化工进展 ›› 2024, Vol. 43 ›› Issue (4): 1840-1850.DOI: 10.16085/j.issn.1000-6613.2023-0571
• 工业催化 • 上一篇
Ni/Sm2O3-CeO2/Al2O3催化剂氧空位对二氧化碳低温甲烷化的影响
郭潇东1,2( ), 毛玉娇1,2, 刘相洋1,2, 邱丽1,2(
), 毛玉娇1,2, 刘相洋1,2, 邱丽1,2( ), 于峰1,2, 闫晓亮1,2(
), 于峰1,2, 闫晓亮1,2( )
)
- 1.太原理工大学化学工程与技术学院,山西 太原 030024
2.太原理工大学省部共建煤基能源清洁高效利用 国家重点实验室,山西 太原 030024
-
收稿日期:2023-04-11修回日期:2023-06-15出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:邱丽,闫晓亮 -
作者简介:郭潇东(1998—),男,硕士研究生,研究方向为镍基催化剂的设计及甲烷化性能。E-mail:2970988405@qq.com。 -
基金资助:国家自然科学基金(22108189)
Effect of oxygen vacancies in Ni/Sm2O3-CeO2/Al2O3 catalyst on CO2 methanation at low temperature
GUO Xiaodong1,2( ), MAO Yujiao1,2, LIU Xiangyang1,2, QIU Li1,2(
), MAO Yujiao1,2, LIU Xiangyang1,2, QIU Li1,2( ), YU Feng1,2, YAN Xiaoliang1,2(
), YU Feng1,2, YAN Xiaoliang1,2( )
)
- 1.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2023-04-11Revised:2023-06-15Online:2024-04-15Published:2024-05-13 -
Contact:QIU Li, YAN Xiaoliang
摘要:
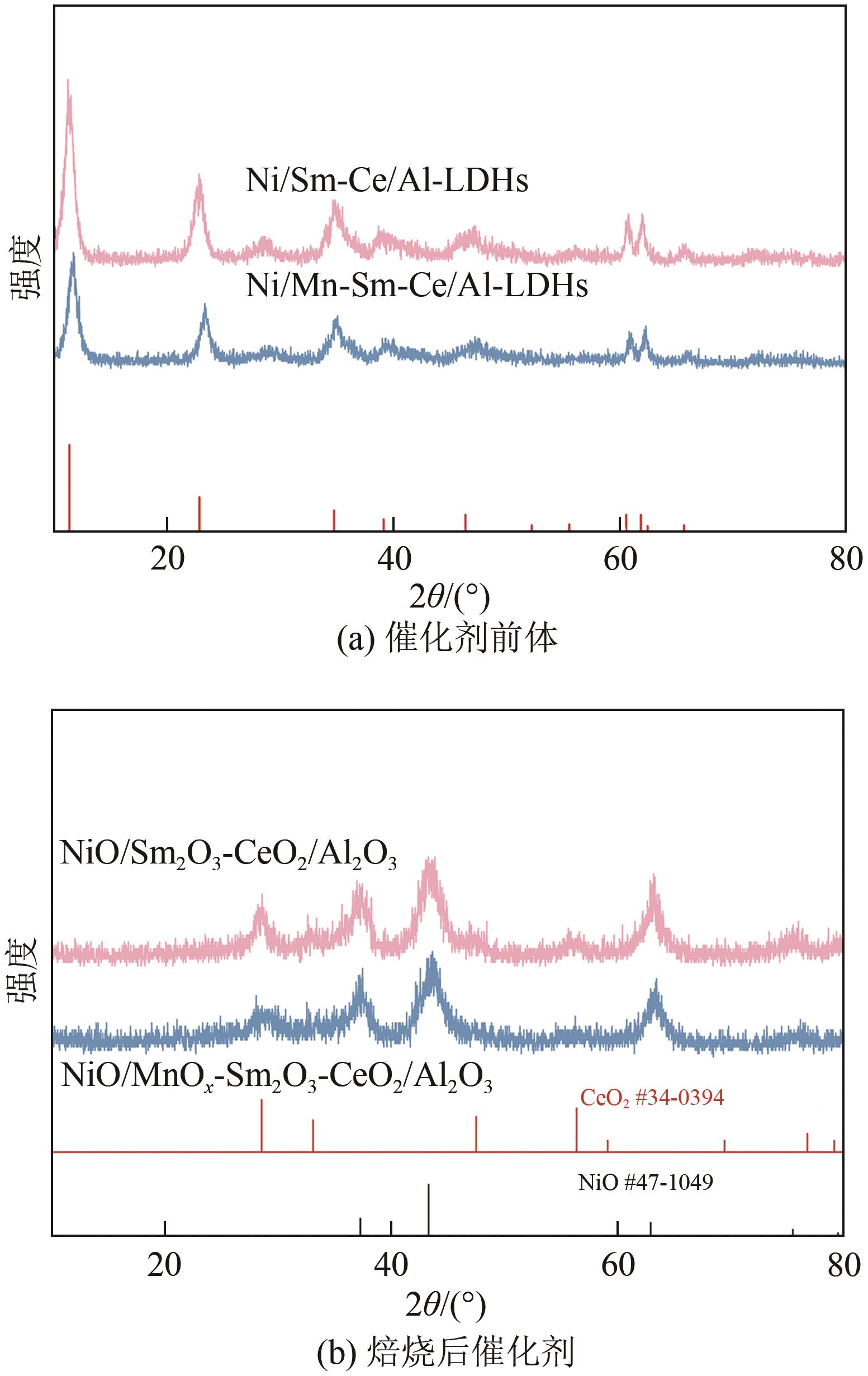
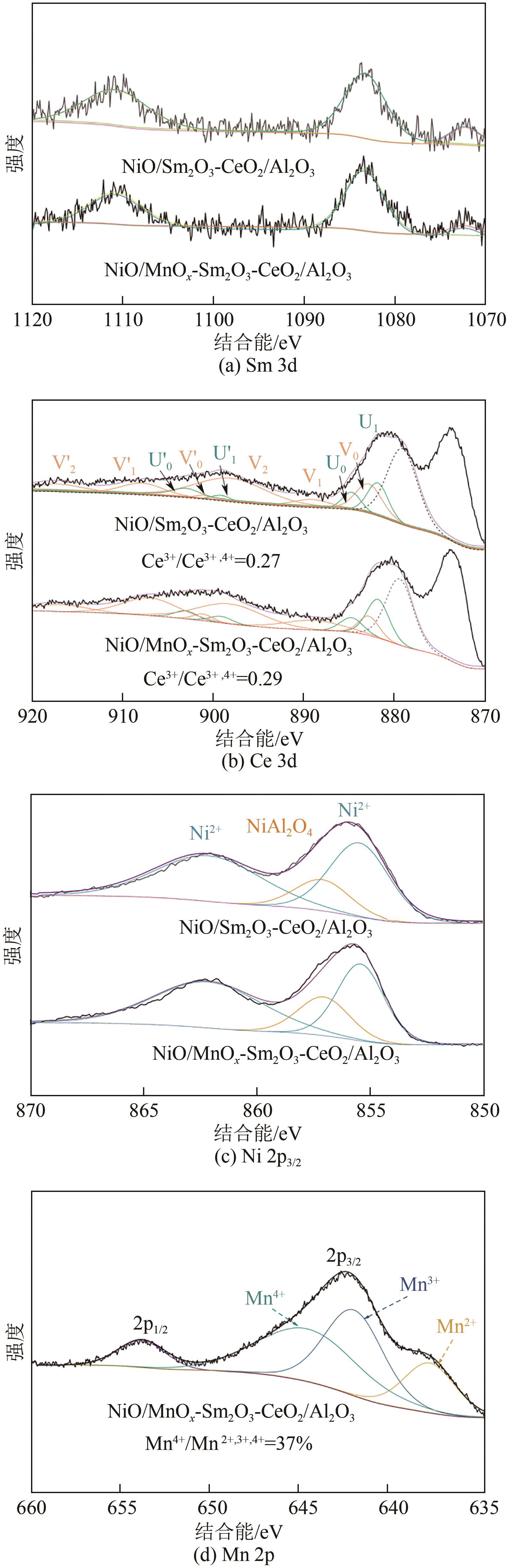
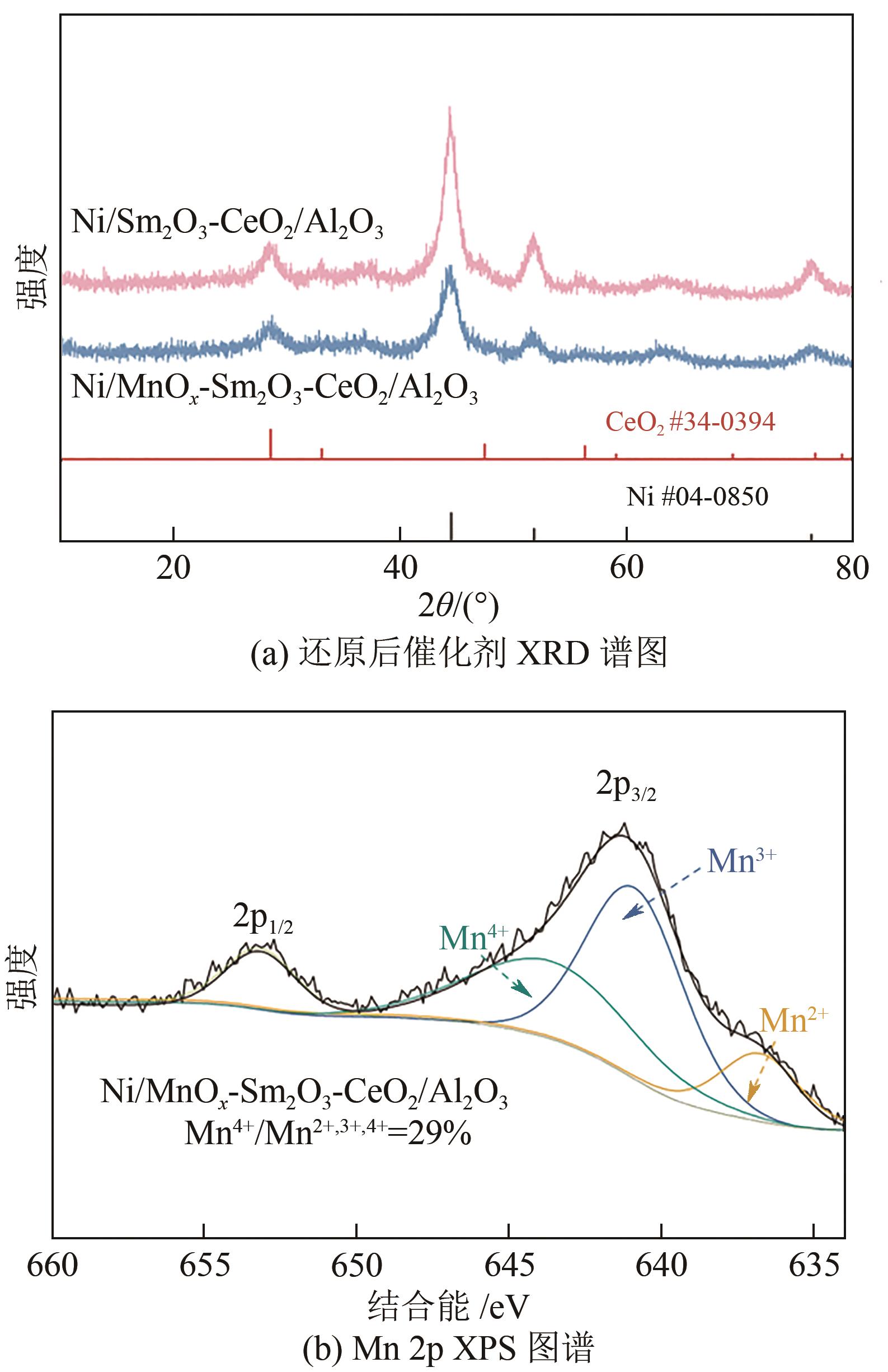
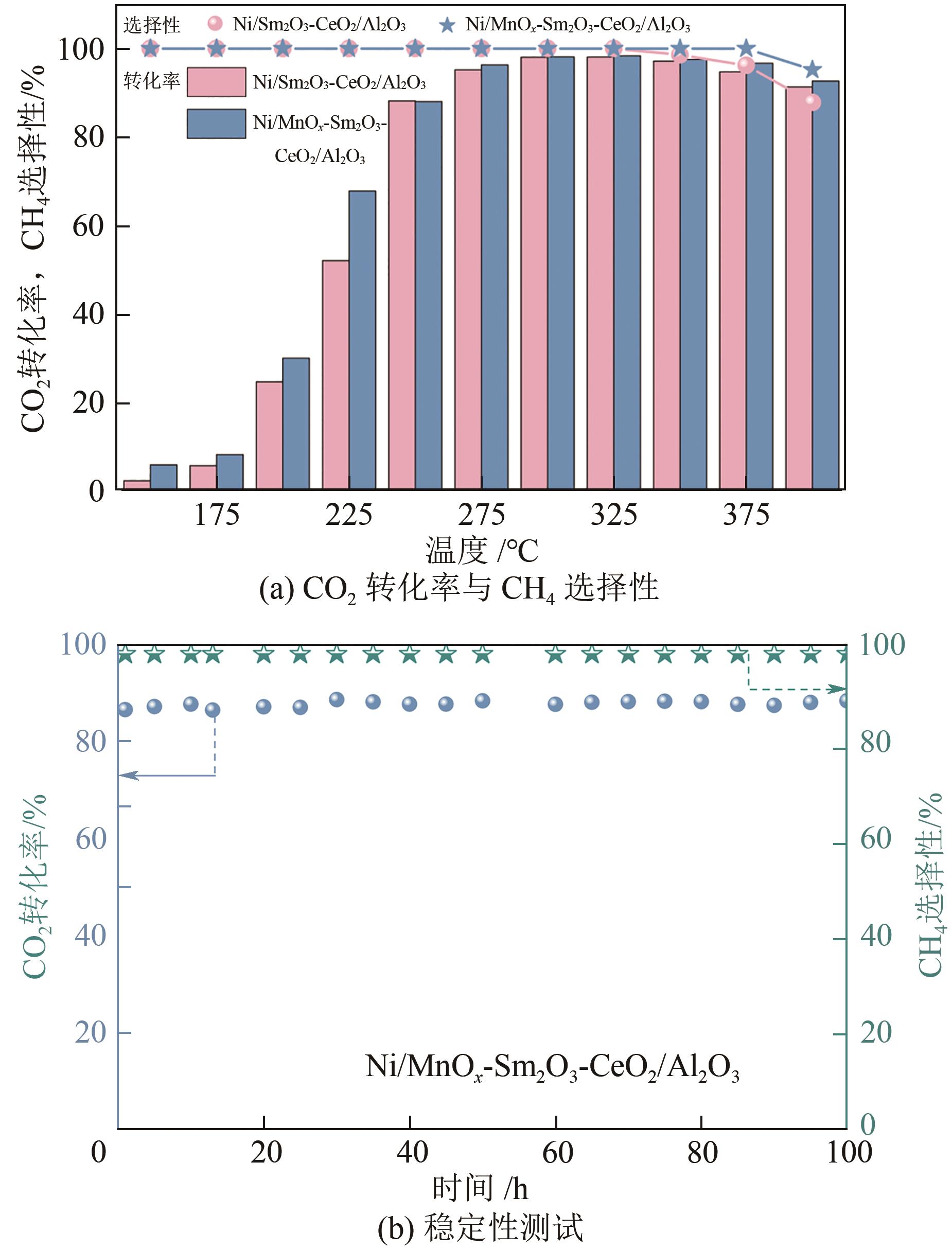
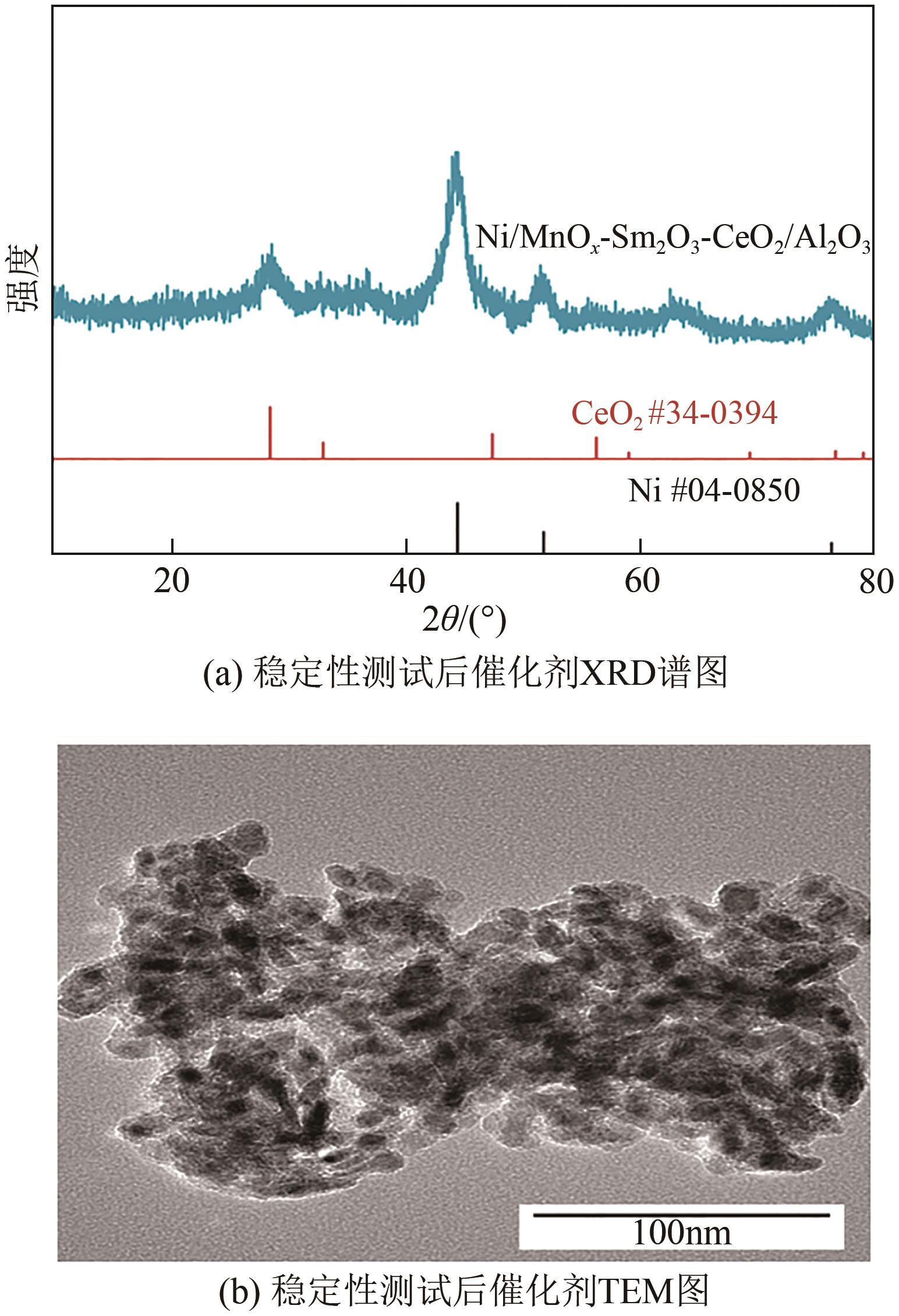
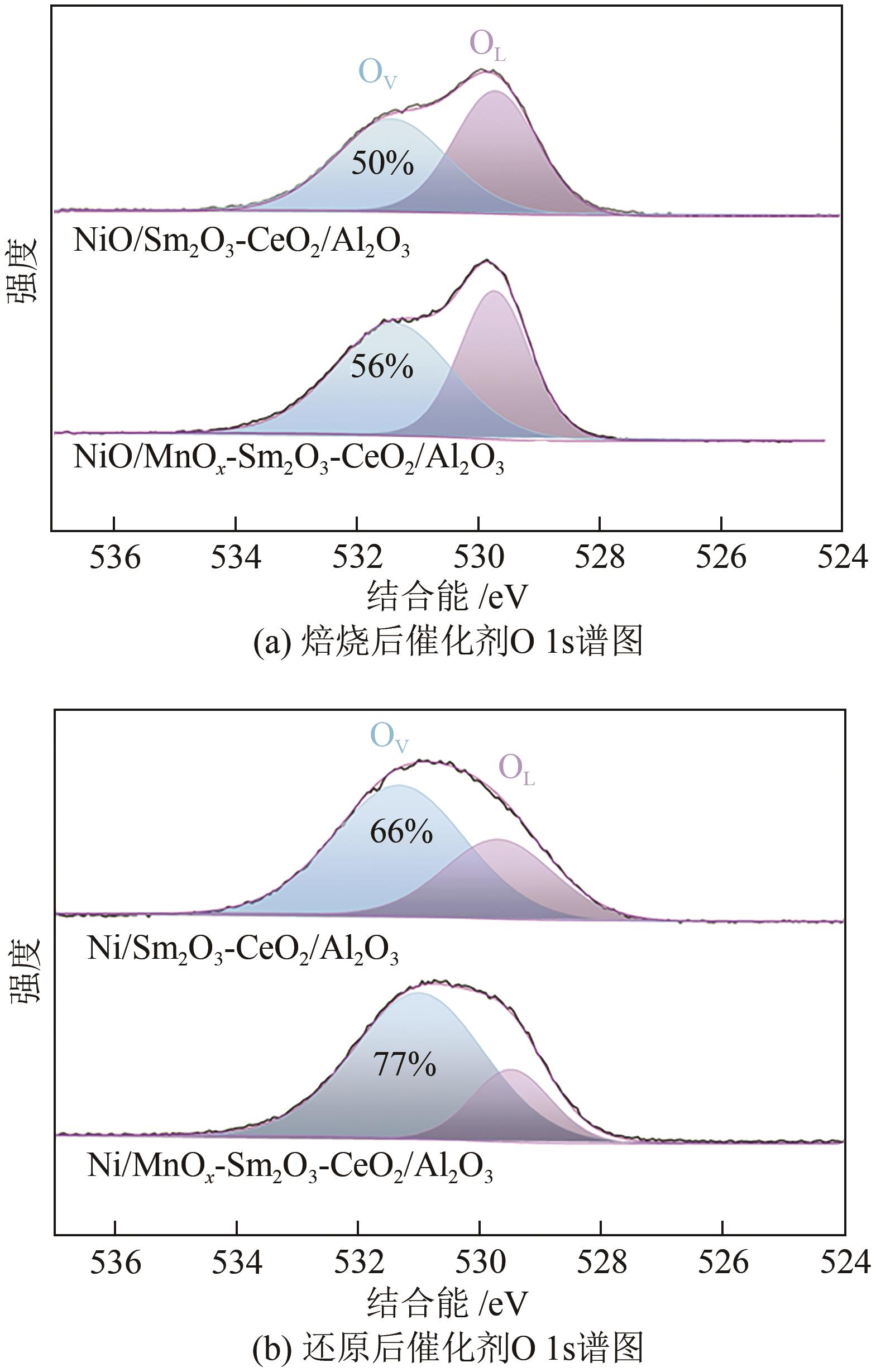
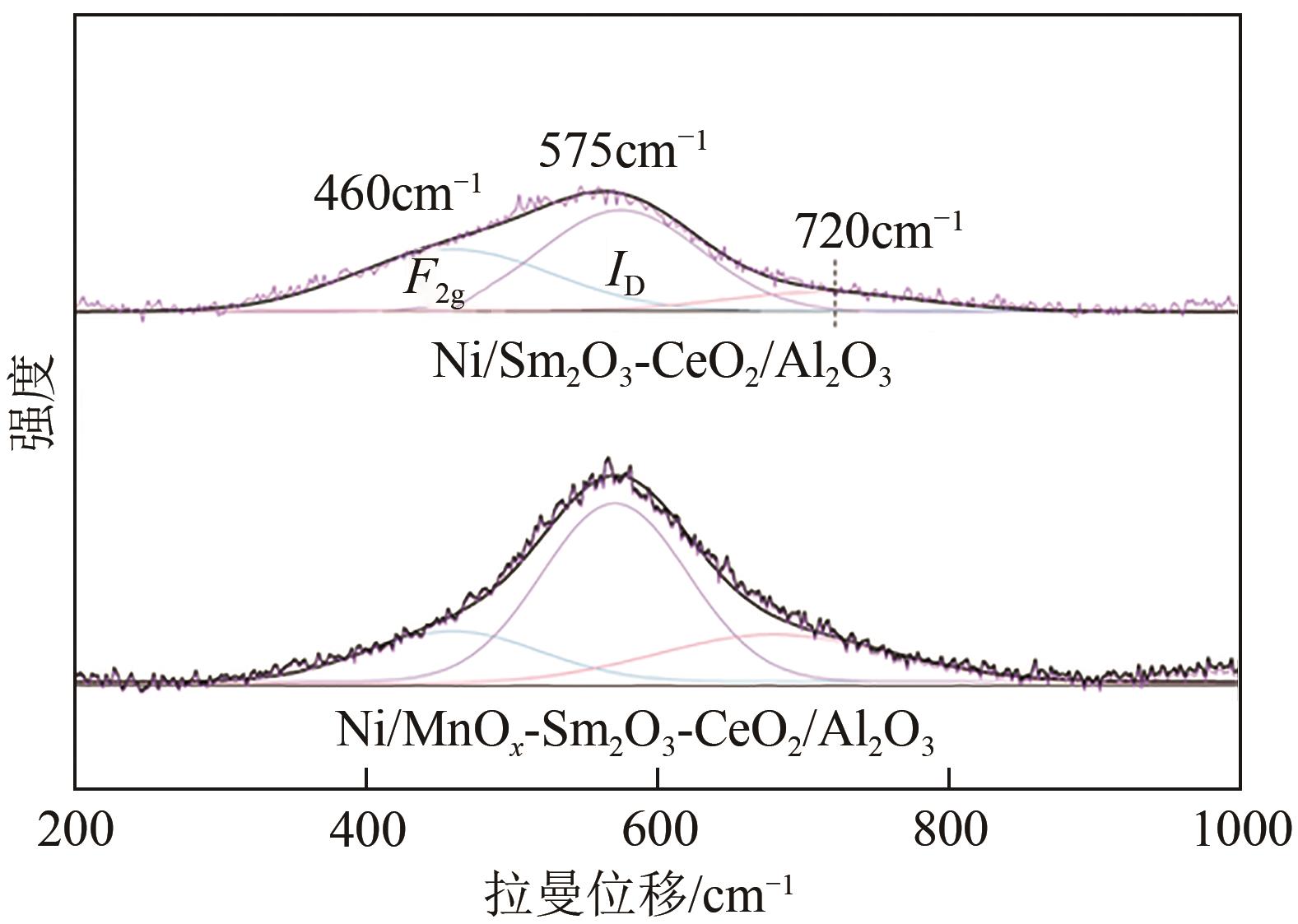
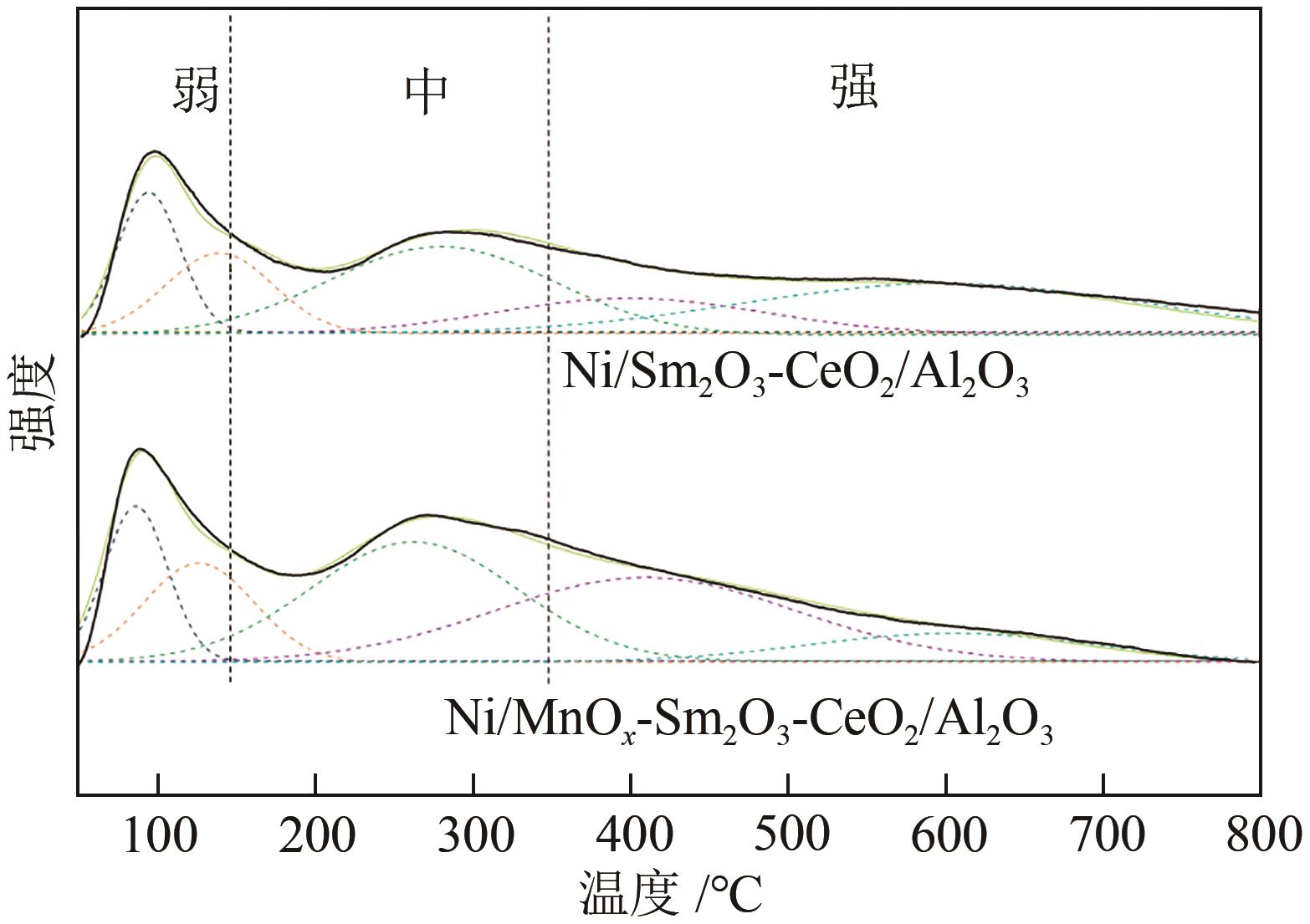
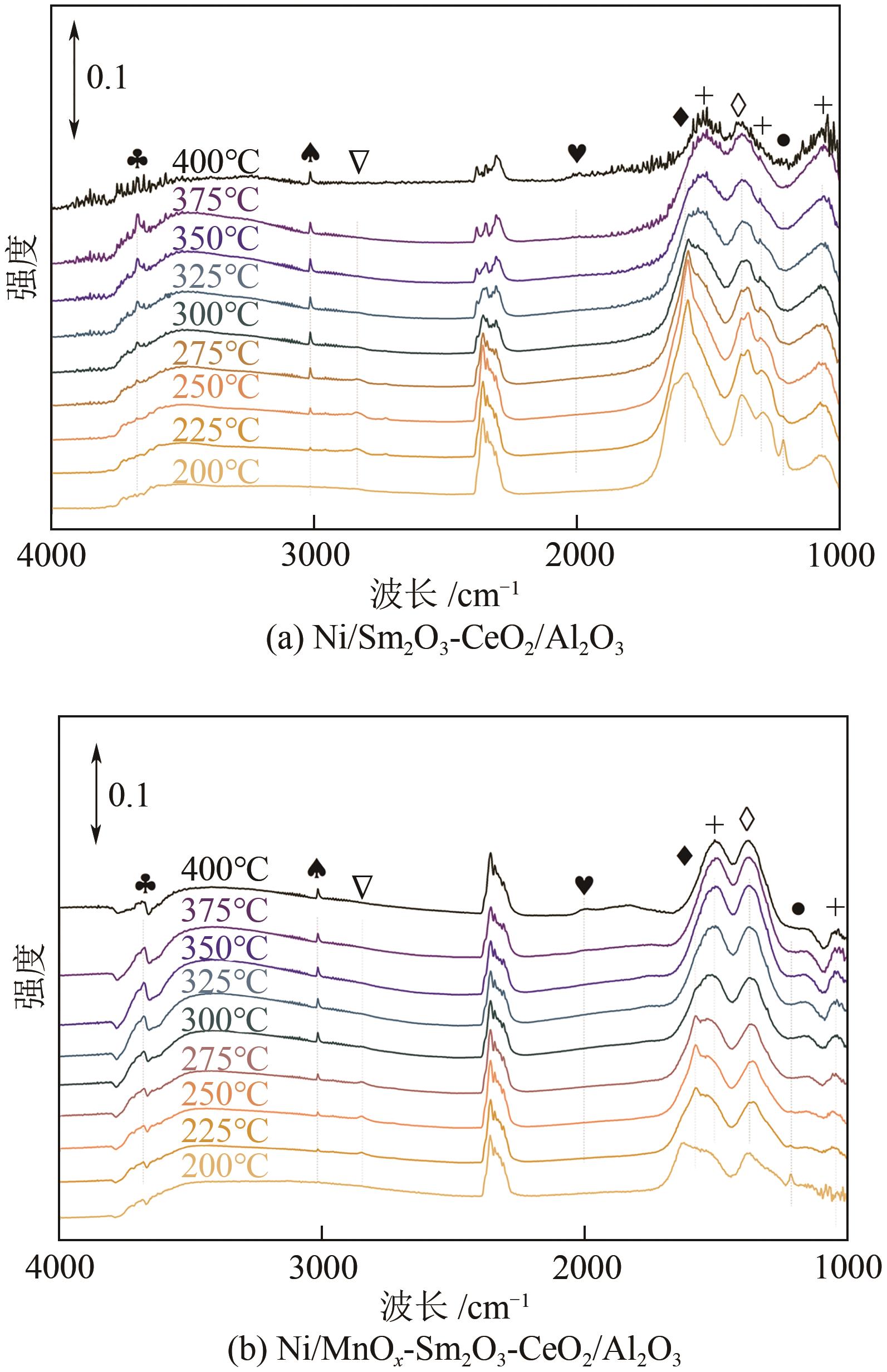
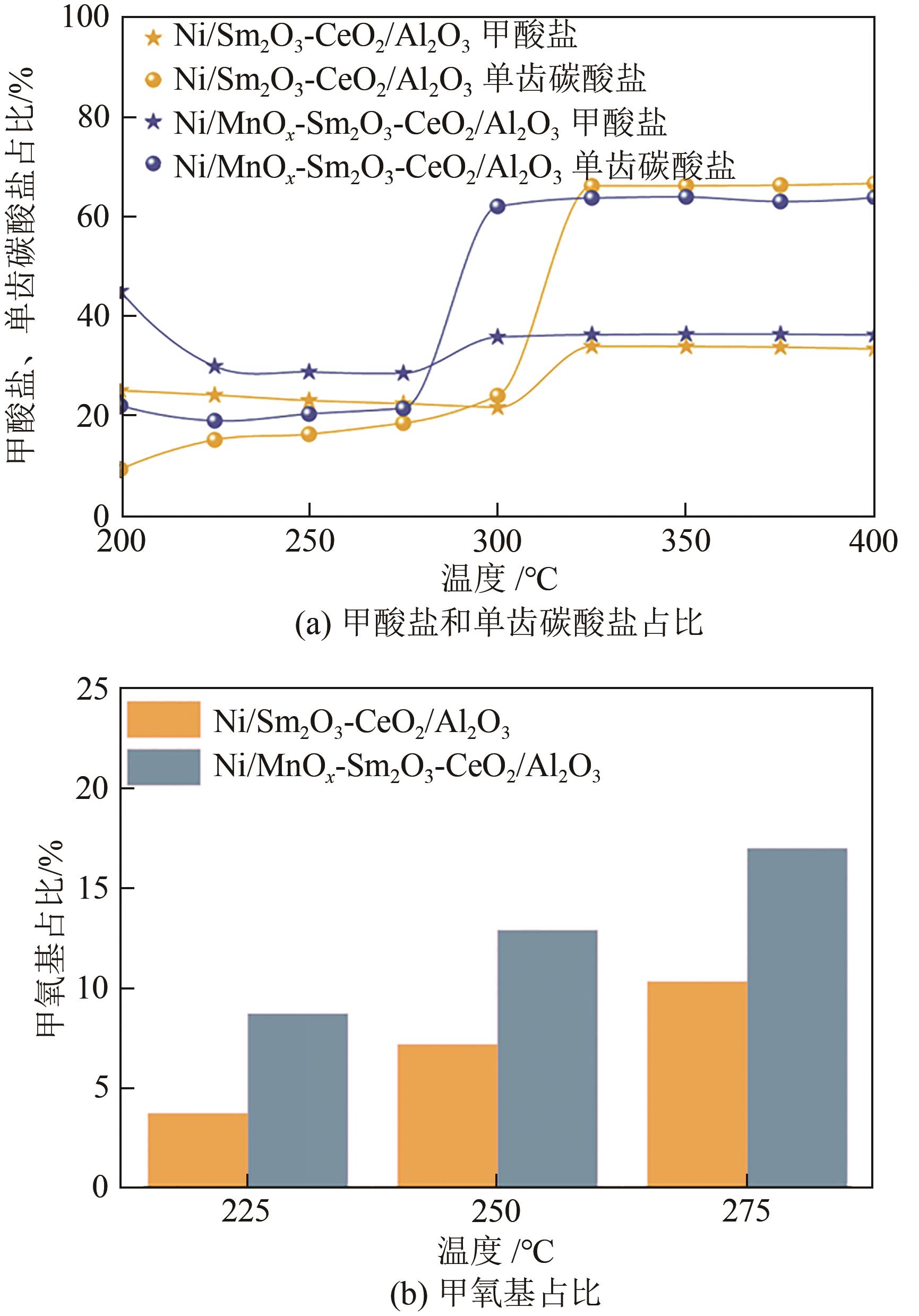
通过水热合成法将Mn、Sm和Ce作为助剂,掺入水滑石(LDHs)前体后经焙烧-还原分别得到Ni/Sm2O3-CeO2/Al2O3和Ni/MnO x -Sm2O3-CeO2/Al2O3催化剂,研究了两种结构催化剂的二氧化碳低温甲烷化反应。研究结果表明,相较于Ni/Sm2O3-CeO2/Al2O3,MnO x 的引入使Ni/MnO x -Sm2O3-CeO2/Al2O3在225℃以下表现出优异的性能,CO2转化率达到68%,CH4选择性达到100%,且在100h内具有良好的稳定性,150℃时TOF为0.087s-1,大于Ni/Sm2O3-CeO2/Al2O3(0.013s-1)。这主要是由于引入MnO x,在保持金属颗粒高度分散的同时,借助MnO x 在催化剂表面连续还原,提高了Ni/MnO x -Sm2O3-CeO2/Al2O3表面氧空位浓度且增加了催化剂的碱性位点,促进了二氧化碳的吸附和活化。原位红外结果进一步表明,低温下催化剂表面氧空位促进甲酸盐和甲氧基中间物种的生成,进而提高了二氧化碳甲烷化活性。
中图分类号:
引用本文
郭潇东, 毛玉娇, 刘相洋, 邱丽, 于峰, 闫晓亮. Ni/Sm2O3-CeO2/Al2O3催化剂氧空位对二氧化碳低温甲烷化的影响[J]. 化工进展, 2024, 43(4): 1840-1850.
GUO Xiaodong, MAO Yujiao, LIU Xiangyang, QIU Li, YU Feng, YAN Xiaoliang. Effect of oxygen vacancies in Ni/Sm2O3-CeO2/Al2O3 catalyst on CO2 methanation at low temperature[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1840-1850.
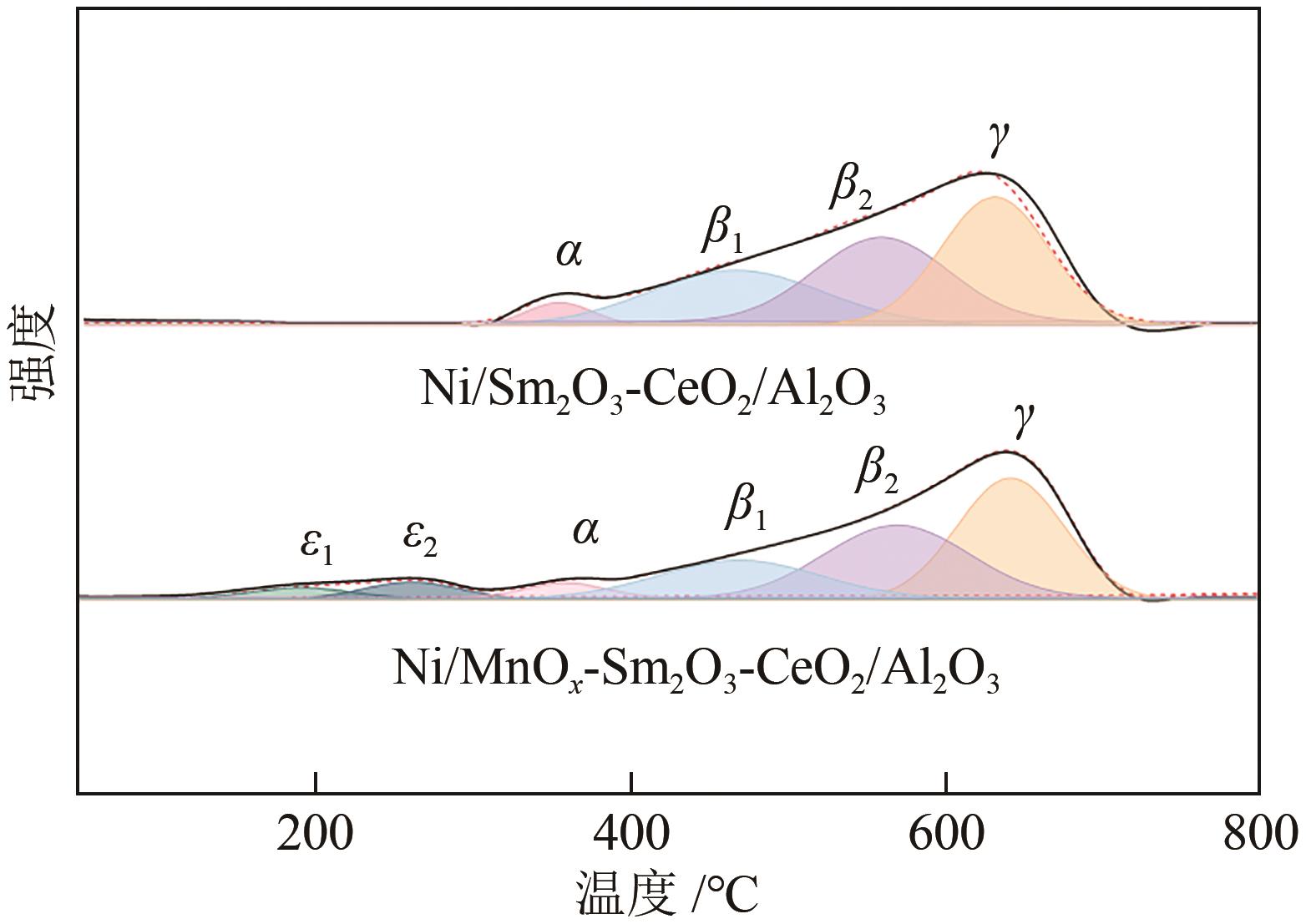
| 催化剂 | H2消耗峰/℃ | |||||
|---|---|---|---|---|---|---|
| ε1 | ε2 | α | β1 | β2 | γ | |
| NiO/Sm2O3-CeO2/Al2O3 | — | — | 356 | 468 | 560 | 632 |
| NiO/MnO x -Sm2O3-CeO2/Al2O3 | 192 | 262 | 360 | 470 | 570 | 642 |
表1 NiO/Sm2O3-CeO2/Al2O3和NiO/MnO x -Sm2O3-CeO2/Al2O3催化剂还原温度
| 催化剂 | H2消耗峰/℃ | |||||
|---|---|---|---|---|---|---|
| ε1 | ε2 | α | β1 | β2 | γ | |
| NiO/Sm2O3-CeO2/Al2O3 | — | — | 356 | 468 | 560 | 632 |
| NiO/MnO x -Sm2O3-CeO2/Al2O3 | 192 | 262 | 360 | 470 | 570 | 642 |
| 催化剂 | 反应温度/℃ | 空速/h-1 | CO2转化率/% | CH4选择性/% | 参考文献 |
|---|---|---|---|---|---|
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 225 | 15000 | 68 | 100 | 本工作 |
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 250 | 15000 | 87.8 | 100 | 本工作 |
| 250 | 40000 | 80.5 | 95.8 | [ | |
| NiCeAl-RMO | 250 | 15000 | 78.6 | 100% | [ |
| 350 | 25000 | 54.5 | 100% | [ | |
| 300 | 3600 | 85.4 | >80% | [ | |
| Ni/ZrO2-Al2O3-0.1 | 280 | 48000 | 84.4 | 99.4 | [ |
| 1%Ru/CeO2 | 300 | 1800 | 86 | 100 | [ |
| Co-Al-O | 250 | 5000 | 74 | 99 | [ |
| Ni-Mn/γ-Al2O3 | 280 | 12000 | 85 | 99 | [ |
| Ni/La-Sm-Ce | 350 | 25000 | 53 | 100 | [ |
| 300 | 30000 | 88.6 | 99% | [ |
表2 不同催化剂在甲烷化反应中的催化性能
| 催化剂 | 反应温度/℃ | 空速/h-1 | CO2转化率/% | CH4选择性/% | 参考文献 |
|---|---|---|---|---|---|
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 225 | 15000 | 68 | 100 | 本工作 |
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 250 | 15000 | 87.8 | 100 | 本工作 |
| 250 | 40000 | 80.5 | 95.8 | [ | |
| NiCeAl-RMO | 250 | 15000 | 78.6 | 100% | [ |
| 350 | 25000 | 54.5 | 100% | [ | |
| 300 | 3600 | 85.4 | >80% | [ | |
| Ni/ZrO2-Al2O3-0.1 | 280 | 48000 | 84.4 | 99.4 | [ |
| 1%Ru/CeO2 | 300 | 1800 | 86 | 100 | [ |
| Co-Al-O | 250 | 5000 | 74 | 99 | [ |
| Ni-Mn/γ-Al2O3 | 280 | 12000 | 85 | 99 | [ |
| Ni/La-Sm-Ce | 350 | 25000 | 53 | 100 | [ |
| 300 | 30000 | 88.6 | 99% | [ |
| 催化剂 | 弱碱性位点 /mmol·g-1 | 中等碱性位点 /mmol·g-1 | 强碱性位点 /mmol·g-1 | 总碱度 /mmol·g-1 |
|---|---|---|---|---|
| Ni/Sm2O3-CeO2/Al2O3 | 0.0081 | 0.0093 | 0.013 | 0.03 |
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 0.013 | 0.015 | 0.021 | 0.049 |
表3 Ni/Sm2O3-CeO2/Al2O3和Ni/MnO x -Sm2O3-CeO2/Al2O3催化剂的CO2-TPD定量计算结果
| 催化剂 | 弱碱性位点 /mmol·g-1 | 中等碱性位点 /mmol·g-1 | 强碱性位点 /mmol·g-1 | 总碱度 /mmol·g-1 |
|---|---|---|---|---|
| Ni/Sm2O3-CeO2/Al2O3 | 0.0081 | 0.0093 | 0.013 | 0.03 |
| Ni/MnO x -Sm2O3-CeO2/Al2O3 | 0.013 | 0.015 | 0.021 | 0.049 |
| 1 | 刘昌俊, 郭秋婷, 叶静云, 等. 二氧化碳转化催化剂研究进展及相关问题思考[J]. 化工学报, 2016, 67(1): 6-13. |
| LIU Changjun, GUO Qiuting, YE Jingyun, et al. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC Journal, 2016, 67(1): 6-13. | |
| 2 | YAN Xiaoliang, SUN Wei, FAN Liming, et al. Nickel@Siloxene catalytic nanosheets for high-performance CO2 methanation[J]. Nature Communications, 2019, 10: 2608. |
| 3 | 周程, 南永永, 查飞, 等. 金属有机骨架材料在二氧化碳加氢中的应用[J]. 燃料化学学报, 2021, 49(10): 1444-1457. |
| ZHOU Cheng, Yongyong NAN, ZHA Fei, et al. Application of metal-organic frameworks in CO2 hydrogenation[J]. Journal of Fuel Chemistry and Technology, 2021, 49(10): 1444-1457. | |
| 4 | SAEIDI Samrand, NAJARI Sara, HESSEL Volker, et al. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions[J]. Progress in Energy and Combustion Science, 2021, 85: 100905. |
| 5 | YE Runping, LI Qiaohong, GONG Weibo, et al. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation [J]. Applied Catalysis B: Environmental, 2020, 268: 118474. |
| 6 | 崔凯凯, 周桂林, 谢红梅. 二氧化碳甲烷化催化剂的研究进展[J]. 化工进展, 2015, 34(3): 724-730, 737. |
| CUI Kaikai, ZHOU Guilin, XIE Hongmei. Research progress in CO2 methanation catalysts[J]. Chemical Industry and Engineering Progress, 2015, 34(3): 724-730, 737. | |
| 7 | WANG Qianqian, CAO Min, FAN Liming, et al. Effects of penta-coordinated Al3+ sites and Ni defective sites on Ni/Al2O3 for CO methanation[J]. AIChE Journal, 2023, 69(5): e17998. |
| 8 | YAN Xiaoliang, YUAN Chen, BAO Jiehua, et al. A Ni-based catalyst with enhanced Ni-support interaction for highly efficient CO methanation[J]. Catalysis Science & Technology, 2018, 8(14): 3474-3483. |
| 9 | HAN Rui, XING Shuang, WANG Yang, et al. Two birds with one stone: MgO promoted Ni-CaO as stable and coke-resistant bifunctional materials for integrated CO2 capture and conversion[J]. Separation and Purification Technology, 2023, 307: 122808. |
| 10 | MA Xiaotong, LI Yingjie, SHI Lei, et al. Fabrication and CO2 capture performance of magnesia-stabilized carbide slag by by-product of biodiesel during calcium looping process[J]. Applied Energy, 2016, 168: 85-95. |
| 11 | AHMAD Waqar, YOUNIS Muhammad Naeem, SHAWABKEH Reyad, et al. Synthesis of lanthanide series (La, Ce, Pr, Eu & Gd) promoted Ni/γ-Al2O3 catalysts for methanation of CO2 at low temperature under atmospheric pressure[J]. Catalysis Communications, 2017, 100: 121-126. |
| 12 | BRANCO Joaquim B, BRITO Pedro E, FERREIRA Ana C. Methanation of CO2 over nickel-lanthanide bimetallic oxides supported on silica[J]. Chemical Engineering Journal, 2020, 380: 122465. |
| 13 | ZHANG Qinwei, XU Ruinian, LIU Ning, et al. In situ Ce-doped catalyst derived from NiCeAl-LDHs with enhanced low-temperature performance for CO2 methanation[J]. Applied Surface Science, 2022, 579: 152204. |
| 14 | ZHU Minghui, TIAN Pengfei, CAO Xinyu, et al. Vacancy engineering of the nickel-based catalysts for enhanced CO2 methanation[J]. Applied Catalysis B: Environmental, 2021, 282: 119561. |
| 15 | LI Shuangshuang, LIU Guilong, ZHANG Siran, et al. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation[J]. Journal of Energy Chemistry, 2020, 43: 155-164. |
| 16 | POLYCHRONOPOULOU K, ZEDAN AF, KATSIOTIS M S, et al. Rapid microwave assisted sol-gel synthesis of CeO2 and Ce x Sm1- x O2 nanoparticle catalysts for CO oxidation[J]. Molecular Catalysis, 2017, 428: 41-55. |
| 17 | SIAKAVELAS G I, CHARISIOU N D, ALKHOORI S, et al. Highly selective and stable nickel catalysts supported on ceria promoted with Sm2O3, Pr2O3 and MgO for the CO2 methanation reaction[J]. Applied Catalysis B: Environmental, 2021, 282: 119562. |
| 18 | 田郡博, 古芳娜, 苏发兵, 等. 二氧化碳甲烷化催化剂及反应机理研究进展[J]. 过程工程学报, 2023, 23(3): 375-395. |
| TIAN Junbo, GU Fangna, SU Fabing, et al. CO2 methanation: Recent advances in catalyst development and reaction mechanistic study[J]. The Chinese Journal of Process Engineering, 2023, 23(3): 375-395. | |
| 19 | KRCHA Matthew D, MAYERNICK Adam D, JANIK Michael J. Periodic trends of oxygen vacancy formation and C—H bond activation over transition metal-doped CeO2 (111) surfaces[J]. Journal of Catalysis, 2012, 293: 103-115. |
| 20 | JIANG Yuexiu, HUANG Tongxia, DONG Lihui, et al. Mn modified Ni/bsentonite for CO2 methanation[J]. Catalysts, 2018, 8(12): 646. |
| 21 | WANG Binxia, ZHANG Xinyue, LIU Yanan, et al. Basic intensity regulation of layered double oxide for CO2 adsorption process at medium temperature in coal gasification[J]. Chemical Engineering Journal, 2022, 446: 136842. |
| 22 | LIN Shuangxi, LI Zhenhua, LI Maoshuai. Tailoring metal-support interactions via tuning CeO2 particle size for enhancing CO2 methanation activity over Ni/CeO2 catalysts[J]. Fuel, 2023, 333: 126369. |
| 23 | XU Qian, HU Shanwei, WANG Weijia, et al. Temperature-induced structural evolution of Sm nanoparticles on Al2O3 thin film: An in situ investigation using SRPES, XPS and STM[J]. Applied Surface Science, 2018, 432: 115-120. |
| 24 | HU Feiyang, JIN Chengkai, WU Rundong, et al. Enhancement of hollow Ni/CeO2-Co3O4 for CO2 methanation: From CO2 adsorption and activation by synergistic effects[J]. Chemical Engineering Journal, 2023, 461: 142108. |
| 25 | ZHANG Min, LI Weiman, WU Xiaofeng, et al. Low-temperature catalytic oxidation of benzene over nanocrystalline Cu-Mn composite oxides by facile sol-gel synthesis[J]. New Journal of Chemistry, 2020, 44(6): 2442-2451. |
| 26 | 马源, 肖晴月, 岳君容, 等. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| MA Yuan, XIAO Qingyue, YUE Junrong, et al. CO x co-methanation over a Ni-based catalyst supported on CeO2-Al2O3 composite[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2421-2428. | |
| 27 | LIN Xueting, LI Shujun, HE Hui, et al. Evolution of oxygen vacancies in MnO x -CeO2 mixed oxides for soot oxidation[J]. Applied Catalysis B: Environmental, 2018, 223: 91-102. |
| 28 | GONZÁLEZ-CASTAÑO M, GONZÁLEZ-ARIAS J, BOBADILLA L F, et al. In-situ DRIFTS steady-state study of CO2 and CO methanation over Ni-promoted catalysts[J]. Fuel, 2023, 338: 127241. |
| 29 | FU Hao, SUN Shaohui, LIAN Honglei. Enhanced low-temperature CO2 methanation over Ni/ZrO2-Al2O3 catalyst: Effect of Al addition on catalytic performance and reaction mechanism[J]. Journal of CO2 Utilization, 2023, 69: 102415. |
| 30 | WANG Chunfen, SUN Hongman, LIU Xiaoqi, et al. Low-temperature CO2 methanation over Ru/CeO2: Investigation into Ru loadings[J]. Fuel, 2023, 345: 128238. |
| 31 | LIU Zhihao, GAO Xinhua, LIU Bo, et al. Highly stable and selective layered Co-Al-O catalysts for low-temperature CO2 methanation[J]. Applied Catalysis B: Environmental, 2022, 310: 121303. |
| 32 | QIN Daxin, XIE Dengbing, ZHENG Heping, et al. In-situ FTIR study of CO2 adsorption and methanation mechanism over bimetallic catalyst at low temperature[J]. Catalysis Letters, 2021, 151: 2894-2905. |
| 33 | SIAKAVELAS G I, CHARISIOU N D, ALKHOORI A, et al. Highly selective and stable Ni/La-M (M=Sm, Pr, and Mg)-CeO2 catalysts for CO2 methanation[J]. Journal of CO2 Utilization, 2021, 51: 101618. |
| 34 | ZHANG Tengfei, WANG Weiwei, GU Fangna, et al. Enhancing the low-temperature CO2 methanation over Ni/La-CeO2 catalyst: The effects of surface oxygen vacancy and basic site on the catalytic performance[J]. Applied Catalysis B: Environmental, 2022, 312: 121385. |
| 35 | 张嘉琪, 林丽娜, 高文桂, 等. CeO2的形貌对CuO/CeO2催化剂CO2加氢制甲醇性能的影响[J]. 化工进展, 2022, 41(8): 4213-4223. |
| ZHANG Jiaqi, LIN Lina, GAO Wengui, et al. Effect of CeO2 morphology on the performance of CuO/CeO2 catalyst for CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4213-4223. | |
| 36 | MONICA S E S, DHAS C R, VENKATESH R,et al. Nebulizer sprayed nickel-manganese (Ni-Mn) mixed metal oxide nanocomposite coatings for high-performance electrochromic device applications[J]. Journal of Solid State Electrochemistry, 2022, 26(5): 1271-1290. |
| 37 | 王国栋, 郭亚飞, 李佳媛, 等. 碱/碱土金属修饰Ni基催化剂的CO2吸附与甲烷化性能[J]. 化工进展, 2021, 40(12): 6925-6933. |
| WANG Guodong, GUO Yafei, LI Jiayuan, et al. CO2 adsorption and methanation performance of nickel-based catalysts modified with alkali/alkaline-earth metals[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6925-6933. | |
| 38 | DROUILLY Charlotte, KRAFFT Jean-Marc, AVERSENG Frédéric, et al. Role of oxygen vacancies in the basicity of ZnO: From the model methylbutynol conversion to the ethanol transformation application[J]. Applied Catalysis A: General, 2013, 453: 121-129. |
| 39 | WANG Xiang, SHI Hui, KWAK Ja Hun, et al. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts: Kinetics and transient DRIFTS-MS studies[J]. ACS Catalysis, 2015, 5(11): 6337-6349. |
| 40 | SCHREITER Norman, KIRCHNER Johann, KURETI Sven. A DRIFTS and TPD study on the methanation of CO2 on Ni/Al2O3 catalyst[J]. Catalysis Communications, 2020, 140: 105988. |
| 41 | FATAH N A A, JALIL A A, SALLEH N F M, et al. Elucidation of cobalt disturbance on Ni/Al2O3 in dissociating hydrogen towards improved CO2 methanation and optimization by response surface methodology (RSM)[J]. International Journal of Hydrogen Energy, 2020, 45(36): 18562-18573. |
| 42 | CÁRDENAS-ARENAS A, QUINDIMIL A, DAVÓ-QUIÑONERO A, et al. Isotopic and in situ DRIFTS study of the CO2 methanation mechanism using Ni/CeO2 and Ni/Al2O3 catalysts[J]. Applied Catalysis B: Environmental, 2020, 265: 118538. |
| 43 | JIA Xinyu, ZHANG Xiaoshan, RUI Ning, et al. Structural effect of Ni/ZrO2 catalyst on CO2 methanation with enhanced activity[J]. Applied Catalysis B: Environmental, 2019, 244: 159-169. |
| [1] | 王彦红, 蒋雷, 薛帅, 李洪伟, 贾玉婷. 预冷通道中超临界甲烷换热特性分析[J]. 化工进展, 2024, 43(4): 1690-1699. |
| [2] | 刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741. |
| [3] | 王红妍, 马子然, 李歌, 马静, 赵春林, 周佳丽, 王磊, 彭胜攀. 燃煤耦合可再生燃料烟气多污染物协同催化脱除研究进展[J]. 化工进展, 2024, 43(4): 1783-1795. |
| [4] | 陈家一, 高帷韬, 阴亚楠, 王诚, 欧阳鸿武, 毛宗强. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| [5] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [6] | 刘雨蓉, 王兴宝, 李文英. 分子筛负载Pt催化剂酸性位点调控及对蒽深度加氢性能的影响[J]. 化工进展, 2024, 43(4): 1832-1839. |
| [7] | 王凯, 叶丁丁, 朱恂, 杨扬, 陈蓉, 廖强. 超亲气泡沫铜纳米线电极电化学还原CO2性能[J]. 化工进展, 2024, 43(3): 1232-1240. |
| [8] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [9] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [10] | 谷星朋, 马红钦, 刘嘉豪. 雷尼镍的磷量子点改性及其催化加氢脱硫性能[J]. 化工进展, 2024, 43(3): 1293-1301. |
| [11] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [12] | 陈风, 王宣德, 黄伟, 王晓东, 王琰. HZSM-22的粒径调控及Pt/HZSM-22的正十二烷加氢异构催化性能[J]. 化工进展, 2024, 43(3): 1309-1317. |
| [13] | 萧垚鑫, 张军, 单锐, 袁浩然, 陈勇. Pt/CaO材料催化糠醇加氢制备戊二醇[J]. 化工进展, 2024, 43(3): 1318-1327. |
| [14] | 李伟杰, 康金灿, 张传明, 林丽娜, 李昌鑫, 朱红平. 锆改性Cu/SiO2催化剂催化3-羟基丙酸甲酯选择性加氢[J]. 化工进展, 2024, 43(3): 1328-1341. |
| [15] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||