化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2714-2722.DOI: 10.16085/j.issn.1000-6613.2023-2064
• 二氧化碳捕集与资源化利用 • 上一篇
电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展
- 江苏大学化学化工学院,江苏 镇江 212013
-
收稿日期:2023-11-18修回日期:2024-02-21出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:施伟东 -
作者简介:解仲凯(1996—),男,博士研究生,研究方向为光催化能源资源转化。E-mail:xzk0702@sina.com。 -
基金资助:国家杰出青年科学基金(22225808)
Research progress of charge polarized photocatalysts in photoconversion carbon dioxide into multi-carbon chemicals
- School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, Jiangsu, China
-
Received:2023-11-18Revised:2024-02-21Online:2024-05-15Published:2024-06-15 -
Contact:SHI Weidong
摘要:
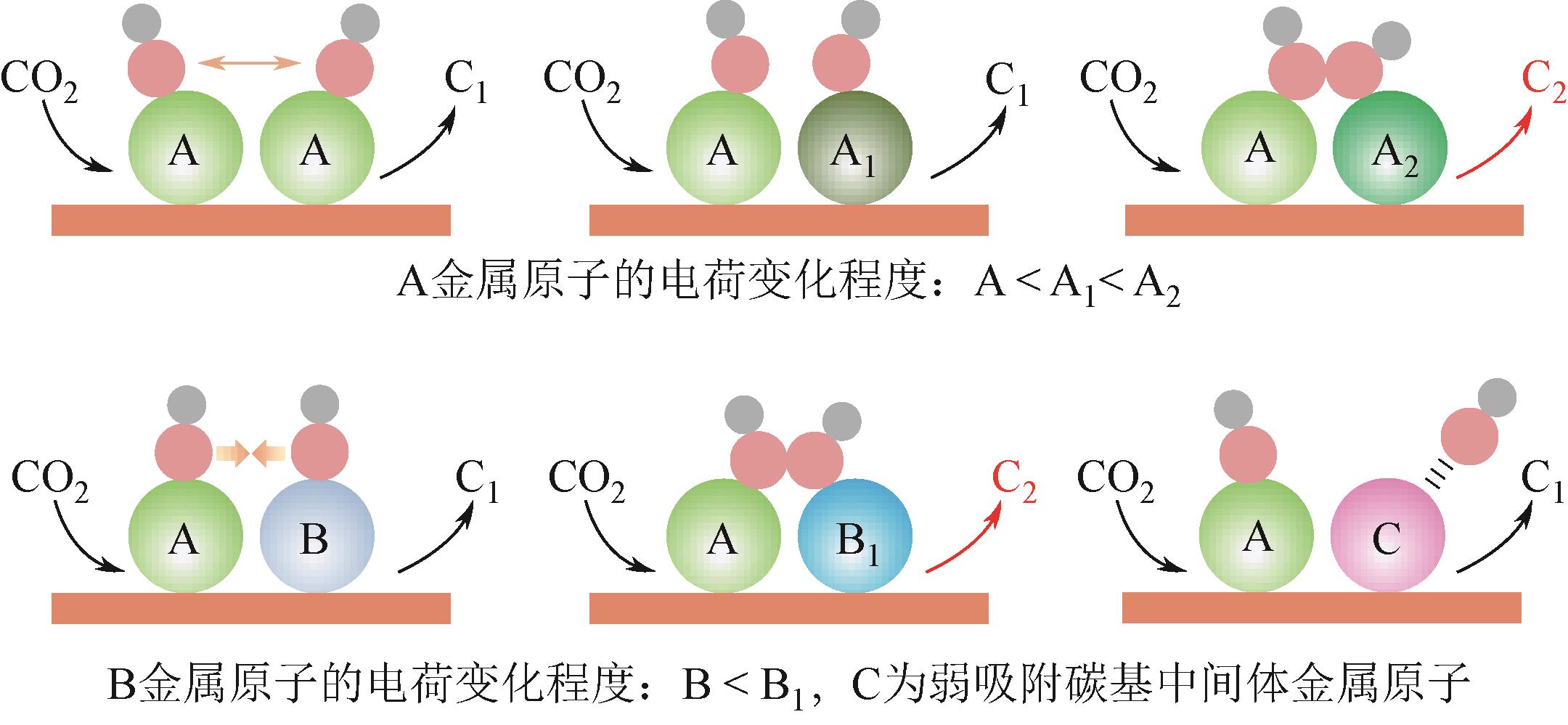
二氧化碳(CO2)光合成高附加值多碳化学品是缓解温室效应和能源危机的极具前景的绿色发展新技术。设计具有电荷极化活性位点的光催化剂能够有效降低C-C偶联反应能垒,进而提高光合成多碳化学品催化选择性和活性。本文综述了光催化CO2还原制C2化学品的相关研究,深入研究电荷不对称位点构筑的主要策略,阐明微观层面上电荷极化效应对C2产物活性和选择性的影响机制,总结出极具前景的高效光催化剂的设计与开发思路,为光催化技术的实际应用提供重要的理论和实践指导。展望未来,应更加注重催化剂在原子层面上的精准调控,开发出更高效、更专一的多碳产物制备系统,助力能源产业结构的低碳转型。
中图分类号:
引用本文
解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722.
XIE Zhongkai, SHI Weidong. Research progress of charge polarized photocatalysts in photoconversion carbon dioxide into multi-carbon chemicals[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2714-2722.
| 光催化剂 | 光催化测试条件 | 光源及光强 | C2化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
| Au1/RP | 50mg光催化剂,0.5g KHCO3, 1~2mL 6mol/L的HCl | 300W氙灯,300mW·cm-2 | 1.32 (C2H6) | [ |
| Bi19S27Cl3 | 10mg光催化剂,20mL水,0.4mg Na2S,2.9mg Na2SO3,CO2 | 420W氙灯,未提及光强 | 5.19 (CH3CH2OH) | [ |
| Cu SAs/UiO-66-NH2 | 100mg光催化剂,50mL水,100µL TEOA,100kPa CO2 | 300W氙灯,未提及光强 | 4.22 (CH3CH2OH) | [ |
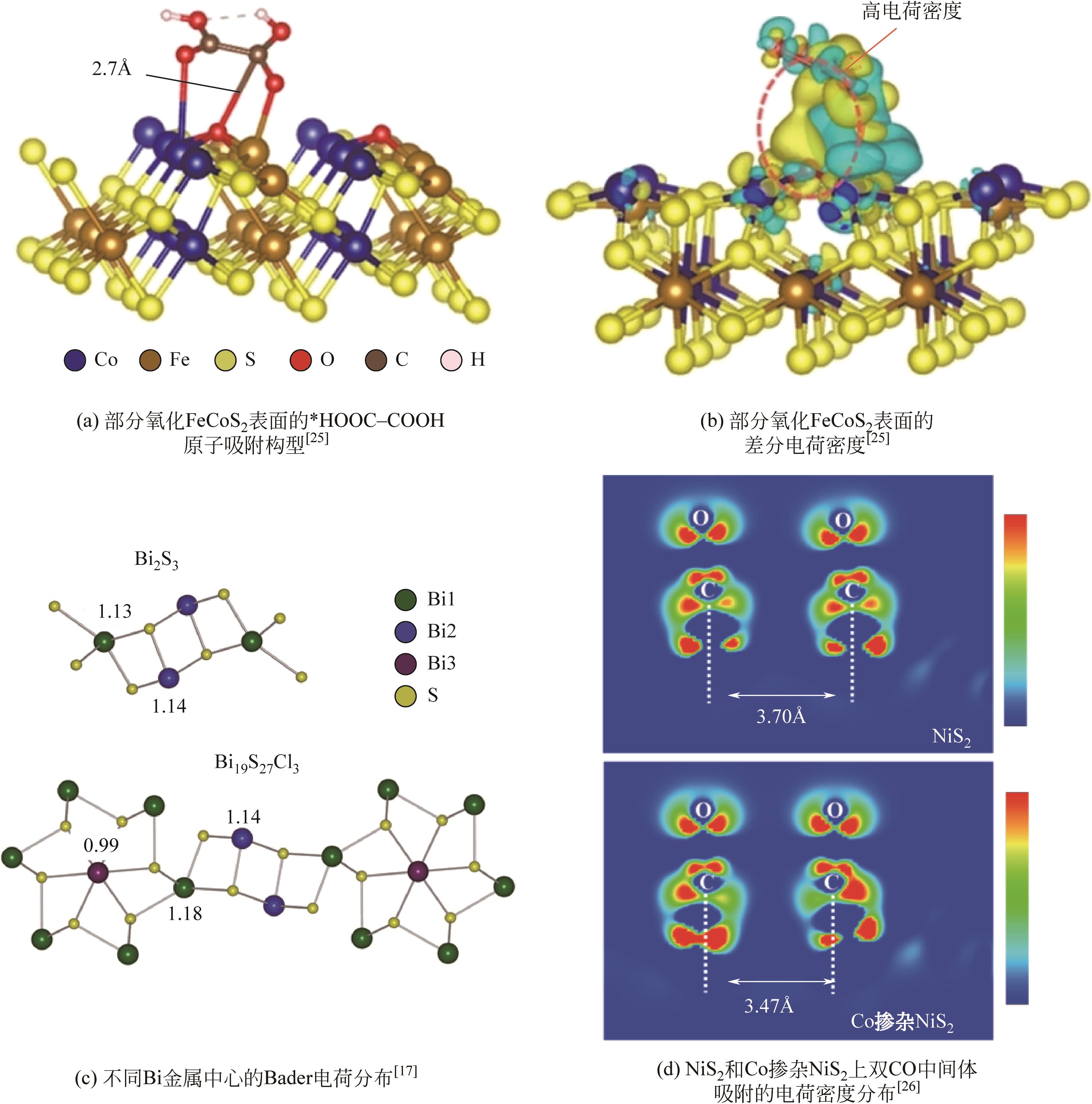
| Co-doped NiS2 | 10mg光催化剂,10kPa CO2 | 300W氙灯,100mW·cm-2 | 2.3 (C2H4) | [ |
| P/Cu SAs@CN | 0.5mg光催化剂,25mL水,3.0mL TEOA,65kPa CO2 | 300W氙灯,未提及光强 | 616.6 (C2H6) | [ |
| CuACs/PCNs | 5mg光催化剂,45mL水,5mL TEOA,100kPa CO2,C30H24Cl2N6Ru·6H2O | 300W氙灯,未提及光强 | 10.2 (C2H4) | [ |
| WO3-x -2 | 5mg光催化剂,0.2mL水,100kPa CO2 | 300W氙灯,未提及光强 | 61.6 (C2H4) | [ |
| CuO X @p-ZnO | 5mg光催化剂,水,50kPa CO2 | 300W氙灯,100mW·cm-2 | 2.7 (C2H4) | [ |
| In2.77S4(P6) | 3mg光催化剂,20mL水,100kPa CO2 | 300W氙灯,110mW·cm-2 | 67.65 (C2H4) | [ |
表1 单核位点光催化剂光催化还原CO2制备C2化学品性能对比
| 光催化剂 | 光催化测试条件 | 光源及光强 | C2化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
| Au1/RP | 50mg光催化剂,0.5g KHCO3, 1~2mL 6mol/L的HCl | 300W氙灯,300mW·cm-2 | 1.32 (C2H6) | [ |
| Bi19S27Cl3 | 10mg光催化剂,20mL水,0.4mg Na2S,2.9mg Na2SO3,CO2 | 420W氙灯,未提及光强 | 5.19 (CH3CH2OH) | [ |
| Cu SAs/UiO-66-NH2 | 100mg光催化剂,50mL水,100µL TEOA,100kPa CO2 | 300W氙灯,未提及光强 | 4.22 (CH3CH2OH) | [ |
| Co-doped NiS2 | 10mg光催化剂,10kPa CO2 | 300W氙灯,100mW·cm-2 | 2.3 (C2H4) | [ |
| P/Cu SAs@CN | 0.5mg光催化剂,25mL水,3.0mL TEOA,65kPa CO2 | 300W氙灯,未提及光强 | 616.6 (C2H6) | [ |
| CuACs/PCNs | 5mg光催化剂,45mL水,5mL TEOA,100kPa CO2,C30H24Cl2N6Ru·6H2O | 300W氙灯,未提及光强 | 10.2 (C2H4) | [ |
| WO3-x -2 | 5mg光催化剂,0.2mL水,100kPa CO2 | 300W氙灯,未提及光强 | 61.6 (C2H4) | [ |
| CuO X @p-ZnO | 5mg光催化剂,水,50kPa CO2 | 300W氙灯,100mW·cm-2 | 2.7 (C2H4) | [ |
| In2.77S4(P6) | 3mg光催化剂,20mL水,100kPa CO2 | 300W氙灯,110mW·cm-2 | 67.65 (C2H4) | [ |
| 光催化剂 | 光催化测试条件 | 光源及光强 | C2化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
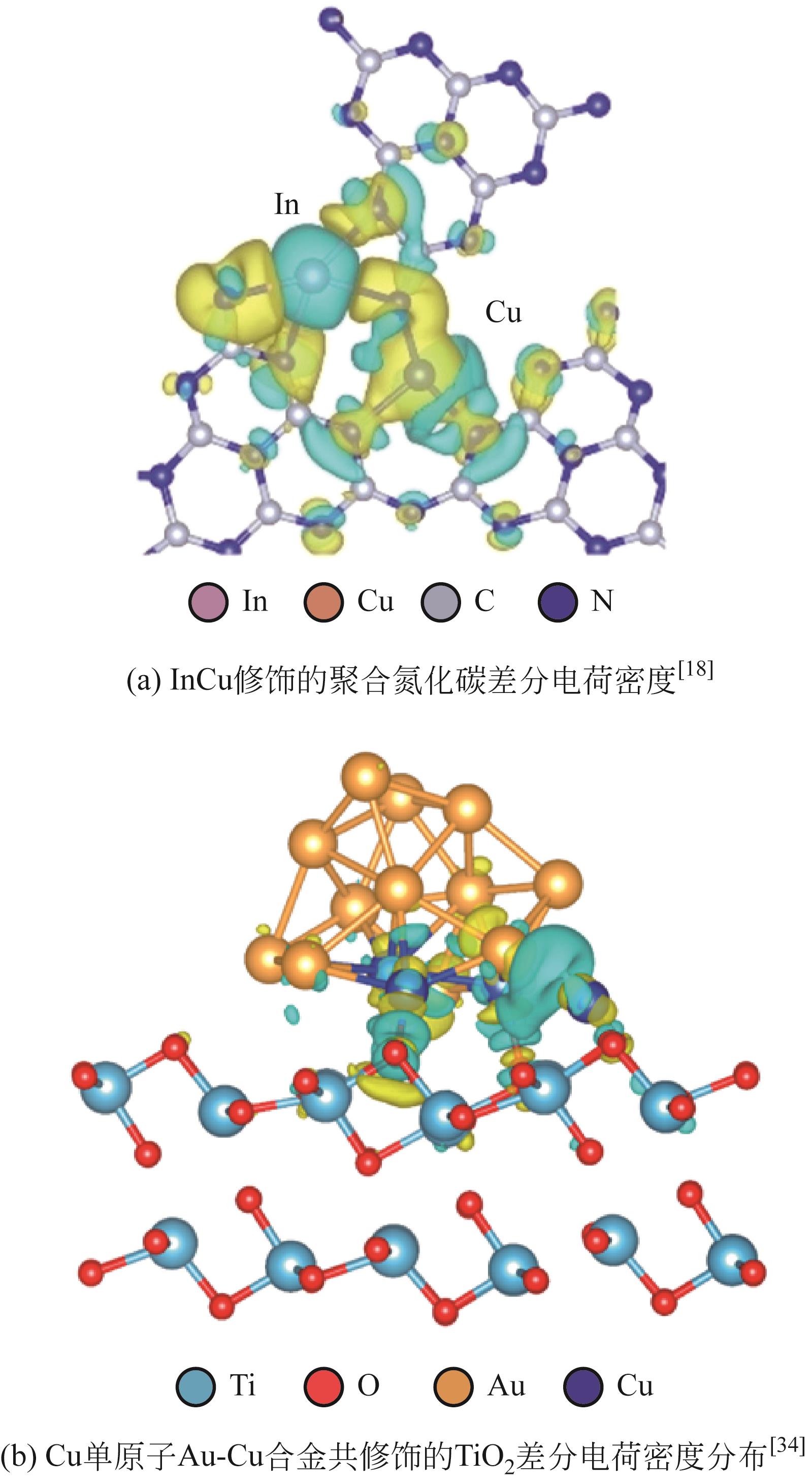
| InCu/PCN | 50mg光催化剂,3mL水,21mL DMF,80kPa CO2 | 300W氙灯,1000mW·cm-2 | 28.5 (CH3CH2OH) | [ |
| CuGaS2 | 5mg光催化剂,20mL水,100kPa CO2,pH=12 | 450W氙灯,未提及光强 | 20.6 (C2H4) | [ |
| FeCoS2 | 10mg光催化剂,100kPa CO2 | 300W氙灯,100mW·cm-2 | 20.1 (C2H4) | [ |
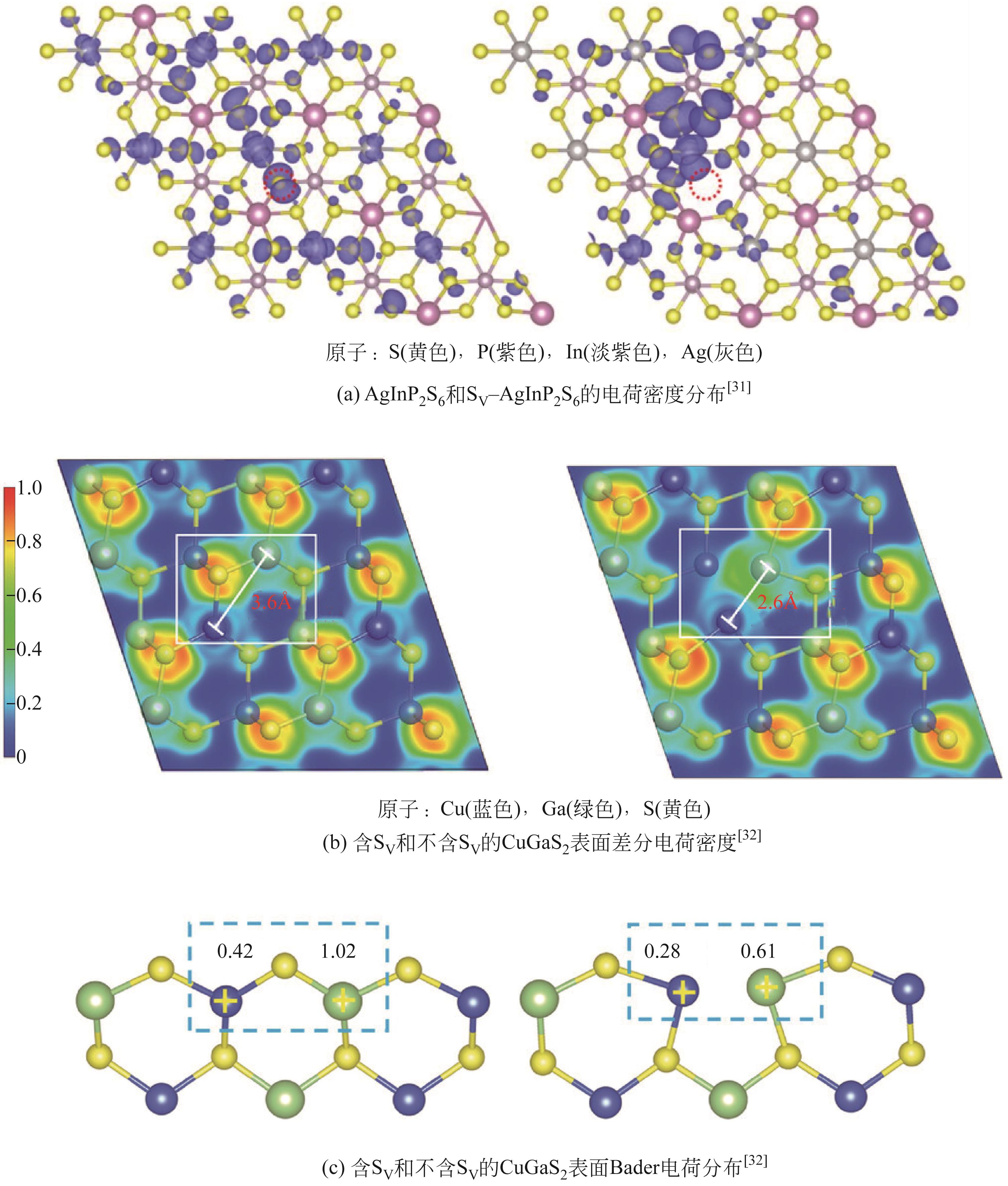
| VS-AgInP2S6 | 4~5mg光催化剂,0.4mL水,100kPa CO2 | 300W氙灯,未提及光强 | 44.3 (C2H4) | [ |
| CuGaS2/Ga2S3 | 20mg光催化剂,3mL水,TEOA,70kPa CO2 | 300W氙灯,未提及光强 | 335.7 (C2H4) | [ |
| Cu0.8Au0.2/TiO2 | 2.0mg光催化剂,0.15mL水,90kPa CO2 | 300W氙灯,500mW·cm-2 | 369.8 (C2H4) | [ |
| Cu0.8Ag0.2/TiO2 | 2.0mg光催化剂,0.15mL水,90kPa CO2 | 300W氙灯,500mW·cm-2 | 1110.6 (C2H4) | [ |
| Pt0.35%-Cu1.00%-BT | 40mg光催化剂,水,CO2 | 300W氙灯,100mW·cm-2 | 25 (C2H6) | [ |
| CuPt/WO3 | 40mg光催化剂,20mL水,HCl,90kPa CO2 | 300W氙灯,未提及光强 | 19.41 (CH3COOH) | [ |
| In2O3/Cu-O3 | 10mg光催化剂,10mL水,CO2 | 300W氙灯,未提及光强 | 20.7 (CH3CH2OH) | [ |
| Cu δ+/CeO2-TiO2 | 10mg光催化剂,30mL水,100kPa CO2 | 300W氙灯,200mW·cm-2 | 4.51 (C2H4) | [ |
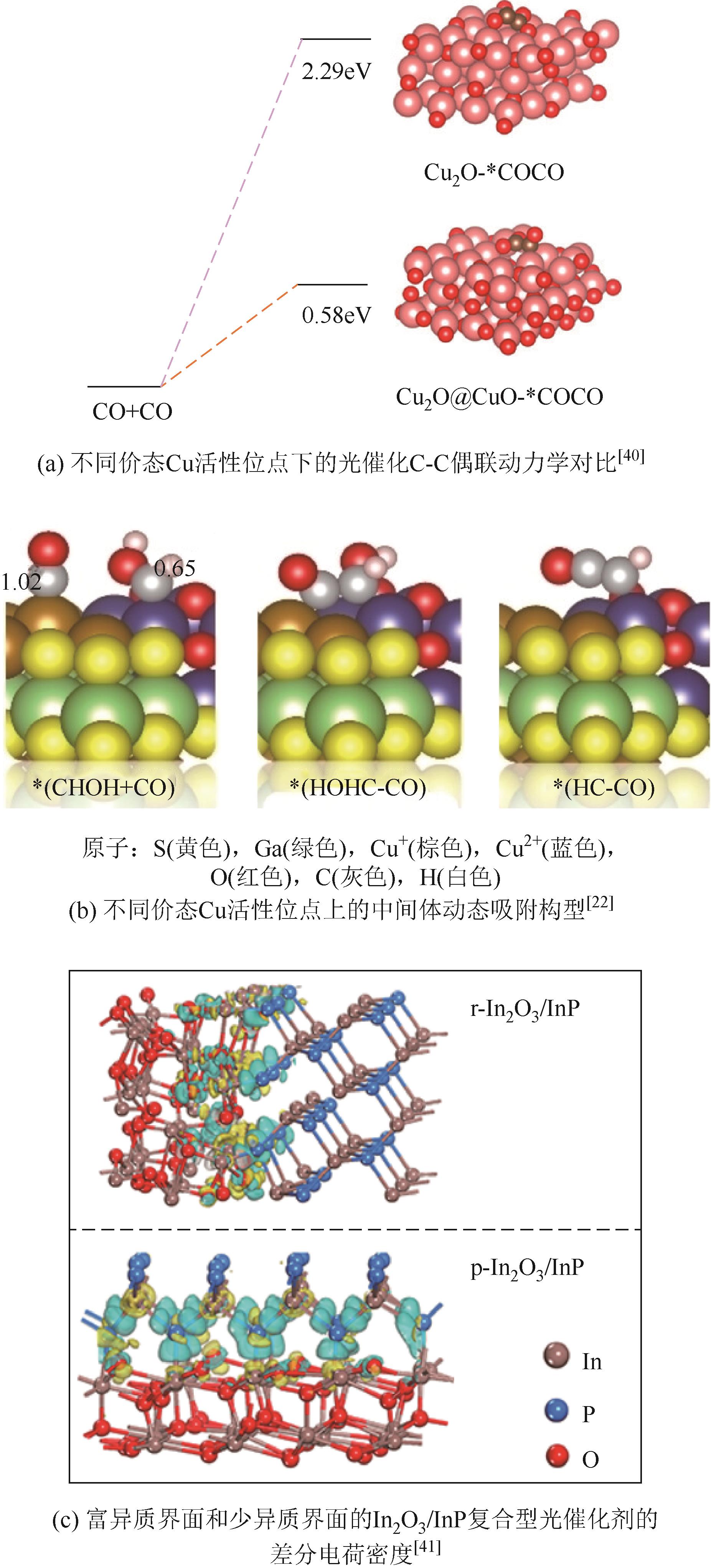
| r-In2O3/InP | 50mg光催化剂,100mL水,80kPa CO2 | 300W氙灯,未提及光强 | 9.67 (CH3COOH) | [ |
| Bi2S3@In2S3 | 5mg光催化剂,1mL水,100kPa CO2 | 300W氙灯,1150mW·cm-2 | 11.81 (C2H4) | [ |
表2 多核位点光催化剂光催化还原CO2制备C2化学品性能对比
| 光催化剂 | 光催化测试条件 | 光源及光强 | C2化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
| InCu/PCN | 50mg光催化剂,3mL水,21mL DMF,80kPa CO2 | 300W氙灯,1000mW·cm-2 | 28.5 (CH3CH2OH) | [ |
| CuGaS2 | 5mg光催化剂,20mL水,100kPa CO2,pH=12 | 450W氙灯,未提及光强 | 20.6 (C2H4) | [ |
| FeCoS2 | 10mg光催化剂,100kPa CO2 | 300W氙灯,100mW·cm-2 | 20.1 (C2H4) | [ |
| VS-AgInP2S6 | 4~5mg光催化剂,0.4mL水,100kPa CO2 | 300W氙灯,未提及光强 | 44.3 (C2H4) | [ |
| CuGaS2/Ga2S3 | 20mg光催化剂,3mL水,TEOA,70kPa CO2 | 300W氙灯,未提及光强 | 335.7 (C2H4) | [ |
| Cu0.8Au0.2/TiO2 | 2.0mg光催化剂,0.15mL水,90kPa CO2 | 300W氙灯,500mW·cm-2 | 369.8 (C2H4) | [ |
| Cu0.8Ag0.2/TiO2 | 2.0mg光催化剂,0.15mL水,90kPa CO2 | 300W氙灯,500mW·cm-2 | 1110.6 (C2H4) | [ |
| Pt0.35%-Cu1.00%-BT | 40mg光催化剂,水,CO2 | 300W氙灯,100mW·cm-2 | 25 (C2H6) | [ |
| CuPt/WO3 | 40mg光催化剂,20mL水,HCl,90kPa CO2 | 300W氙灯,未提及光强 | 19.41 (CH3COOH) | [ |
| In2O3/Cu-O3 | 10mg光催化剂,10mL水,CO2 | 300W氙灯,未提及光强 | 20.7 (CH3CH2OH) | [ |
| Cu δ+/CeO2-TiO2 | 10mg光催化剂,30mL水,100kPa CO2 | 300W氙灯,200mW·cm-2 | 4.51 (C2H4) | [ |
| r-In2O3/InP | 50mg光催化剂,100mL水,80kPa CO2 | 300W氙灯,未提及光强 | 9.67 (CH3COOH) | [ |
| Bi2S3@In2S3 | 5mg光催化剂,1mL水,100kPa CO2 | 300W氙灯,1150mW·cm-2 | 11.81 (C2H4) | [ |
| 光催化剂 | 光催化测试条件 | 光源及光强 | C1化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
| BP-Bi24O31Br10 | 30mg光催化剂,50mL水,80kPa CO2 | 300W氙灯,未提及光强 | 39.8 (CO) | [ |
| VBiO-Bi24O31Br10 | 30mg光催化剂,50mL水,80kPa CO2 | 300W氙灯,300mW·cm-2 | 24.9 (CO) | [ |
| VW-Bi2WO6 | 10mg光催化剂,1.7g NaHCO3,15mL 4mol/L的H2SO4 | 300W氙灯,未提及光强 | 30.62 (CO) | [ |
| Cu1.95S1-x | 10mg光催化剂,10mL水,100kPa CO2 | 300W氙灯,未提及光强 | 13.63 (CH4) | [ |
| Ni:CdS | 催化剂,5mL水,1mL三乙醇胺,100kPa CO2 | 300W氙灯,未提及光强 | 9.5 (CO) | [ |
| Mn-CsPbBr3 | 2mg光催化剂,10mL水,100kPa CO2 | 300W氙灯,100mW·cm-2 | 45.4 (CO)/约3.5 (CH4) | [ |
| Mo/TiO2 | 光催化剂,20uL水,20mL CO2 | 300W氙灯,未提及光强 | 8.2 (CO)/约9.8 (CH4) | [ |
| Ni-BiOBr | 5mg光催化剂,1mL水,5mg [Ru(bpy)3]Cl2·6H2O,1mL三乙醇胺,4mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 378.7 (CO) μ | [ |
| PtRu/TiO2 | 100mg光催化剂,2mL水,CO2 | 300W氙灯,80mW·cm-2 | 38.7 (CH4) | [ |
| SnS2/Pt3Co | 20mg光催化剂,100mL水,140kPa CO2 | 300W氙灯,未提及光强 | 27.34 (CO)/约14.02 (CH4) | [ |
| TiO2-AuCu-V | 15mg光催化剂,1mL水,150kPa CO2 | 300W氙灯,未提及光强 | 3.4 (CO)/约33.5 (CH4) | [ |
| Pd7Cu1-TiO2 | 5mg光催化剂,1mL水,200kPa CO2 | 300W氙灯,未提及光强 | 19.6 (CH4) | [ |
| TiO2-Pd | 15mg光催化剂,2mL水,150kPa CO2 | 300W氙灯,未提及光强 | 12.6 (CO)/约3.0 (CH4) | [ |
| Au-TiO2 | 50mg光催化剂,0.084g NaHCO3,0.6mL 4mol/L的HCl | 300W氙灯,未提及光强 | 70.34 (CO)/约19.75 (CH4) | [ |
| Ni/SOM-ZIF-8 | 3mg光催化剂,2mL水,10mg [Ru(bpy)3]Cl2·6H2O,2mL三乙醇胺,6mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 4.2 (CO) mmol/(g·h) | [ |
| Co1/ZnO | 1mg光催化剂,4mL水,10mg [Ru(bpy)3]Cl2·6H2O,4mL三乙醇胺,16mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 22.5 (CO) mmol/(g·h) | [ |
表3 含极化和非极化电荷光催化剂光催化还原CO2制备C1化学品性能对比
| 光催化剂 | 光催化测试条件 | 光源及光强 | C1化学品产率/μmol·g-1·h-1 | 参考文献 |
|---|---|---|---|---|
| BP-Bi24O31Br10 | 30mg光催化剂,50mL水,80kPa CO2 | 300W氙灯,未提及光强 | 39.8 (CO) | [ |
| VBiO-Bi24O31Br10 | 30mg光催化剂,50mL水,80kPa CO2 | 300W氙灯,300mW·cm-2 | 24.9 (CO) | [ |
| VW-Bi2WO6 | 10mg光催化剂,1.7g NaHCO3,15mL 4mol/L的H2SO4 | 300W氙灯,未提及光强 | 30.62 (CO) | [ |
| Cu1.95S1-x | 10mg光催化剂,10mL水,100kPa CO2 | 300W氙灯,未提及光强 | 13.63 (CH4) | [ |
| Ni:CdS | 催化剂,5mL水,1mL三乙醇胺,100kPa CO2 | 300W氙灯,未提及光强 | 9.5 (CO) | [ |
| Mn-CsPbBr3 | 2mg光催化剂,10mL水,100kPa CO2 | 300W氙灯,100mW·cm-2 | 45.4 (CO)/约3.5 (CH4) | [ |
| Mo/TiO2 | 光催化剂,20uL水,20mL CO2 | 300W氙灯,未提及光强 | 8.2 (CO)/约9.8 (CH4) | [ |
| Ni-BiOBr | 5mg光催化剂,1mL水,5mg [Ru(bpy)3]Cl2·6H2O,1mL三乙醇胺,4mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 378.7 (CO) μ | [ |
| PtRu/TiO2 | 100mg光催化剂,2mL水,CO2 | 300W氙灯,80mW·cm-2 | 38.7 (CH4) | [ |
| SnS2/Pt3Co | 20mg光催化剂,100mL水,140kPa CO2 | 300W氙灯,未提及光强 | 27.34 (CO)/约14.02 (CH4) | [ |
| TiO2-AuCu-V | 15mg光催化剂,1mL水,150kPa CO2 | 300W氙灯,未提及光强 | 3.4 (CO)/约33.5 (CH4) | [ |
| Pd7Cu1-TiO2 | 5mg光催化剂,1mL水,200kPa CO2 | 300W氙灯,未提及光强 | 19.6 (CH4) | [ |
| TiO2-Pd | 15mg光催化剂,2mL水,150kPa CO2 | 300W氙灯,未提及光强 | 12.6 (CO)/约3.0 (CH4) | [ |
| Au-TiO2 | 50mg光催化剂,0.084g NaHCO3,0.6mL 4mol/L的HCl | 300W氙灯,未提及光强 | 70.34 (CO)/约19.75 (CH4) | [ |
| Ni/SOM-ZIF-8 | 3mg光催化剂,2mL水,10mg [Ru(bpy)3]Cl2·6H2O,2mL三乙醇胺,6mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 4.2 (CO) mmol/(g·h) | [ |
| Co1/ZnO | 1mg光催化剂,4mL水,10mg [Ru(bpy)3]Cl2·6H2O,4mL三乙醇胺,16mL CH3CN,100kPa CO2 | 300W氙灯,未提及光强 | 22.5 (CO) mmol/(g·h) | [ |
| 4 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 5 | SHARMA Yogeshkumar, SINGH Bhaskar, UPADHYAY Siddhnath. Advancements in development and characterization of biodieset: A review[J]. Fuel, 2008, 87(12): 2355-2373. |
| 6 | KOSARI Mohammadreza, Alvin M H LIM, SHAO Yu, et al. Thermocatalytic CO2 conversion by siliceous matter: A review[J]. Journal of Materials Chemistry A, 2023, 11(4): 1593-1633. |
| 7 | LU Tianrui, XU Ting, ZHU Shaojun, et al. Electrocatalytic CO2 reduction to ethylene: From advanced catalyst design to industrial applications[J]. Advanced Materials, 2023, 35(52): e2310433. |
| 8 | SAKIMOTO Kelsey K, WONG Andrew Barnabas, YANG Peidong. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 9 | Inwhan ROH, YU Sunmoon, LIN Chung-Kuan, et al. Photoelectrochemical CO2 reduction toward multicarbon products with silicon nanowire photocathodes interfaced with copper nanoparticles[J]. Journal of the American Chemical Society, 2022, 144(18): 8002-8006. |
| 10 | Honghui OU, LI Guosheng, REN Wei, et al. Atomically dispersed Au-assisted C-C coupling on red phosphorus for CO2 photoreduction to C2H6 [J]. Journal of the American Chemical Society, 2022, 144(48): 22075-22082. |
| 11 | XIONG Xuyang, MAO Chengliang, YANG Zhaojun, et al. Photocatalytic CO2 reduction to CO over Ni single atoms supported on defect-rich zirconia[J]. Advanced Energy Materials, 2020, 10(46): 2002928. |
| 12 | XU Jiaqi, JU Zhengyu, ZHANG Wei, et al. Efficient infrared-light-driven CO2 reduction over ultrathin metallic Ni-doped CoS2 nanosheets[J]. Angewandte Chemie International Edition, 2021, 60(16): 8705-8709. |
| 13 | WANG Xianyi, WANG Yingshuo, GAO Meichao, et al. BiVO4/Bi4Ti3O12 heterojunction enabling efficient photocatalytic reduction of CO2 with H2O to CH3OH and CO[J]. Applied Catalysis B: Environmental, 2020, 270: 118876. |
| 14 | WANG Ying, SHANG Xiaotong, SHEN Jinni, et al. Direct and indirect Z-scheme heterostructure-coupled photosystem enabling cooperation of CO2 reduction and H2O oxidation[J]. Nature Communications, 2020, 11(1): 3043. |
| 15 | DOKANIA Abhay, RAMIREZ Adrian, BAVYKINA Anastasiya, et al. Heterogeneous catalysis for the valorization of CO2: Role of bifunctional processes in the production of chemicals[J]. ACS Energy Letters, 2019, 4(1): 167-176. |
| 16 | MENG Dongli, ZHANG Mengdi, SI Duanhui, et al. Highly selective tandem electroreduction of CO2 to ethylene over atomically isolated nickel-nitrogen site/copper nanoparticle catalysts[J]. Angewandte Chemie International Edition, 2021, 60(48): 25485-25492. |
| 17 | Kousik DAS, Risov DAS, RIYAZ Mohd, et al. Intrinsic charge polarization in Bi19S27Cl3 nanorods promotes selective C-C coupling reaction during photoreduction of CO2 to ethanol[J]. Advanced Materials, 2023, 35(5): 2205994. |
| 18 | SHI Hainan, WANG Haozhi, ZHOU Yichen, et al. Atomically dispersed indium-copper dual-metal active sites promoting C-C coupling for CO2 photoreduction to ethanol[J]. Angewandte Chemie International Edition, 2022, 61(40): e202208904. |
| 19 | WANG Gang, HE Chunting, HUANG Rong, et al. Photoinduction of Cu single atoms decorated on UiO-66-NH2 for enhanced photocatalytic reduction of CO2 to liquid fuels[J]. Journal of the American Chemical Society, 2020, 142(45): 19339-19345. |
| 20 | ZHENG Tingting, JIANG Kun, Na TA, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst[J]. Joule, 2019, 3(1): 265-278. |
| 21 | FENG Manman, WU Xuemei, CHENG Huiyuan, et al. Well-defined Fe-Cu diatomic sites for efficient catalysis of CO2 electroreduction[J]. Journal of Materials Chemistry A, 2021, 9(42): 23817-23827. |
| 22 | CHAKRABORTY Subhajit, Risov DAS, RIYAZ Mohd, et al. Wurtzite CuGaS2 with an in-situ-formed CuO layer photocatalyzes CO2 conversion to ethylene with high selectivity[J]. Angewandte Chemie International Edition, 2023, 62(9): e202216613. |
| 23 | WU Yang, HU Qinyuan, CHEN Qingxia, et al. Fundamentals and challenges of engineering charge polarized active sites for CO2 photoreduction toward C2 products[J]. Accounts of Chemical Research, 2023, 56(18): 2500-2513. |
| 24 | ALBERO Josep, PENG Yong, GARCIA Hermenegildo. Photocatalytic CO2 reduction to C2+ products[J]. ACS Catalysis, 2020, 10(10): 5734-5749. |
| 25 | WU Yang, CHEN Qingxia, ZHU Juncheng, et al. Selective CO2-to-C2H4 photoconversion enabled by oxygen-mediated triatomic sites in partially oxidized bimetallic sulfide[J]. Angewandte Chemie International Edition, 2023, 62(15): 2301075. |
| 26 | SHAO Weiwei, LI Xiaodong, ZHU Juncheng, et al. Metal n +-metal δ + pair sites steer C-C coupling for selective CO2 photoreduction to C2 hydrocarbons[J]. Nano Research, 2022, 15(3): 1882-1891. |
| 27 | WANG Gang, CHEN Zhe, WANG Tao, et al. P and Cu dual sites on graphitic carbon nitride for photocatalytic CO2 reduction to hydrocarbon fuels with high C2H6 evolution[J]. Angewandte Chemie International Edition, 2022, 61(40): e202210789. |
| 1 | PETER Sebastian C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis[J]. ACS Energy Letters, 2018, 3(7): 1557-1561. |
| 2 | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 3 | ZHOU Yansong, WANG Zhitong, HUANG Lei, et al. Engineering 2D photocatalysts toward carbon dioxide reduction[J]. Advanced Energy Materials, 2021, 11(8): 2003159. |
| 28 | XIE Wenke, LI Kuangjun, LIU Xuanhe, et al. P-mediated Cu-N4 sites in carbon nitride realizing CO2 photoreduction to C2H4 with selectivity modulation[J]. Advanced Materials, 2023, 35(3): e2208132. |
| 29 | YU Hongjian, CHEN Fang, LI Xiaowei, et al. Synergy of ferroelectric polarization and oxygen vacancy to promote CO2 photoreduction[J]. Nature Communications, 2021, 12(1): 4594. |
| 30 | CAO Yuehan, GUO Lan, DAN Meng, et al. Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction[J]. Nature Communications, 2021, 12(1): 1675. |
| 31 | GAO Wa, LI Shi, HE Huichao, et al. Vacancy-defect modulated pathway of photoreduction of CO2 on single atomically thin AgInP2S6 sheets into olefiant gas[J]. Nature Communications, 2021, 12(1): 4747. |
| 32 | WANG Junyan, YANG Chen, MAO Liang, et al. Regulating the metallic Cu-Ga bond by S vacancy for improved photocatalytic CO2 reduction to C2H4 [J]. Advanced Functional Materials, 2023, 33(28): 2213901. |
| 33 | LU Changhai, LI Juan, YAN Jiahao, et al. Surface plasmon resonance and defects on tungsten oxides synergistically boost high-selective CO2 reduction for ethylene[J]. Applied Materials Today, 2020, 20: 100744. |
| 34 | YU Yangyang, DONG Xing’an, CHEN Peng, et al. Synergistic effect of Cu single atoms and Au-Cu alloy nanoparticles on TiO2 for efficient CO2 photoreduction[J]. ACS Nano, 2021, 15(9): 14453-14464. |
| 35 | YU Yangyang, HE Ye, YAN Ping, et al. Boosted C-C coupling with Cu-Ag alloy sub-nanoclusters for CO2-to-C2H4 photosynthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(44): e2307320120. |
| 36 | SORCAR Saurav, HWANG Yunju, LEE Jaewoong, et al. CO2, water, and sunlight to hydrocarbon fuels: A sustained sunlight to fuel (Joule-to-Joule) photoconversion efficiency of 1%[J]. Energy & Environmental Science, 2019, 12(9): 2685-2696. |
| 37 | ZENG Di, WANG Haipeng, ZHU Xiaodi, et al. Photocatalytic conversion of CO2 to acetic acid by CuPt/WO3: Chloride enhanced C-C coupling mechanism[J]. Applied Catalysis B: Environmental, 2023, 323: 122177. |
| 38 | GONG Shuaiqi, NI Baoxin, HE Xiaoyang, et al. Electronic modulation of a single-atom-based tandem catalyst boosts CO2 photoreduction to ethanol[J]. Energy & Environmental Science, 2023, 16(12): 5956-5969. |
| 39 | WANG Ting, CHEN Liang, CHEN Cong, et al. Engineering catalytic interfaces in Cu δ +/CeO2-TiO2 photocatalysts for synergistically boosting CO2 reduction to ethylene[J]. ACS Nano, 2022, 16(2): 2306-2318. |
| 40 | WANG Wei, DENG Chaoyuan, XIE Shijie, et al. Photocatalytic C-C coupling from carbon dioxide reduction on copper oxide with mixed-valence copper(Ⅰ)/copper(Ⅱ)[J]. Journal of the American Chemical Society, 2021, 143(7): 2984-2993. |
| 41 | GONG Shuaiqi, NIU Yanli, LIU Xuan, et al. Selective CO2 photoreduction to acetate at asymmetric ternary bridging sites[J]. ACS Nano, 2023, 17(5): 4922-4932. |
| 42 | Risov DAS, PAUL Ratul, PARUI Arko, et al. Engineering the charge density on an In2.77S4/porous organic polymer hybrid photocatalyst for CO2-to-ethylene conversion reaction[J]. Journal of the American Chemical Society, 2023, 145(1): 422-435. |
| 43 | YAN Ke, WU Donghai, WANG Ting, et al. Highly selective ethylene production from solar-driven CO2 reduction on the Bi2S3@In2S3 catalyst with In-SV-Bi active sites[J]. ACS Catalysis, 2023, 13(4): 2302-2312. |
| 44 | DI Jun, ZHU Xingwang, HAO Gazi, et al. Vacancy pair-induced charge rebalancing with surface and interfacial dual polarization for CO2 photoreduction[J]. ACS Catalysis, 2022, 12(24): 15728-15736. |
| 45 | DI Jun, CHEN Chao, ZHU Chao, et al. Surface local polarization induced by bismuth-oxygen vacancy pairs tuning non-covalent interaction for CO2 photoreduction[J]. Advanced Energy Materials, 2021, 11(41): 2102389. |
| 46 | WANG Yinghui, HU Jingcong, GE Teng, et al. Gradient cationic vacancies enabling inner-to-outer tandem homojunctions: Strong local internal electric field and reformed basic sites boosting CO2 photoreduction[J]. Advanced Materials, 2023, 35(31): e2302538. |
| 47 | SHI Xian, DAI Weidong, DONG Xing’an, et al. Dual Cu and S vacancies boost CO2 photomethanation on Cu1.95S1- x : Vacancy-regulated selective photocatalysis[J]. Applied Catalysis B: Environmental, 2023, 339: 123147. |
| 48 | WANG Jin, XIA Tong, WANG Lei, et al. Enabling visible-light-driven selective CO2 reduction by doping quantum dots: Trapping electrons and suppressing H2 evolution[J]. Angewandte Chemie International Edition, 2018, 57(50): 16447-16451. |
| 49 | LIN Cheng-Chieh, LIU Tingran, LIN Sin-Rong, et al. Spin-polarized photocatalytic CO2 reduction of Mn-doped perovskite nanoplates[J]. Journal of the American Chemical Society, 2022, 144(34): 15718-15726. |
| 50 | FENG Shuaijun, ZHAO Jie, BAI Yujie, et al. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping[J]. Journal of CO2 Utilization, 2020, 38: 1-9. |
| 51 | WANG Yiqiao, XIE Yu, YU Shuohan, et al. Ni doping in unit cell of BiOBr to increase dipole moment and induce spin polarization for promoting CO2 photoreduction via enhanced build-in electric field[J]. Applied Catalysis B: Environmental, 2023, 327: 122420. |
| 52 | WEI Yuechang, WU Xingxing, ZHAO Yilong, et al. Efficient photocatalysts of TiO2 nanocrystals-supported PtRu alloy nanoparticles for CO2 reduction with H2O: Synergistic effect of Pt-Ru[J]. Applied Catalysis B: Environmental, 2018, 236: 445-457. |
| 53 | YIN Shikang, LIU Yun, ZHOU Weiqiang, et al. Nanocluster-mediated electron-hole separation for efficient CO2 photoreduction[J]. Chemical Engineering Journal, 2023, 477: 147292. |
| 54 | LIU Qian, CHEN Qin, LI Tianyu, et al. Vacancy engineering of AuCu cocatalysts for improving the photocatalytic conversion of CO2 to CH4 [J]. Journal of Materials Chemistry A, 2019, 7(47): 27007-27015. |
| 55 | LONG Ran, LI Yu, LIU Yan, et al. Isolation of Cu atoms in Pd lattice: Forming highly selective sites for photocatalytic conversion of CO2 to CH4 [J]. Journal of the American Chemical Society, 2017, 139(12): 4486-4492. |
| 56 | ZHU Yuzhen, XU Zaixiang, JIANG Wenya, et al. Engineering on the edge of Pd nanosheet cocatalysts for enhanced photocatalytic reduction of CO2 to fuels[J]. Journal of Materials Chemistry A, 2017, 5(6): 2619-2628. |
| 57 | WANG Rui, SHEN Jun, SUN Kouhua, et al. Enhancement in photocatalytic activity of CO2 reduction to CH4 by 0D/2D Au/TiO2 plasmon heterojunction[J]. Applied Surface Science, 2019, 493: 1142-1149. |
| 58 | LIU Zhiguo, CHEN Ziyu, LI Mingyang, et al. Construction of single Ni atom-immobilized ZIF-8 with ordered hierarchical pore structures for selective CO2 photoreduction[J]. ACS Catalysis, 2023, 13(10): 6630-6640. |
| 59 | MA Zhentao, WANG Qingyu, LIU Limin, et al. Low-coordination environment design of single Co atoms for efficient CO2 photoreduction[J]. Nano Research, 2024. 17(5): 3745-3751. |
| [1] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [2] | 江安迪, 丁雪兴, 王世鹏, 丁俊华, 力宁. 超临界CO2干气密封热动力学性能研究进展[J]. 化工进展, 2024, 43(5): 2354-2369. |
| [3] | 韩伟, 韩恒文, 程薇, 汤玮健. 碳中和目标驱动下生物质燃料技术研究进展[J]. 化工进展, 2024, 43(5): 2463-2474. |
| [4] | 冯勇强, 王洁茹, 王超娴, 李芳, 苏婉婷, 孙宇, 赵彬然. γ-Al2O3 负载的Ni、Fe、Cu对介质阻挡放电等离子体转化CO2/CH4的影响[J]. 化工进展, 2024, 43(5): 2705-2713. |
| [5] | 廖昌建, 张可伟, 王晶, 曾翔宇, 金平, 刘志禹. 直接空气捕集二氧化碳技术研究进展[J]. 化工进展, 2024, 43(4): 2031-2048. |
| [6] | 孙伟吉, 刘浪, 方治余, 朱梦博, 解耿, 何伟, 高宇恒. 改性镁渣的湿法碳酸化工艺[J]. 化工进展, 2024, 43(4): 2161-2173. |
| [7] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [8] | 郭潇东, 毛玉娇, 刘相洋, 邱丽, 于峰, 闫晓亮. Ni/Sm2O3-CeO2/Al2O3催化剂氧空位对二氧化碳低温甲烷化的影响[J]. 化工进展, 2024, 43(4): 1840-1850. |
| [9] | 王凯, 叶丁丁, 朱恂, 杨扬, 陈蓉, 廖强. 超亲气泡沫铜纳米线电极电化学还原CO2性能[J]. 化工进展, 2024, 43(3): 1232-1240. |
| [10] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [11] | 徐泽文, 王明, 王强, 侯影飞. 胺基材料在二氧化碳分离膜领域研究进展[J]. 化工进展, 2024, 43(3): 1374-1386. |
| [12] | 张瑞凯, 张会书, 郑龙云, 曾爱武. CO2吸收过程中气相分压对Rayleigh对流传质特性的影响[J]. 化工进展, 2024, 43(2): 913-924. |
| [13] | 朱兵国, 巩楷刚, 彭斌. 垂直管内高质量流速超临界CO2换热特性[J]. 化工进展, 2024, 43(2): 937-947. |
| [14] | 于笑笑, 巢艳红, 刘海燕, 朱文帅, 刘植昌. D-A共轭聚合强化光电性能及光催化CO2转化[J]. 化工进展, 2024, 43(1): 292-301. |
| [15] | 杨梦茹, 彭琴, 常玉龙, 邱淑兴, 张溅波, 江霞. 生物炭替代煤粉/焦炭高炉炼铁碳减排技术研究进展[J]. 化工进展, 2024, 43(1): 490-500. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||