化工进展 ›› 2024, Vol. 43 ›› Issue (4): 1810-1822.DOI: 10.16085/j.issn.1000-6613.2023-0603
• 工业催化 • 上一篇
粉体光催化全水分解技术研究进展
吴晨赫1( ), 刘彧旻1(
), 刘彧旻1( ), 杨昕旻1, 崔记伟1, 姜韶堃2(
), 杨昕旻1, 崔记伟1, 姜韶堃2( ), 叶金花1, 刘乐全1(
), 叶金花1, 刘乐全1( )
)
- 1.天津大学材料科学与工程学院,天津 300072
2.邯郸净化设备研究所,河北 邯郸 056000
-
收稿日期:2023-04-14修回日期:2023-05-28出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:姜韶堃,刘乐全 -
作者简介:吴晨赫(1998—),女,硕士研究生,研究方向为光催化。E-mail:wuchenhe2021@tju.edu.cn
刘彧旻(2004—),男,本科生,研究方向为光催化水分解。E-mail:liuyumin919@tju.edu.cn。 -
基金资助:国家重点研发计划(2021YFA1500800);国家自然科学基金(22072106)
Particulate photocatalysts for light-driven overall water splitting
WU Chenhe1( ), LIU Yumin1(
), LIU Yumin1( ), YANG Xinmin1, CUI Jiwei1, JIANG Shaokun2(
), YANG Xinmin1, CUI Jiwei1, JIANG Shaokun2( ), YE Jinhua1, LIU Lequan1(
), YE Jinhua1, LIU Lequan1( )
)
- 1.School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China
2.Purification Equipment Research Institute of Handan, Handan 056000, Hebei, China
-
Received:2023-04-14Revised:2023-05-28Online:2024-04-15Published:2024-05-13 -
Contact:JIANG Shaokun, LIU Lequan
摘要:
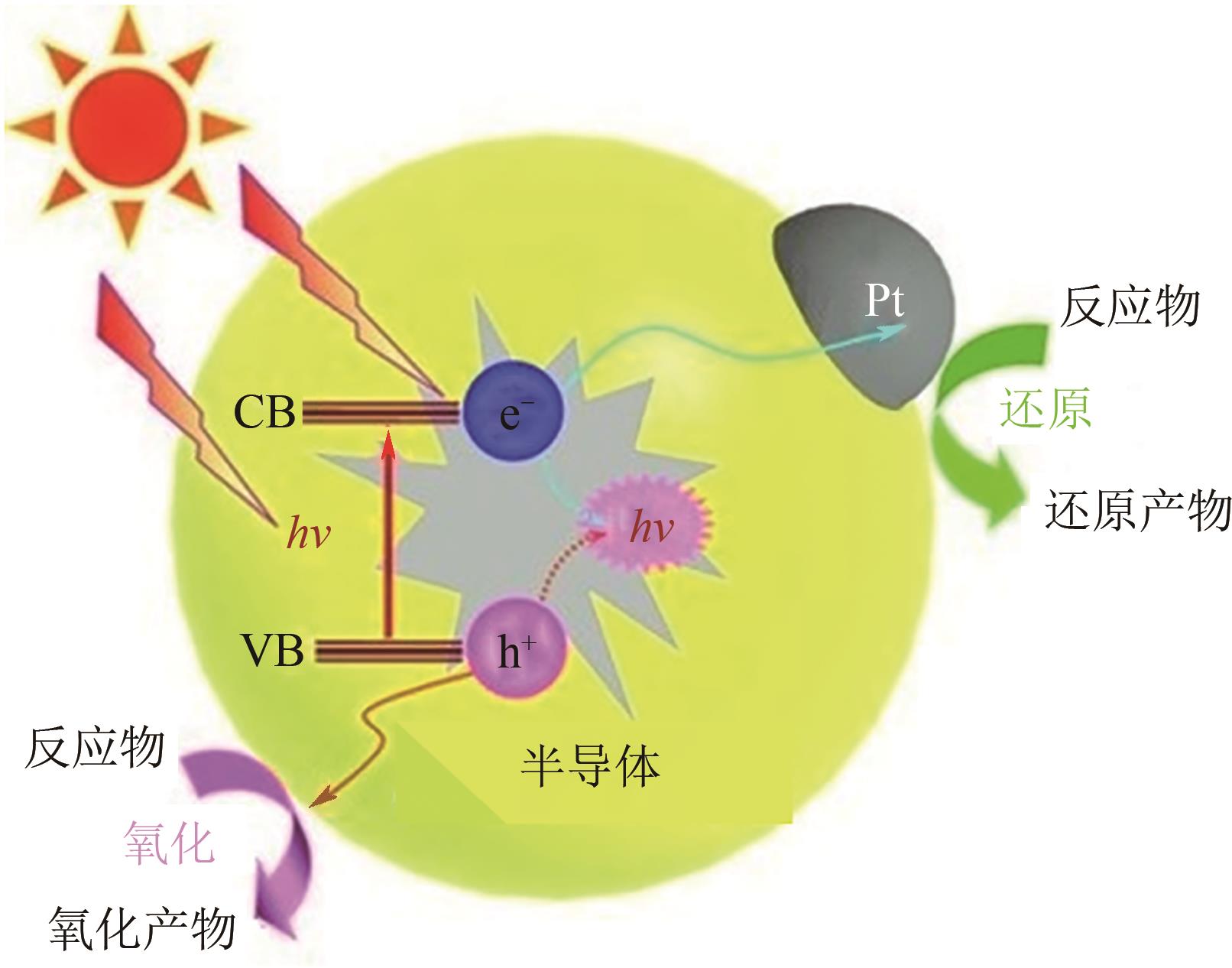
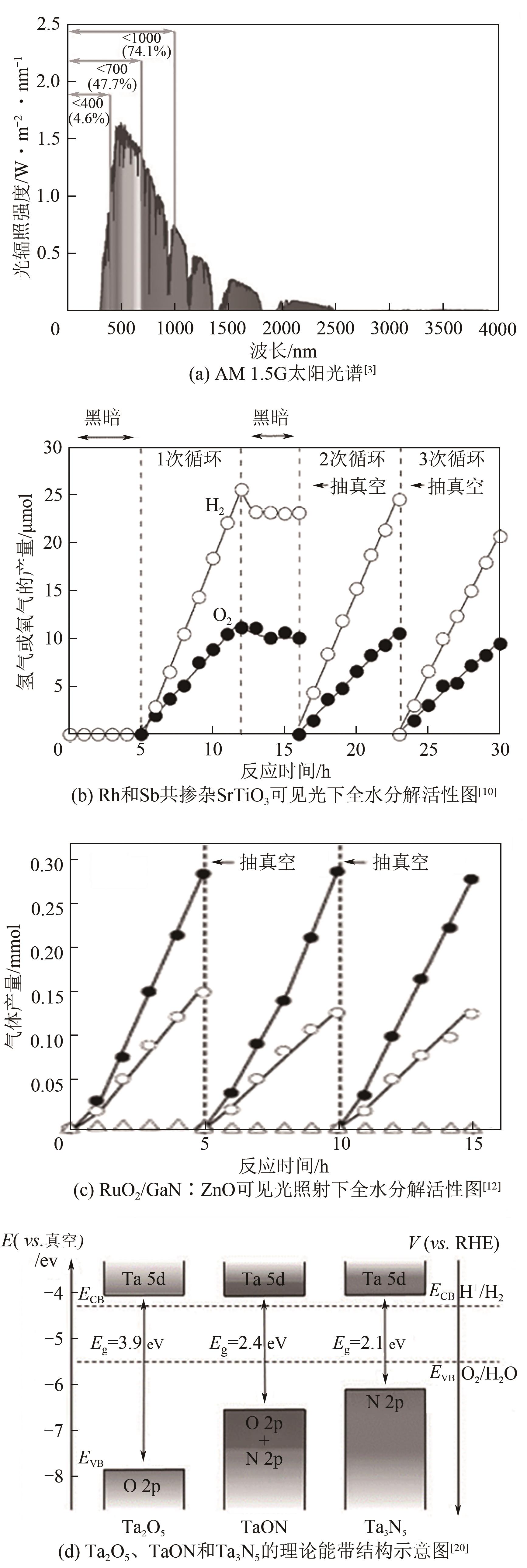
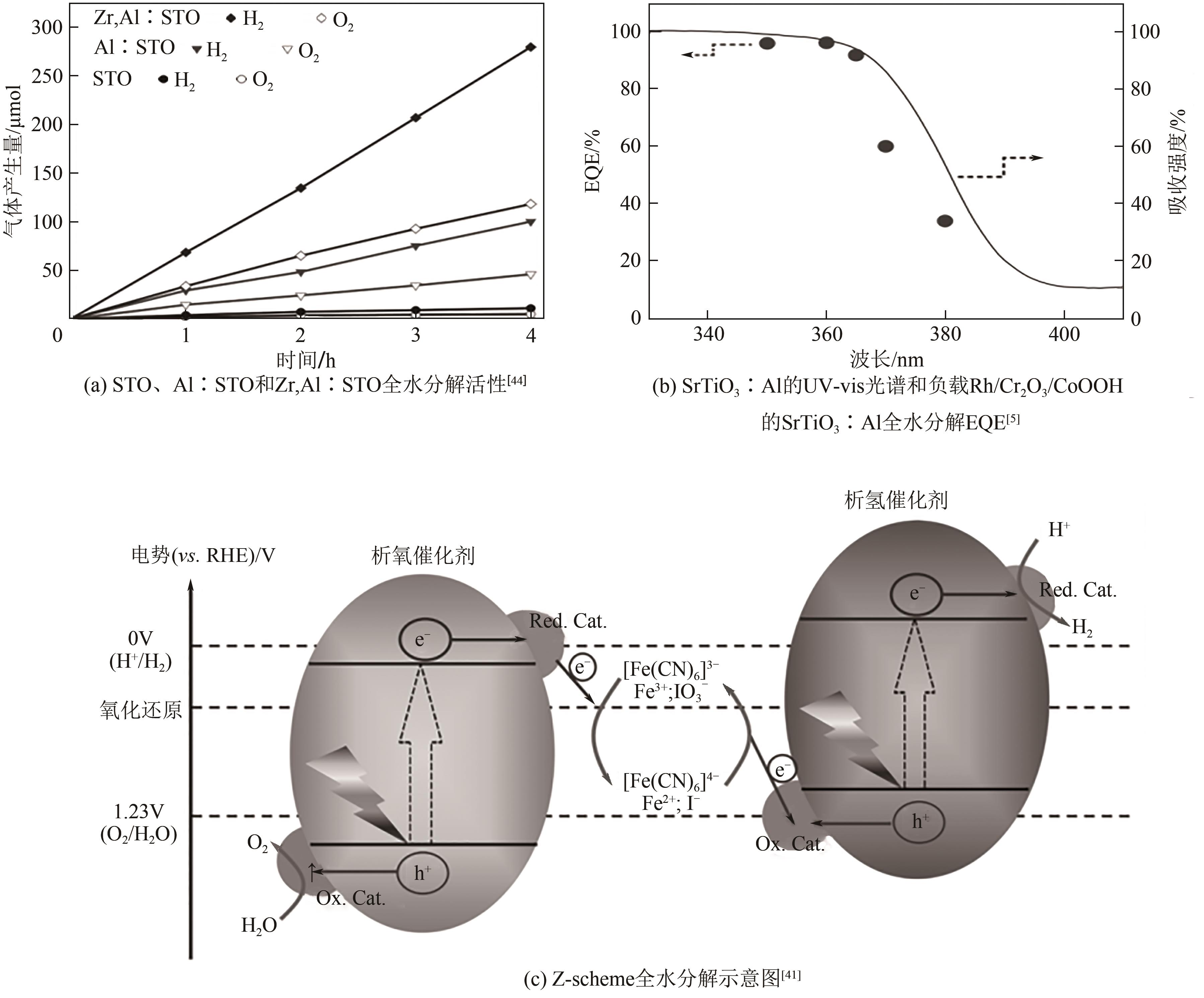
光催化全水分解制氢可以直接将太阳能转变为绿色氢能,该技术具有过程简单、成本低等优势,受到广泛关注的同时展现出了良好的应用前景。半导体光催化剂的性能是光催化全水分解技术发展的核心因素,目前该领域主要围绕光催化反应的三个基本步骤对其性能进行提升:光吸收、载流子分离与迁移以及表面反应。本文从光催化基本原理出发,围绕以上三方面概述了应对相应挑战的有效策略与近年来的研究进展,在此基础上总结了设计、制备高效光催化全水分解材料的重要方法,分析了当前影响该水分解制氢技术工业化应用的难点,指出该领域的核心问题是开发高效的窄带隙光催化材料,同时未来需着重解决逆反应严重、催化剂稳定性不足以及大规模实施过程中的氢氧混合气体分离等技术问题。
中图分类号:
引用本文
吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822.
WU Chenhe, LIU Yumin, YANG Xinmin, CUI Jiwei, JIANG Shaokun, YE Jinhua, LIU Lequan. Particulate photocatalysts for light-driven overall water splitting[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1810-1822.
| 催化剂 | 吸光范围/nm | 反应溶液 | 效率 | 参考文献 |
|---|---|---|---|---|
| NiO-NaTaO3∶La | <300 | 纯水 | AQY:56%@270nm | [ |
| Rh0.5Cr1.5O3-Ga2O3∶Zn | <280 | 纯水 | AQY:71%@254nm | [ |
| Rh0.5Cr1.5O3-AgTaO3 | <364 | 纯水 | AQY:40%@340nm | [ |
| Pt@C/CoOOH-SrTiO3 | <385 | 纯水 | AQY:44%@360nm | [ |
| Rh/Cr2O3/CoOOH-SrTiO3∶Al | <385 | 纯水 | AQY:95.9%@360nm,STH:0.65% | [ |
| PtO x /CoO x -(g-C3N4) | <440 | 纯水 | AQY:0.3%@405nm | [ |
| Pt/Co(OH)2-(Cl-g-C3N4) | <440 | 纯水 | AQY:6.9%@420nm | [ |
| RhCrO3-(Ga1-x Zn x )(N1-x O x ) | <470 | 硫酸溶液(pH=4.5) | AQY:5.9%@420~440nm | [ |
| IrO2-SrTiO3∶Rh,Sb | <500 | 硫酸溶液(pH=3) | AQY:0.1%@420nm | [ |
| NiO x -InTaO4∶Ni | <540 | 纯水 | AQY:0.66%@402nm | [ |
| RhCrO x -LaMg1/3Ta2/3O2N | <590 | 纯水 | AQY:0.03%@(440±30)nm | [ |
| Rh/Cr2O3-Ta3N5/KTaO3 | <600 | 纯水 | AQY:0.22%@(420±25)nm,STH:0.014% | [ |
| Ru/Cr2O3/IrO2-TaON∶Zr | <600 | NaOH溶液(pH=8) | AQY:0.66%@420nm,STH:0.009% | [ |
| Ru/CrO x /IrO2-SrTaO2N | <600 | NaOH溶液(pH=8) | AQY:0.34%@(429±30)nm,STH:0.0063% | [ |
| Rh/Cr2O3/Co3O4-InGaN/GaN | <630 | 纯水 | STH:9.2%(343K),0.5%(303K) | [ |
| RhCrO3/Ir, FeCoO x - (ZrO2/TaON)/BiVO4 | ZrO2/TaON<530 BiVO4<530 | Na3PO4溶液(pH=6.8) 离子对[Fe(CN)6]3-/4- | AQY:12.3%@(420±10)nm, STH:0.6% | [ |
| Ru/Cr2O3-SrTiO3∶La, Rh/Au/BiVO4∶Mo | SrTiO3∶La,Rh<520 BiVO4∶Mo<520 | 纯水 | AQY:30%@419nm,STH:1.1% | [ |
| Ru/RuO x -SrTiO3∶La, Rh/C/BiVO4∶Mo | SrTiO3∶La,Rh<520 BiVO4∶Mo<520 | 纯水 | STH:1.2%(331K,10kPa), 1%(331K,91kPa) | [ |
| Pt/Co(OH)2-BDCNN350/ BDCNN425 | BDCNN350<470 BDCNN425<580 | 纯水 | AQY:23.52%@420nm, STH:1.16% | [ |
表1 光催化全水分解粉体光催化剂
| 催化剂 | 吸光范围/nm | 反应溶液 | 效率 | 参考文献 |
|---|---|---|---|---|
| NiO-NaTaO3∶La | <300 | 纯水 | AQY:56%@270nm | [ |
| Rh0.5Cr1.5O3-Ga2O3∶Zn | <280 | 纯水 | AQY:71%@254nm | [ |
| Rh0.5Cr1.5O3-AgTaO3 | <364 | 纯水 | AQY:40%@340nm | [ |
| Pt@C/CoOOH-SrTiO3 | <385 | 纯水 | AQY:44%@360nm | [ |
| Rh/Cr2O3/CoOOH-SrTiO3∶Al | <385 | 纯水 | AQY:95.9%@360nm,STH:0.65% | [ |
| PtO x /CoO x -(g-C3N4) | <440 | 纯水 | AQY:0.3%@405nm | [ |
| Pt/Co(OH)2-(Cl-g-C3N4) | <440 | 纯水 | AQY:6.9%@420nm | [ |
| RhCrO3-(Ga1-x Zn x )(N1-x O x ) | <470 | 硫酸溶液(pH=4.5) | AQY:5.9%@420~440nm | [ |
| IrO2-SrTiO3∶Rh,Sb | <500 | 硫酸溶液(pH=3) | AQY:0.1%@420nm | [ |
| NiO x -InTaO4∶Ni | <540 | 纯水 | AQY:0.66%@402nm | [ |
| RhCrO x -LaMg1/3Ta2/3O2N | <590 | 纯水 | AQY:0.03%@(440±30)nm | [ |
| Rh/Cr2O3-Ta3N5/KTaO3 | <600 | 纯水 | AQY:0.22%@(420±25)nm,STH:0.014% | [ |
| Ru/Cr2O3/IrO2-TaON∶Zr | <600 | NaOH溶液(pH=8) | AQY:0.66%@420nm,STH:0.009% | [ |
| Ru/CrO x /IrO2-SrTaO2N | <600 | NaOH溶液(pH=8) | AQY:0.34%@(429±30)nm,STH:0.0063% | [ |
| Rh/Cr2O3/Co3O4-InGaN/GaN | <630 | 纯水 | STH:9.2%(343K),0.5%(303K) | [ |
| RhCrO3/Ir, FeCoO x - (ZrO2/TaON)/BiVO4 | ZrO2/TaON<530 BiVO4<530 | Na3PO4溶液(pH=6.8) 离子对[Fe(CN)6]3-/4- | AQY:12.3%@(420±10)nm, STH:0.6% | [ |
| Ru/Cr2O3-SrTiO3∶La, Rh/Au/BiVO4∶Mo | SrTiO3∶La,Rh<520 BiVO4∶Mo<520 | 纯水 | AQY:30%@419nm,STH:1.1% | [ |
| Ru/RuO x -SrTiO3∶La, Rh/C/BiVO4∶Mo | SrTiO3∶La,Rh<520 BiVO4∶Mo<520 | 纯水 | STH:1.2%(331K,10kPa), 1%(331K,91kPa) | [ |
| Pt/Co(OH)2-BDCNN350/ BDCNN425 | BDCNN350<470 BDCNN425<580 | 纯水 | AQY:23.52%@420nm, STH:1.16% | [ |
| 1 | TONG Hua, OUYANG Shuxin, BI Yingpu, et al. Nano-photocatalytic materials: Possibilities and challenges[J]. Advanced Materials, 2012, 24(2): 229-251. |
| 2 | KEENE Sam, BALA CHANDRAN Rohini, ARDO Shane. Calculations of theoretical efficiencies for electrochemically-mediated tandem solar water splitting as a function of bandgap energies and redox shuttle potential[J]. Energy & Environmental Science, 2019, 12(1): 261-272. |
| 3 | WANG Qian, DOMEN Kazunari. Particulate photocatalysts for light-driven water splitting: Mechanisms, challenges, and design strategies[J]. Chemical Reviews, 2020, 120(2): 919-985. |
| 4 | SHANER Matthew R, ATWATER Harry A, LEWIS Nathan S, et al. A comparative technoeconomic analysis of renewable hydrogen production using solar energy[J]. Energy & Environmental Science, 2016, 9(7): 2354-2371. |
| 5 | TAKATA Tsuyoshi, JIANG Junzhe, SAKATA Yoshihisa, et al. Photocatalytic water splitting with a quantum efficiency of almost unity[J]. Nature, 2020, 581(7809): 411-414. |
| 6 | BORGARELLO Enrico, KIWI John, GRAETZEL Michael, et al. Visible light induced water cleavage in colloidal solutions of chromium-doped titanium dioxide particles[J]. Journal of the American Chemical Society, 1982, 104(11): 2996-3002. |
| 7 | Alam KHAN M, Seong Ihl WOO, YANG O Bong. Hydrothermally stabilized Fe(Ⅲ) doped titania active under visible light for water splitting reaction[J]. International Journal of Hydrogen Energy, 2008, 33(20): 5345-5351. |
| 8 | KONTA Ryoko, ISHII Tatsuya, KATO Hideki, et al. Photocatalytic activities of noble metal ion doped SrTiO3 under visible light irradiation[J]. The Journal of Physical Chemistry B, 2004, 108(26): 8992-8995. |
| 9 | MOSS Benjamin, WANG Qian, BUTLER Keith T, et al. Linking in situ charge accumulation to electronic structure in doped SrTiO3 reveals design principles for hydrogen-evolving photocatalysts[J]. Nature Materials, 2021, 20(4): 511-517. |
| 10 | ASAI Rikako, NEMOTO Hiroaki, JIA Qingxin, et al. A visible light responsive rhodium and antimony-codoped SrTiO3 powdered photocatalyst loaded with an IrO2 cocatalyst for solar water splitting[J]. Chemical Communications, 2014, 50(19): 2543-2546. |
| 11 | ZOU Zhigang, YE Jinhua, SAYAMA Kazuhiro, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst[J]. Nature, 2001, 414(6864): 625-627. |
| 12 | MAEDA Kazuhiko, TAKATA Tsuyoshi, HARA Michikazu, et al. GaN∶ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting[J]. Journal of the American Chemical Society, 2005, 127(23): 8286-8287. |
| 13 | OHNO Tomoyuki, BAI Lu, HISATOMI Takashi, et al. Photocatalytic water splitting using modified GaN∶ZnO solid solution under visible light: Long-time operation and regeneration of activity[J]. Journal of the American Chemical Society, 2012, 134(19): 8254-8259. |
| 14 | MAEDA Kazuhiko, TERAMURA Kentaro, DOMEN Kazunari. Effect of post-calcination on photocatalytic activity of (Ga1- x Zn x )(N1- x O x ) solid solution for overall water splitting under visible light[J]. Journal of Catalysis, 2008, 254(2): 198-204. |
| 15 | LEE Yungi, TERAMURA Kentaro, HARA Michikazu, et al. Modification of (Zn1+ x Ge)(N2O x ) solid solution as a visible light driven photocatalyst for overall water splitting [J]. Chemistry of Materials, 2007, 19(8): 2120-2127. |
| 16 | PAN Chengsi, TAKATA Tsuyoshi, NAKABAYASHI Mamiko, et al. A complex perovskite-type oxynitride: The first photocatalyst for water splitting operable at up to 600nm[J]. Angewandte Chemie International Edition, 2015, 54(10): 2955-2959. |
| 17 | SEO Jeongsuk, ISHIZUKA Daisaku, HISATOMI Takashi, et al. Effect of Mg2+ substitution on the photocatalytic water splitting activity of LaMg x Nb1- x O1+3 x N2-3 x [J]. Journal of Materials Chemistry A, 2021, 9(13): 8655-8662. |
| 18 | WANG Zheng, LI Can, DOMEN Kazunari. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting[J]. Chemical Society Reviews, 2019, 48(7): 2109-2125. |
| 19 | WANG Qian, NAKABAYASHI Mamiko, HISATOMI Takashi, et al. Oxysulfide photocatalyst for visible-light-driven overall water splitting[J]. Nature Materials, 2019, 18(8): 827-832. |
| 20 | DE RESPINIS Moreno, FRAVVENTURA Maria, ABDI Fatwa F, et al. Oxynitrogenography: Controlled synthesis of single-phase tantalum oxynitride photoabsorbers[J]. Chemistry of Materials, 2015, 27(20): 7091-7099. |
| 21 | WANG Zheng, INOUE Yasunobu, HISATOMI Takashi, et al. Overall water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles[J]. Nature Catalysis, 2018, 1(10): 756-763. |
| 22 | XIAO Jiadong, NISHIMAE Shinji, VEQUIZO Junie Jhon M, et al. Enhanced overall water splitting by a zirconium-doped TaON-based photocatalyst[J]. Angewandte Chemie International Edition, 2022, 61(17): e202116573. |
| 23 | CHEN Kaihong, XIAO Jiadong, VEQUIZO Junie Jhon M, et al. Overall water splitting by a SrTaO2N-based photocatalyst decorated with an Ir-promoted Ru-based cocatalyst[J]. Journal of the American Chemical Society, 2023, 145(7): 3839-3843. |
| 24 | 王文霞, 刘小丰, 陈浠, 等. 多孔g-C3N4基光催化材料的制备及应用研究进展[J]. 化工进展, 2022, 41(1): 300-309. |
| WANG Wenxia, LIU Xiaofeng, CHEN Xi, et al. Research advances of synthesis and applications of porous g-C3N4-based photocatalyst[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 300-309. | |
| 25 | CHENG Cheng, MAO Liuhao, KANG Xing, et al. A high-cyano groups-content amorphous-crystalline carbon nitride isotype heterojunction photocatalyst for high-quantum-yield H2 production and enhanced CO2 reduction[J]. Applied Catalysis B: Environmental, 2023, 331: 122733. |
| 26 | CHENG Cheng, SHI Jinwen, MAO Liuhao, et al. Ultrathin porous graphitic carbon nitride from recrystallized precursor toward significantly enhanced photocatalytic water splitting[J]. Journal of Colloid and Interface Science, 2023, 637: 271-282. |
| 27 | WANG Xinchen, MAEDA Kazuhiko, CHEN Xiufang, et al. Polymer semiconductors for artificial photosynthesis: Hydrogen evolution by mesoporous graphitic carbon nitride with visible light[J]. Journal of the American Chemical Society, 2009, 131(5): 1680-1681. |
| 28 | ZHANG Guigang, LAN Zhi’an, LIN Lihua, et al. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents[J]. Chemical Science, 2016, 7(5): 3062-3066. |
| 29 | XU Beibei, FU Xiaobin, YOU Xiaomeng, et al. Synergistic promotion of single-atom Co surrounding a PtCo alloy based on a g-C3N4 nanosheet for overall water splitting[J]. ACS Catalysis, 2022, 12(12): 6958-6967. |
| 30 | ZHAO Daming, CHEN Jie, DONG Chung-Li, et al. Interlayer interaction in ultrathin nanosheets of graphitic carbon nitride for efficient photocatalytic hydrogen evolution[J]. Journal of Catalysis, 2017, 352: 491-497. |
| 31 | ZHAO Daming, WANG Yiqing, DONG Chungli, et al. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting[J]. Nature Energy, 2021, 6(4): 388-397. |
| 32 | WU Ji, LIU Zhonghuan, LIN Xinyu, et al. Breaking through water-splitting bottlenecks over carbon nitride with fluorination[J]. Nature Communications, 2022, 13(1): 6999. |
| 33 | WANG Defa, PIERRE Adrien, KIBRIA Md Golam, et al. Wafer-level photocatalytic water splitting on GaN nanowire arrays grown by molecular beam epitaxy[J]. Nano Letters, 2011, 11(6): 2353-2357. |
| 34 | ALOTAIBI B, NGUYEN H P T, ZHAO S, et al. Highly stable photoelectrochemical water splitting and hydrogen generation using a double-band InGaN/GaN core/shell nanowire photoanode[J]. Nano Letters, 2013, 13(9): 4356-4361. |
| 35 | ZHOU Peng, NAVID Ishtiaque Ahmed, MA Yongjin, et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting[J]. Nature, 2023, 613(7942): 66-70. |
| 36 | KATO Hideki, ASAKURA Kiyotaka, KUDO Akihiko. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure[J]. Journal of the American Chemical Society, 2003, 125(10): 3082-3089. |
| 37 | SAKATA Yoshihisa, HAYASHI Takuya, YASUNAGA Ryō, et al. Remarkably high apparent quantum yield of the overall photocatalytic H2O splitting achieved by utilizing Zn ion added Ga2O3 prepared using dilute CaCl2 solution[J]. Chemical Communications, 2015, 51(65): 12935-12938. |
| 38 | WATANABE Kenta, IWASE Akihide, KUDO Akihiko. Solar water splitting over Rh0.5Cr1.5O3-loaded AgTaO3 of a valence-band-controlled metal oxide photocatalyst[J]. Chemical Science, 2020, 11(9): 2330-2334. |
| 39 | YANG Xinmin, CUI Jiwei, LIU Xiaolu, et al. Boosting photocatalytic overall water splitting over single-layer graphene coated metal cocatalyst[J]. Applied Catalysis B: Environmental, 2023, 325: 122369. |
| 40 | ZHANG Qiqi, CHEN Xin, YANG Zhongshan, et al. Precisely tailoring nitrogen defects in carbon nitride for efficient photocatalytic overall water splitting[J]. ACS Applied Materials & Interfaces, 2022, 14(3): 3970-3979. |
| 41 | QI Yu, ZHANG Jiangwei, KONG Yuan, et al. Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting[J]. Nature Communications, 2022, 13(1): 484. |
| 42 | WANG Qian, HISATOMI Takashi, JIA Qingxin, et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%[J]. Nature Materials, 2016, 15(6): 611-615. |
| 43 | WANG Qian, HISATOMI Takashi, SUZUKI Yohichi, et al. Particulate photocatalyst sheets based on carbon conductor layer for efficient Z-scheme pure-water splitting at ambient pressure[J]. Journal of the American Chemical Society, 2017, 139(4): 1675-1683. |
| 44 | CUI Jiwei, YANG Xinmin, YANG Zhongshan, et al. Zr-Al co-doped SrTiO3 with suppressed charge recombination for efficient photocatalytic overall water splitting[J]. Chemical Communications, 2021, 57(81): 10640-10643. |
| 45 | Hao LYU, HISATOMI Takashi, GOTO Yosuke, et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000h of constant illumination[J]. Chemical Science, 2019, 10(11): 3196-3201. |
| 46 | TAN Huaqiao, ZHAO Zhao, ZHU Wanbin, et al. Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3 [J]. ACS Applied Materials & Interfaces, 2014, 6(21): 19184-19190. |
| 47 | MU Linchao, ZHAO Yue, LI Ailong, et al. Enhancing charge separation on high symmetry SrTiO3 exposed with anisotropic facets for photocatalytic water splitting[J]. Energy & Environmental Science, 2016, 9(7): 2463-2469. |
| 48 | NISHIYAMA Hiroshi, YAMADA Taro, NAKABAYASHI Mamiko, et al. Photocatalytic solar hydrogen production from water on a 100m2 scale[J]. Nature, 2021, 598: 304-307. |
| 49 | FU Bing, WU Zhijiao, CAO Shuang, et al. Effect of aspect ratios of rutile TiO2 nanorods on overall photocatalytic water splitting performance[J]. Nanoscale, 2020, 12(8): 4895-4902. |
| 50 | LIN Lihua, LIN Zhiyou, ZHANG Jian, et al. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting[J]. Nature Catalysis, 2020, 3(8): 649-655. |
| 51 | FANG Wenjian, JIANG Zhi, YU Lei, et al. Novel dodecahedron BiVO4∶YVO4 solid solution with enhanced charge separation on adjacent exposed facets for highly efficient overall water splitting[J]. Journal of Catalysis, 2017, 352: 155-159. |
| 52 | ZHAO Gang, HAO Shuhua, GUO Jinghua, et al. Design of p-n homojunctions in metal-free carbon nitride photocatalyst for overall water splitting[J]. Chinese Journal of Catalysis, 2021, 42(3): 501-509. |
| 53 | JIA Yushuai, SHEN Shuai, WANG Donge, et al. Composite Sr2TiO4/SrTiO3(La,Cr) heterojunction based photocatalyst for hydrogen production under visible light irradiation[J]. Journal of Materials Chemistry A, 2013, 1(27): 7905-7912. |
| 54 | BARD Allen J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors[J]. Journal of Photochemistry, 1979, 10(1): 59-75. |
| 55 | CHEN Shanshan, QI Yu, HISATOMI Takashi, et al. Efficient visible-light-driven Z-scheme overall water splitting using a MgTa2O6- x N y /TaON heterostructure photocatalyst for H2 evolution[J]. Angewandte Chemie-International Edition, 2015, 54(29): 8498-8501. |
| 56 | TADA Hiroaki, MITSUI Tomohiro, KIYONAGA Tomokazu, et al. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system[J]. Nature Materials, 2006, 5(10): 782-786. |
| 57 | WU Xiuqin, ZHAO Juan, WANG Liping, et al. Carbon dots as solid-state electron mediator for BiVO4/CDs/CdS Z-scheme photocatalyst working under visible light[J]. Applied Catalysis B: Environmental, 2017, 206: 501-509. |
| 58 | IWASE Akihide, Yun Hau NG, ISHIGURO Yoshimi, et al. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light[J]. Journal of the American Chemical Society, 2011, 133(29): 11054-11057. |
| 59 | WANG Xuewen, LIU Gang, CHEN Zhigang, et al. Enhanced photocatalytic hydrogen evolution by prolonging the lifetime of carriers in ZnO/CdS heterostructures[J]. Chemical Communications, 2009(23): 3452-3454. |
| 60 | YU Jiaguo, WANG Shuhan, Jingxiang LOW, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics, 2013, 15(39): 16883-16890. |
| 61 | SASAKI Yasuyoshi, NEMOTO Hiroaki, SAITO Kenji, et al. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator[J]. The Journal of Physical Chemistry C, 2009, 113(40): 17536-17542. |
| 62 | DING Yao, WEI Dingqiong, HE Rong, et al. Rational design of Z-scheme PtS-ZnIn2S4/WO3-MnO2 for overall photo-catalytic water splitting under visible light[J]. Applied Catalysis B: Environmental, 2019, 258: 117948. |
| 63 | WANG Zhiliang, WANG Lianzhou. Progress in designing effective photoelectrodes for solar water splitting[J]. Chinese Journal of Catalysis, 2018, 39(3): 369-378. |
| 64 | JIAO Yan, ZHENG Yao, JARONIEC Mietek, et al. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions[J]. Chemical Society Reviews, 2015, 44(8): 2060-2086. |
| 65 | INDRA Arindam, ACHARJYA Amitava, MENEZES Prashanth W, et al. Boosting visible-light-driven photocatalytic hydrogen evolution with an integrated nickel phosphide-carbon nitride system[J]. Angewandte Chemie-International Edition, 2017, 56(6): 1653-1657. |
| 66 | CHENG Cheng, MAO Liuhao, SHI Jinwen, et al. NiCo2O4 nanosheets as a novel oxygen-evolution-reaction cocatalyst in situ bonded on the g-C3N4 photocatalyst for excellent overall water splitting[J]. Journal of Materials Chemistry A, 2021, 9(20): 12299-12306. |
| 67 | WANG Ting, LIU Lequan, GE Ge, et al. Two-dimensional titanium oxide nanosheets rich in titanium vacancies as an efficient cocatalyst for photocatalytic water oxidation[J]. Journal of Catalysis, 2018, 367: 296-305. |
| 68 | GE Ge, LIU Min, LIU Chao, et al. Ultrathin FeOOH nanosheets as an efficient cocatalyst for photocatalytic water oxidation[J]. Journal of Materials Chemistry A, 2019, 7(15): 9222-9229. |
| 69 | WOLFF Christian M, FRISCHMANN Peter D, SCHULZE Marcus, et al. All-in-one visible-light-driven water splitting by combining nanoparticulate and molecular co-catalysts on CdS nanorods[J]. Nature Energy, 2018, 3(10): 862-869. |
| 70 | QIU Bocheng, DU Mengmeng, MA Yingxin, et al. Integration of redox cocatalysts for artificial photosynthesis[J]. Energy & Environmental Science, 2021, 14(10): 5260-5288. |
| 71 | MAEDA Kazuhiko, XIONG Anke, YOSHINAGA Taizo, et al. Photocatalytic overall water splitting promoted by two different cocatalysts for hydrogen and oxygen evolution under visible light[J]. Angewandte Chemie International Edition, 2010, 49(24): 4096-4099. |
| 72 | ZHANG Jijie, BAI Tianyu, HUANG Hui, et al. Metal-organic-framework-based photocatalysts optimized by spatially separated cocatalysts for overall water splitting[J]. Advanced Materials, 2020, 32(49): e2004747. |
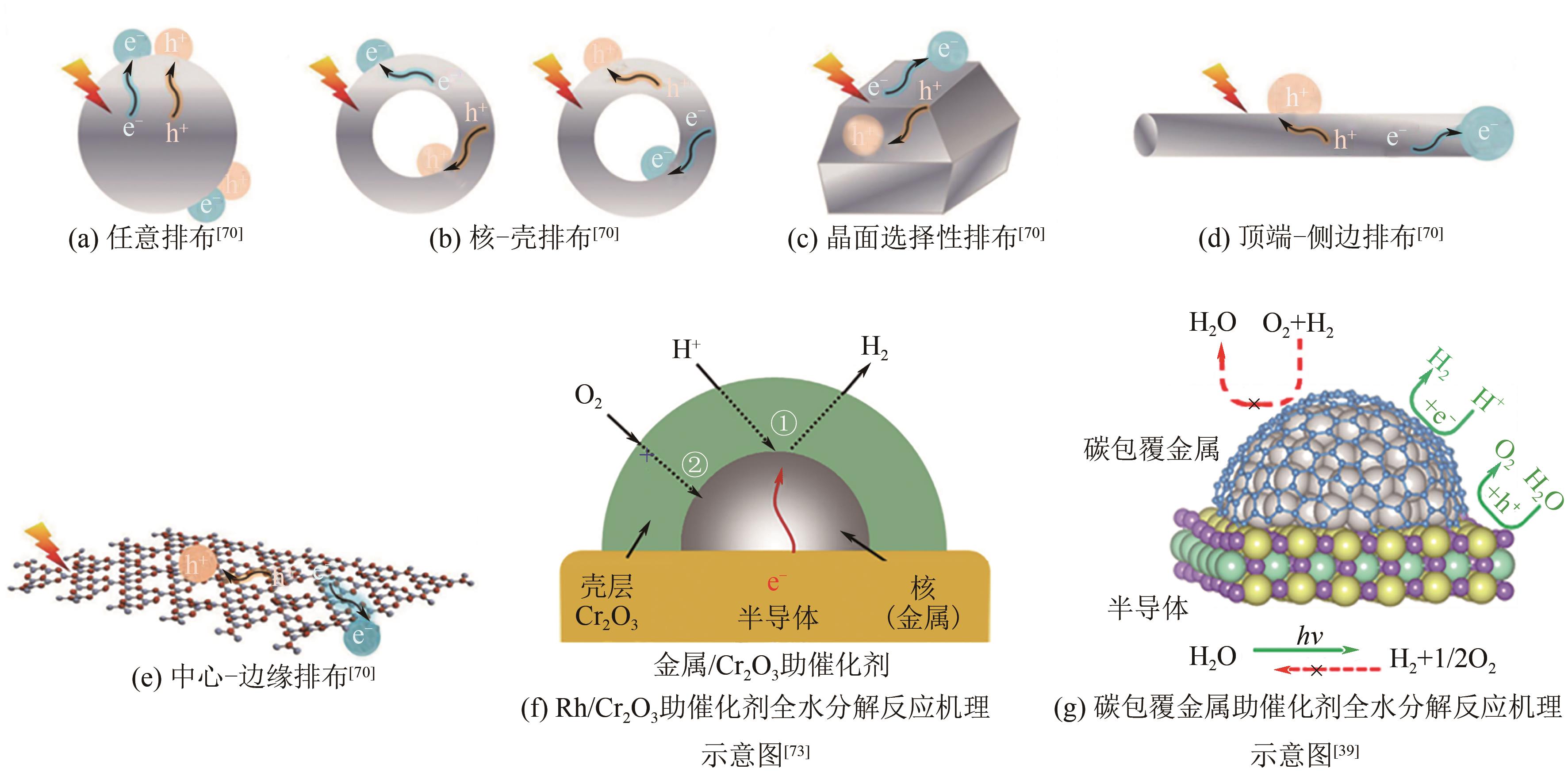
| 73 | CHEN Shanshan, QI Yu, LI Can, et al. Surface strategies for particulate photocatalysts toward artificial photosynthesis[J]. Joule, 2018, 2(11): 2260-2288. |
| 74 | LI Zheng, LI Rengui, JING Huijuan, et al. Blocking the reverse reactions of overall water splitting on a Rh/GaN-ZnO photocatalyst modified with Al2O3 [J]. Nature Catalysis, 2023, 6(1): 80-88. |
| 75 | MAEDA Kazuhiko, TERAMURA Kentaro, LU Daling, et al. Noble-metal/Cr2O3 core/shell nanoparticles as a cocatalyst for photocatalytic overall water splitting[J]. Angewandte Chemie International Edition, 2006, 45(46): 7806-7809. |
| 76 | TAKATA Tsuyoshi, PAN Chengsi, NAKABAYASHI Mamiko, et al. Fabrication of a core-shell-type photocatalyst via photodeposition of group Ⅳ and Ⅴ transition metal oxyhydroxides: An effective surface modification method for overall water splitting[J]. Journal of the American Chemical Society, 2015, 137(30): 9627-9634. |
| 77 | DOMEN Kazunari, NAITO Shuichi, SOMA Mitsuyuki, et al. Photocatalytic decomposition of water vapour on an NiO-SrTiO3 catalyst[J]. Journal of the Chemical Society, Chemical Communications, 1980 (12): 543-544. |
| 78 | Ryu ABE, SAYAMA Kazuhiro, ARAKAWA Hironori. Significant effect of iodide addition on water splitting into H2 and O2 over Pt-loaded TiO2 photocatalyst: Suppression of backward reaction[J]. Chemical Physics Letters, 2003, 371(3/4): 360-364. |
| 79 | BERTO Tobias F, SANWALD Kai E, Paige BYERS J, et al. Enabling overall water splitting on photocatalysts by CO-covered noble metal co-catalysts[J]. The Journal of Physical Chemistry Letters, 2016, 7(21): 4358-4362. |
| 80 | LI Yuhang, XING Jun, CHEN Zongjia, et al. Unidirectional suppression of hydrogen oxidation on oxidized platinum clusters[J]. Nature Communications, 2013, 4(1): 2500. |
| 81 | QURESHI Muhammad, GARCIA-ESPARZA Angel T, JEANTELOT Gabriel, et al. Catalytic consequences of ultrafine Pt clusters supported on SrTiO3 for photocatalytic overall water splitting[J]. Journal of Catalysis, 2019, 376: 180-190. |
| 82 | TIAN Bin, YANG Baojun, LI Jian, et al. Water splitting by CdS/Pt/WO3-CeO x photocatalysts with assisting of artificial blood perfluorodecalin[J]. Journal of Catalysis, 2017, 350: 189-196. |
| 83 | ZHANG Xuqiang, JIAN Lijuan, WANG Li, et al. Inhibition of H2-O2 recombination using amino functional groups to anchor oxygen for enhancing photocatalytic hydrogen generation[J]. Applied Surface Science, 2022, 571: 151304. |
| [1] | 刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741. |
| [2] | 王红妍, 马子然, 李歌, 马静, 赵春林, 周佳丽, 王磊, 彭胜攀. 燃煤耦合可再生燃料烟气多污染物协同催化脱除研究进展[J]. 化工进展, 2024, 43(4): 1783-1795. |
| [3] | 陈家一, 高帷韬, 阴亚楠, 王诚, 欧阳鸿武, 毛宗强. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| [4] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [5] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [6] | 谷星朋, 马红钦, 刘嘉豪. 雷尼镍的磷量子点改性及其催化加氢脱硫性能[J]. 化工进展, 2024, 43(3): 1293-1301. |
| [7] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [8] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| [9] | 刘斌, 王勇军, 吕汪洋, 陈文兴. 高稳定性钛系聚酯催化剂TiOC@SiO2的制备及应用[J]. 化工进展, 2024, 43(3): 1395-1402. |
| [10] | 王雄, 康文倩, 任悦, 乔彤森, 张鹏, 黄安平, 李广全. 多孔有机聚合物中试制备及其在聚烯烃催化剂中的应用[J]. 化工进展, 2024, 43(3): 1412-1417. |
| [11] | 闫守成, 张慧华, 徐倩倩, 王煜坤. 石墨烯复合载体催化剂在柴油车尾气NO脱除中的应用[J]. 化工进展, 2024, 43(3): 1456-1465. |
| [12] | 董冰岩, 李贞栋, 王佩祥, 涂文娟, 谭艳雯, 张芹. DBD等离子体耦合BiOI催化材料降解苯甲羟肟酸的特性与机制[J]. 化工进展, 2024, 43(3): 1565-1575. |
| [13] | 陈晓贞, 刘丽, 杨成敏, 郑步梅, 尹晓莹, 孙进, 姚运海, 段为宇. 氧化铝基加氢脱硫催化剂研究进展[J]. 化工进展, 2024, 43(2): 948-961. |
| [14] | 丁康, 何军桥, 陈元捷, 杨霞珍, 刘化章, 霍超. Ru/Ba-MgO氨合成催化剂模板棉纤维的盐酸处理对催化性能的影响[J]. 化工进展, 2024, 43(2): 962-970. |
| [15] | 王达锐, 孙洪敏, 王一棪, 唐智谋, 李芮, 范雪研, 杨为民. 分子筛催化反应过程高效化的技术进展[J]. 化工进展, 2024, 43(1): 1-18. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||