化工进展 ›› 2024, Vol. 43 ›› Issue (3): 1318-1327.DOI: 10.16085/j.issn.1000-6613.2023-0448
• 工业催化 • 上一篇
Pt/CaO材料催化糠醇加氢制备戊二醇
萧垚鑫1,2( ), 张军2,3,4(
), 张军2,3,4( ), 单锐2,3,4, 袁浩然2,3,4(
), 单锐2,3,4, 袁浩然2,3,4( ), 陈勇1,2,3,4
), 陈勇1,2,3,4
- 1.华南农业大学生物质工程研究院,广东 广州 510642
2.中国科学院广州能源研究所,广东 广州 510640
3.中国科学院可再生能源重点实验室,广东 广州 510640
4.广东省新能源和可再生能源研究开发与应用重点 实验室,广东 广州 510640
-
收稿日期:2023-03-23修回日期:2023-05-22出版日期:2024-03-10发布日期:2024-04-11 -
通讯作者:袁浩然 -
作者简介:萧垚鑫(1998—),男,硕士研究生,研究方向为生物质高值资源化利用。E-mail:xiao19980129@yahoo.com
张军(1987—),男,博士,副研究员,研究方向为生物质/有机固废高值资源化利用。E-mail:zhangjun@ms.giec.ac.cn。 -
基金资助:国家自然科学基金面上项目(51976222);能源清洁利用国家重点实验室开放基金(ZJU-CEU2020023);中国科学院青年创新促进会会员(2023367)
Catalytic hydrogenation of furfuryl alcohol into pentanediol over Pt/CaO materials
XIAO Yaoxin1,2( ), ZHANG Jun2,3,4(
), ZHANG Jun2,3,4( ), SHAN Rui2,3,4, YUAN Haoran2,3,4(
), SHAN Rui2,3,4, YUAN Haoran2,3,4( ), CHEN Yong1,2,3,4
), CHEN Yong1,2,3,4
- 1.Institute of Biomass Engineering, South China Agricultural University, Guangzhou 510642, Guangdong, China
2.Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences (CAS), Guangzhou 510640, Guangdong, China
3.CAS Key Laboratory of Renewable Energy, Guangzhou 510640, Guangdong, China
4.Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou 510640, Guangdong, China
-
Received:2023-03-23Revised:2023-05-22Online:2024-03-10Published:2024-04-11 -
Contact:YUAN Haoran
摘要:
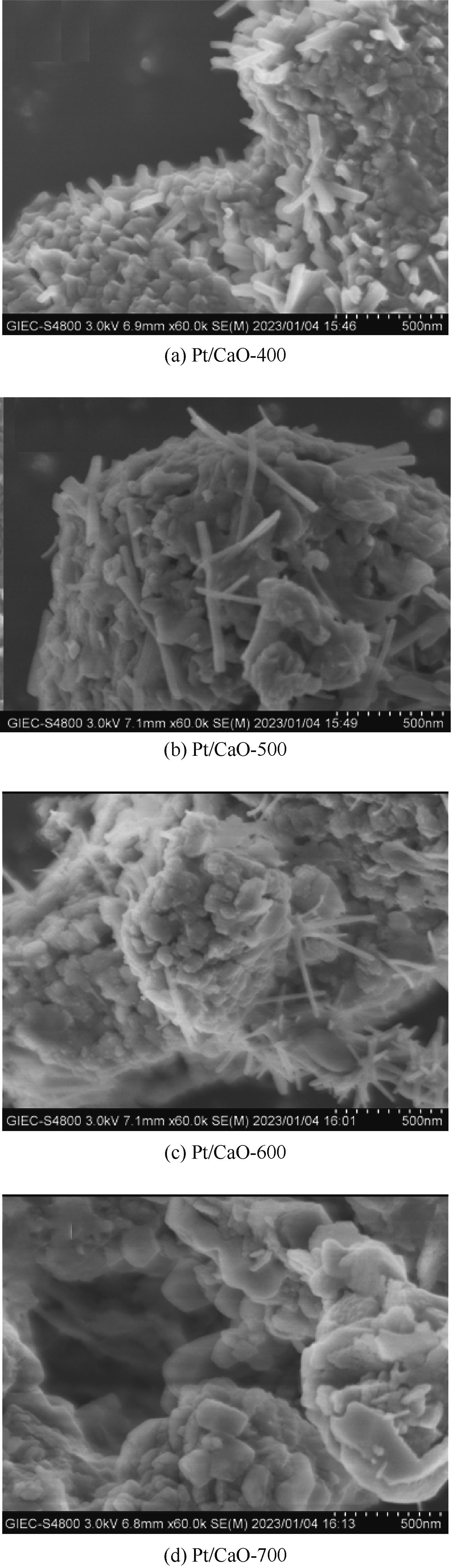
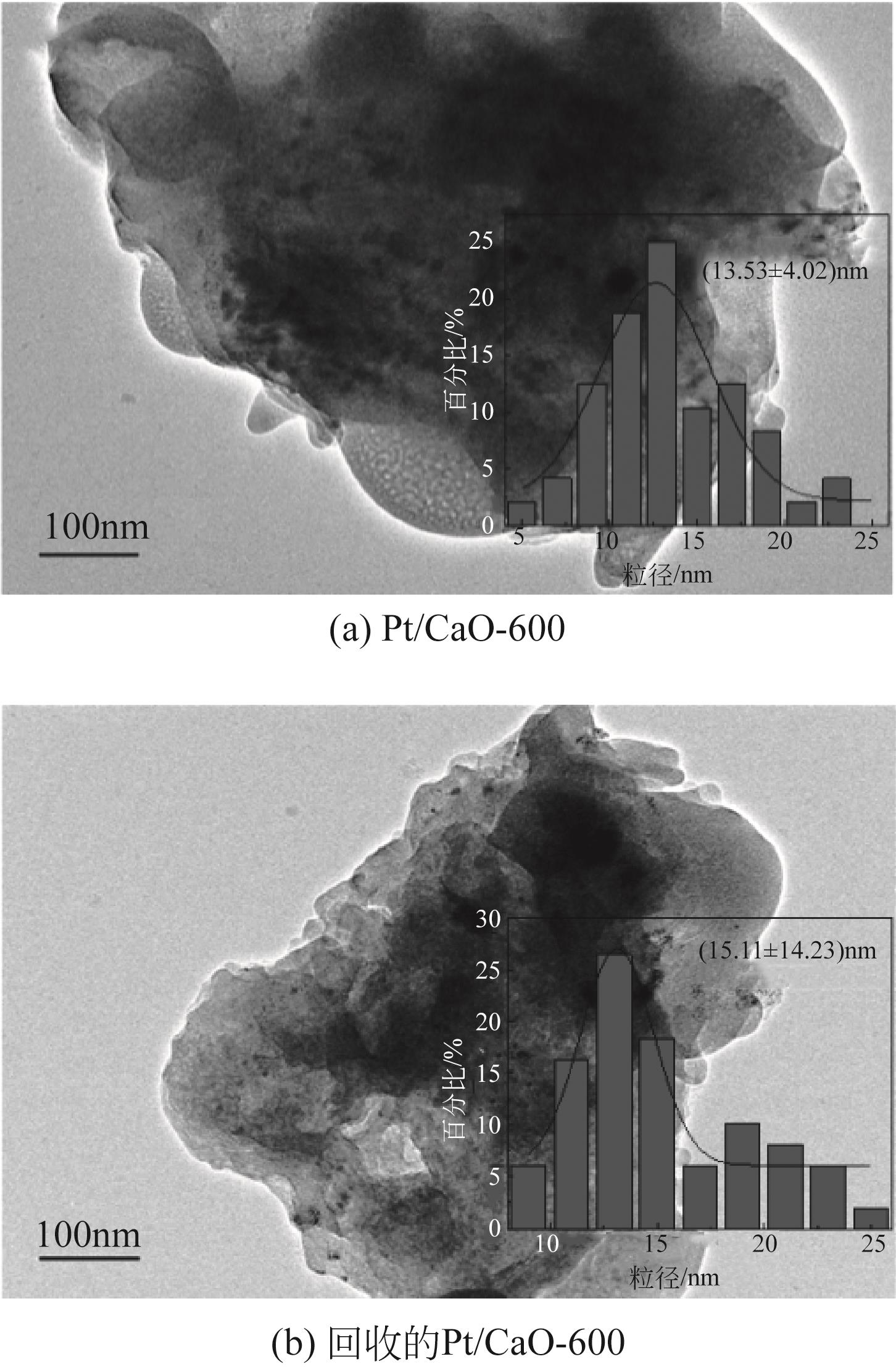
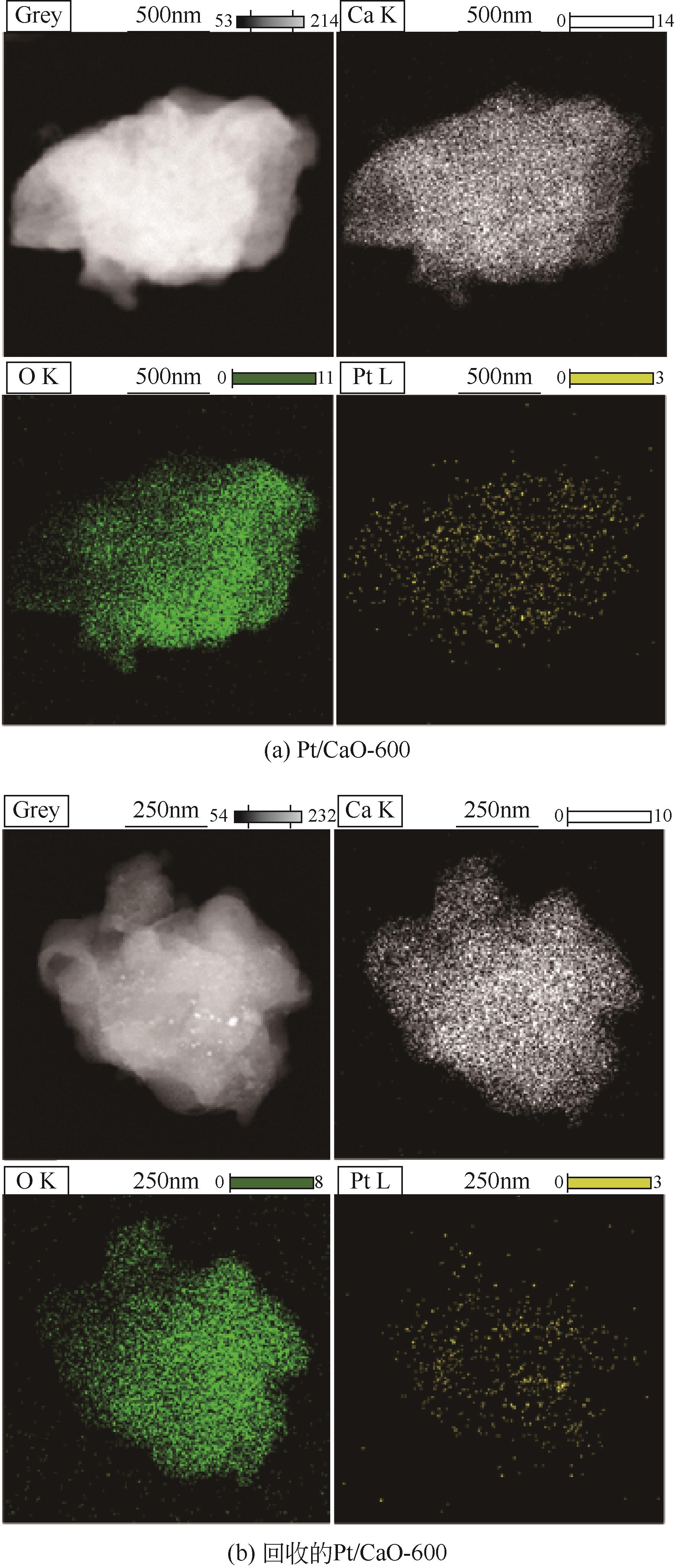
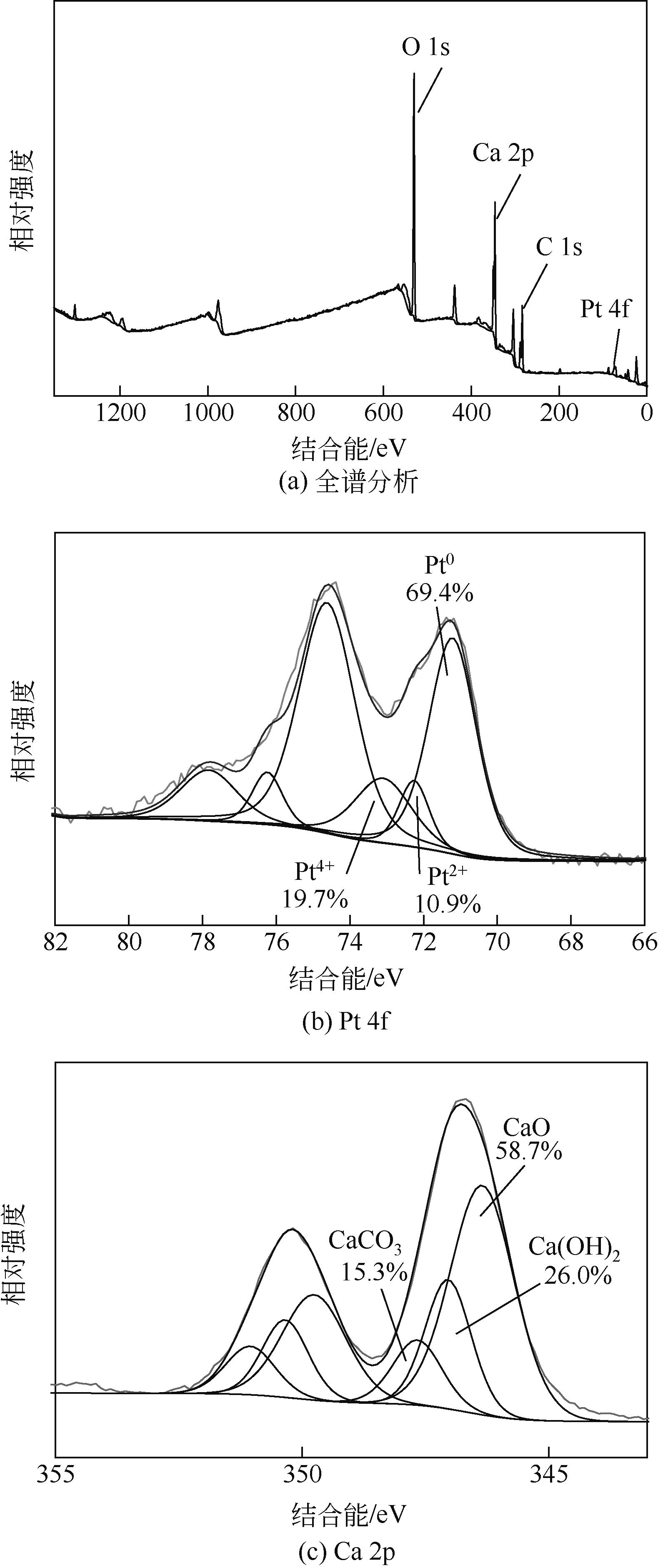
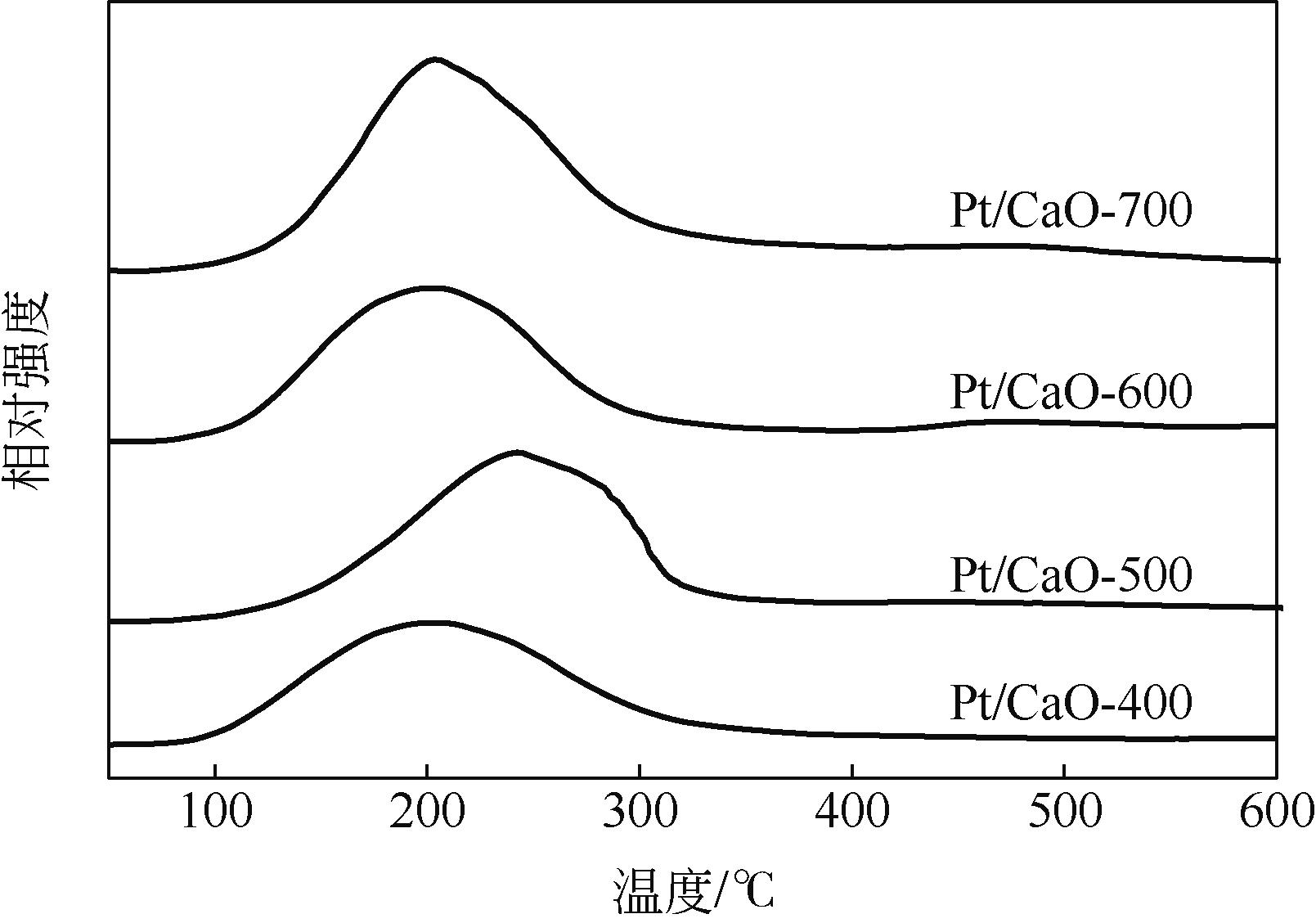
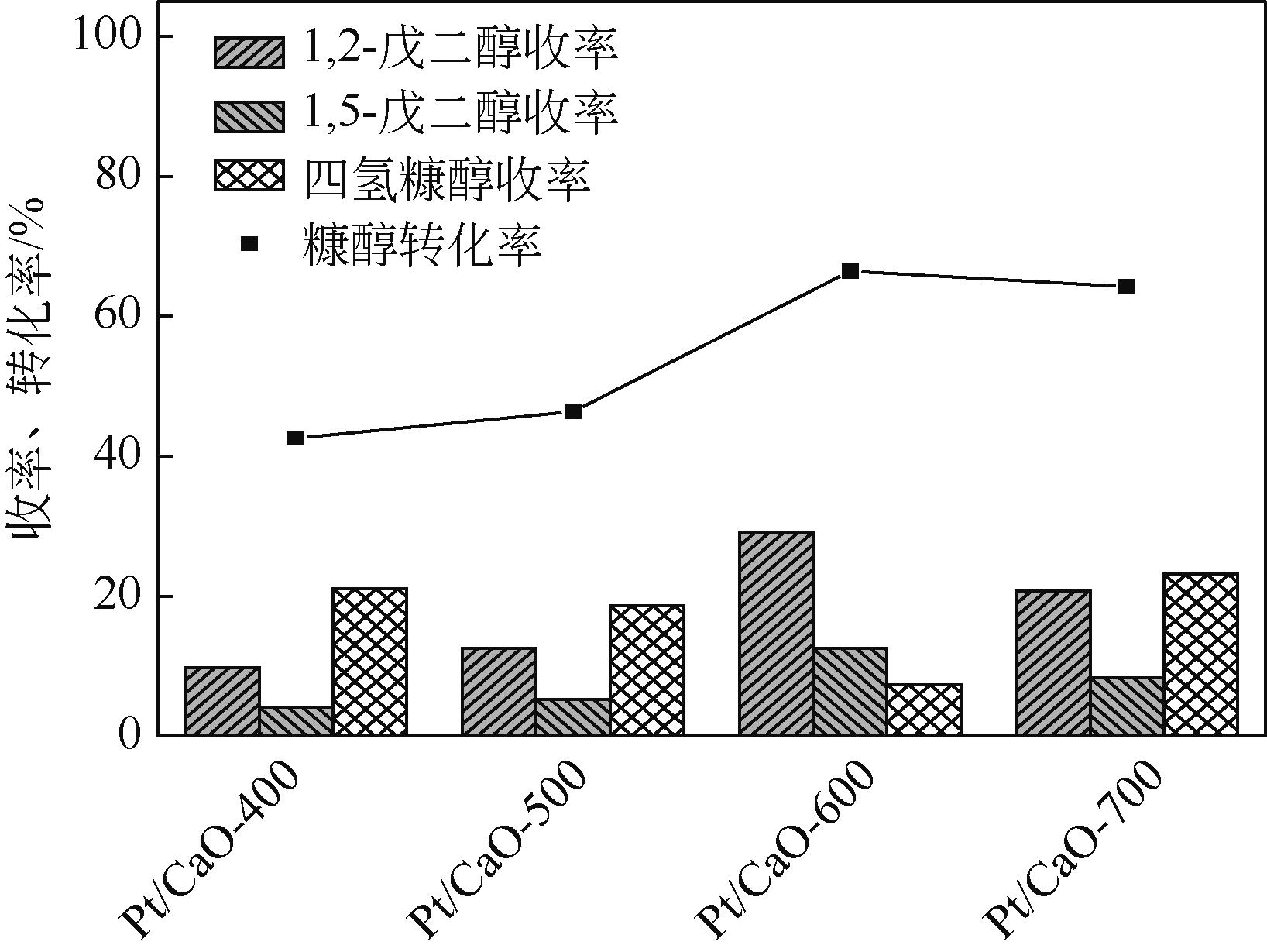
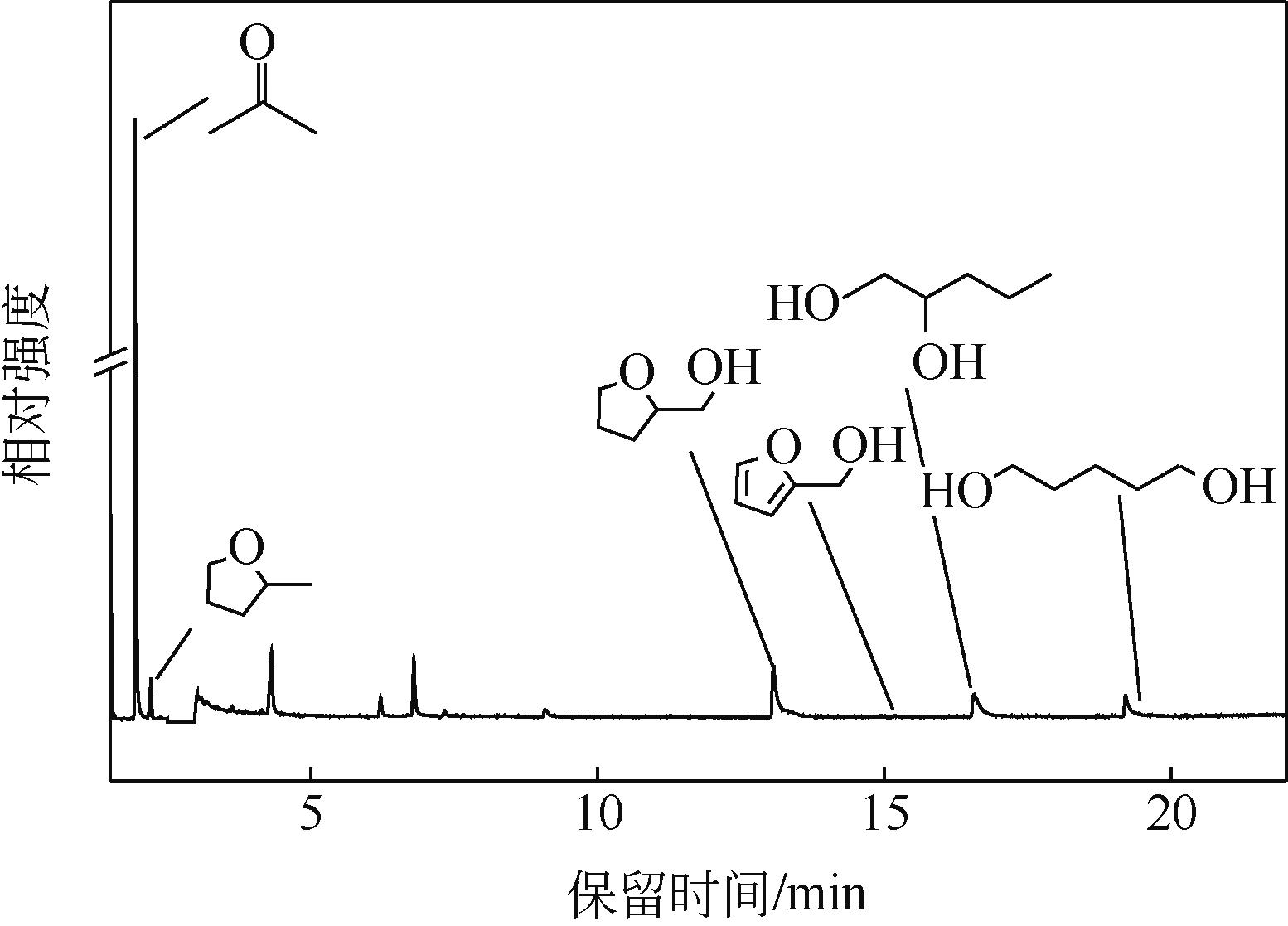
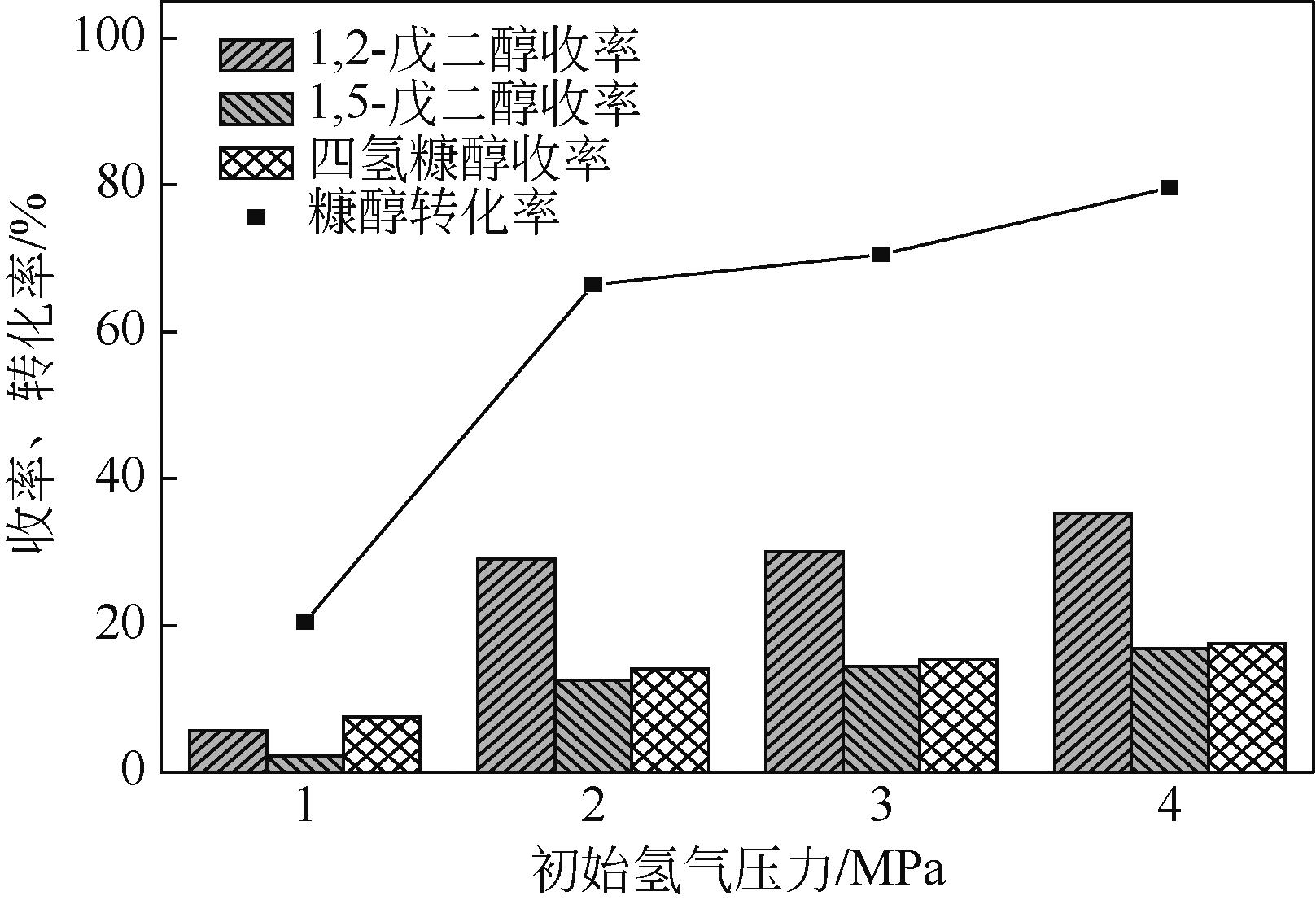
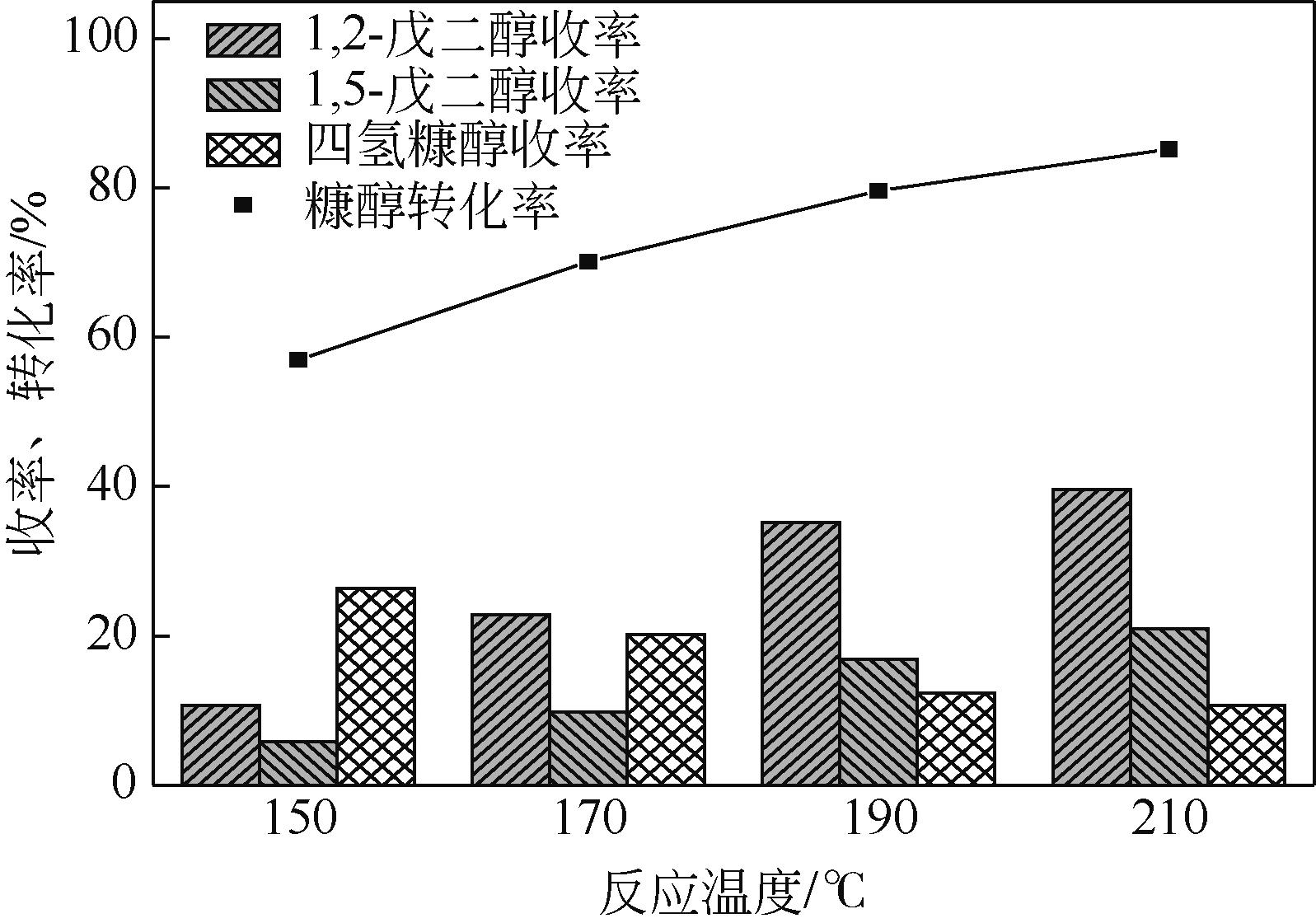
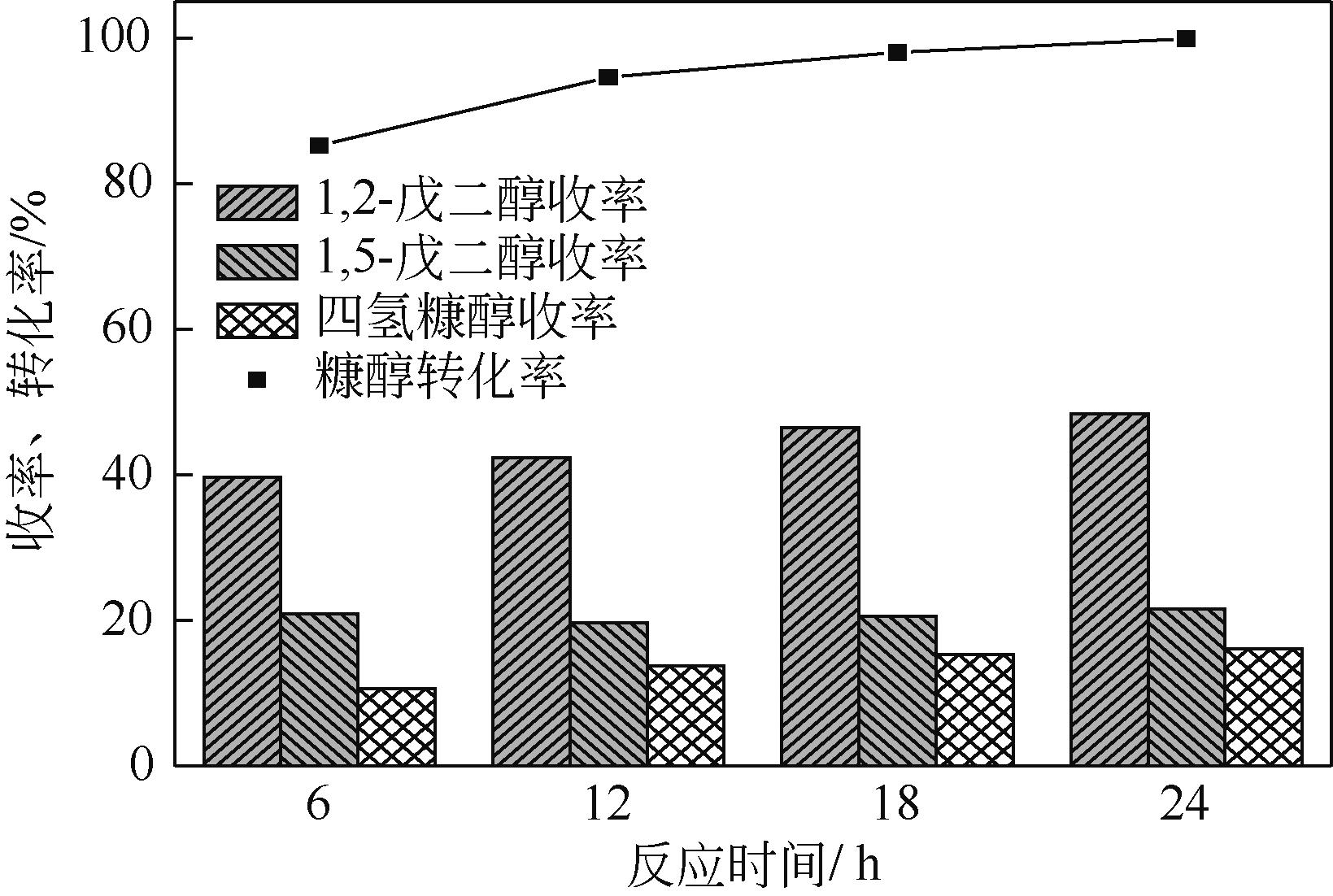
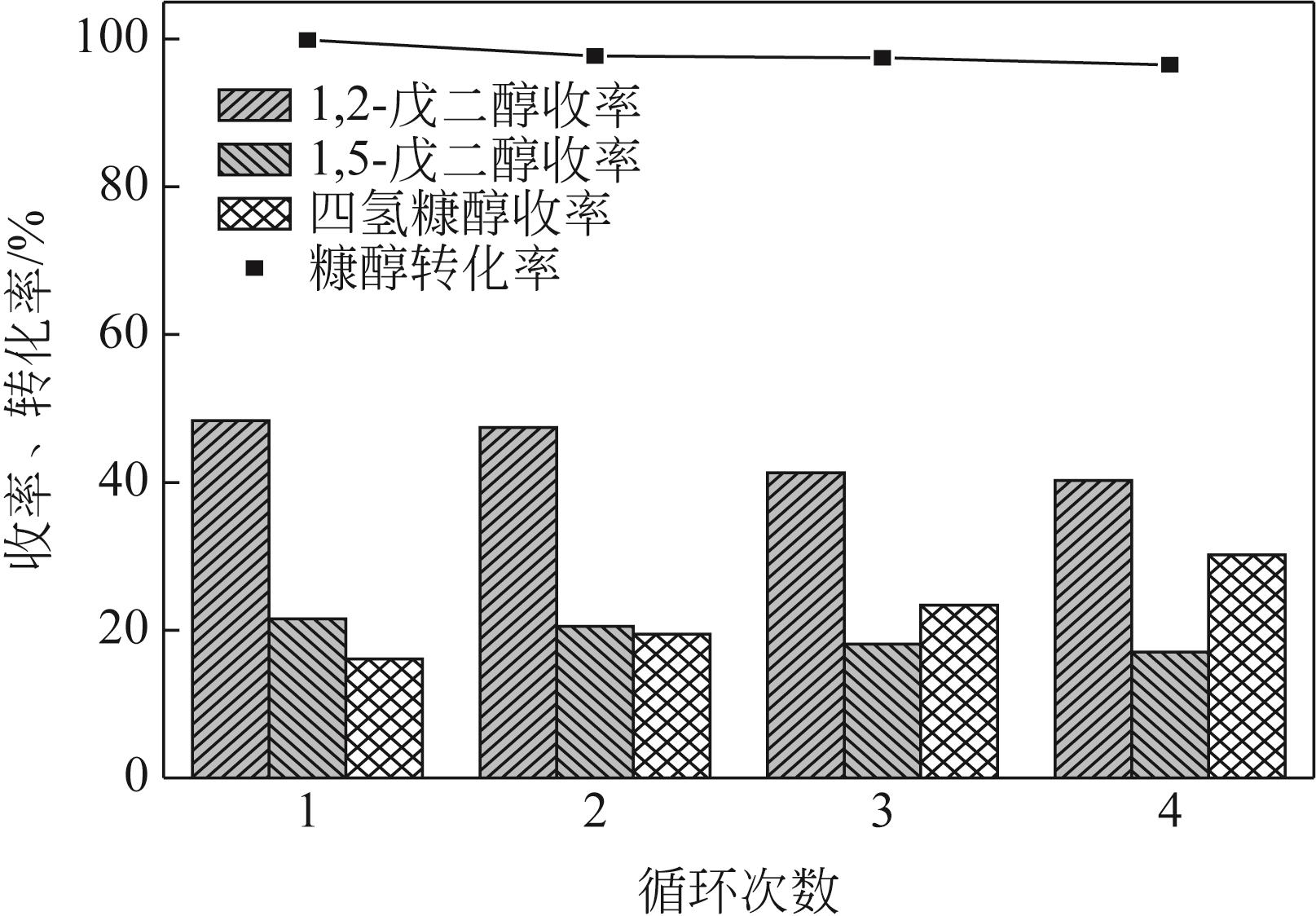
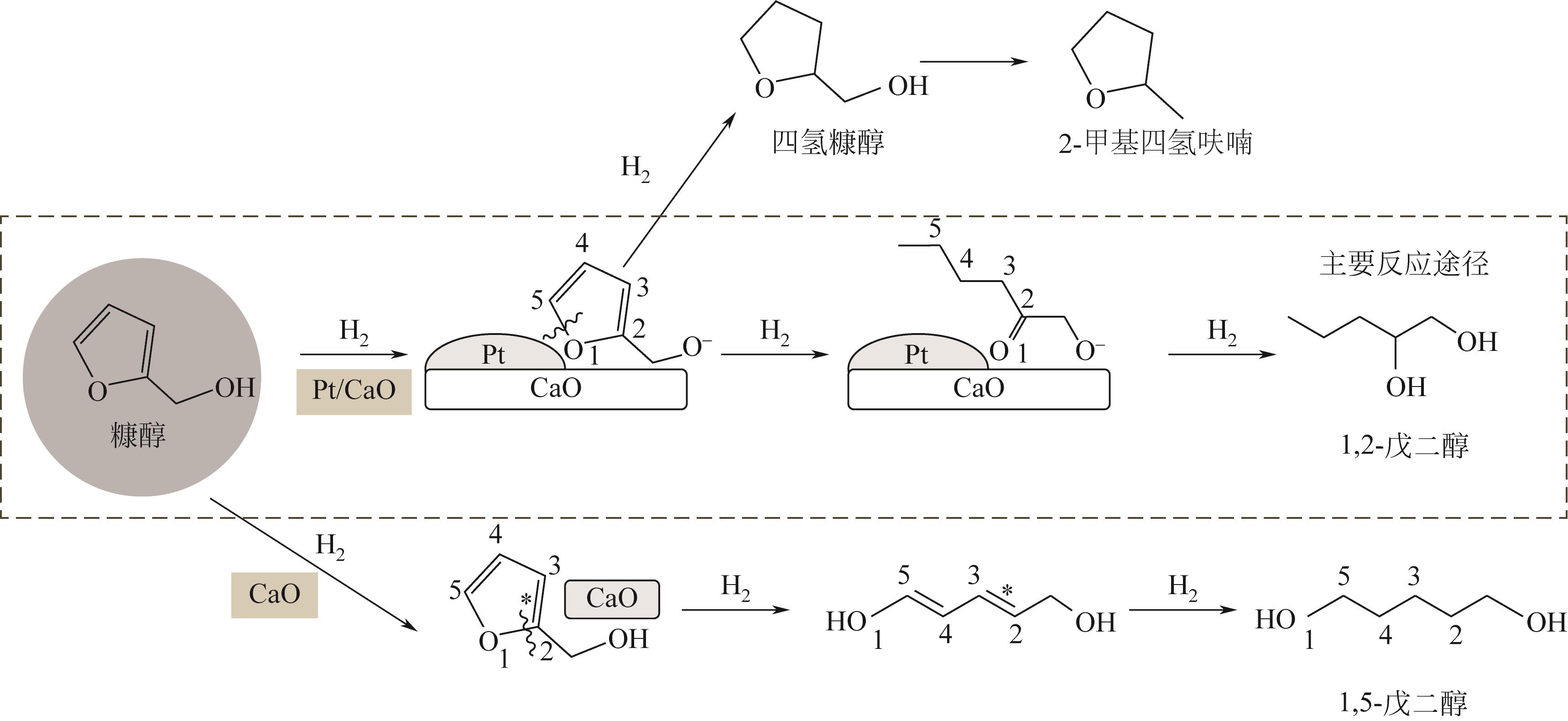
通过浸渍法制备出一系列氧化钙负载铂金属催化材料,用于生物质基糠醇选择性加氢制备戊二醇研究。研究采用X射线衍射仪、扫描电子显微镜、CO2-程序升温脱附、N2等温吸附-脱附、X射线光电子能谱、热重-差热分析法等一系列表征手段,对合成的催化剂样品物理化学性质进行了系统表征。实验结果表明,Pt/CaO-600催化剂表现出优异的催化活性,在4MPa氢压、210℃反应温度条件下,糠醇转化率为99.8%,1,2-戊二醇和1,5-戊二醇收率分别可达48.6%和21.5%。催化剂的优异性能可归因于作为载体的CaO提供了适宜的碱性位点,有利于糠醇开环反应的发生,进而提高了糠醇转化率与戊二醇选择性。同时,催化剂还表现出了优异的循环使用性能,经重复使用4次后,其催化糠醇转化率可达96.5%,1,2-戊二醇和1,5-戊二醇的收率分别为40.3%和17.0%。
中图分类号:
引用本文
萧垚鑫, 张军, 单锐, 袁浩然, 陈勇. Pt/CaO材料催化糠醇加氢制备戊二醇[J]. 化工进展, 2024, 43(3): 1318-1327.
XIAO Yaoxin, ZHANG Jun, SHAN Rui, YUAN Haoran, CHEN Yong. Catalytic hydrogenation of furfuryl alcohol into pentanediol over Pt/CaO materials[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1318-1327.
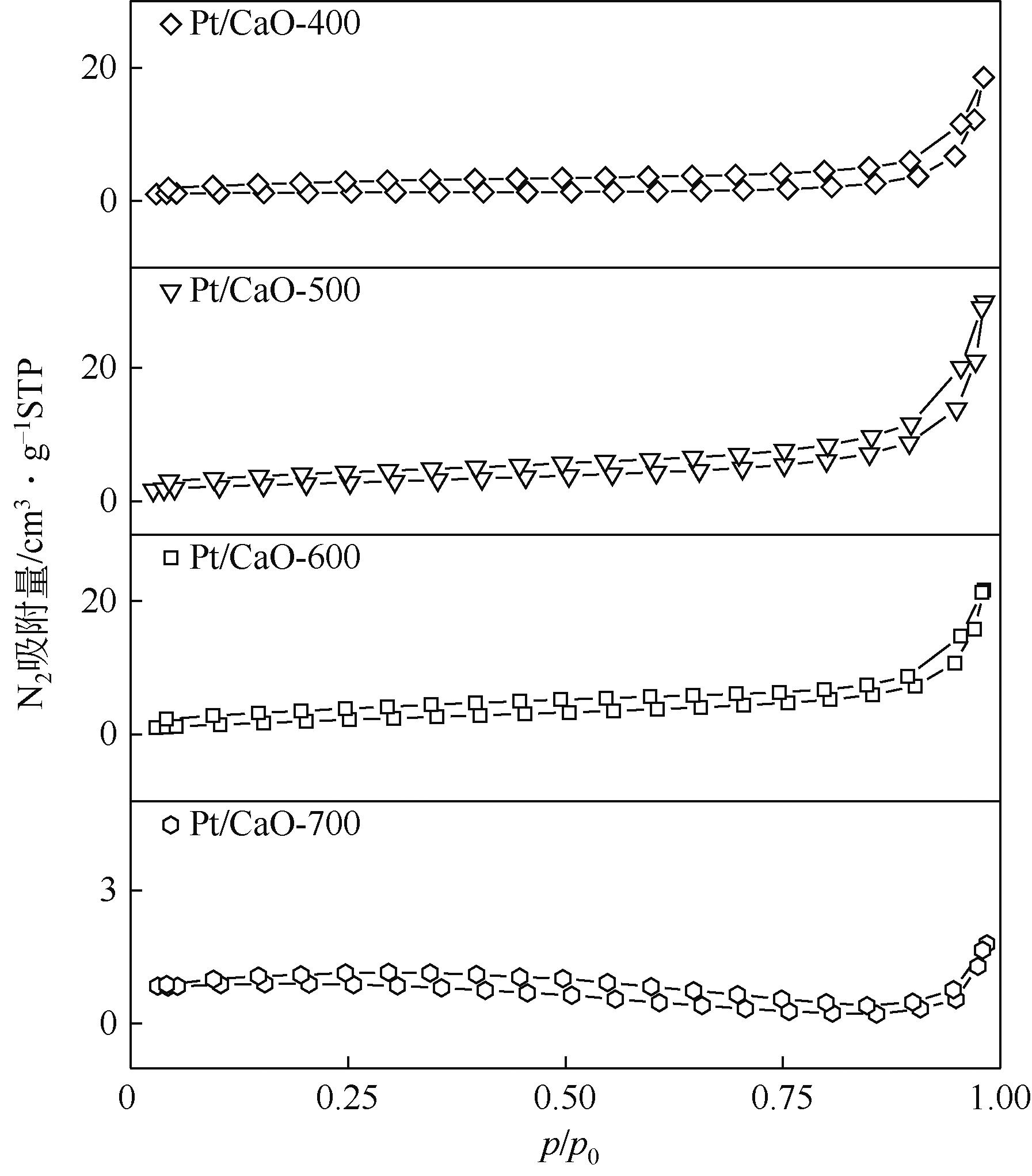
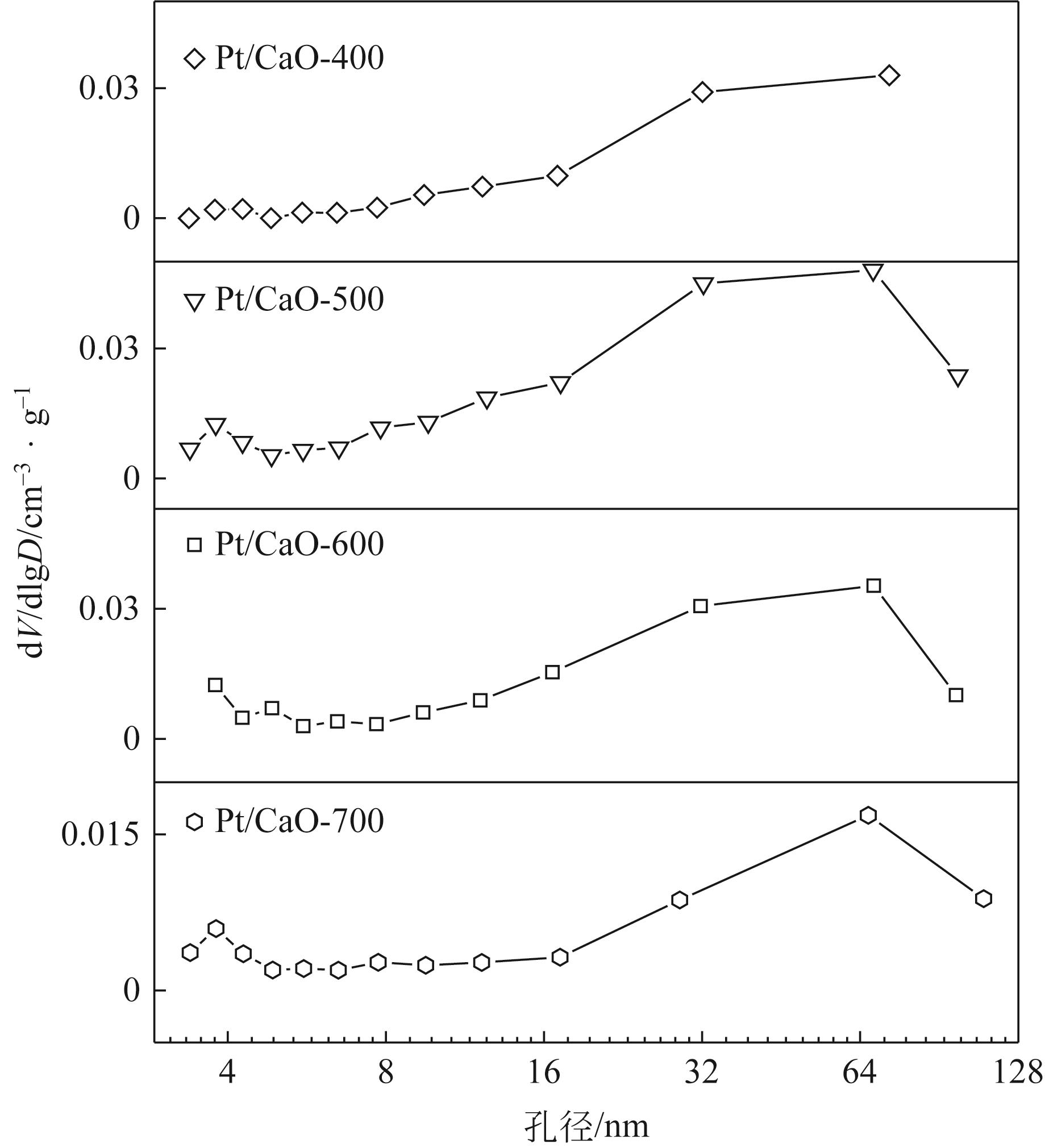
| 样品 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 碱度/μmol·g-1 |
|---|---|---|---|---|
| Pt/CaO-400 | 48.47 | 23.70 | 0.29 | 37.59 |
| Pt/CaO-500 | 93.62 | 19.75 | 0.46 | 50.14 |
| Pt/CaO-600 | 76.72 | 17.46 | 0.33 | 55.45 |
| Pt/CaO-700 | 5.70 | 19.48 | 0.002 | 63.45 |
表1 催化剂结构信息和碱性参数
| 样品 | 比表面积/m2·g-1 | 平均孔径/nm | 孔容/cm3·g-1 | 碱度/μmol·g-1 |
|---|---|---|---|---|
| Pt/CaO-400 | 48.47 | 23.70 | 0.29 | 37.59 |
| Pt/CaO-500 | 93.62 | 19.75 | 0.46 | 50.14 |
| Pt/CaO-600 | 76.72 | 17.46 | 0.33 | 55.45 |
| Pt/CaO-700 | 5.70 | 19.48 | 0.002 | 63.45 |
| 1 | SHARMA H K, XU C B, QIN W S. Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: An overview[J]. Waste and Biomass Valorization, 2019, 10(2): 235-251. |
| 2 | UBANDO A T, FELIX C B, CHEN W H. Biorefineries in circular bioeconomy: A comprehensive review[J]. Bioresource Technology, 2020, 299: 122585. |
| 3 | KUMAR B, BHARDWAJ N, AGRAWAL K, et al. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept[J]. Fuel Process Technology, 2020, 199: 106244. |
| 4 | CHIO C L, SAIN M, QIN W S. Lignin utilization: A review of lignin depolymerization from various aspects[J]. Renewable & Sustainable Energy Reviews, 2019, 107: 232-249. |
| 5 | AN Z D, LI J. Recent advances in the catalytic transfer hydrogenation of furfural to furfuryl alcohol over heterogeneous catalysts[J]. Green Chemistry, 2022, 24(5): 1780-1808. |
| 6 | ZHANG X, XU S Q, LI Q F, et al. Recent advances in the conversion of furfural into bio-chemicals through chemo- and bio-catalysis[J]. RSC Advances, 2021, 11(43): 27042-27058. |
| 7 | 陆强, 朱锡锋, 李全新, 等. 生物质快速热解制备液体燃料[J]. 化学进展, 2007, 19(7/8): 1064-1071. |
| LU Qiang, ZHU Xifeng, LI Quanxin, et al. Biomass fast pyrolysis for liquid fuels[J]. Progress in Chemistry, 2007, 19(7/8): 1064-1071. | |
| 8 | 张军, 李丹妮, 袁浩然, 等. 生物质基糠醛和5-羟甲基糠醛加氢转化研究进展[J]. 燃料化学学报, 2021, 49(12): 1752-1767. |
| ZHANG Jun, LI Danni, YUAN Haoran, et al. Advances on the catalytic hydrogenation of biomass-derived furfural and 5-hydroxymethylfurfural[J]. Journal of Fuel Chemistry and Technology, 2021, 49(12): 1752-1767. | |
| 9 | ZHANG M, YANG J H. Selective hydrogenation of furfural: Pure silica supported metal catalysts[J]. ChemistrySelect, 2022, 7(9): e202200013. |
| 10 | YE L, HAN Y W, WANG X T, et al. Recent progress in furfural production from hemicellulose and its derivatives: Conversion mechanism, catalytic system, solvent selection[J]. Molecular Catalysis, 2021, 515: 111899. |
| 11 | ZHAO L, SUN Z F, ZHANG C C, et al. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives[J]. Bioresource Technology, 2022, 343: 126123. |
| 12 | 杨启悦, 吴巧妹, 邱佳容, 等. 生物基平台化合物催化转化制备糠醇[J]. 化学进展, 2022, 34(8): 1748-1759. |
| YANG Qiyue, WU Qiaomei, QIU Jiarong, et al. Catalytic conversion of bio-based platform compounds to fufuryl alcohol[J]. Progress in Chemistry, 2022, 34(8): 1748-1759. | |
| 13 | 李梦雨, 杨鹏, 常春, 等. 糠醛渣高值化利用的研究进展[J]. 林产化学与工业, 2021, 41(6): 117-126. |
| LI Mengyu, YANG Peng, CHANG Chun, et al. Research progress in high-value utilization of furfural residue[J]. Chemistry and Industry of Forest Products, 2021, 41(6): 117-126. | |
| 14 | BECERRA M L, PRIETO G A, RENDUELES M, et al. Biological transformations of furanic platform molecules to obtain biomass-derived furans: A review[J]. Biomass Conversion and Biorefinery. 2022, 12: 1-19. |
| 15 | 萧垚鑫, 张军, 胡升, 等. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352. |
| XIAO Yaoxin, ZHANG Jun, HU Sheng, et al. Cu-Zn catalyzed hydrogenation of furfural with methanol as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1341-1352. | |
| 16 | 高芳芳, 陈静, 黄志威, 等. 生物质基呋喃衍生物选择氢解制备戊二醇和己二醇研究进展[J]. 分子催化, 2018, 32(3): 276-293. |
| GAO Fangfang, CHEN Jing, HUANG Zhiwei, et al. Recent advances in the selective hydrogenolysis of biomass-based furan derivatives to pentanediols and hexanediol[J]. Journal of Molecular Catalysis(China), 2018, 32(3): 276-293. | |
| 17 | MIZUGAKI T, YAMAKAWA T, NAGATSU Y, et al. Direct transformation of furfural to 1,2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(10): 2243-2247. |
| 18 | LIU H L, HUANG Z W, ZHAO F, et al. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2-and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst[J]. Catalysis Science & Technology, 2016, 6(3): 668-671. |
| 19 | KOSO S, FURIKADO I, SHIMAO A, et al. Chemoselective hydrogenolysis of tetrahydrofurfuryl alcohol to 1,5-pentanediol[J]. Chemical Communications, 2009, (15): 2035-2037. |
| 20 | 樊冬娜, 刘晓然, 王喜成, 等. 生物基糠醛催化转化制备戊二醇的研究进展[J]. 化工进展, 2018, 37(3): 938-946. |
| FAN Dongna, LIU Xiaoran, WANG Xucheng, et al. Catalytic conversion of biomass-derived furfural into pentanediols[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 938-946. | |
| 21 | 徐保明, 唐强, 罗岩, 等. 1,2-戊二醇的新合成方法及工艺优化研究[J]. 高校化学工程学报, 2014, 28(1): 92-97. |
| XU Baoming, TANG Qiang, LUO Yan, et al. Study on the new synthetic method of 1,2-pentanediol and process optimization thereof[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(1): 92-97. | |
| 22 | MA R F, WU X P, TONG T, et al. The critical role of water in the ring opening of furfural alcohol to 1,2-pentanediol[J]. ACS Catalysis, 2017, 7(1): 333-337. |
| 23 | TAYLOR M J, DURNDELL L J, ISAACS M A, et al. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions[J]. Applied Catalysis B-Environmental, 2016, 180: 580-585. |
| 24 | 崔鸿劼. 碱金属盐修饰中高温CO2固体吸附材料的构效关系和吸附机理研究[D]. 上海: 华东理工大学, 2021. |
| CUI Hongjie. Structure-perfomance relationship and sorption mechanisms of alkali metal salt-promoted solid sorbents for intermediate- and high-temperature CO2 capture[D]. Shanghai: East China University of Science and Technology, 2021. | |
| 25 | HARISH, KUMARI S, PARIHAR J, et al. Synthesis, characterization, and antibacterial activity of calcium hydroxide nanoparticles against gram-positive and gram-negative bacteria[J]. ChemistrySelect, 2022, 7(37): e202203094. |
| 26 | GRANADOS M L, POVES M D Z, ALONSO D M, et al. Biodiesel from sunflower oil by using activated calcium oxide[J]. Applied Catalysis B-Environmental, 2007, 73(3): 317-326. |
| 27 | LEE H, KIM W I, JUNG K D, et al. Effect of Cu promoter and alumina phases on Pt/Al2O3 for propane dehydrogenation[J]. Korean Journal of Chemical Engineering, 2017, 34(5): 1337-1345. |
| 28 | MEI Y, XU J, ZHANG Y, et al. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution[J]. Bioresource Technology, 2021, 325: 124732. |
| 29 | CAO Y L, ZHANG H P, LIU K K, et al. Biowaste-derived bimetallic Ru-MoO x catalyst for the direct hydrogenation of furfural to tetrahydrofurfuryl alcohol[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(15): 12858-12866. |
| 30 | 冯占雄, 汪云, 马强, 等. 连续管道微波技术制备Pt/C催化剂及其氧还原性能[J].化工进展, 2022, 41(12): 6377-6384. |
| FENG Zhanxiong, WANG Yun, MA Qiang, et al. Preparation of Pt/C catalyst by continuous pipeline microwave technology and its oxygen reduction performance[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6377-6384. | |
| 31 | 张敏, 冯彩霞, 金振声, 等. 空气气氛中Pt和TiO2间强相互作用的STS和XPS研究[J]. 催化学报, 2005(6): 508-512. |
| ZHANG Min, FENG Caixia, JIN Zhensheng, et al. STS and XPS study of the strong interaction between pt and TiO2 under air atmosphere[J]. Chinese Journal Of Catalysis, 2005(6): 508-512. | |
| 32 | TANG Y, GU X, MENG M, et al. Direct Henry reactions with modified calcium oxide as solid catalyst[J]. Research on Chemical Intermediates, 2013, 39(8): 3715-3725. |
| 33 | NAKAGAWA Y, TAMURA M, TOMISHIGE K. Catalytic reduction of biomass-derived furanic compounds with hydrogen[J]. ACS Catalysis, 2013, 3(12): 2655-2668. |
| 34 | NAKAGAWA Y, TAKADA K, TAMURA M, et al. Total hydrogenation of furfural and 5-hydroxymethylfurfural over supported Pd-Ir alloy catalyst[J]. ACS Catalysis, 2014, 4(8): 2718-2726. |
| 35 | XU W J, WANG H F, LIU X H, et al. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst[J]. Chemical Communications, 2011, 47(13): 3924-3926. |
| [1] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [2] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [3] | 谷星朋, 马红钦, 刘嘉豪. 雷尼镍的磷量子点改性及其催化加氢脱硫性能[J]. 化工进展, 2024, 43(3): 1293-1301. |
| [4] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [5] | 陈风, 王宣德, 黄伟, 王晓东, 王琰. HZSM-22的粒径调控及Pt/HZSM-22的正十二烷加氢异构催化性能[J]. 化工进展, 2024, 43(3): 1309-1317. |
| [6] | 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 氧化铝基加氢脱硫催化剂研究进展[J]. 化工进展, 2024, 43(2): 948-961. |
| [7] | 丁康, 何军桥, 陈元捷, 杨霞珍, 刘化章, 霍超. Ru/Ba-MgO氨合成催化剂模板棉纤维的盐酸处理对催化性能的影响[J]. 化工进展, 2024, 43(2): 962-970. |
| [8] | 王达锐, 孙洪敏, 王一棪, 唐智谋, 李芮, 范雪研, 杨为民. 分子筛催化反应过程高效化的技术进展[J]. 化工进展, 2024, 43(1): 1-18. |
| [9] | 苏梦军, 刘剑, 辛靖, 陈禹霏, 张海洪, 韩龙年, 朱元宝, 李洪宝. 气液混合强化在固定床加氢过程中的应用进展[J]. 化工进展, 2024, 43(1): 100-110. |
| [10] | 罗芬, 杨晓琪, 段方麟, 李小江, 吴亮, 徐铜文. 双极膜研究进展及应用展望[J]. 化工进展, 2024, 43(1): 145-163. |
| [11] | 盖宏伟, 张辰君, 屈晶莹, 孙怀禄, 脱永笑, 王斌, 金旭, 张茜, 冯翔, CHEN De. 有机液体储氢技术催化脱氢过程强化研究进展[J]. 化工进展, 2024, 43(1): 164-185. |
| [12] | 张家昊, 李盈盈, 徐彦琳, 尹佳滨, 张吉松. 微反应器中连续还原胺化反应的研究进展[J]. 化工进展, 2024, 43(1): 186-197. |
| [13] | 王立华, 蔡苏杭, 江文涛, 罗倩, 罗勇, 陈建峰. 微纳尺度气液传质强化油品催化加氢反应[J]. 化工进展, 2024, 43(1): 19-33. |
| [14] | 王玉杰, 张艳梅, 栾金义, 赵之平. 酶催化固碳过程及其强化技术研究进展[J]. 化工进展, 2024, 43(1): 232-245. |
| [15] | 衡霖宇, 邓卓然, 程道建, 魏彬, 赵利强. 高通量合成装置强化金属催化剂制备过程的研究进展[J]. 化工进展, 2024, 43(1): 246-259. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||