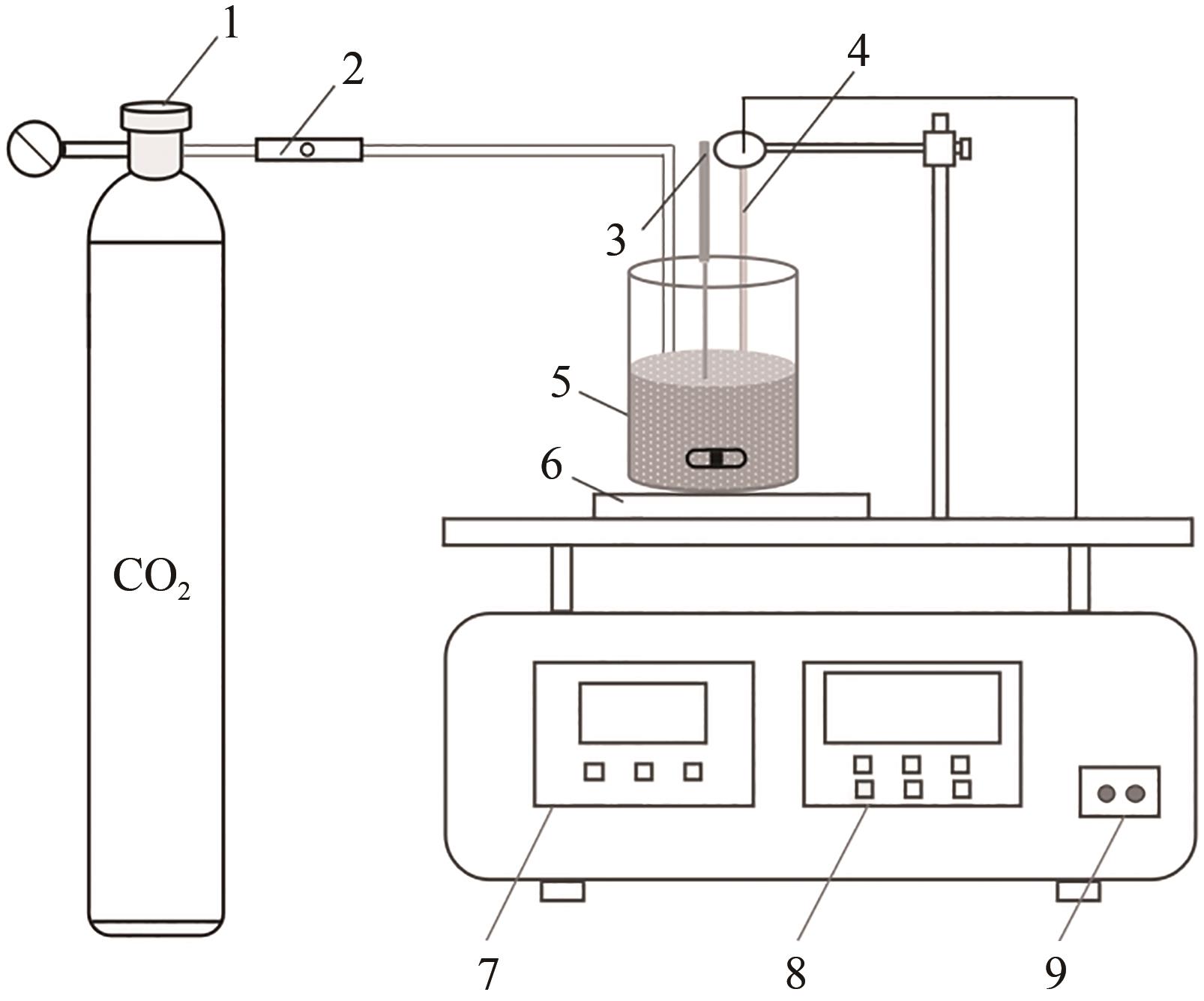
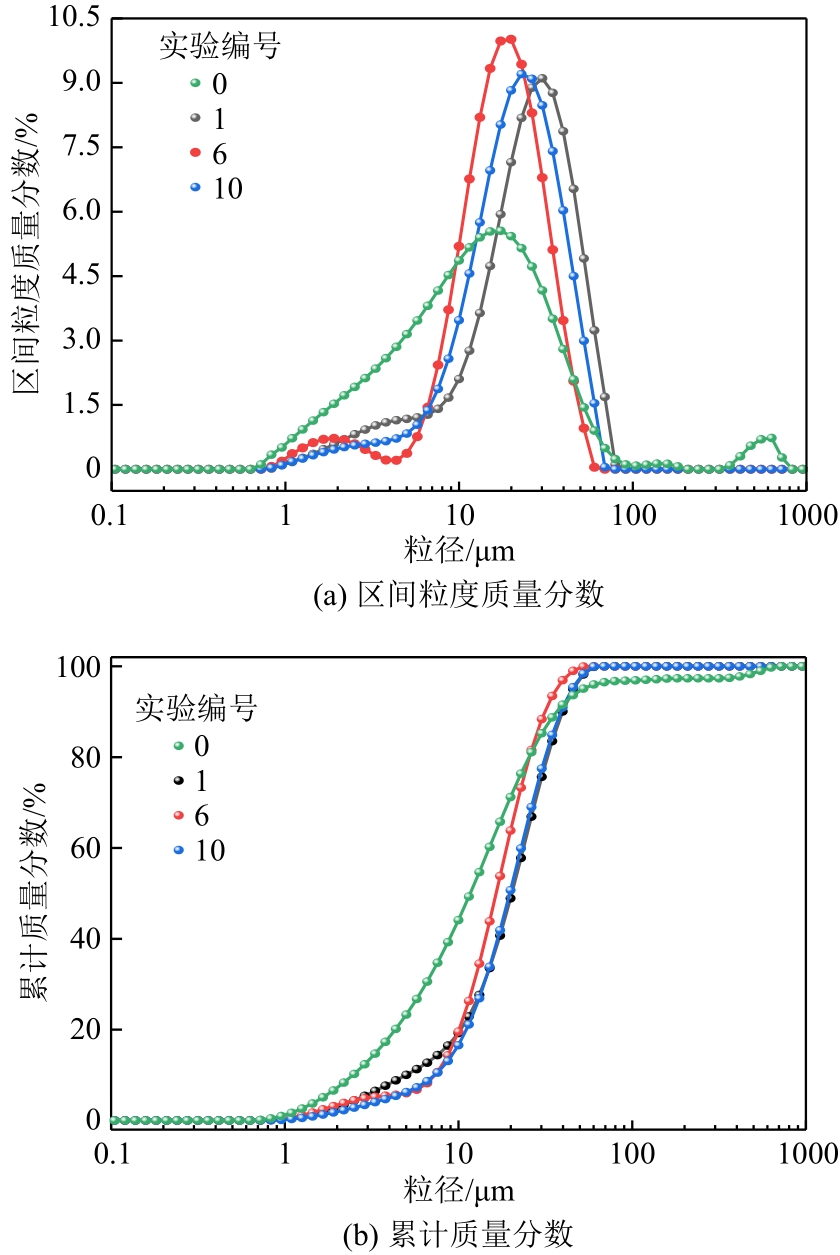
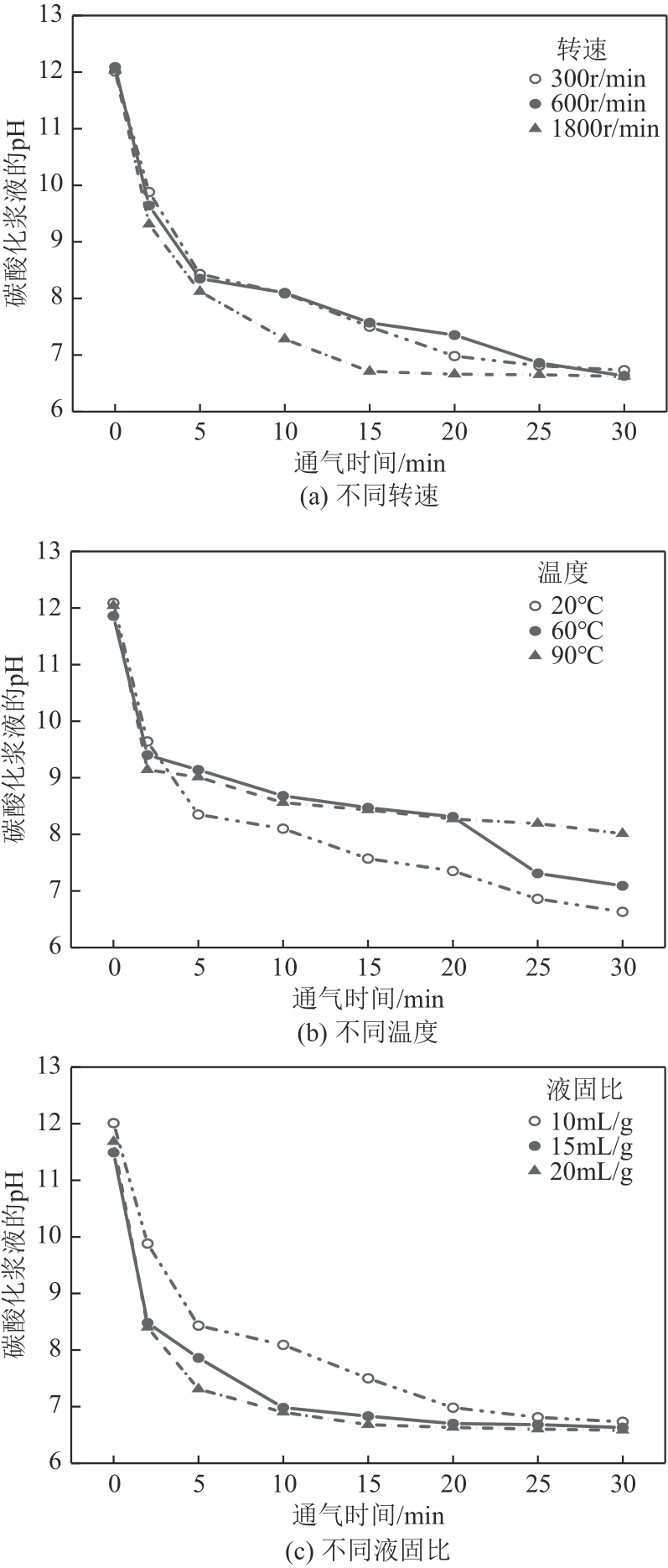
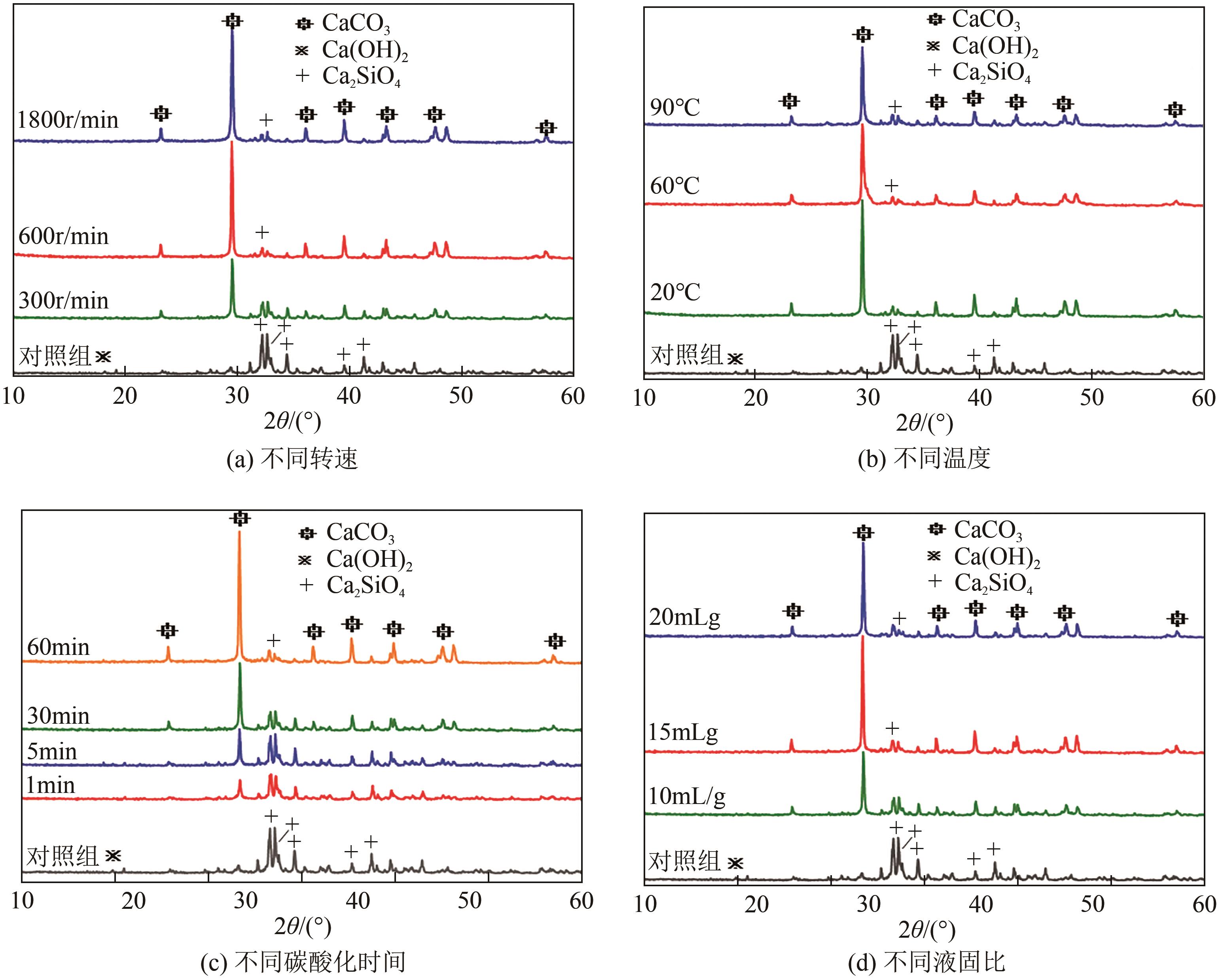
化工进展 ›› 2024, Vol. 43 ›› Issue (4): 2161-2173.DOI: 10.16085/j.issn.1000-6613.2023-0660
• 资源与环境化工 • 上一篇
改性镁渣的湿法碳酸化工艺
孙伟吉1,2( ), 刘浪1,2(
), 刘浪1,2( ), 方治余1,2, 朱梦博1,2, 解耿1,2, 何伟1,2, 高宇恒1,2
), 方治余1,2, 朱梦博1,2, 解耿1,2, 何伟1,2, 高宇恒1,2
- 1.西安科技大学能源学院, 陕西 西安 710054
2.西安科技大学矿山功能性充填技术研究中心, 陕西 西安 710054
-
收稿日期:2023-04-23修回日期:2023-09-01出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:刘浪 -
作者简介:孙伟吉(1994—),男,博士研究生,研究方向为采空区CO2封存。E-mail:sunweji0825@163.com。 -
基金资助:陕西省重点研发计划——“两链”融合重点专项(2023-LL-QY-07);国家自然科学基金面上项目(52222404);国家自然科学基金青年基金(52204175)
Technique of wet carbonation of modified magnesium slag
SUN Weiji1,2( ), LIU Lang1,2(
), LIU Lang1,2( ), FANG Zhiyu1,2, ZHU Mengbo1,2, XIE Geng1,2, HE Wei1,2, GAO Yuheng1,2
), FANG Zhiyu1,2, ZHU Mengbo1,2, XIE Geng1,2, HE Wei1,2, GAO Yuheng1,2
- 1.College of Energy Engineering, Xi’an University of Science and Technology, Xi’an 710054, Shaanxi, China
2.Research Center for Functional Backfill Technology in Mine, Xi’an University of Science and Technology, Xi’an 710054, Shaanxi, China
-
Received:2023-04-23Revised:2023-09-01Online:2024-04-15Published:2024-05-13 -
Contact:LIU Lang
摘要:
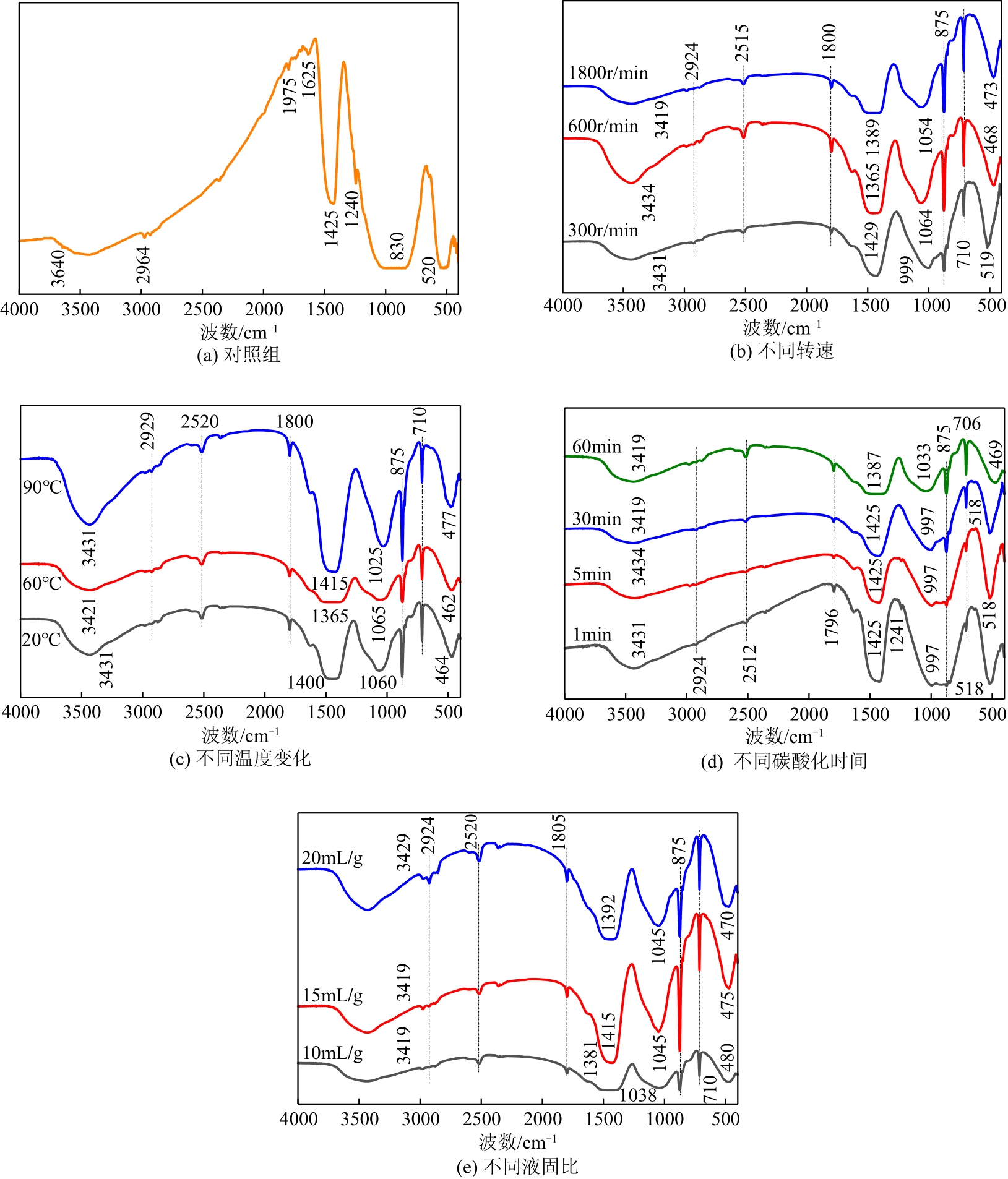
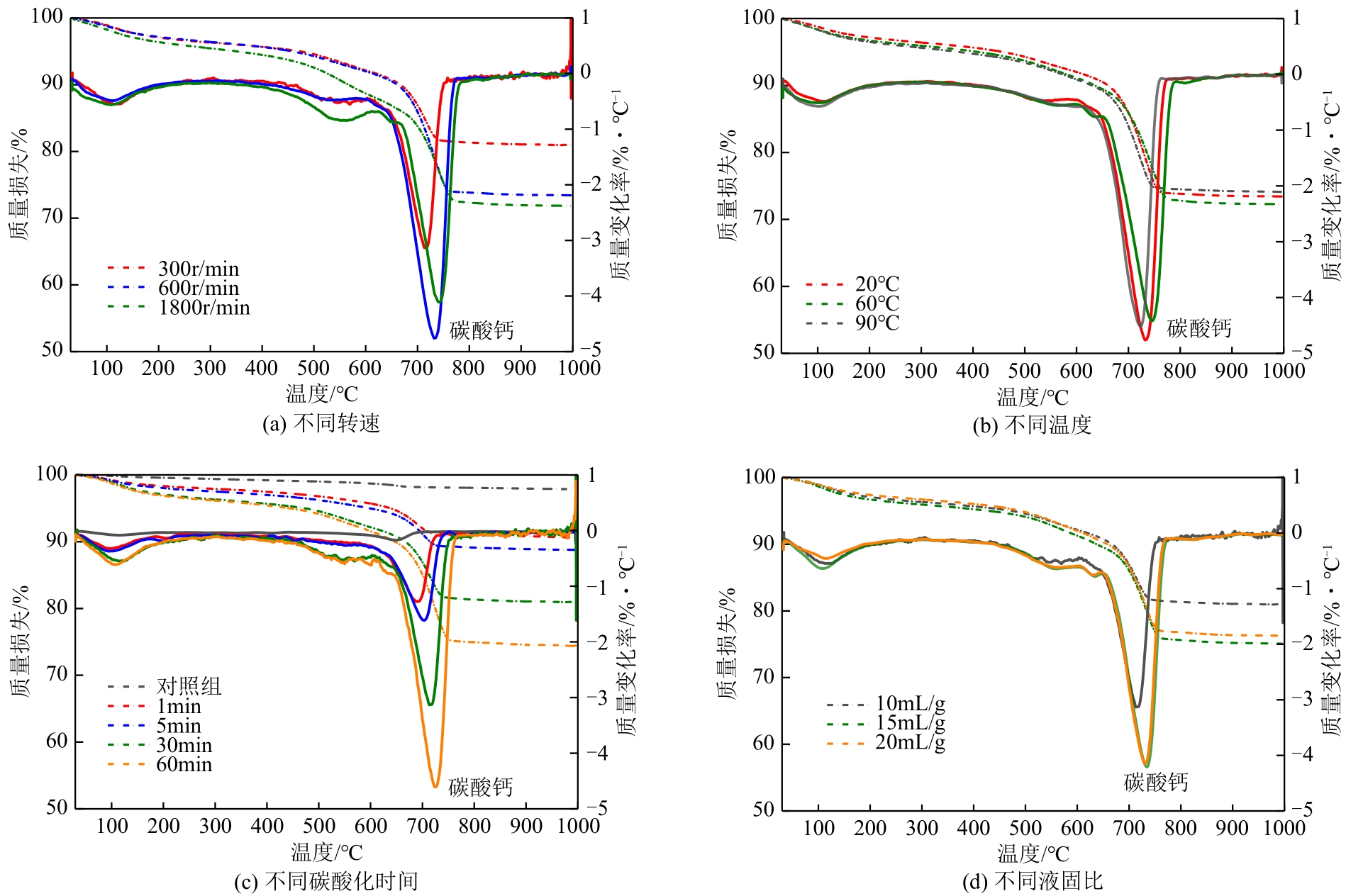
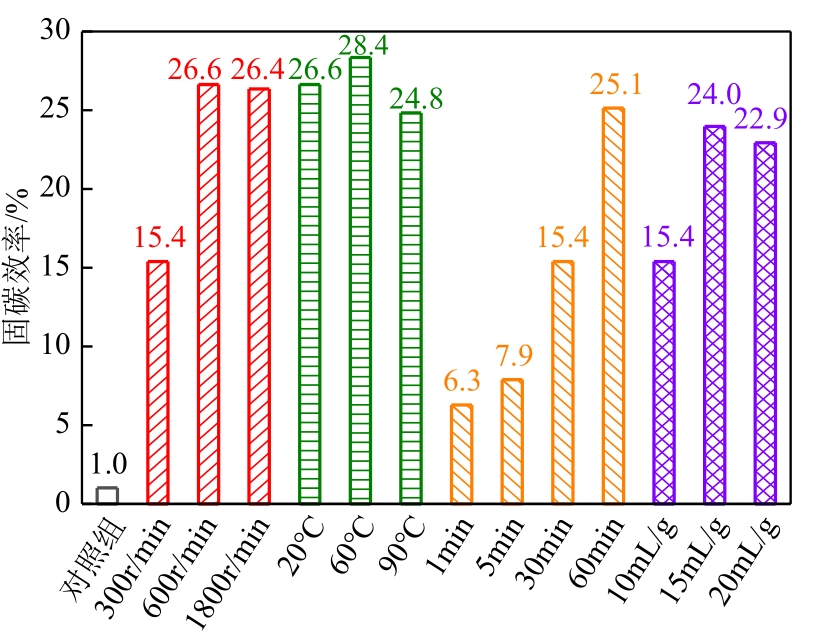
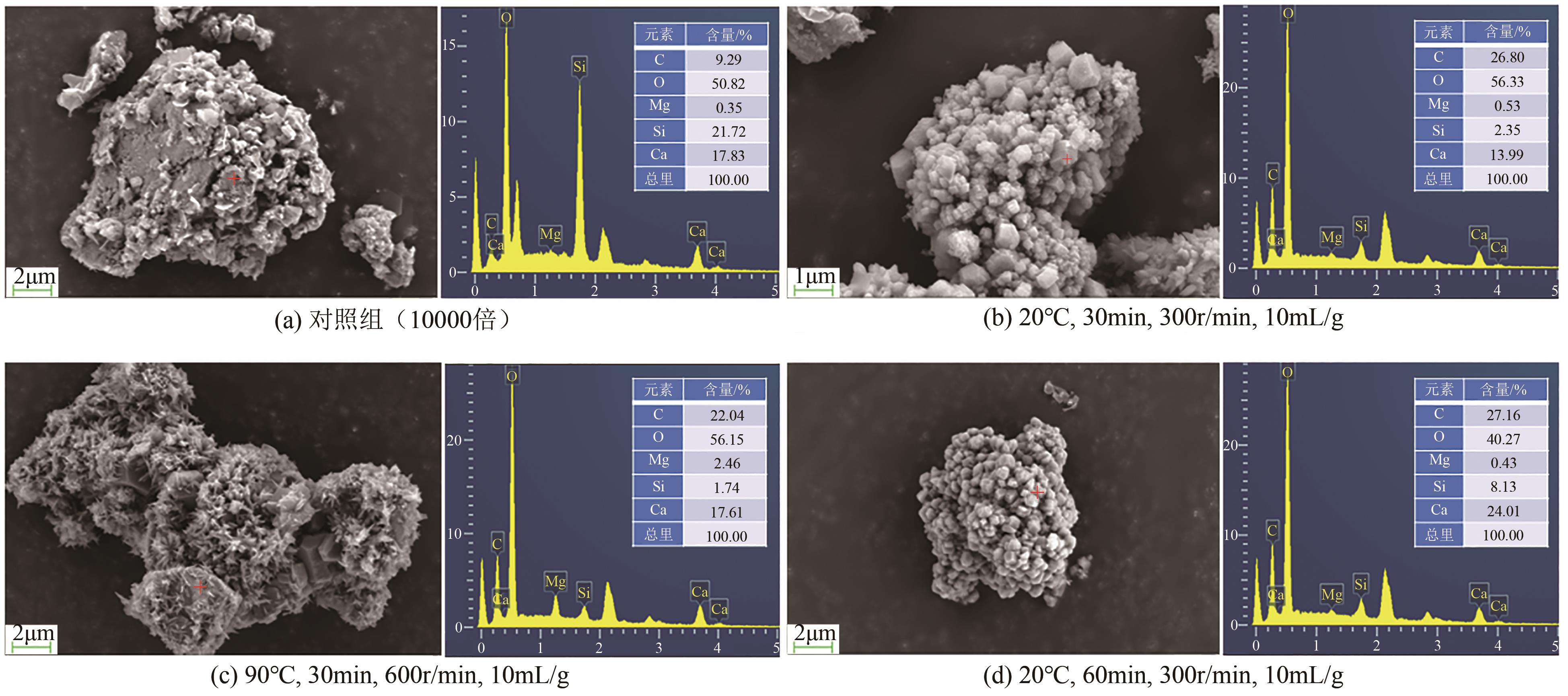
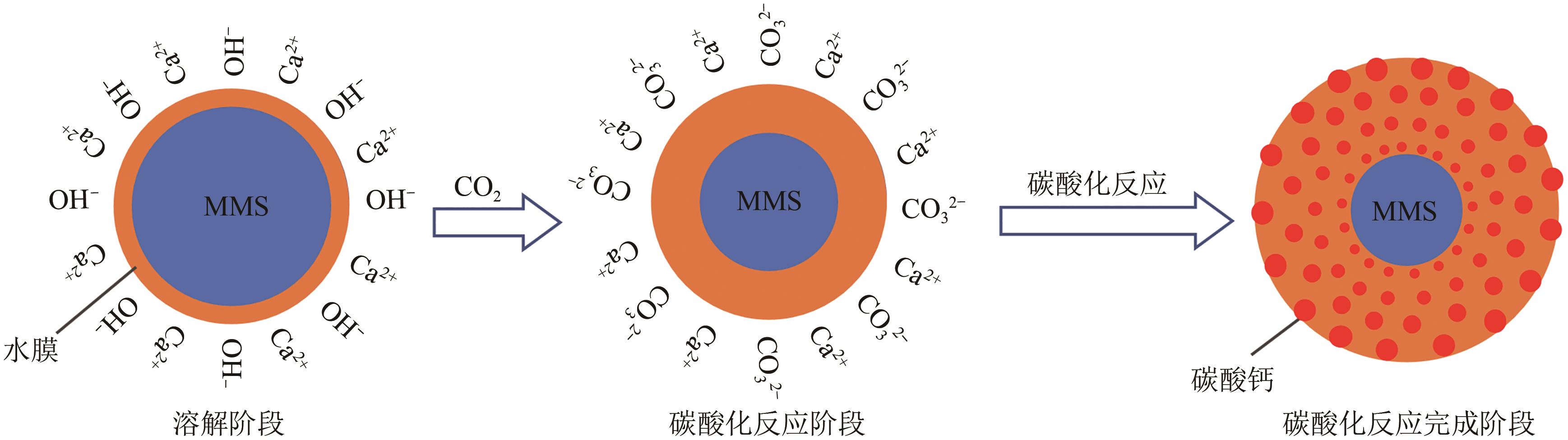
碱基工业固废的碳酸化反应是一种矿化吸收CO2并减少温室气体排放的固碳策略之一。本研究首次引入湿法碳酸化工艺,通过借助X射线衍射、红外光谱测试、热重分析等实验测试,重点探究了温度变化、搅拌速度、碳酸化时间和液固比例对改性镁渣碳酸化反应的影响,进而讨论了4种因素下改性镁渣的碳酸化产物与固碳效率。结果表明,改性镁渣的矿物成分主要以β-C2S为主,是一种具有良好储存CO2能力的工业固废,碳酸化产物主要以方解石型碳酸钙晶体和二氧化硅凝胶为主。碳酸化反应后,改性镁渣碳酸化产物的粒径显著增大,比表面积减小,粒径分布变均匀,粒径分布范围变窄。当碳酸化反应温度为60℃、通气时间为30min、液固比为10mL/g、搅拌速度为600r/min时,改性镁渣的固碳效率达到最大(28.4%),即1kg改性镁渣可以矿化吸收0.284kg CO2。因此,湿法碳酸化工艺对改性镁渣的回收利用及CO2捕集均具有很大的应用潜力。
中图分类号:
引用本文
孙伟吉, 刘浪, 方治余, 朱梦博, 解耿, 何伟, 高宇恒. 改性镁渣的湿法碳酸化工艺[J]. 化工进展, 2024, 43(4): 2161-2173.
SUN Weiji, LIU Lang, FANG Zhiyu, ZHU Mengbo, XIE Geng, HE Wei, GAO Yuheng. Technique of wet carbonation of modified magnesium slag[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2161-2173.
| 化学成分 | MMS/% |
|---|---|
| SiO2 | 19.21 |
| Al2O3 | 0.82 |
| CaO | 41.18 |
| MgO | 3.78 |
| Fe2O3 | 2.59 |
| SO3 | 0.02 |
| P2O5 | <0.01 |
| MnO | <0.01 |
表1 MMS的化学组成 (质量分数)
| 化学成分 | MMS/% |
|---|---|
| SiO2 | 19.21 |
| Al2O3 | 0.82 |
| CaO | 41.18 |
| MgO | 3.78 |
| Fe2O3 | 2.59 |
| SO3 | 0.02 |
| P2O5 | <0.01 |
| MnO | <0.01 |
| 实验编号 | 转子速度 /r·min-1 | 温度 /℃ | 通气时间 /min | 液固比 /mL·g-1 | 通气速率 /L·min-1 |
|---|---|---|---|---|---|
| 0(对照组) | 300 | 20 | 30 | 10 | 0 |
| 1 | 300 | 20 | 30 | 10 | 1 |
| 2 | 600 | 20 | 30 | 10 | 1 |
| 3 | 1800 | 20 | 30 | 10 | 1 |
| 4 | 600 | 20 | 30 | 10 | 1 |
| 5 | 600 | 60 | 30 | 10 | 1 |
| 6 | 600 | 90 | 30 | 10 | 1 |
| 7 | 300 | 20 | 1 | 10 | 1 |
| 8 | 300 | 20 | 5 | 10 | 1 |
| 9 | 300 | 20 | 30 | 10 | 1 |
| 10 | 300 | 20 | 60 | 10 | 1 |
| 11 | 300 | 20 | 30 | 10 | 1 |
| 12 | 300 | 20 | 30 | 15 | 1 |
| 13 | 300 | 20 | 30 | 20 | 1 |
表2 改性镁渣湿法碳酸化实验方案
| 实验编号 | 转子速度 /r·min-1 | 温度 /℃ | 通气时间 /min | 液固比 /mL·g-1 | 通气速率 /L·min-1 |
|---|---|---|---|---|---|
| 0(对照组) | 300 | 20 | 30 | 10 | 0 |
| 1 | 300 | 20 | 30 | 10 | 1 |
| 2 | 600 | 20 | 30 | 10 | 1 |
| 3 | 1800 | 20 | 30 | 10 | 1 |
| 4 | 600 | 20 | 30 | 10 | 1 |
| 5 | 600 | 60 | 30 | 10 | 1 |
| 6 | 600 | 90 | 30 | 10 | 1 |
| 7 | 300 | 20 | 1 | 10 | 1 |
| 8 | 300 | 20 | 5 | 10 | 1 |
| 9 | 300 | 20 | 30 | 10 | 1 |
| 10 | 300 | 20 | 60 | 10 | 1 |
| 11 | 300 | 20 | 30 | 10 | 1 |
| 12 | 300 | 20 | 30 | 15 | 1 |
| 13 | 300 | 20 | 30 | 20 | 1 |
| 实验编号 | D10/μm | D50/μm | D90/μm | 均匀性系数 | 比表面积/m2·g-1 |
|---|---|---|---|---|---|
| 0 | 2.470 | 11.696 | 36.712 | 1.980 | 0.978 |
| 1 | 5.731 | 23.315 | 45.640 | 0.508 | 0.493 |
| 6 | 7.353 | 16.490 | 31.418 | 0.457 | 0.578 |
| 10 | 7.323 | 19.739 | 38.884 | 0.487 | 0.490 |
表3 碳酸化反应后MMS粒径的变化
| 实验编号 | D10/μm | D50/μm | D90/μm | 均匀性系数 | 比表面积/m2·g-1 |
|---|---|---|---|---|---|
| 0 | 2.470 | 11.696 | 36.712 | 1.980 | 0.978 |
| 1 | 5.731 | 23.315 | 45.640 | 0.508 | 0.493 |
| 6 | 7.353 | 16.490 | 31.418 | 0.457 | 0.578 |
| 10 | 7.323 | 19.739 | 38.884 | 0.487 | 0.490 |
| 矿物成分 | 实验条件 | 衍射峰位置 | 总峰面积 | 总峰高度 |
|---|---|---|---|---|
| CaCO3 | 300r/min | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 23633 | 2448 |
| 600r/min | 40211 | 4540 | ||
| 1800r/min | 44253 | 4410 | ||
| CaCO3 | 20℃ | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 40211 | 4540 |
| 60℃ | 41428 | 3109 | ||
| 90℃ | 31045 | 3026 | ||
| CaCO3 | 1min | 23.1°、29.5°、36.0°、39.5°、3.2°、47.6°、57.5° | 9964 | 777 |
| 5min | 13848 | 1334 | ||
| 30min | 23633 | 2448 | ||
| 60min | 41980 | 4540 | ||
| CaCO3 | 10mL/g | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 23633 | 2448 |
| 15mL/g | 36961 | 4261 | ||
| 20mL/g | 30727 | 3440 |
表4 MMS碳酸化产物的衍射峰特征
| 矿物成分 | 实验条件 | 衍射峰位置 | 总峰面积 | 总峰高度 |
|---|---|---|---|---|
| CaCO3 | 300r/min | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 23633 | 2448 |
| 600r/min | 40211 | 4540 | ||
| 1800r/min | 44253 | 4410 | ||
| CaCO3 | 20℃ | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 40211 | 4540 |
| 60℃ | 41428 | 3109 | ||
| 90℃ | 31045 | 3026 | ||
| CaCO3 | 1min | 23.1°、29.5°、36.0°、39.5°、3.2°、47.6°、57.5° | 9964 | 777 |
| 5min | 13848 | 1334 | ||
| 30min | 23633 | 2448 | ||
| 60min | 41980 | 4540 | ||
| CaCO3 | 10mL/g | 23.1°、29.5°、36.0°、39.5°、43.2°、47.6°、57.5° | 23633 | 2448 |
| 15mL/g | 36961 | 4261 | ||
| 20mL/g | 30727 | 3440 |
| 1 | CHEN Wanqi, TANG Haoyue, HE Li, et al. Co-effect assessment on regional air quality: A perspective of policies and measures with greenhouse gas reduction potential[J]. The Science of the Total Environment, 2022, 851: 158119. |
| 2 | ZHANG Yixiang, FU Bowen. Impact of China’s establishment of ecological civilization pilot zones on carbon dioxide emissions[J]. Journal of Environmental Management, 2023, 325: 116652. |
| 3 | DAVIS Steven J, CALDEIRA Ken, Damon MATTHEWS H. Future CO2 emissions and climate change from existing energy infrastructure[J]. Science, 2010, 329(5997): 1330-1333. |
| 4 | YANG Yang, XU Wenqing, WANG Yan, et al. Progress of CCUS technology in the iron and steel industry and the suggestion of the integrated application schemes for China[J]. Chemical Engineering Journal, 2022, 450: 138438. |
| 5 | 王秋华, 吴嘉帅, 张卫风. 碱性工业固废矿化封存二氧化碳研究进展[J]. 化工进展, 2023, 42(3): 1572-1582. |
| WANG Qiuhua, WU Jiashuai, ZHANG Weifeng. Research progress of alkaline industrial solid wastes mineralization for carbon dioxide sequestration[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1572-1582. | |
| 6 | MONTEIRO Juliana, ROUSSANALY Simon. CCUS scenarios for the cement industry: Is CO2 utilization feasible?[J]. Journal of CO2 Utilization, 2022, 61: 102015. |
| 7 | SEIFRITZ W. CO2 disposal by means of silicates[J]. Nature, 1990, 345(6275): 486. |
| 8 | LI Yingjie, SUN Rongyue, LIU Changtian, et al. CO2 capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles[J]. International Journal of Greenhouse Gas Control, 2012, 9: 117-123. |
| 9 | CHEN Zhimin, LI Rui, ZHENG Xianming, et al. Carbon sequestration of steel slag and carbonation for activating RO phase[J]. Cement and Concrete Research, 2021, 139: 106271. |
| 10 | LUO Zhongtao, WANG Yu, YANG Guangjun, et al. Effect of curing temperature on carbonation behavior of steel slag compacts[J]. Construction and Building Materials, 2021, 291: 123369. |
| 11 | QIN Ling, GAO Xiaojian. Recycling of waste autoclaved aerated concrete powder in Portland cement by accelerated carbonation[J]. Waste Management, 2019, 89: 254-264. |
| 12 | 赵雯涵, 吴水木, 李英杰. 钙基工业固废循环捕集CO2性能研究进展[J]. 煤炭学报, 2022, 47(11): 3926-3935. |
| ZHAO Wenhan, WU Shuimu, LI Yingjie. Research progress on CO2 capture performance of calcium-based industrial solid waste recycling[J]. Journal of China Coal Society, 2022, 47(11): 3926-3935. | |
| 13 | 李文秀, 杨宇航, 黄艳, 等. 二氧化碳矿化高钙基固废制备微细碳酸钙研究进展[J]. 化工进展, 2023, 42(4): 2047-2057. |
| LI Wenxiu, YANG Yuhang, HUANG Yan, et al. Preparation of ultrafine calcium carbonate by CO2 mineralization using high calcium-based solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2047-2057. | |
| 14 | GHOULEH Zaid, GUTHRIE Roderick I L, SHAO Yixin. Production of carbonate aggregates using steel slag and carbon dioxide for carbon-negative concrete[J]. Journal of CO2 Utilization, 2017, 18: 125-138. |
| 15 | MA Zhuohui, LIAO H, WANG Li, et al. Effects of iron/silicon/magnesium/aluminum on CaO carbonation of CO2 in steel slag-based building materials during carbonation curing[J]. Construction and Building Materials, 2021, 298: 123889. |
| 16 | 任国宏, 廖洪强, 高宏宇, 等. 粉煤灰-电石渣制浆矿化的固碳增强特性[J]. 材料导报, 2019, 33(21): 3556-3560. |
| REN Guohong, LIAO Hongqiang, GAO Hongyu, et al. Carbon dioxide-fixing and compression strength enhancing characteristics of mineralized immobilization of fly ash-calcium carbide slag slurry[J]. Materials Reports, 2019, 33(21): 3556-3560. | |
| 17 | MIAO Endong, ZHENG Xufan, XIONG Zhuo, et al. Kinetic modeling of direct aqueous mineral carbonation using carbide slag in a stirred tank reactor[J]. Fuel, 2022, 315: 122837. |
| 18 | LIU Lili, JI Yongsheng, GAO Furong, et al. Study on high-efficiency CO2 absorption by fresh cement paste[J]. Construction and Building Materials, 2021, 270: 121364. |
| 19 | LI Yisha, MEHDIZADEH Hamideh, MO Kim Hung, et al. Co-utilization of aqueous carbonated basic oxygen furnace slag (BOFS) and carbonated filtrate in cement pastes considering reaction duration effect[J]. Cement and Concrete Composites, 2023, 138: 104988. |
| 20 | HALMANN M, FREI A, STEINFELD A. Magnesium production by the pidgeon process involving dolomite calcination and MgO silicothermic reduction: thermodynamic and environmental analyses[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2146-2154. |
| 21 | 唐洋洋, 李林波, 王超, 等. 镁渣资源化利用新进展[J]. 现代化工, 2020, 40(12): 63-67. |
| TANG Yangyang, LI Linbo, WANG Chao, et al. New progress in reutilization of magnesium slag[J]. Modern Chemical Industry, 2020, 40(12): 63-67. | |
| 22 | XU Jilei, LIU Jinhui, GUO Dong, et al. Mechanism of slag pellets sticking on the wall of reduction pot in magnesium production by Pidgeon process[J]. Journal of Magnesium and Alloys, 2022: https://doi.org/10.1016/j.jma.2022.10.016. |
| 23 | 刘浪, 阮仕山, 方治余, 等. 镁渣的改性及其在矿山充填领域的应用探索[J]. 煤炭学报, 2021, 46(12): 3833-3845. |
| LIU Lang, RUAN Shishan, FANG Zhiyu, et al. Modification of magnesium slag and its application in the field of mine filling[J]. Journal of China Coal Society, 2021, 46(12): 3833-3845. | |
| 24 | 孙伟吉, 刘浪, 徐龙华, 等. 改性镁渣基矿用复合胶凝材料的水化性能[J]. 中南大学学报(自然科学版), 2022, 53(10): 4057-4070. |
| SUN Weiji, LIU Lang, XU Longhua, et al. Hydration properties of modified magnesium slag-based composite cementitious materials for mining[J]. Journal of Central South University (Science and Technology), 2022, 53(10): 4057-4070. | |
| 25 | RUAN Shishan, LIU Lang, ZHU Mengbo, et al. Application of desulfurization gypsum as activator for modified magnesium slag-fly ash cemented paste backfill material[J]. Science of The Total Environment, 2023, 869: 161631. |
| 26 | WANG Dan, CHANG Jun. Comparison on accelerated carbonation of β-C2S, Ca(OH)2, and C4AF: Reaction degree, multi-properties, and products[J]. Construction and Building Materials, 2019, 224: 336-347. |
| 27 | WANG Dan, FANG Yanfeng, ZHANG Yangyang, et al. Changes in mineral composition, growth of calcite crystal, and promotion of physico-chemical properties induced by carbonation of β-C2S[J]. Journal of CO2 Utilization, 2019, 34: 149-162. |
| 28 | 伊元荣, 马忠乐, 杜昀聪, 等. 不同温度下精炼渣碳酸化微观结构变化[J]. 钢铁研究学报, 2021, 33(2): 127-135. |
| YI Yuanrong, MA Zhongle, DU Yuncong, et al. Microstructure changes of refining slag under carbonization at different reaction temperatures[J]. Journal of Iron and Steel Research, 2021, 33(2): 127-135. | |
| 29 | LI Yemei, PEI Silu, PAN Shuyuan, et al. Carbonation and utilization of basic oxygen furnace slag coupled with concentrated water from electrodeionization[J]. Journal of CO2 Utilization, 2018, 25: 46-55. |
| 30 | METZ V, GANOR J. Stirring effect on kaolinite dissolution rate[J]. Geochimica et Cosmochimica Acta, 2001, 65(20): 3475-3490. |
| 31 | DUAN Zhenhao, SUN Rui. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar[J]. Chemical Geology, 2003, 193(3/4): 257-271. |
| 32 | LI Yemei, PEI Silu, PAN Shuyuan, et al. Carbonation and utilization of basic oxygen furnace slag coupled with concentrated water from electrodeionization[J]. Journal of CO2 Utilization, 2018, 25: 46-55. |
| 33 | DROUET E, POYET S, LE BESCOP P, et al. Carbonation of hardened cement pastes: Influence of temperature[J]. Cement and Concrete Research, 2019, 115: 445-459. |
| 34 | JI Long, YU Hai, WANG Xiaolong, et al. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal[J]. Fuel Processing Technology, 2017, 156: 429-437. |
| 35 | TAN Yicheng, LIU Zhichao, WANG Fazhou. Effect of temperature on the carbonation behavior of γ-C2S compacts[J]. Cement and Concrete Composites, 2022, 133: 104652. |
| 36 | 沈鹤鸣, 吴灿彬, 李志华, 等. 氢氧化钙的固碳功能性研究—CO2浓度与碳化时间的影响[J]. 功能材料, 2020, 51(1): 1115-1119. |
| SHEN Heming, WU Canbin, LI Zhihua, et al. Carbon sequestration functionality of calcium hydroxide—Effect of CO2 concentration and carbonation time[J]. Journal of Functional Materials, 2020, 51(1): 1115-1119. | |
| 37 | 郑鹏, 李蔚玲, 郭亚飞, 等. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| ZHENG Peng, LI Weiling, GUO Yafei, et al. Analysis of carbide slag accelerated carbonation in bubble column and response surface optimization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1528-1538. | |
| 38 | ZHANG Yunhua, WANG Ruoxin, LIU Zhiyi, et al. A novel carbonate binder from waste hydrated cement paste for utilization of CO2 [J]. Journal of CO2 Utilization, 2019, 32: 276-280. |
| 39 | RAUTARAY Debabrata, SAINKAR S R, SASTRY Murali. Thermally evaporated aerosol OT thin films as templates for the room temperature synthesis of aragonite crystals[J]. Chemistry of Materials, 2003, 15(14): 2809-2814. |
| 40 | CHEN Z X, CHU S H, LEE Y S, et al. Coupling effect of γ-dicalcium silicate and slag on carbonation resistance of low carbon materials[J]. Journal of Cleaner Production, 2020, 262: 121385. |
| 41 | ASHRAF Warda, OLEK Jan. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials[J]. Journal of Materials Science, 2016, 51(13): 6173-6191. |
| 42 | CHEN Z X, CHU S H, ISHAK S, et al. Roles of particle packing and water coating thickness in carbonation and strength of γ-dicalcium silicate-based low carbon materials[J]. Journal of Cleaner Production, 2022, 358: 131735. |
| 43 | WEI Xinlei, NI Wen, ZHANG Siqi, et al. Influence of the key factors on the performance of steel slag-desulphurisation gypsum-based hydration-carbonation materials[J]. Journal of Building Engineering, 2022, 45: 103591. |
| 44 | ZHANG Jiake, SHI Caijun, LI Yake, et al. Performance enhancement of recycled concrete aggregates through carbonation[J]. Journal of Materials in Civil Engineering, 2015, 27(11): 04015029. |
| 45 | LEE Seung-Woo, KIM Yong-Jae, LEE Yun-Hee, et al. Behavior and characteristics of amorphous calcium carbonate and calcite using CaCO3 film synthesis[J]. Materials & Design, 2016, 112: 367-373. |
| 46 | MO Liwu, HAO Yuanyuan, LIU Yunpeng, et al. Preparation of calcium carbonate binders via CO2 activation of magnesium slag[J]. Cement and Concrete Research, 2019, 121: 81-90. |
| 47 | WANG Dan, XIONG Cang, LI Wenzheng, et al. Growth of calcium carbonate induced by accelerated carbonation of tricalcium silicate[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(39): 14718-14731. |
| 48 | CHANG Jun, JIANG Ting, CUI Kai. Influence on compressive strength and CO2 capture after accelerated carbonation of combination β-C2S with γ-C2S[J]. Construction and Building Materials, 2021, 312: 125359. |
| [1] | 高飞, 刘志松, 潘珂珂, 刘敏敏, 代斌, 但建明, 于锋. 蛭石基FeCeO x 催化剂及CO选择性催化还原NO[J]. 化工进展, 2024, 43(4): 1851-1862. |
| [2] | 李开瑞, 高照华, 刘甜甜, 李静, 魏海生. 还原温度调变Rh/FePO4催化剂喹啉选择加氢性能[J]. 化工进展, 2024, 43(3): 1342-1349. |
| [3] | 徐泽文, 王明, 王强, 侯影飞. 胺基材料在二氧化碳分离膜领域研究进展[J]. 化工进展, 2024, 43(3): 1374-1386. |
| [4] | 楚振普, 陈禹蒙, 李俊国, 孙庆轩, 刘科. 废旧锂离子电池负极石墨循环再生的研究进展[J]. 化工进展, 2024, 43(3): 1524-1534. |
| [5] | 张瑞凯, 张会书, 郑龙云, 曾爱武. CO2吸收过程中气相分压对Rayleigh对流传质特性的影响[J]. 化工进展, 2024, 43(2): 913-924. |
| [6] | 王达锐, 孙洪敏, 王一棪, 唐智谋, 李芮, 范雪研, 杨为民. 分子筛催化反应过程高效化的技术进展[J]. 化工进展, 2024, 43(1): 1-18. |
| [7] | 苏梦军, 刘剑, 辛靖, 陈禹霏, 张海洪, 韩龙年, 朱元宝, 李洪宝. 气液混合强化在固定床加氢过程中的应用进展[J]. 化工进展, 2024, 43(1): 100-110. |
| [8] | 张家昊, 李盈盈, 徐彦琳, 尹佳滨, 张吉松. 微反应器中连续还原胺化反应的研究进展[J]. 化工进展, 2024, 43(1): 186-197. |
| [9] | 任鹏锟, 仲兆平, 杨宇轩, 张杉, 杜浩然, 李骞. 改性海泡石对污泥热解过程中重金属的控制[J]. 化工进展, 2024, 43(1): 541-550. |
| [10] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [11] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [12] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [15] | 阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||