via intermediate confinement for selective CO2 electroreduction to C2+ fuels[J]. Journal of the American Chemical Society, 2020, 142(13): 6400-6408. |
| 54 | WANG Lei, NITOPI Stephanie, WONG Andrew B, et al. Electrochemically converting carbon monoxide to liquid fuels by directing selectivity with electrode surface area[J]. Nature Catalysis, 2019, 2(8): 702-708. |
| 55 | MORALES-GUIO Carlos G, CAVE Etosha R, NITOPI Stephanie A, et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst[J]. Nature Catalysis, 2018, 1(10): 764-771. |
| 56 | REN Dan, Bridget Su-Hui ANG, Boon Siang YEO. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived Cu x Zn catalysts[J]. ACS Catalysis, 2016, 6(12): 8239-8247. |
| 57 | LI Yuguang C, WANG Ziyun, YUAN Tiange, et al. Binding site diversity promotes CO2 electroreduction to ethanol[J]. Journal of the American Chemical Society, 2019, 141(21): 8584-8591. |
| 58 | LUO Mingchuan, WANG Ziyun, LI Yuguang C, et al. Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen[J]. Nature Communications, 2019, 10: 5814. |
| 59 | LI Jun, XU Aoni, LI Fengwang, et al. Enhanced multi-carbon alcohol electroproduction from CO via modulated hydrogen adsorption[J]. Nature Communications, 2020, 11: 3685. |
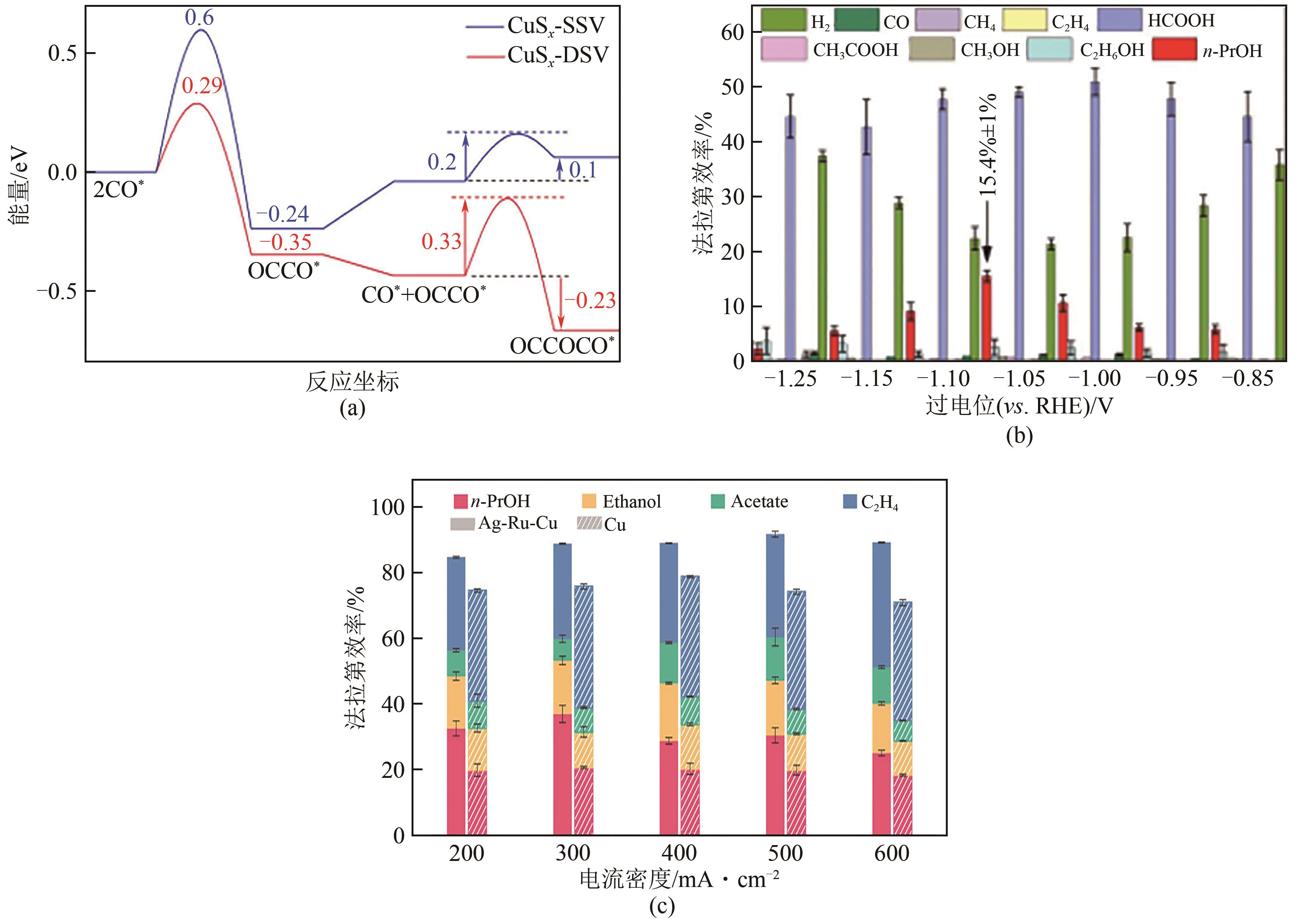
| 60 | PENG Chen, LUO Gan, ZHANG Junbo, et al. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol[J]. Nature Communications, 2021, 12: 1580. |
| 61 | WANG Xue, Pengfei OU, OZDEN Adnan, et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag-Ru-Cu catalyst[J]. Nature Energy, 2022, 7(2): 170-176. |
| 62 | RAHAMAN Motiar, DUTTA Abhijit, ZANETTI Alberto, et al. Electrochemical reduction of CO2 into multicarbon alcohols on activated Cu mesh catalysts: An identical location (IL) study[J]. ACS Catalysis, 2017, 7(11): 7946-7956. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [7] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [8] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [9] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [12] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||