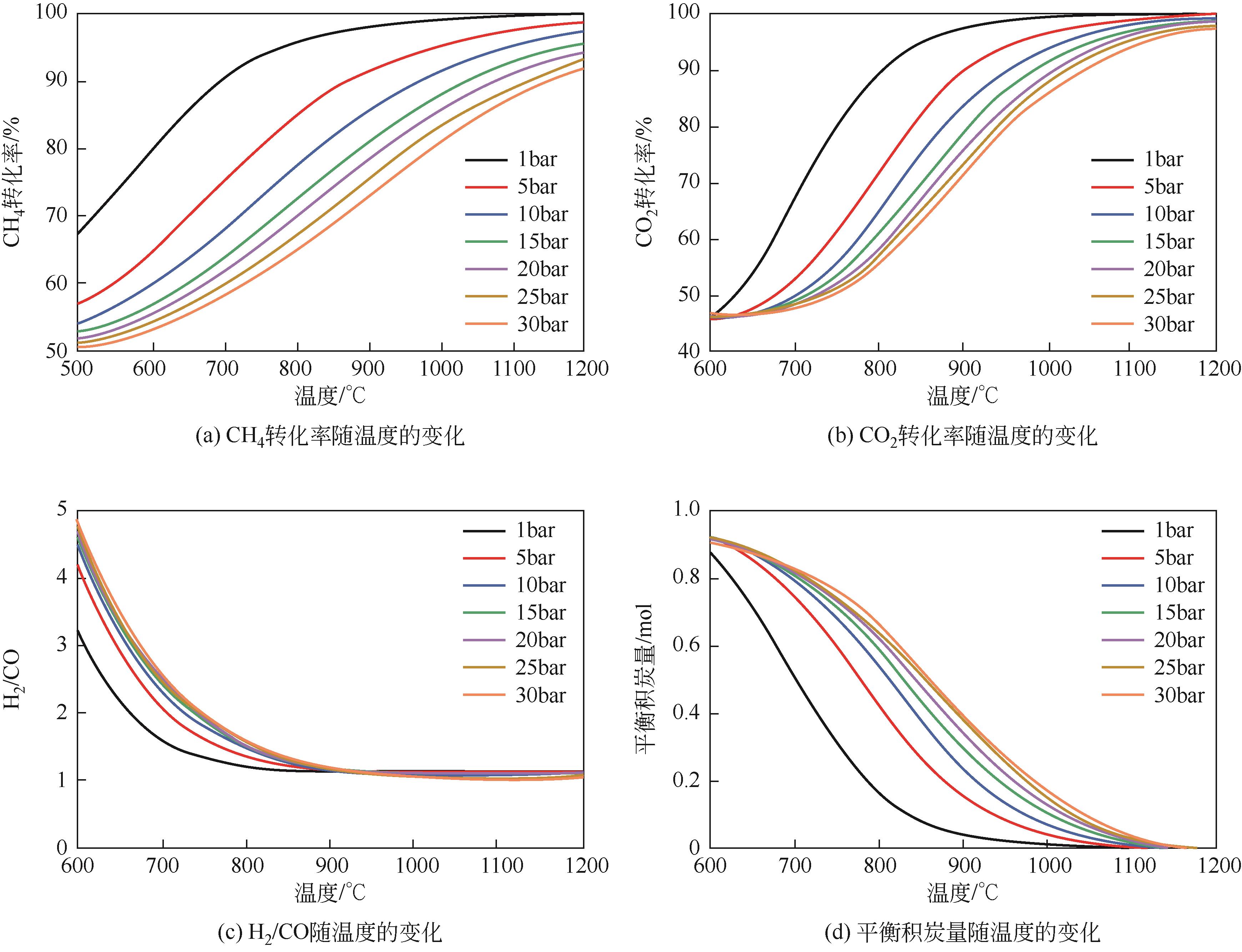
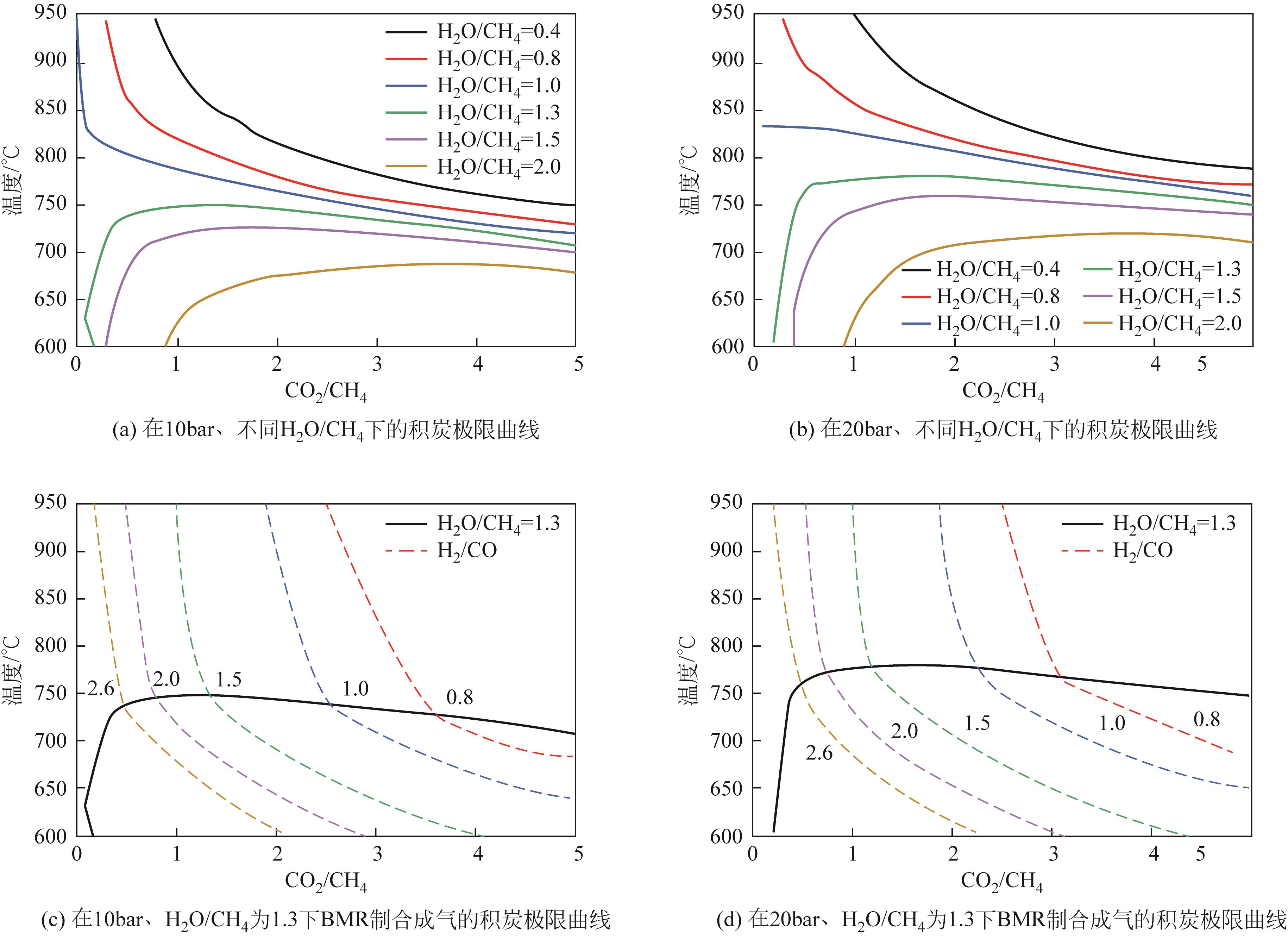
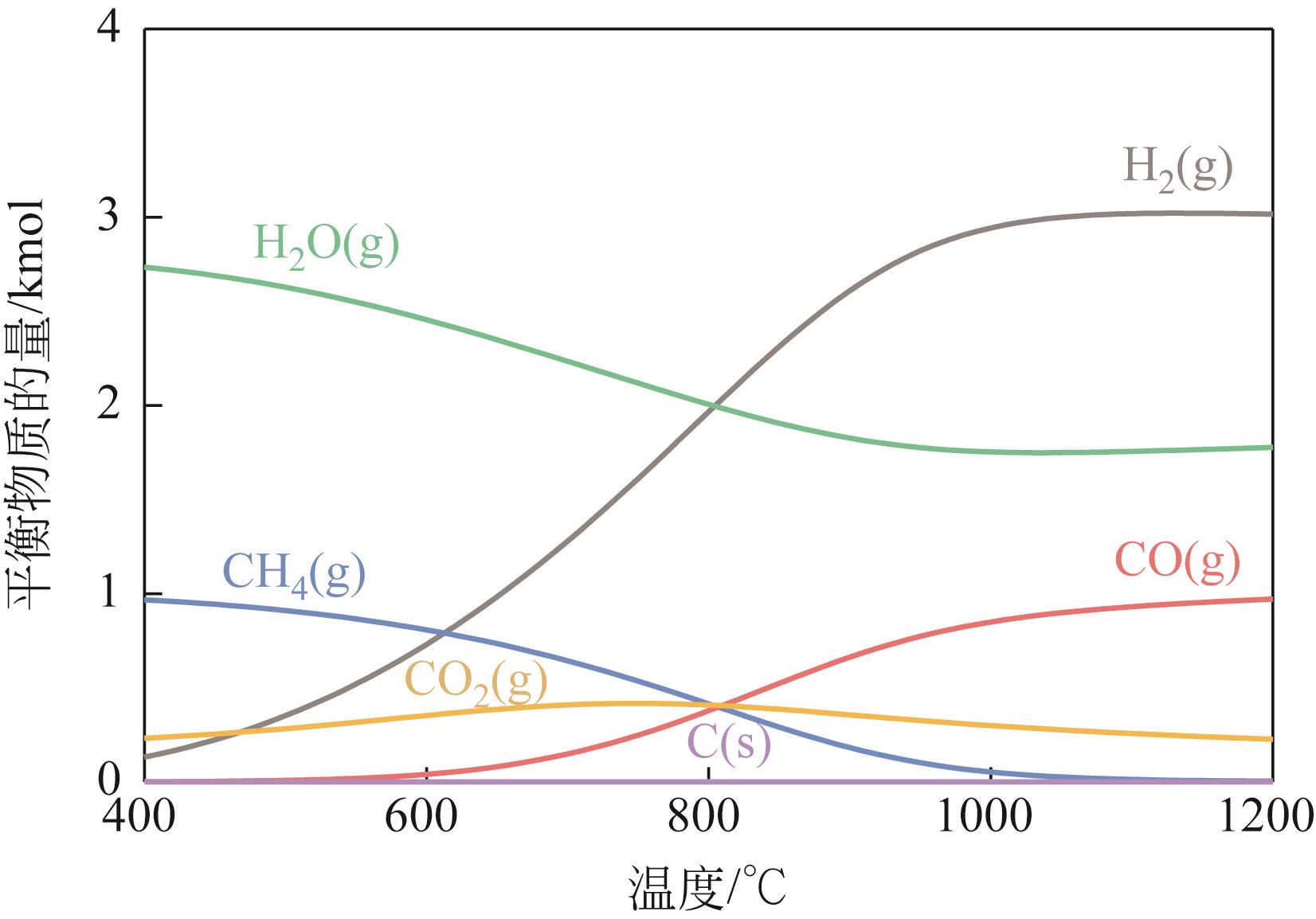
| 1 |
USMAN Muhammad, WAN DAUD W M A, ABBAS Hazzim F. Dry reforming of methane: Influence of process parameters—A review[J]. Renewable and Sustainable Energy Reviews, 2015, 45: 710-744.
|
| 2 |
TANG Pei, ZHU Qingjun, WU Zhaoxuan, et al. Methane activation: The past and future[J]. Energy & Environmental Science, 2014, 7(8): 2580-2591.
|
| 3 |
SCHULZ Hans. Short history and present trends of Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 1999, 186(1/2): 3-12.
|
| 4 |
Mark E DRY. The Fischer-Tropsch process: 1950—2000[J]. Catalysis Today, 2002, 71(3/4): 227-241.
|
| 5 |
OLAH George A, GOEPPERT Alain, CZAUN Miklos, et al. Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO-2H2) for methanol and hydrocarbon synthesis[J]. Journal of the American Chemical Society, 2013, 135(2): 648-650.
|
| 6 |
CHOUDHARY Vasant R, MAMMAN Ajit S. Simultaneous oxidative conversion and CO2 or steam reforming of methane to syngas over CoO-NiO-MgO catalyst[J]. Journal of Chemical Technology & Biotechnology, 1998, 73(4): 345-350.
|
| 7 |
HEGARTY M E S, O’CONNOR A M, ROSS J R H. Syngas production from natural gas using ZrO2-supported metals[J]. Catalysis Today, 1998, 42(3): 225-232.
|
| 8 |
CHOUDHARY Vasant R, RAJPUT Amarjeet M. Simultaneous carbon dioxide and steam reforming of methane to syngas over NiO-CaO catalyst[J]. Industrial & Engineering Chemistry Research, 1996, 35(11): 3934-3939.
|
| 9 |
SANTOS Bruno A V, LOUREIRO José M, RIBEIRO Ana M, et al. Methanol production by bi-reforming[J]. The Canadian Journal of Chemical Engineering, 2015, 93(3): 510-526.
|
| 10 |
CHOUDHARY Vasant R, MONDAL Kartick C. CO2 reforming of methane combined with steam reforming or partial oxidation of methane to syngas over NdCoO3 perovskite-type mixed metal-oxide catalyst[J]. Applied Energy, 2006, 83(9): 1024-1032.
|
| 11 |
NORONHA F B, SHAMSI A, TAYLOR C, et al. Catalytic performance of Pt/ZrO2 and Pt/Ce-ZrO2 catalysts on CO2 reforming of CH4 coupled with steam reforming or under high pressure[J]. Catalysis Letters, 2003, 90(1): 13-21.
|
| 12 |
AMIN Nor Aishah Saidina, Tung Chun YAW. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with partial oxidation of methane to syngas[J]. International Journal of Hydrogen Energy, 2007, 32(12): 1789-1798.
|
| 13 |
LI Yunhua, WANG Yaquan, ZHANG Xiangwen, et al. Thermodynamic analysis of autothermal steam and CO2 reforming of methane[J]. International Journal of Hydrogen Energy, 2008, 33(10): 2507-2514.
|
| 14 |
SONG Chunshan, PAN Wei. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios[J]. Catalysis Today, 2004, 98(4): 463-484.
|
| 15 |
ZHU J, ZHANG D, KING K D. Reforming of CH4 by partial oxidation: Thermodynamic and kinetic analyses[J]. Fuel, 2001, 80(7): 899-905.
|
| 16 |
陈亚男, 胡科峰, 潘牧. C-H-O三元热力学图在甲烷重整积炭研究中的研究进展[J]. 化工进展, 2015, 34(S1): 60-65.
|
|
CHEN Yanan, HU Kefeng, PAN Mu. Progress about thermodynamic analysis of carbon deposition in methane reforming with C-H-O triangular diagrams[J]. Chemical Industry and Engineering Progress, 2015, 34(S1): 60-65.
|
| 17 |
YANG Yu, LIU Jing, SHEN Weifeng, et al. High-efficiency utilization of CO2 in the methanol production by a novel parallel-series system combining steam and dry methane reforming[J]. Energy, 2018, 158: 820-829.
|
| 18 |
CHEN Chong, JIN Yuqi, YAN Jianhua, et al. Simulation of municipal solid waste gasification in two different types of fixed bed reactors[J]. Fuel, 2013, 103: 58-63.
|
| 19 |
HARDING S T, FLOUDAS C A. Phase stability with cubic equations of state: Global optimization approach[J]. AIChE Journal, 2000, 46(7): 1422-1440.
|
| 20 |
Smith J M, Van N H C, Abbott M M. Introduction to chemical engineering thermodynamics[M]. 6th ed. Boston: McGraw-Hill, 2001
|
| 21 |
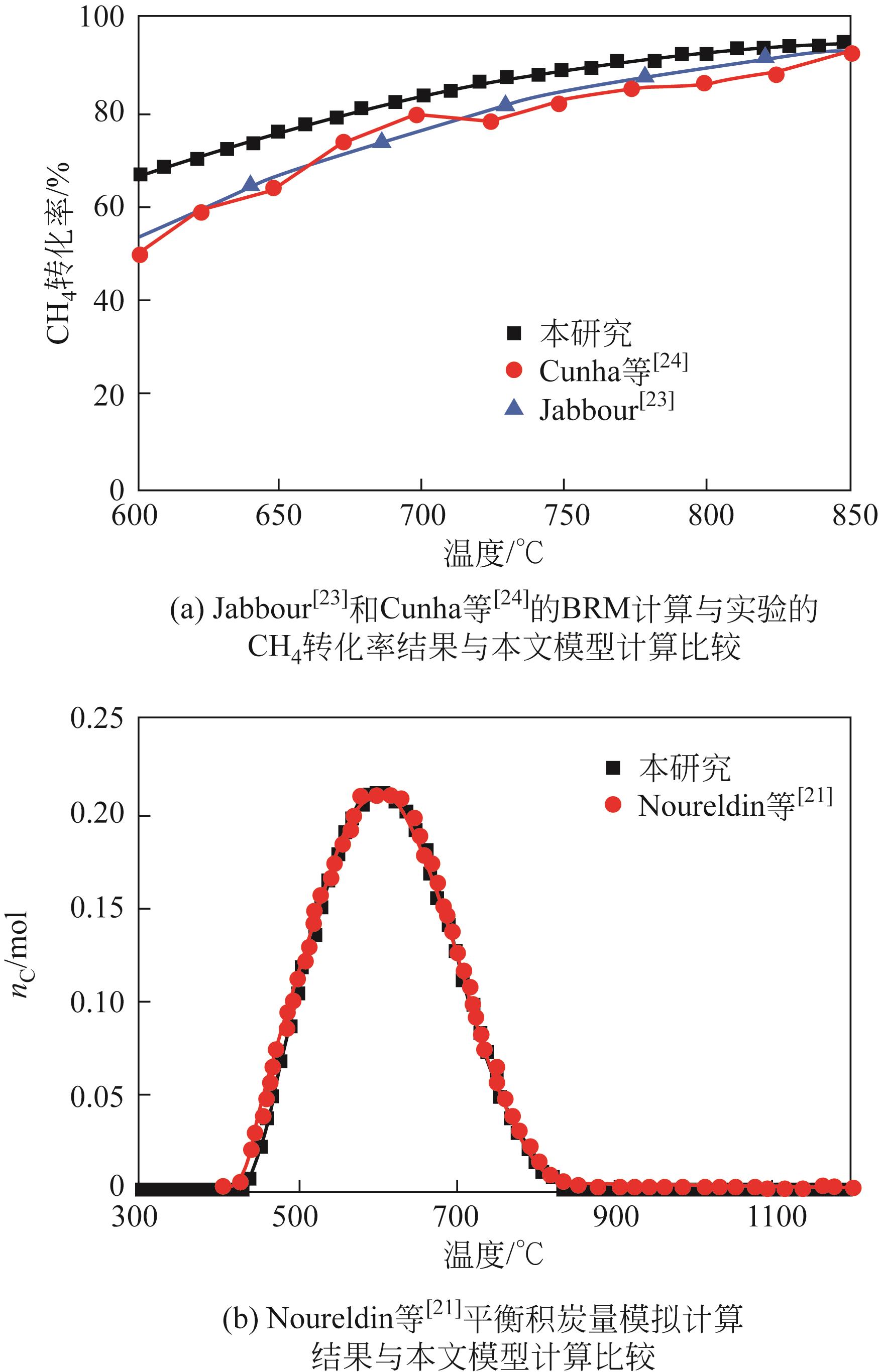
NOURELDIN Mohamed M B, ELBASHIR Nimir O, EL-HALWAGI Mahmoud M. Optimization and selection of reforming approaches for syngas generation from natural/shale gas[J]. Industrial & Engineering Chemistry Research, 2014, 53(5): 1841-1855.
|
| 22 |
PENG Dingyu, ROBINSON Donald B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15(1): 59-64.
|
| 23 |
JABBOUR Karam. Tuning combined steam and dry reforming of methane for “metgas” production: A thermodynamic approach and state-of-the-art catalysts[J]. Journal of Energy Chemistry, 2020, 48: 54-91.
|
| 24 |
CUNHA A F, MATA T M, CAETANO N S, et al. Catalytic bi-reforming of methane for carbon dioxide ennoblement[J]. Energy Reports, 2020, 6: 74-79.
|
| 25 |
WITTICH Knut, Michael KRÄMER, BOTTKE Nils, et al. Catalytic dry reforming of methane: Insights from model systems[J]. ChemCatChem, 2020, 12(8): 2130-2147.
|
| 26 |
NEMATOLLAHI Behzad, REZAEI Mehran, LAY Ebrahim Nemati, et al. Thermodynamic analysis of combined reforming process using Gibbs energy minimization method: In view of solid carbon formation[J]. Journal of Natural Gas Chemistry, 2012, 21(6): 694-702.
|
| 27 |
THEOFANIDIS Stavros Alexandros, GALVITA Vladimir V, POELMAN Hilde, et al. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe[J]. ACS Catalysis, 2015, 5(5): 3028-3039.
|
| 28 |
SEPEHRI Soodeh, REZAEI Mehran. Ce promoting effect on the activity and coke formation of Ni catalysts supported on mesoporous nanocrystalline γ-Al2O3 in autothermal reforming of methane[J]. International Journal of Hydrogen Energy, 2017, 42(16): 11130-11138.
|
| 29 |
HAGHIGHI Mohammad, SUN Zhi-Qiang, WU Jin-Hu, et al. On the reaction mechanism of CO2 reforming of methane over a bed of coal char[J]. Proceedings of the Combustion Institute, 2007, 31(2): 1983-1990.
|
| 30 |
DEROUANE E G. Combinatorial catalysis and high throughput catalyst design and testing[M]. Dordrecht: Kluwer Academic Publishers, 2000.
|
| 31 |
PAKHARE Devendra, SPIVEY James. A review of dry (CO2) reforming of methane over noble metal catalysts[J]. Chemical Society Reviews, 2014, 43(22): 7813-7837.
|
| 32 |
Jens ROSTRUP-NIELSEN, CHRISTIANSEN Lars J. Concepts in syngas manufacture[M]. London: Imperial College Press, 2011.
|
 ), 王昊2, 王健宇3, 朱卡克1(
), 王昊2, 王健宇3, 朱卡克1( ), 刘志成4(
), 刘志成4( )
)
 ), WANG Hao2, WANG Jianyu3, ZHU Kake1(
), WANG Hao2, WANG Jianyu3, ZHU Kake1( ), LIU Zhicheng4(
), LIU Zhicheng4( )
)