化工进展 ›› 2023, Vol. 42 ›› Issue (8): 4058-4075.DOI: 10.16085/j.issn.1000-6613.2023-0894
长波长驱动光开关染料分子研究进展
- 华东理工大学化学与分子工程学院,精细化工研究所,上海 200237
-
收稿日期:2023-05-30修回日期:2023-08-14出版日期:2023-08-15发布日期:2023-09-19 -
通讯作者:张隽佶 -
作者简介:张志伟(1980—),博士,研究方向为光致变色染料。E-mail:zhiweizhang@ecust.edu.cn。 -
基金资助:国家自然科学基金(22122803)
Recent progress of long-wavelength-light-driven photoswitches
ZHANG Zhiwei( ), YANG Weixin, ZHANG Junji(
), YANG Weixin, ZHANG Junji( )
)
- Institute of Fine Chemicals, School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2023-05-30Revised:2023-08-14Online:2023-08-15Published:2023-09-19 -
Contact:ZHANG Junji
摘要:
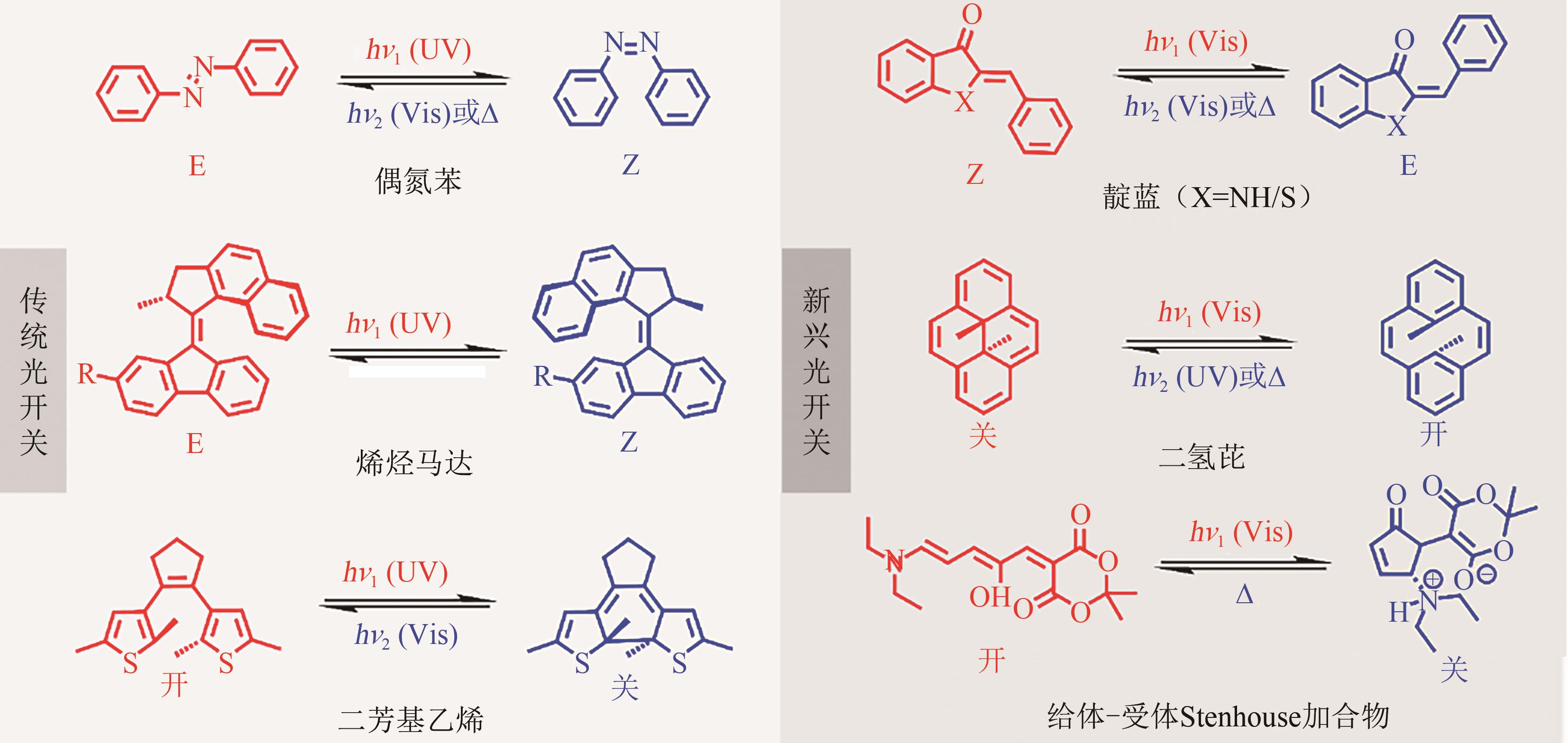
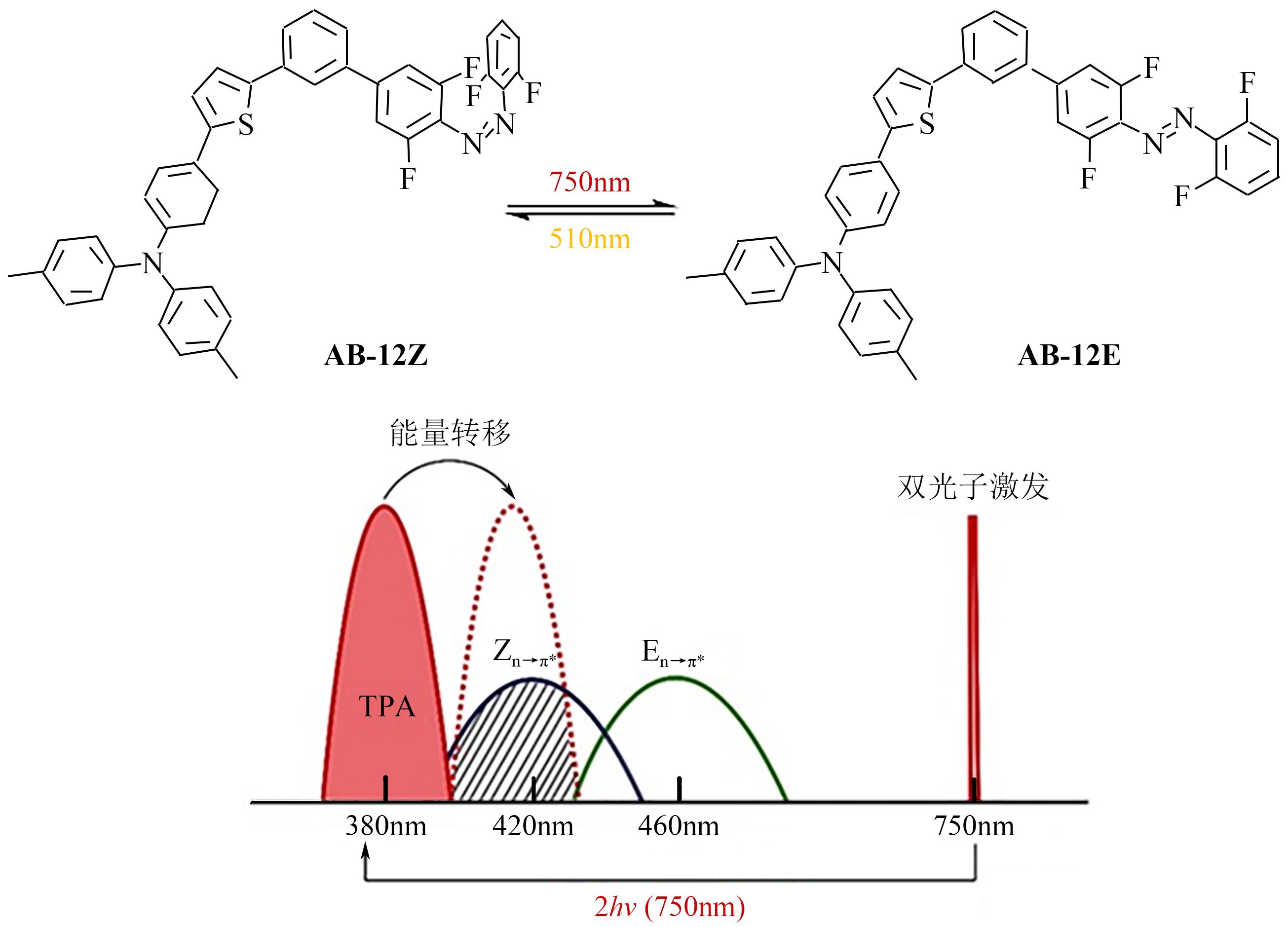
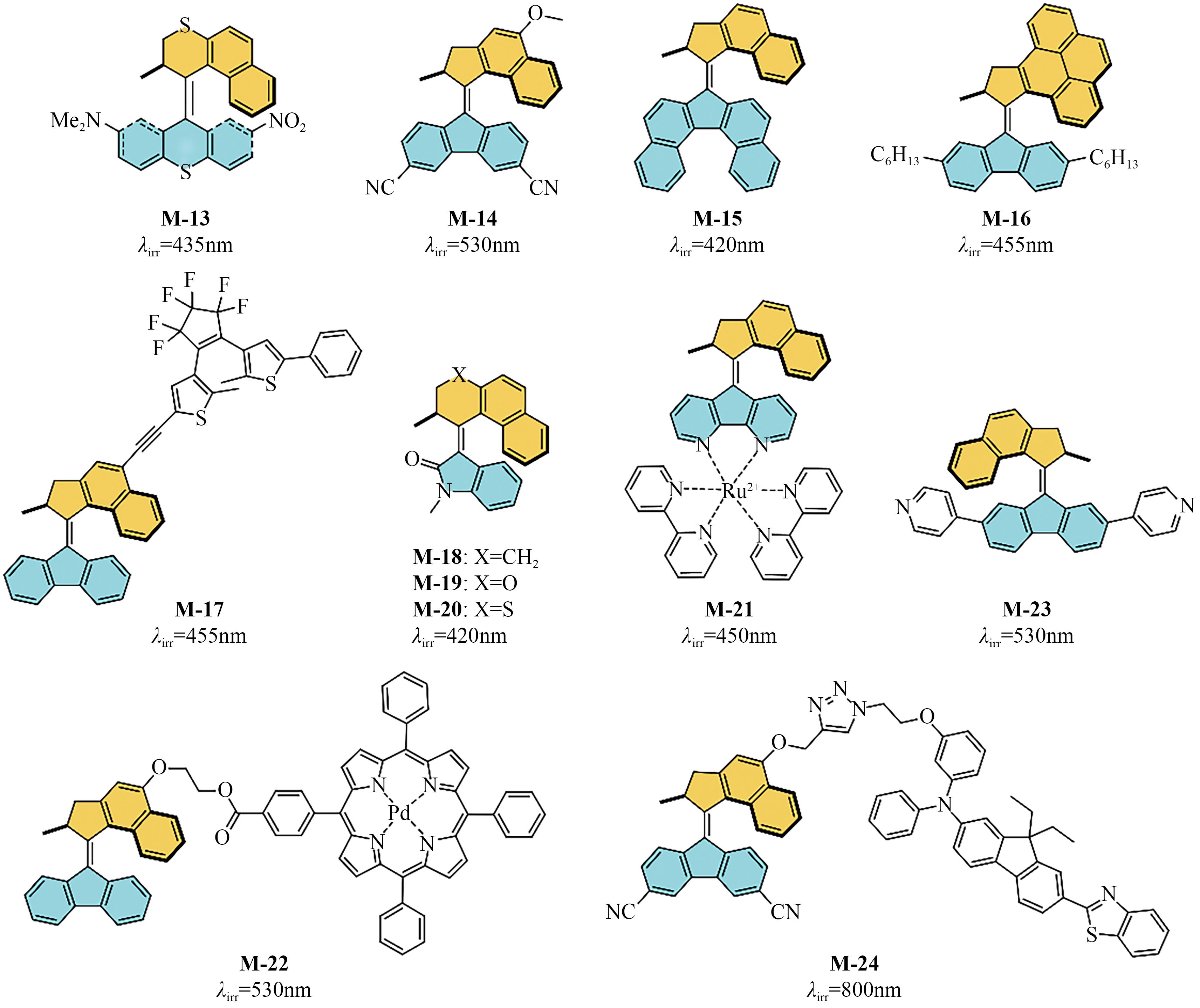
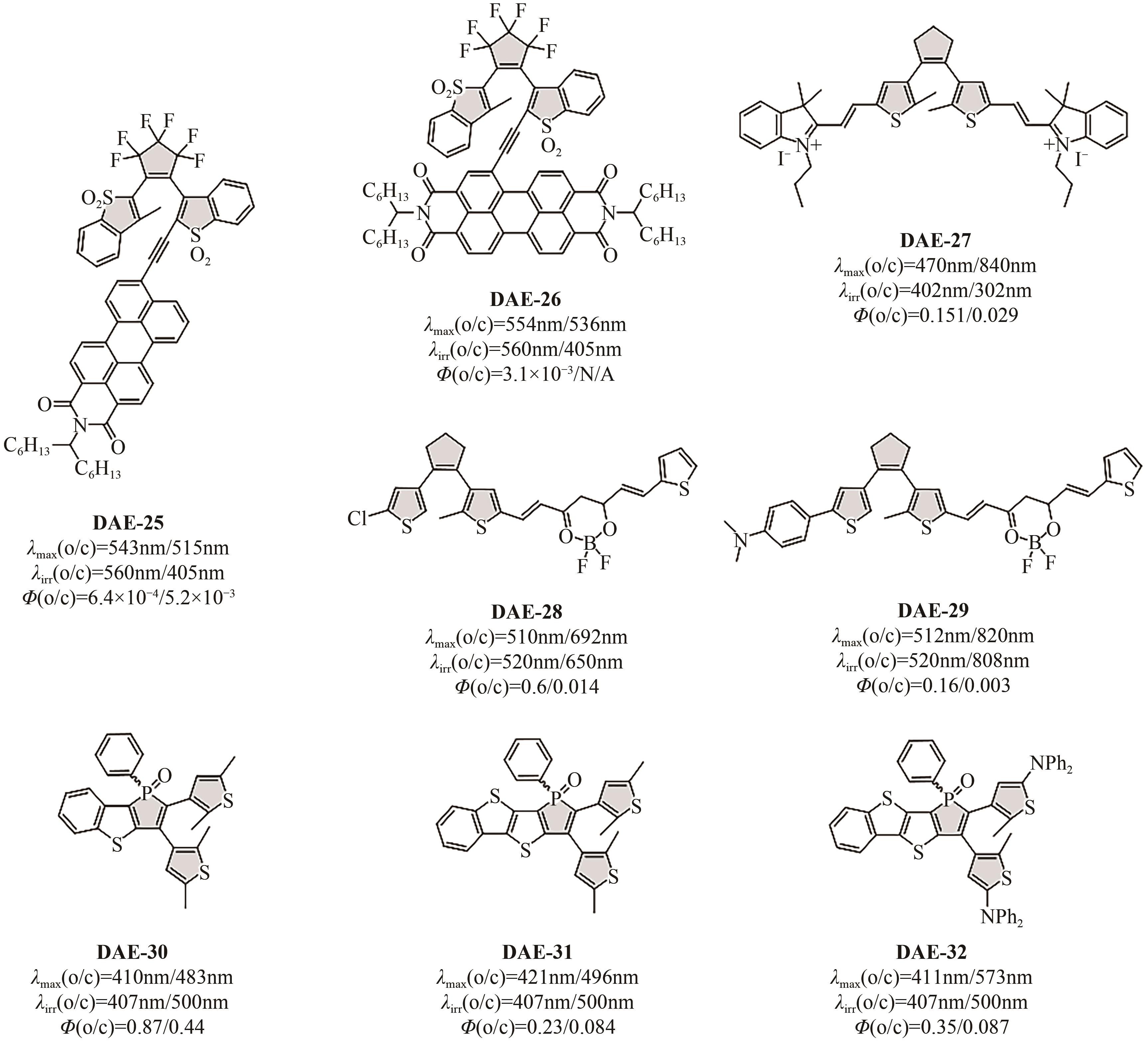
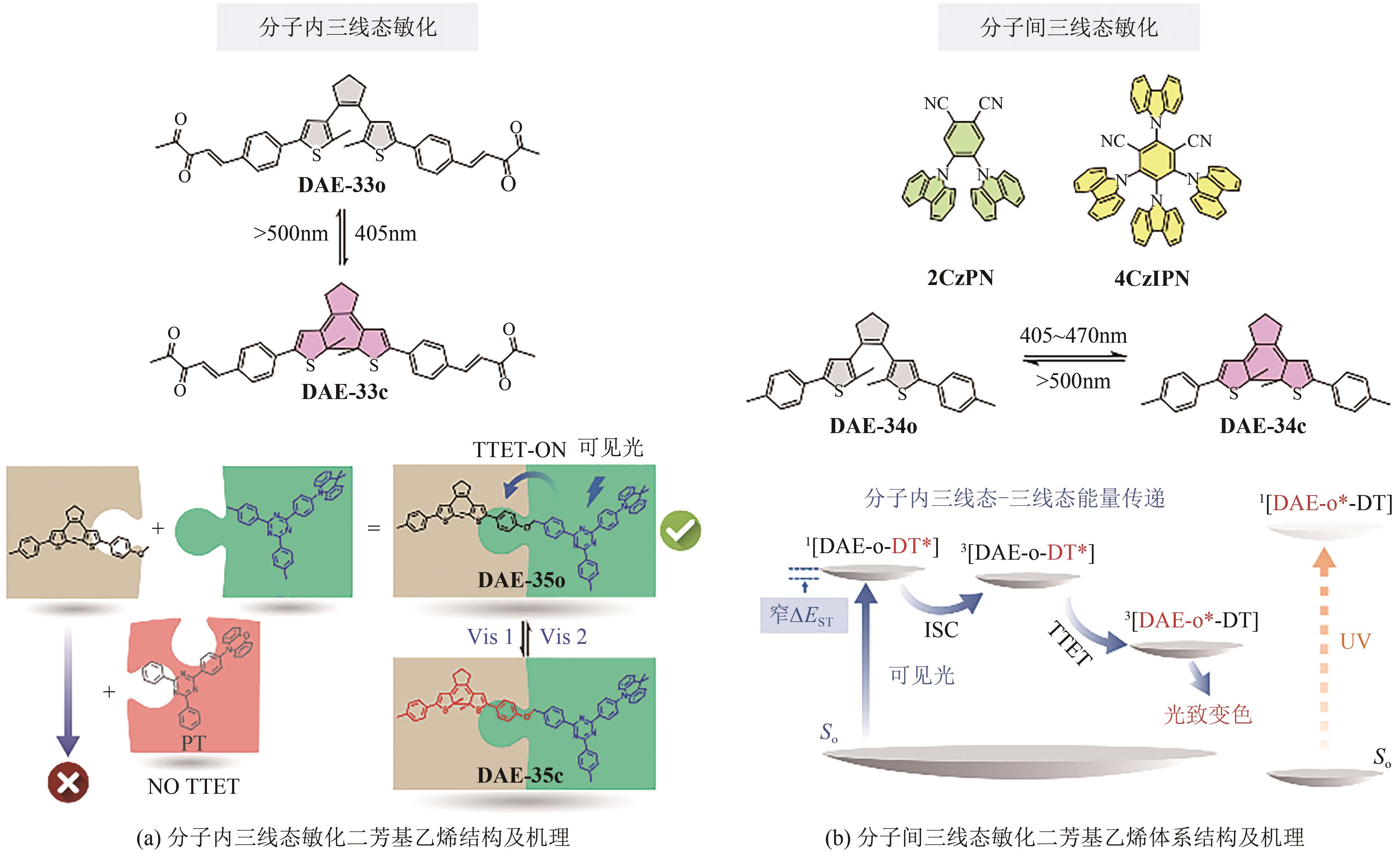
光开关染料分子可以在光照条件下于不同状态间发生可逆异构化,为高时空分辨远程控制材料和生物系统提供了独特的机会。然而,传统的光开关染料分子不可避免地需要紫外光激发,紫外光具有高损伤、高耗能、毒性大、组织穿透力差等弊端,导致其在生物领域应用受限。可见光/近红外光低能耗、低损伤且组织穿透力强,更能满足生物应用需求。因此,开发长波长驱动的光开关染料分子对推进其在超分辨成像、光药理学等新兴交叉学科领域应用具有重要意义。本文详细总结了近年来传统和新兴光开关染料分子在红移激发波长方面取得的最新研究进展,并针对转换效率、异构化量子产率、热力学稳定性以及抗疲劳性等指标对其光开关性能进行评价与讨论。最后对可见光/近红外光开关染料分子在未来发展过程中可能面临的机遇和挑战进行了展望。
中图分类号:
引用本文
张志伟, 杨伟鑫, 张隽佶. 长波长驱动光开关染料分子研究进展[J]. 化工进展, 2023, 42(8): 4058-4075.
ZHANG Zhiwei, YANG Weixin, ZHANG Junji. Recent progress of long-wavelength-light-driven photoswitches[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4058-4075.
| 48 | LI Ziyong, DAI Yijie, LU Zhiqiang, et al. Efficient green light-excited switches based on dithienylethenes with BF2-doped π-conjugated systems[J]. Chemical Communications, 2019, 55(89): 13430-13433. |
| 49 | WU Nathan Man-Wai, Maggie NG, Wai Han LAM, et al. Photochromic heterocycle-fused thieno[3,2-b]phosphole oxides as visible light switches without sacrificing photoswitching efficiency[J]. Journal of the American Chemical Society, 2017, 139(42): 15142-15150. |
| 50 | FREDRICH Sebastian, Robert GÖSTL, HERDER Martin, et al. Switching diarylethenes reliably in both directions with visible light[J]. Angewandte Chemie International Edition, 2016, 55(3): 1208-1212. |
| 51 | ZHANG Zhiwei, ZHANG Junji, WU Bin, et al. Diarylethenes with a narrow singlet-triplet energy gap sensitizer: A simple strategy for efficient visible-light photochromism[J]. Advanced Optical Materials, 2018, 6(6): 1700847. |
| 52 | ZHANG Zhiwei, WANG Wenhui, JIN Peipei, et al. A building-block design for enhanced visible-light switching of diarylethenes[J]. Nature Communications, 2019, 10: 4232. |
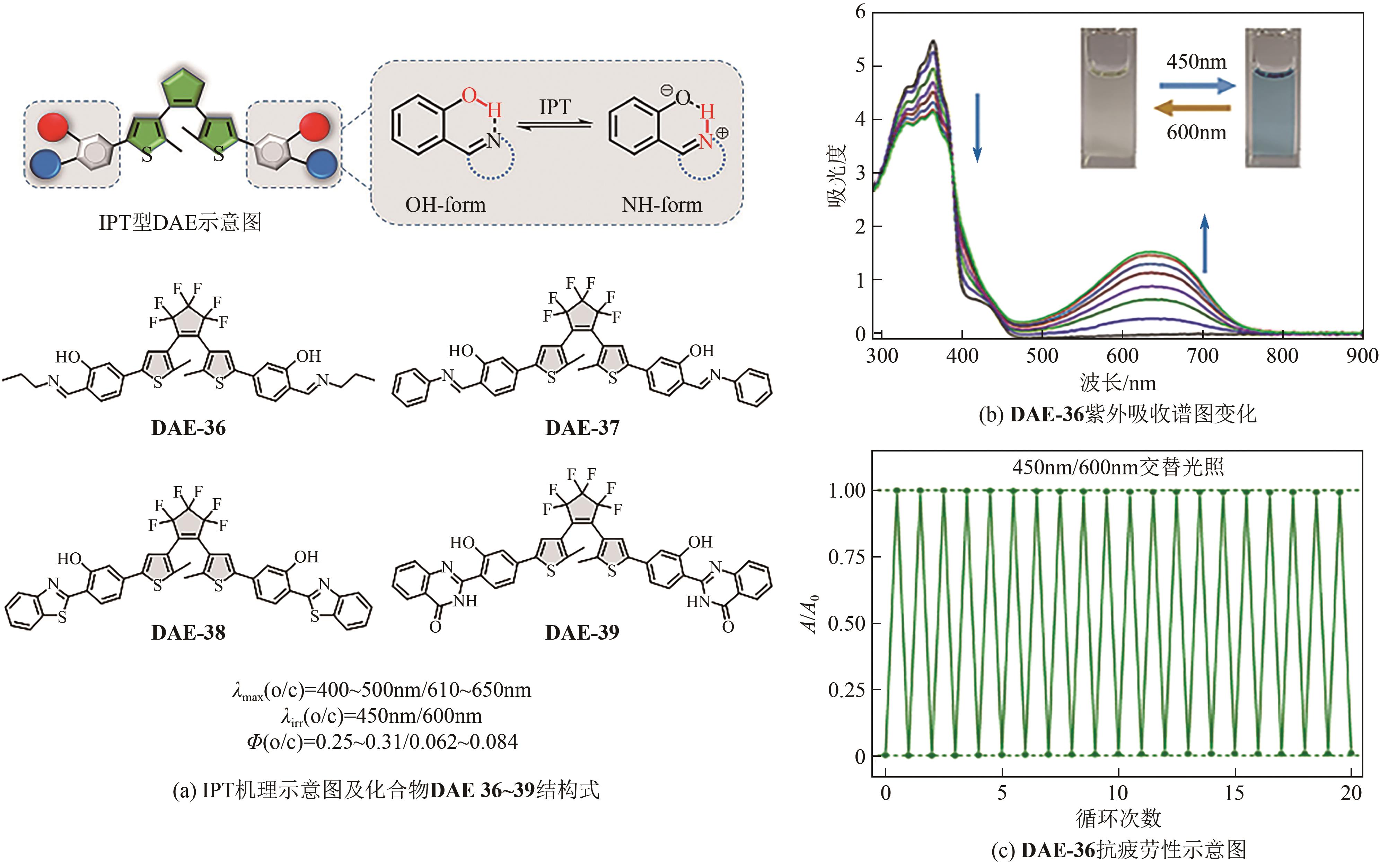
| 53 | XI Hancheng, ZHANG Zhipeng, ZHANG Weiwei, et al. All-visible-light-activated dithienylethenes induced by intramolecular proton transfer[J]. Journal of the American Chemical Society, 2019, 141(46): 18467-18474. |
| 54 | BOYER John-Christopher, CARLING Carl-Johan, GATES Byron D, et al. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity[J]. Journal of the American Chemical Society, 2010, 132(44): 15766-15772. |
| 55 | MORI Kazuya, ISHIBASHI Yukihide, MATSUDA Hirohisa, et al. One-color reversible control of photochromic reactions in a diarylethene derivative: Three-photon cyclization and two-photon cycloreversion by a near-infrared femtosecond laser pulse at 1.28μm[J]. Journal of the American Chemical Society, 2011, 133(8): 2621-2625. |
| 56 | DITTMANN Marc, GRAUPNER Franziska F, MAERZ Benjamin, et al. Photostability of 4, 4’-dihydroxythioindigo, a mimetic of indigo[J]. Angewandte Chemie International Edition, 2014, 53(2): 591-594. |
| 57 | HUANG Chung-Yang, BONASERA Aurelio, HRISTOV Lachezar, et al. N,N’-disubstituted indigos as readily available red-light photoswitches with tunable thermal half-lives[J]. Journal of the American Chemical Society, 2017, 139(42): 15205-15211. |
| 58 | PETERMAYER Christian, THUMSER Stefan, KINK Florian, et al. Hemiindigo: Highly bistable photoswitching at the biooptical window[J]. Journal of the American Chemical Society, 2017, 139(42): 15060-15067. |
| 59 | MAERZ Benjamin, WIEDBRAUK Sandra, OESTERLING Sven, et al. Making fast photoswitches faster—Using Hammett analysis to understand the limit of donor-acceptor approaches for faster hemithioindigo photoswitches[J]. Chemistry-A European Journal, 2014, 20(43): 13984-13992. |
| 60 | KINK Florian, COLLADO Marina Polo, WIEDBRAUK Sandra, et al. Frontispiece: Bistable photoswitching of hemithioindigo with green and red light: Entry point to advanced molecular digital information processing[J]. Chemistry-A European Journal, 2017, 23(26): 6237-6243. |
| 61 | ZWEIG Joshua E, NEWHOUSE Timothy R. Isomer-specific hydrogen bonding as a design principle for bidirectionally quantitative and redshifted hemithioindigo photoswitches[J]. Journal of the American Chemical Society, 2017, 139(32): 10956-10959. |
| 62 | MITCHELL Reginald H, BRKIC Zinka, SAURO Vittorio A, et al. A photochromic, electrochromic, thermochromic Ru complexed benzannulene: an organometallic example of the dimethyldihydropyrene-metacyclophanediene valence isomerization[J]. Journal of the American Chemical Society, 2003, 125(25): 7581-7585. |
| 63 | KLAUE Kristin, GARMSHAUSEN Yves, HECHT Stefan. Taking photochromism beyond visible: Direct one-photon NIR photoswitches operating in the biological window[J]. Angewandte Chemie International Edition, 2018, 57(5): 1414-1417. |
| 64 | KLAUE Kristin, HAN Wenjie, LIESFELD Pauline, et al. Donor-acceptor dihydropyrenes switchable with near-infrared light[J]. Journal of the American Chemical Society, 2020, 142(27): 11857-11864. |
| 65 | LERCH Michael M, SZYMANSKI Wiktor, FERINGA Ben L. The (photo)chemistry of Stenhouse photoswitches: Guiding principles and system design[J]. Chemical Society Reviews, 2018, 47(6): 1910-1937. |
| 66 | HELMY Sameh, LEIBFARTH Frank A, Saemi OH, et al. Photoswitching using visible light: A new class of organic photochromic molecules[J]. Journal of the American Chemical Society, 2014, 136(23): 8169-8172. |
| 67 | HEMMER James R, POELMA Saemi O, TREAT Nicolas, et al. Tunable visible and near infrared photoswitches[J]. Journal of the American Chemical Society, 2016, 138(42): 13960-13966. |
| 68 | HEMMER James R, PAGE Zachariah A, CLARK Kyle D, et al. Controlling dark equilibria and enhancing donor-acceptor stenhouse adduct photoswitching properties through carbon acid design[J]. Journal of the American Chemical Society, 2018, 140(33): 10425-10429. |
| 69 | LERCH Michael M, DI DONATO Mariangela, LAURENT Adèle D, et al. Solvent effects on the actinic step of donor-acceptor Stenhouse adduct photoswitching[J]. Angewandte Chemie International Edition, 2018, 57(27): 8063-8068. |
| 70 | KUMAR Kamlesh, KNIE Christopher, David BLÉGER, et al. A chaotic self-oscillating sunlight-driven polymer actuator[J]. Nature Communications, 2016, 7: 11975. |
| 71 | WANG Yingying, LI Mengwei, YAN Chunmei, et al. Diazocine as a versatile building block enables excellent photoswitching and chromic properties in self-assembled organogels[J]. CCS Chemistry, 2022, 4(2): 704-712. |
| 1 | SINESHCHEKOV Oleg A, SPUDICH John L. Sensory rhodopsin signaling in green flagellate algae[M]//Handbook of Photosensory Receptors. Weinheim, FRG: Wiley-VCH Verlag GmbH & Co. KGaA, 2005: 25-42. |
| 2 | CARBONELL Carlos, VALLES Daniel, WONG Alexa M, et al. Polymer brush hypersurface photolithography[J]. Nature Communications, 2020, 11: 1244. |
| 3 | PHAM Thanh Chung, van Nghia NGUYEN, CHOI Yeonghwan, et al. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy[J]. Chemical Reviews, 2021, 121(21): 13454-13619. |
| 4 | CRESPI Stefano, SIMETH Nadja A, Burkhard KÖNIG. Heteroaryl azo dyes as molecular photoswitches[J]. Nature Reviews Chemistry, 2019, 3(3): 133-146. |
| 5 | ZHANG Junji, TIAN He. The endeavor of diarylethenes: New structures, high performance, and bright future[J]. Advanced Optical Materials, 2018, 6(6): 1701278. |
| 6 | TIAN He, ZHANG Junji. Photochromic materials: preparation, properties and applications[M]. New York, John Wiley & Sons Inc., 2016. |
| 7 | WU Yue, XIE Yongshu, ZHANG Qiong, et al. Quantitative photoswitching in bis(dithiazole)ethene enables modulation of light for encoding optical signals[J]. Angewandte Chemie International Edition, 2014, 53(8): 2090-2094. |
| 8 | Jana VOLARIĆ, SZYMANSKI Wiktor, SIMETH Nadja A, et al. Molecular photoswitches in aqueous environments[J]. Chemical Society Reviews, 2021, 50(22): 12377-12449. |
| 9 | ZHAO Hui, Soumyo SEN, UDAYABHASKARARAO T, et al. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks[J]. Nature Nanotechnology, 2016, 11(1): 82-88. |
| 10 | HOU Lili, ZHANG Xiaoyan, COTELLA Giovanni F, et al. Optically switchable organic light-emitting transistors[J]. Nature Nanotechnology, 2019, 14(4): 347-353. |
| 11 | JIA Chuancheng, Migliore Agostino, XIN Na, et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity[J]. Science, 2016, 352(6292): 1443-1445. |
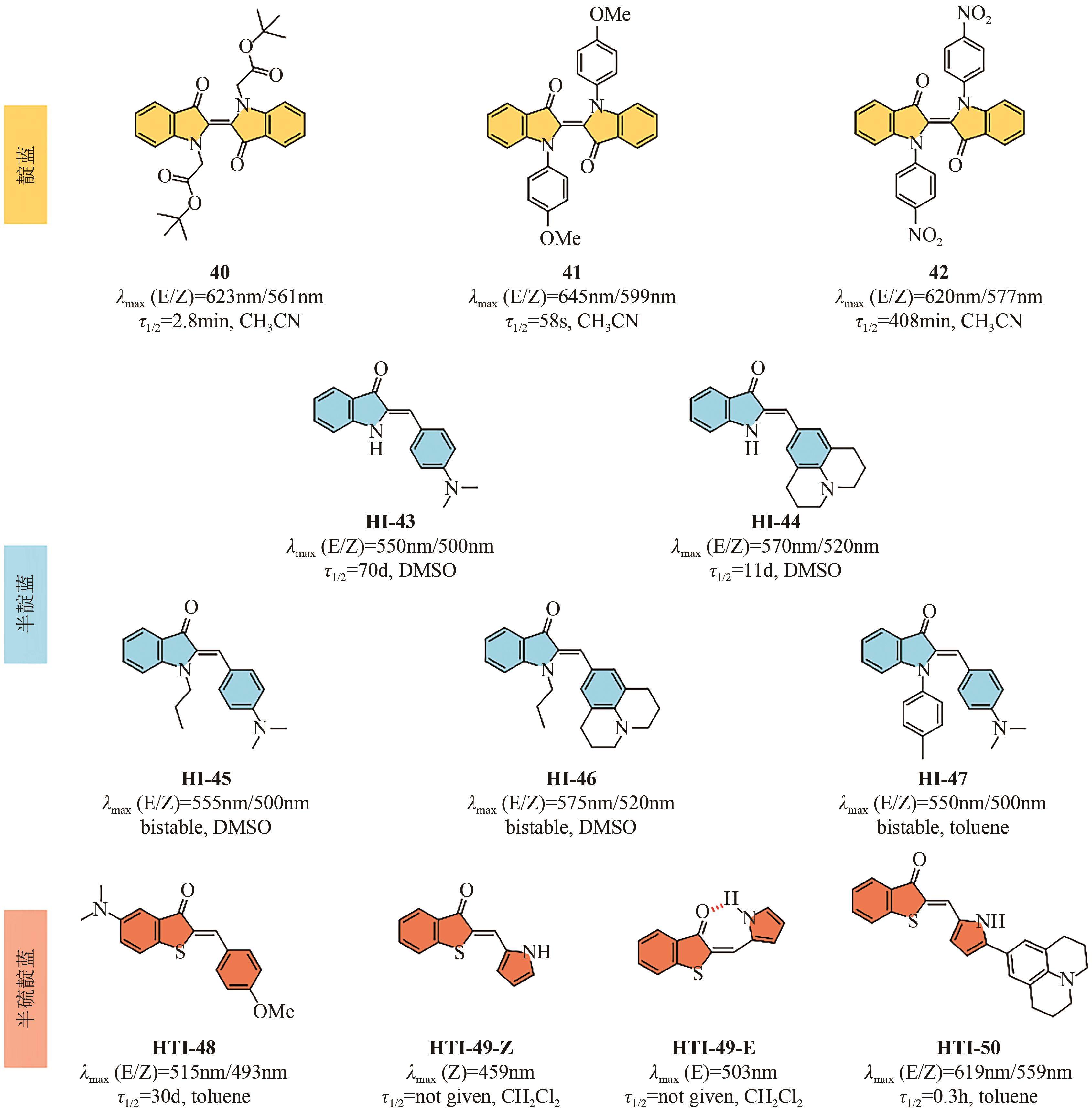
| 12 | EISENREICH Fabian, KATHAN Michael, DALLMANN Andre, et al. A photoswitchable catalyst system for remote-controlled (co)polymerization in situ [J]. Nature Catalysis, 2018, 1(7): 516-522. |
| 13 | XU Wencong, SUN Shaodong, WU Si. Photoinduced reversible solid-to-liquid transitions for photoswitchable materials[J]. Angewandte Chemie International Edition, 2019, 58(29): 9712-9740. |
| 14 | Wiktor SZYMAŃSKI, BEIERLE John M, KISTEMAKER Hans A V, et al. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches[J]. Chemical Reviews, 2013, 113(8): 6114-6178. |
| 15 | LUBBE Anouk S, SZYMANSKI Wiktor, FERINGA Ben L. Recent developments in reversible photoregulation of oligonucleotide structure and function[J]. Chemical Society Reviews, 2017, 46(4): 1052-1079. |
| 16 | LI Ziyuan, LIU Yingya, LI Yuanjie, et al. High-preservation single-cell operation through a photo-responsive hydrogel-nanopipette system[J]. Angewandte Chemie International Edition, 2021, 60(10): 5157-5161. |
| 17 | Katharina HÜLL, MORSTEIN Johannes, TRAUNER Dirk. In vivo photopharmacology[J]. Chemical Reviews, 2018, 118(21): 10710-10747. |
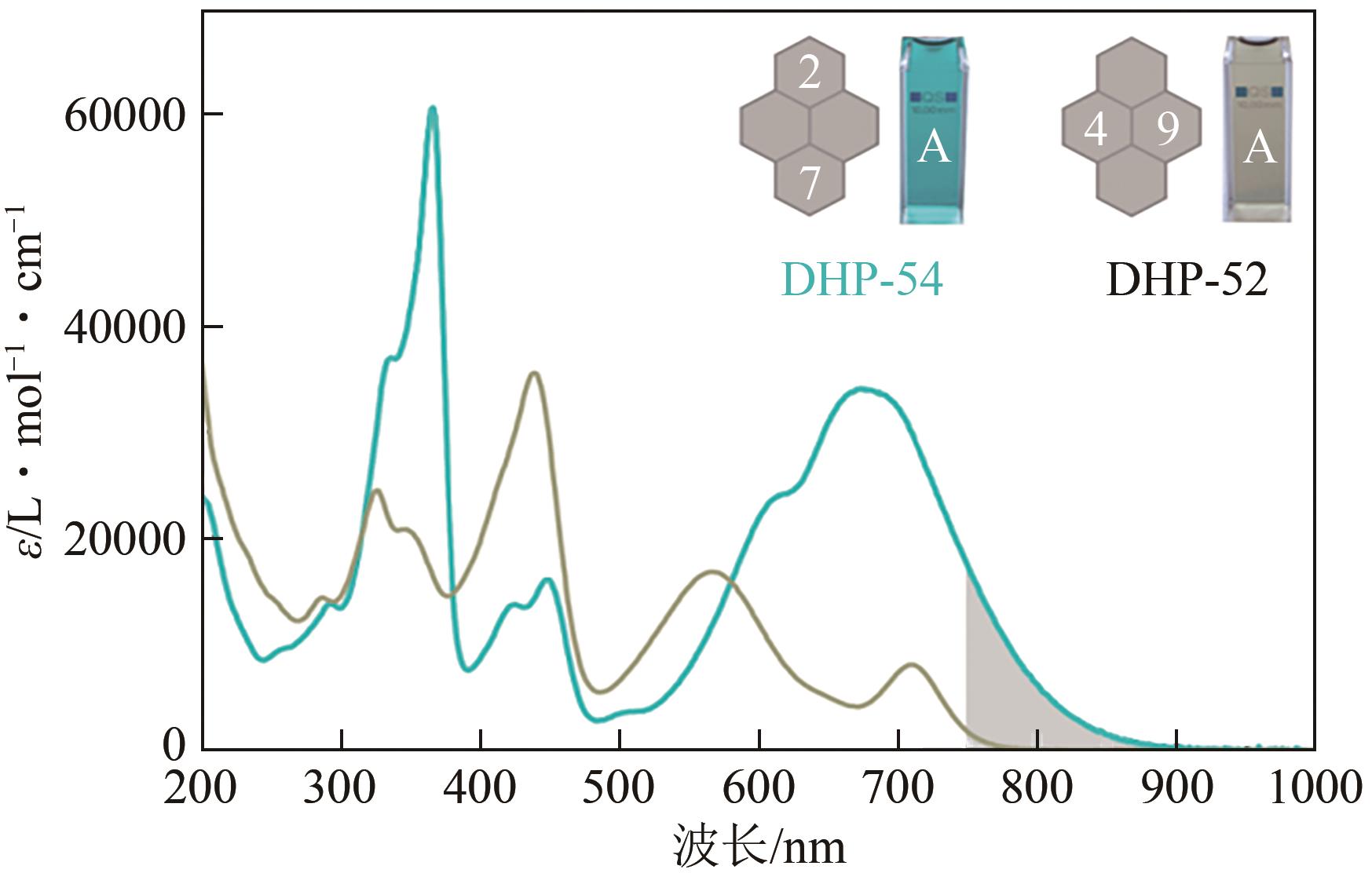
| 18 | FUCHTER Matthew J. On the promise of photopharmacology using photoswitches: A medicinal chemist’s perspective[J]. Journal of Medicinal Chemistry, 2020, 63(20): 11436-11447. |
| 19 | CHAI Xianzhi, HAN Haihao, SEDGWICK Adam C, et al. Photochromic fluorescent probe strategy for the super-resolution imaging of biologically important biomarkers[J]. Journal of the American Chemical Society, 2020, 142(42): 18005-18013. |
| 20 | KALKA Katrin, MERK Hans, MUKHTAR Hasan. Photodynamic therapy in dermatology[J]. Journal of the American Academy of Dermatology, 2000, 42(3): 389-413. |
| 21 | FRAZIER Claude C. Photodynamic therapy in dermatology[J]. International Journal of Dermatology, 1996, 35(5): 312-316. |
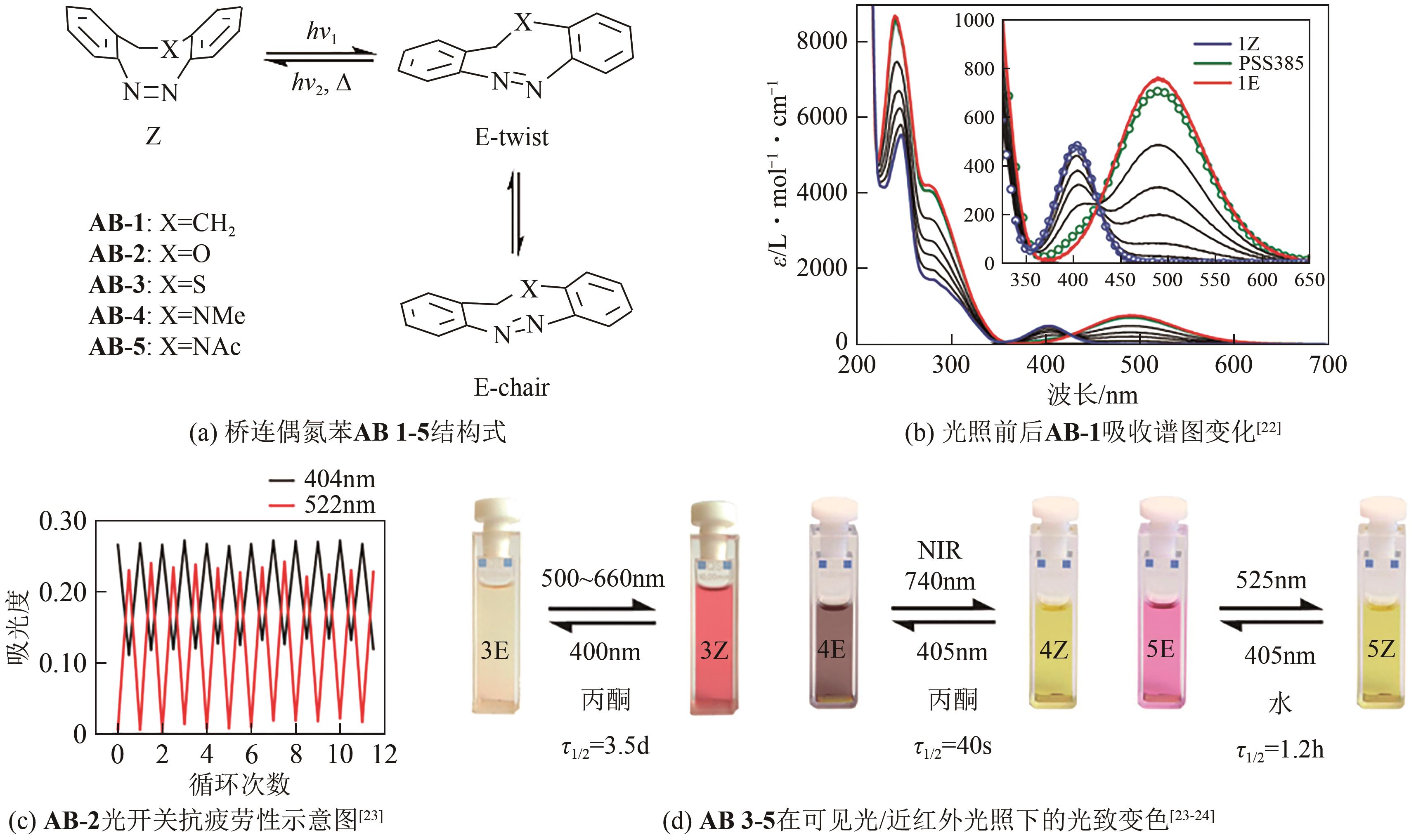
| 22 | SIEWERTSEN R, NEUMANN H, BUCHHEIM-STEHN B, et al. Highly efficient reversible Z-E photoisomerization of a bridged azobenzene with visible light through resolved S(1)(n pi*) absorption bands[J]. Journal of the American Chemical Society, 2009, 131(43): 15594-15595. |
| 23 | HAMMERICH Melanie, Christian SCHÜTT, Cosima STÄHLER, et al. Heterodiazocines: Synthesis and photochromic properties, Trans to Cis switching within the bio-optical window[J]. Journal of the American Chemical Society, 2016, 138(40): 13111-13114. |
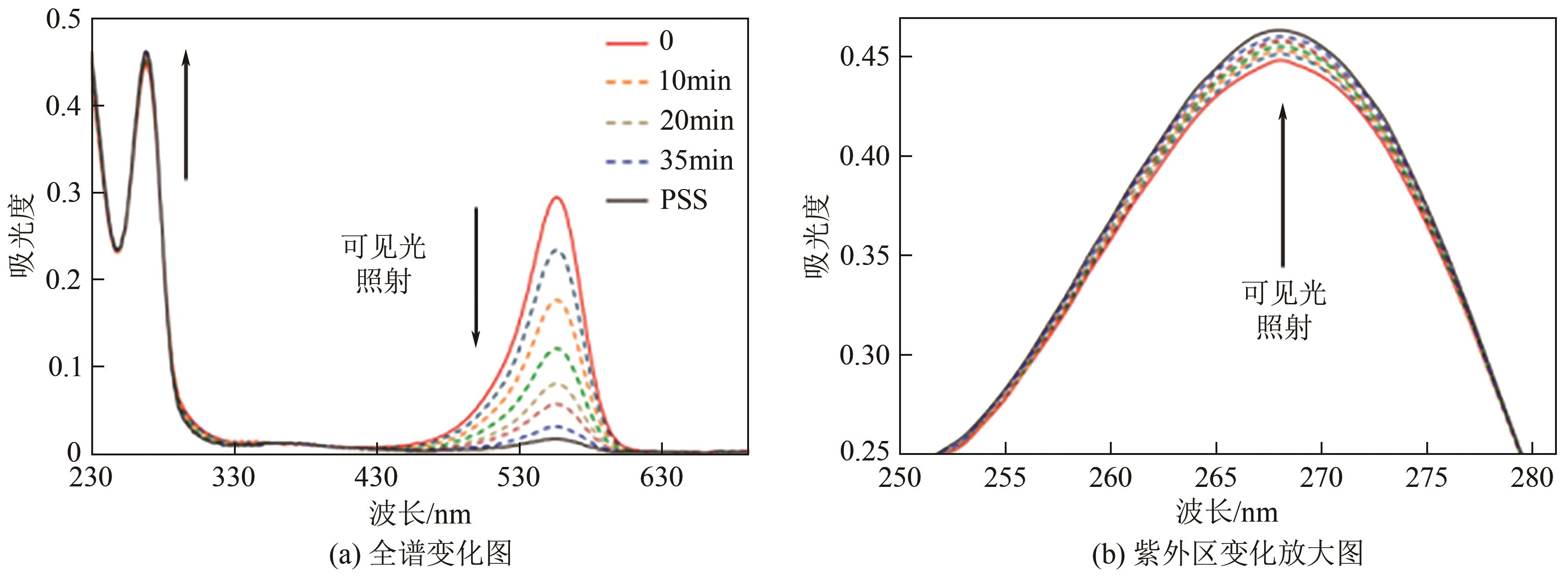
| 24 | LENTES Pascal, STADLER Eduard, Fynn RÖHRICHT, et al. Nitrogen bridged diazocines: Photochromes switching within the near-infrared region with high quantum yields in organic solvents and in water[J]. Journal of the American Chemical Society, 2019, 141(34): 13592-13600. |
| 72 | ZHAO Yunhan, MA Liangwei, HUANG Zizhao, et al. Visible light activated organic room-temperature phosphorescence based on triplet-to-singlet Förster-resonance energy transfer[J]. Advanced Optical Materials, 2022, 10(8): 2102701. |
| 73 | LI Ziyuan, WANG Chen, LI Jiang, et al. Functional DNA structures and their biomedical applications[J]. CCS Chemistry, 2020, 2(5): 707-728. |
| 74 | ZHENG Zhigang, HU Honglong, ZHANG Zhipeng, et al. Digital photoprogramming of liquid-crystal superstructures featuring intrinsic chiral photoswitches[J]. Nature Photonics, 2022, 16(3): 226-234. |
| 75 | VELEMA Willem. A, SZYMAŃSKI Wiktor, FERINGA Ben L, Photopharmacology: Beyond proof of principle[J]. Journal of the American Chemical Society, 2014, 136(6): 2178-2191. |
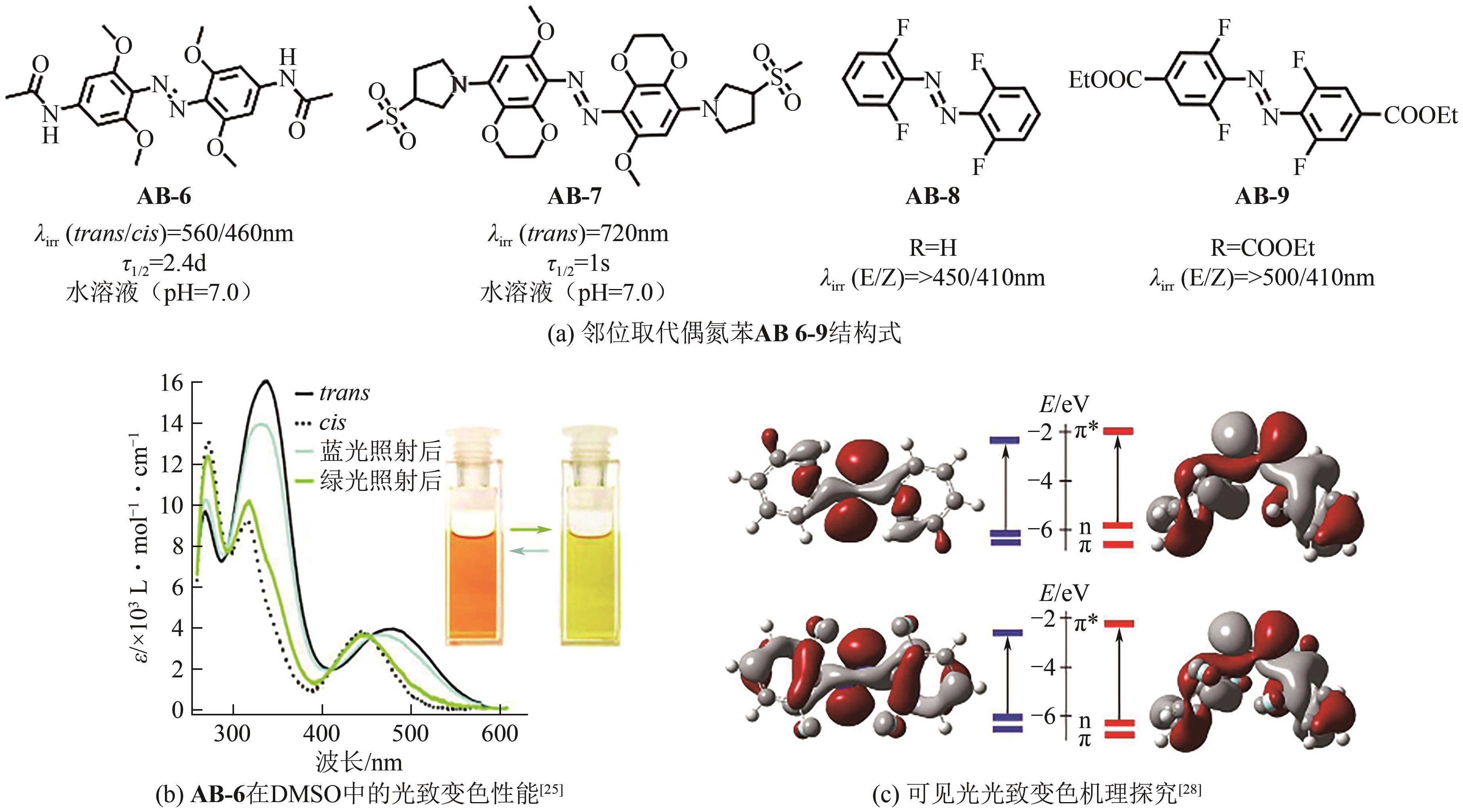
| 25 | BEHARRY Andrew A, SADOVSKI Oleg, Andrew WOOLLEY G. Azobenzene photoswitching without ultraviolet light[J]. Journal of the American Chemical Society, 2011, 133(49): 19684-19687. |
| 26 | SAMANTA Subhas, BEHARRY Andrew A, SADOVSKI Oleg, et al. Photoswitching azo compounds in vivo with red light[J]. Journal of the American Chemical Society, 2013, 135(26): 9777-9784. |
| 27 | DONG Mingxin, BABALHAVAEJI Amirhossein, COLLINS Catherine V, et al. Near-infrared photoswitching of azobenzenes under physiological conditions[J]. Journal of the American Chemical Society, 2017, 139(38): 13483-13486. |
| 28 | David BLÉGER, SCHWARZ Jutta, BROUWER Albert M, et al. O-fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light[J]. Journal of the American Chemical Society, 2012, 134(51): 20597-20600. |
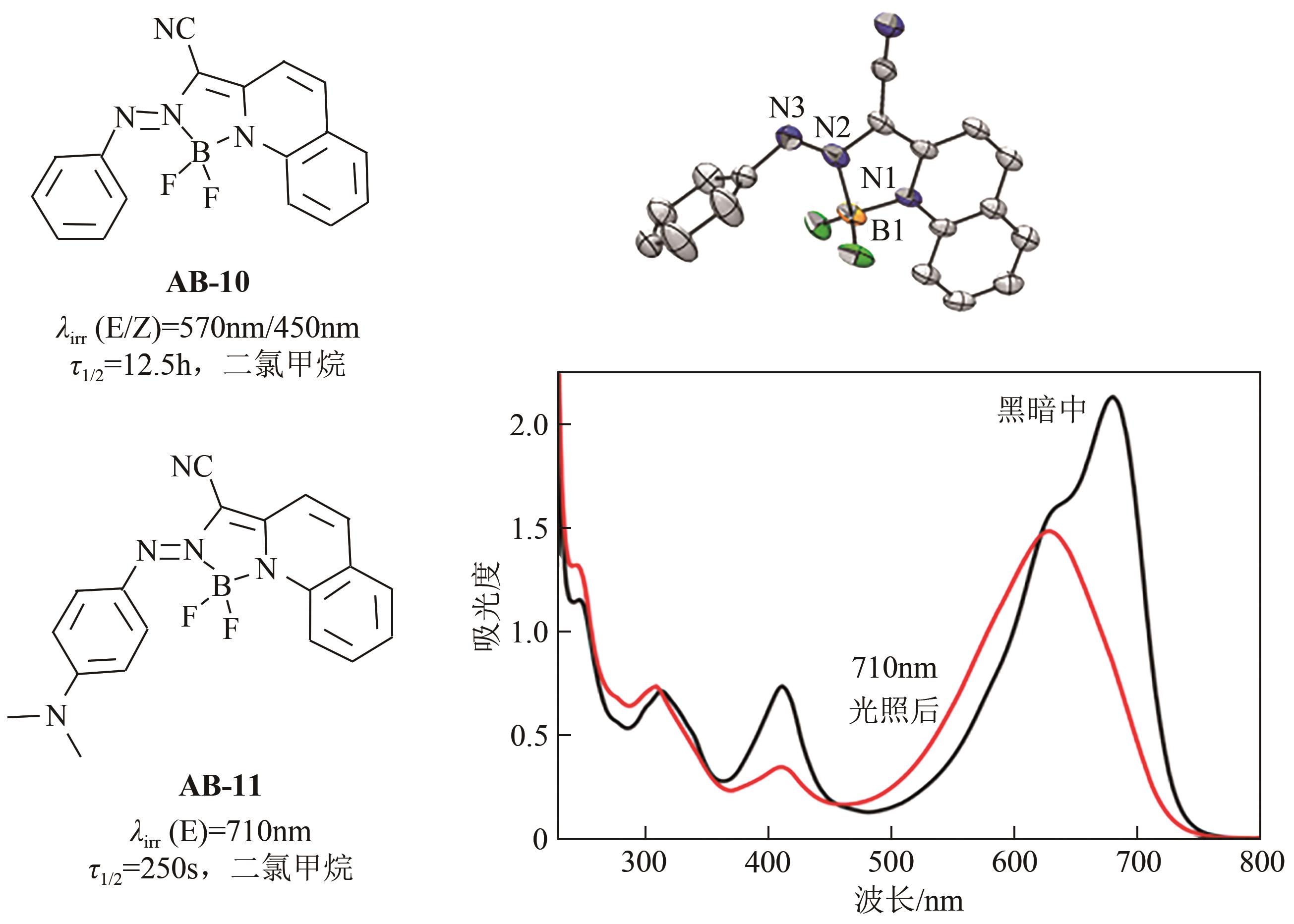
| 29 | YANG Yin, HUGHES Russell P, APRAHAMIAN Ivan. Visible light switching of a BF2-coordinated azo compound[J]. Journal of the American Chemical Society, 2012, 134(37): 15221-15224. |
| 30 | YANG Yin, HUGHES Russell P, APRAHAMIAN Ivan. Near-infrared light activated azo-BF2 switches[J]. Journal of the American Chemical Society, 2014, 136(38): 13190-13193. |
| 31 | WALKER Edwin, RENTZEPIS Peter M. A new dimension[J]. Nature Photonics, 2008, 2(7): 406-408. |
| 32 | MORENO Javier, GERECKE Mario, GRUBERT Lutz, et al. Sensitized two-NIR-photon Z→E isomerization of a visible-light-addressable bistable azobenzene derivative[J]. Angewandte Chemie International Edition, 2016, 55(4): 1544-1547. |
| 33 | KOUMURA N, ZIJLSTRA R W, VAN DELDEN R A, et al. Light-driven monodirectional molecular rotor[J]. Nature, 1999, 401(6749): 152-155. |
| 34 | WANG Jiaobing, FERINGA Ben L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor[J]. Science, 2011, 331(6023): 1429-1432. |
| 35 | VAN DELDEN Richard A, KOUMURA Nagatoshi, SCHOEVAARS Annemarie, et al. A donor-acceptor substituted molecular motor: Unidirectional rotation driven by visible light[J]. Organic & Biomolecular Chemistry, 2003, 1(1): 33-35. |
| 36 | PFEIFER Lukas, Maximilian SCHERÜBL, FELLERT Maximilian, et al. Photoefficient 2nd generation molecular motors responsive to visible light[J]. Chemical Science, 2019, 10(38): 8768-8773. |
| 37 | SHI Zhaotao, HU Yixiong, HU Zhubin, et al. Visible-light-driven rotation of molecular motors in discrete supramolecular metallacycles[J]. Journal of the American Chemical Society, 2021, 143(1): 442-452. |
| 38 | van LEEUWEN Thomas, Jasper POL, ROKE Diederik, et al. Visible-light excitation of a molecular motor with an extended aromatic core[J]. Organic Letters, 2017, 19(6): 1402-1405. |
| 39 | ROKE Diederik, FERINGA Ben L, WEZENBERG Sander J. A visible-light-driven molecular motor based on pyrene[J]. Helvetica Chimica Acta, 2019, 102(2): e1800221. |
| 40 | ROKE Diederik, STUCKHARDT Constantin, DANOWSKI Wojciech, et al. Light-gated rotation in a molecular motor functionalized with a dithienylethene switch[J]. Angewandte Chemie International Edition, 2018, 57(33): 10515-10519. |
| 41 | ROKE Diederik, Metin SEN, DANOWSKI Wojciech, et al. Visible-light-driven tunable molecular motors based on oxindole[J]. Journal of the American Chemical Society, 2019, 141(18): 7622-7627. |
| 42 | WEZENBERG Sander J, CHEN Kuang-Yen, FERINGA Ben L. Visible-light-driven photoisomerization and increased rotation speed of a molecular motor acting as a ligand in a ruthenium(Ⅱ) complex[J]. Angewandte Chemie International Edition, 2015, 54(39): 11457-11461. |
| 43 | CNOSSEN Arjen, HOU Lili, POLLARD Michael M, et al. Driving unidirectional molecular rotary motors with visible light by intra- and intermolecular energy transfer from palladium porphyrin[J]. Journal of the American Chemical Society, 2012, 134(42): 17613-17619. |
| 44 | DANOWSKI Wojciech, CASTIGLIONI Fabio, SARDJAN Andy S, et al. Visible-light-driven rotation of molecular motors in a dual-function metal-organic framework enabled by energy transfer[J]. Journal of the American Chemical Society, 2020, 142(19): 9048-9056. |
| 45 | PFEIFER Lukas, HOANG Nong V, Maximilian SCHERÜBL, et al. Powering rotary molecular motors with low-intensity near-infrared light[J]. Science Advances, 2020, 6(44): eabb6165. |
| 46 | FUKAMINATO Tuyoshi, HIROSE Takashi, Takao DOI, et al. Molecular design strategy toward diarylethenes that photoswitch with visible light[J]. Journal of the American Chemical Society, 2014, 136(49): 17145-17154. |
| 47 | HU Fang, CAO Meijiao, MA Xiang, et al. Visible-light-dependent photocyclization: Design, synthesis, and properties of a cyanine-based dithienylethene[J]. The Journal of Organic Chemistry, 2015, 80(15): 7830-7835. |
| [1] | 多佳, 姚国栋, 王英霁, 曾旭, 金滨滨. 改性Au-TiO2光降解废水中诺氟沙星的影响[J]. 化工进展, 2023, 42(2): 624-630. |
| [2] | 张会霞, 周立山, 张程蕾, 钱光磊, 谢陈鑫, 朱令之. Bi2S3/TiO2纳米锥光阳极的制备及其光电催化降解土霉素[J]. 化工进展, 2023, 42(10): 5548-5557. |
| [3] | 刘海成, 孟无霜, 黄哲, 尤雨, 花瑞琪, 曹梦茹. WO3/BiOCl0.7I0.3光催化剂的制备及其光催化降解机理[J]. 化工进展, 2023, 42(1): 255-264. |
| [4] | 陈毓, 王佳佳, 汤琳. 漂浮型氮化碳光催化剂CNx@mEP的制备及性能[J]. 化工进展, 2022, 41(12): 6477-6488. |
| [5] | 赵文霞, 赵玉, 柴子茹, 张硕, 王世欣, 焦志杰. N2等离子体改性介孔TiO2的可见光催化性能及机理[J]. 化工进展, 2022, 41(11): 5820-5829. |
| [6] | 罗菊香, 程德书, 李明春, 辛梅华. 室温可见光引发聚合诱导自组装制备P2VP-b-PSt纳米材料[J]. 化工进展, 2021, 40(5): 2676-2684. |
| [7] | 胡丽萍, 黄生权, 田淑华, 黄延盛, 胡流云, 李璇, 舒逸聃, 王学重. 近红外光谱技术在中草药口服液在线质量监控中的模型建立和模型转移[J]. 化工进展, 2020, 39(8): 3263-3272. |
| [8] | 李丽妹, 王梅阁, 杨秋生, 段中余. 均三嗪衍生物比色识别Cu2+及荧光识别Zn2+[J]. 化工进展, 2020, 39(8): 3287-3292. |
| [9] | 余传明, 曾圣威, 江金娥, 林雯毓, 邓翠儿, 李泳. 铜基化合物的可见光催化研究进展[J]. 化工进展, 2020, 39(6): 2411-2421. |
| [10] | 朱恩权, 马玉花, 艾尼娃·木尼热. 红磷光催化材料的研究进展[J]. 化工进展, 2019, 38(s1): 139-143. |
| [11] | 于雪,包青青,姜超,张思霞,张丰旗,陈驰,陶娅妮,张跃伟. 花菁类近红外荧光探针在生物检测中的应用研究进展[J]. 化工进展, 2019, 38(12): 5492-5503. |
| [12] | 曹丽丽, 蒋善庆, 凌泽玉, 汪楚乔, 许霞, 王利平. 水热合成Ag+掺杂SrTiO3可见光催化降解四环素性能和机制[J]. 化工进展, 2018, 37(11): 4500-4508. |
| [13] | 贺凯迅, 曹鹏飞. 基于智能优化算法的软测量模型建模样本优选及应用[J]. 化工进展, 2018, 37(07): 2516-2523. |
| [14] | 李晶晶, 周昭露, 黄生权, 鲁亮, 黄延盛, 田淑华, FALOLA Akinola A, 李璇, 李杰, 舒逸聃, 王学重. 近红外光谱技术应用于中草药口服液在线质量控制的化学计量学建模[J]. 化工进展, 2018, 37(05): 1923-1932. |
| [15] | 龚久炎, 宋文东, 陈嘉琳, 李世杰, 蔡璐, 纪丽丽. Ag/AgBr-硅藻土复合光催化剂的制备及其可见光光催化性能[J]. 化工进展, 2017, 36(09): 3309-3315. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||