化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2723-2738.DOI: 10.16085/j.issn.1000-6613.2023-2207
• 二氧化碳捕集与资源化利用 • 上一篇
CeO2载体在CO2加氢制甲醇中的应用和研究进展
周运桃1,2( ), 王洪星1,2, 李新刚1(
), 王洪星1,2, 李新刚1( ), 崔丽凤2(
), 崔丽凤2( )
)
- 1.化学工程联合国家重点实验室,天津化学化工协同创新中心,天津市应用催化科学与工程重点实验室,天津大学化工学院,天津 300354
2.山东华鲁恒升化工股份有限公司,山东 德州 253024
-
收稿日期:2023-12-15修回日期:2024-03-01出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:李新刚,崔丽凤 -
作者简介:周运桃(1988—),男,博士,研究方向为催化反应。E-mail:zhouyuntao1005@163.com。 -
基金资助:国家重点研发计划(2022YFB4101800)
Application and research progress of CeO2 support in CO2 hydrogenation to methanol
ZHOU Yuntao1,2( ), WANG Hongxing1,2, LI Xingang1(
), WANG Hongxing1,2, LI Xingang1( ), CUI Lifeng2(
), CUI Lifeng2( )
)
- 1.State Key Laboratory of Chemical Engineering, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin Key Laboratory of Applied Catalysis Science and Engineering, School of Chemical Engineering & Technology, Tianjin University, Tianjin 300354, China
2.Shandong Hualu Hengsheng Chemical Co. , Ltd. , Dezhou 253024, Shandong, China
-
Received:2023-12-15Revised:2024-03-01Online:2024-05-15Published:2024-06-15 -
Contact:LI Xingang, CUI Lifeng
摘要:
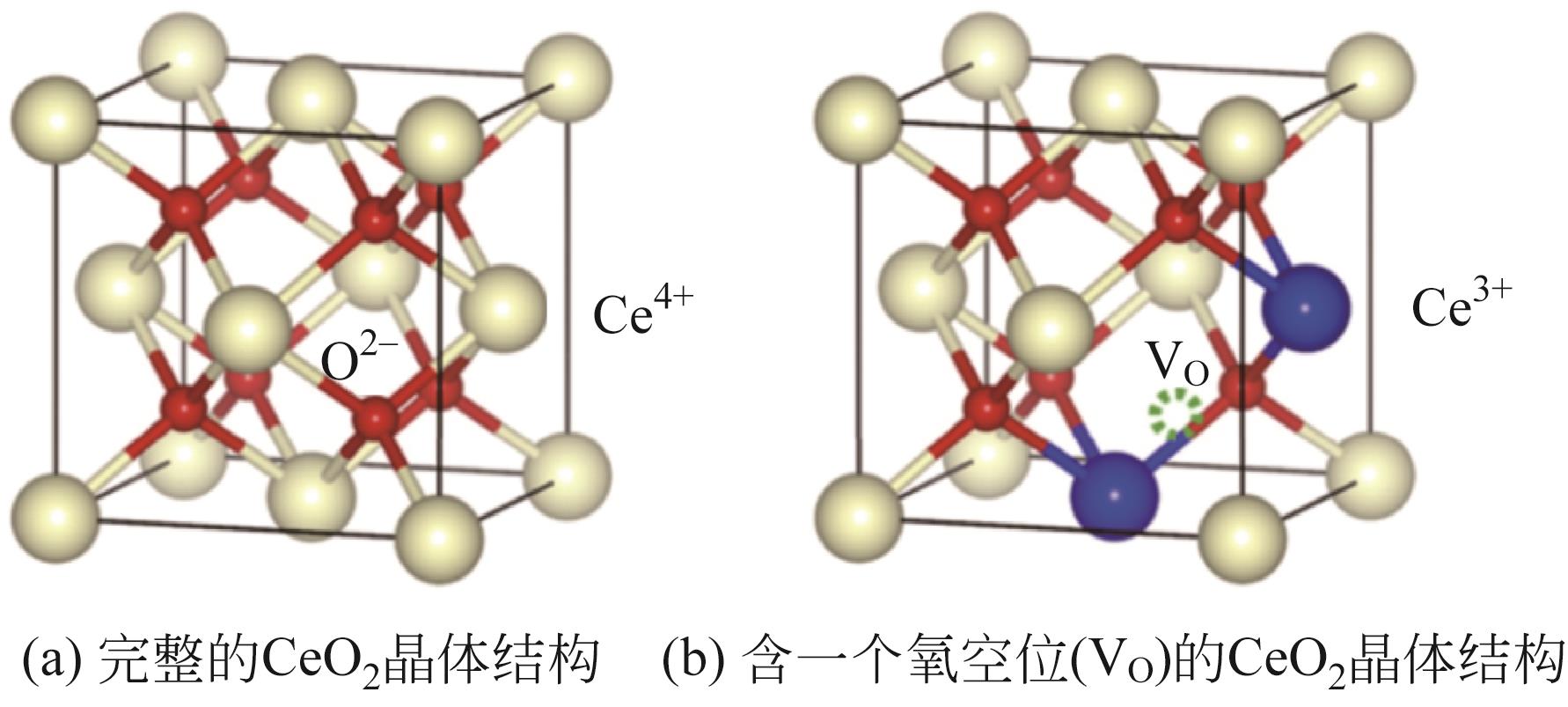
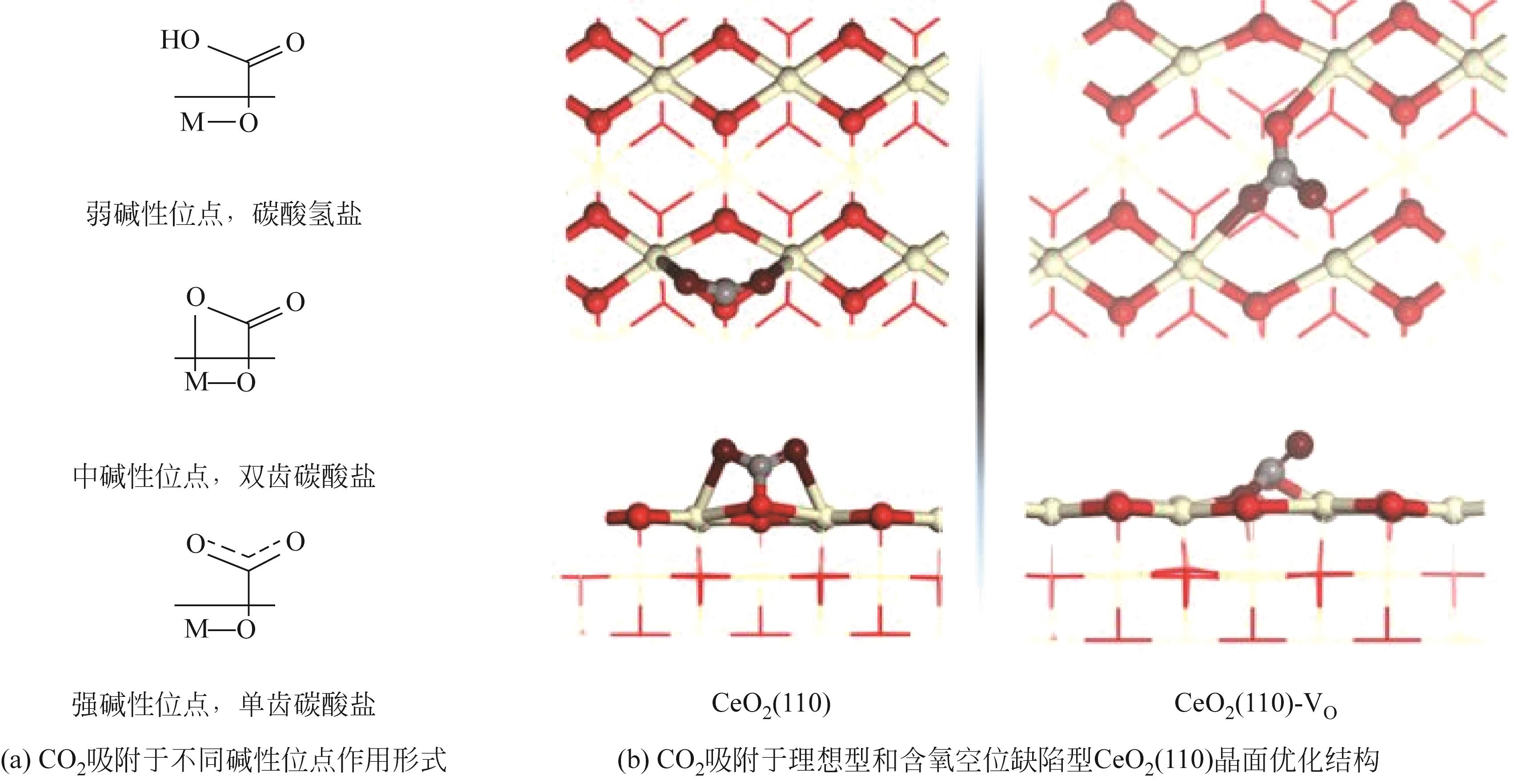
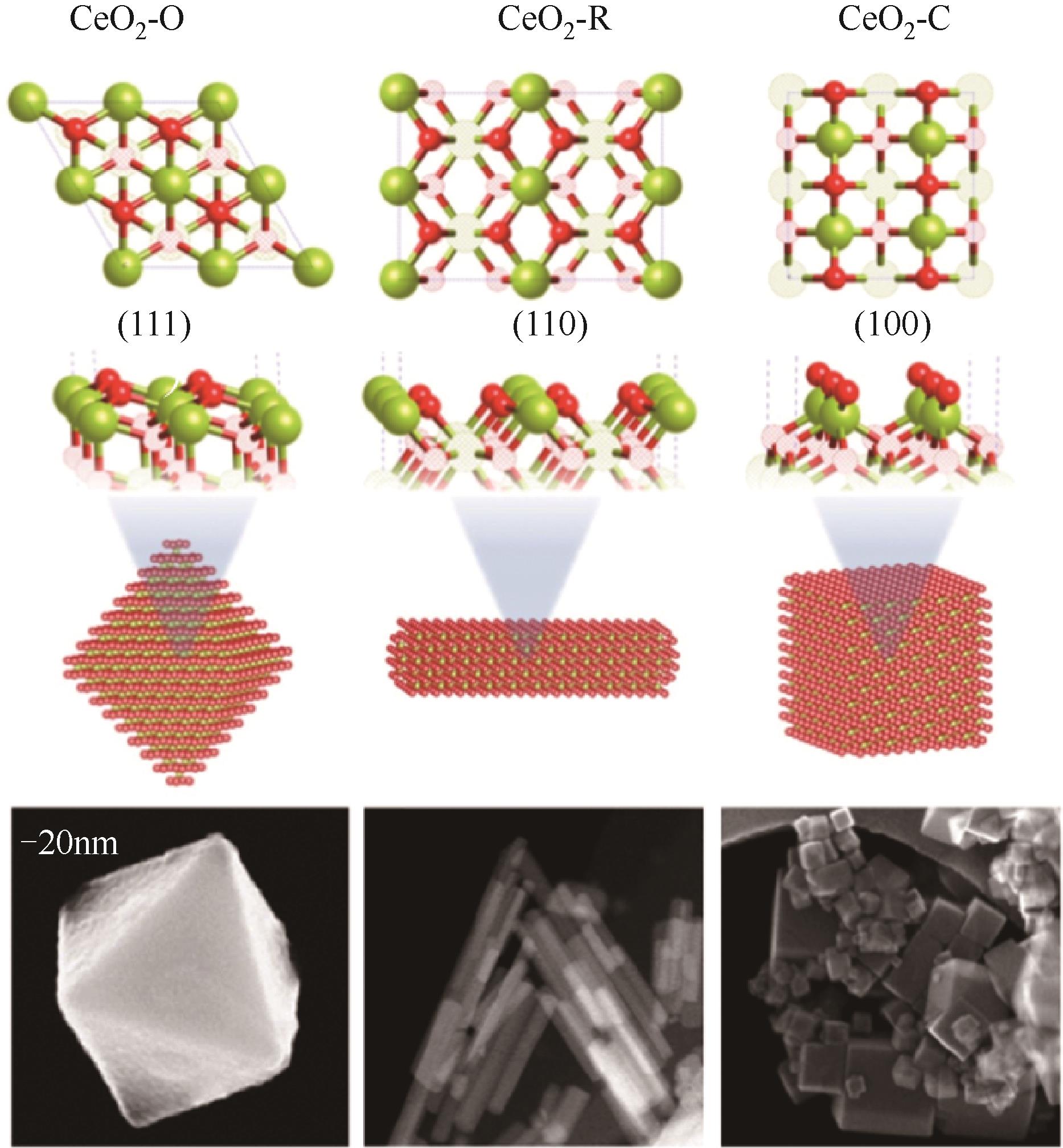
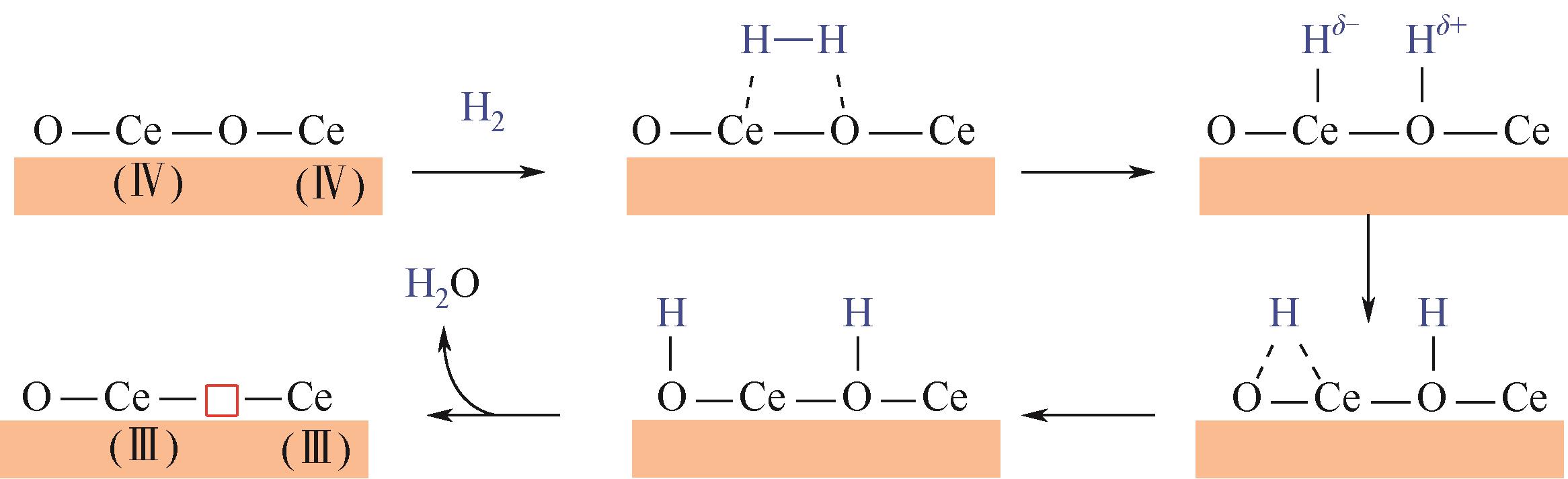
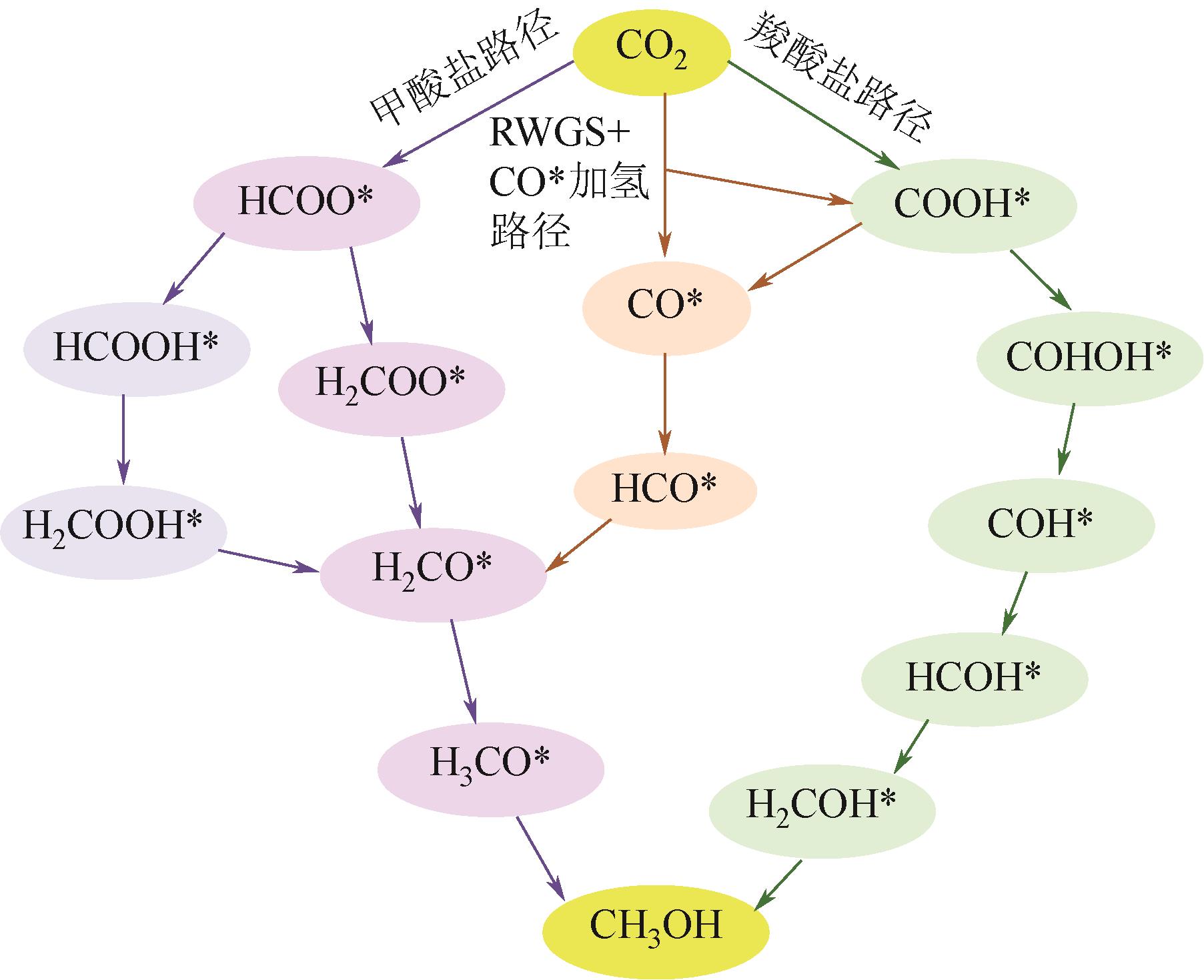
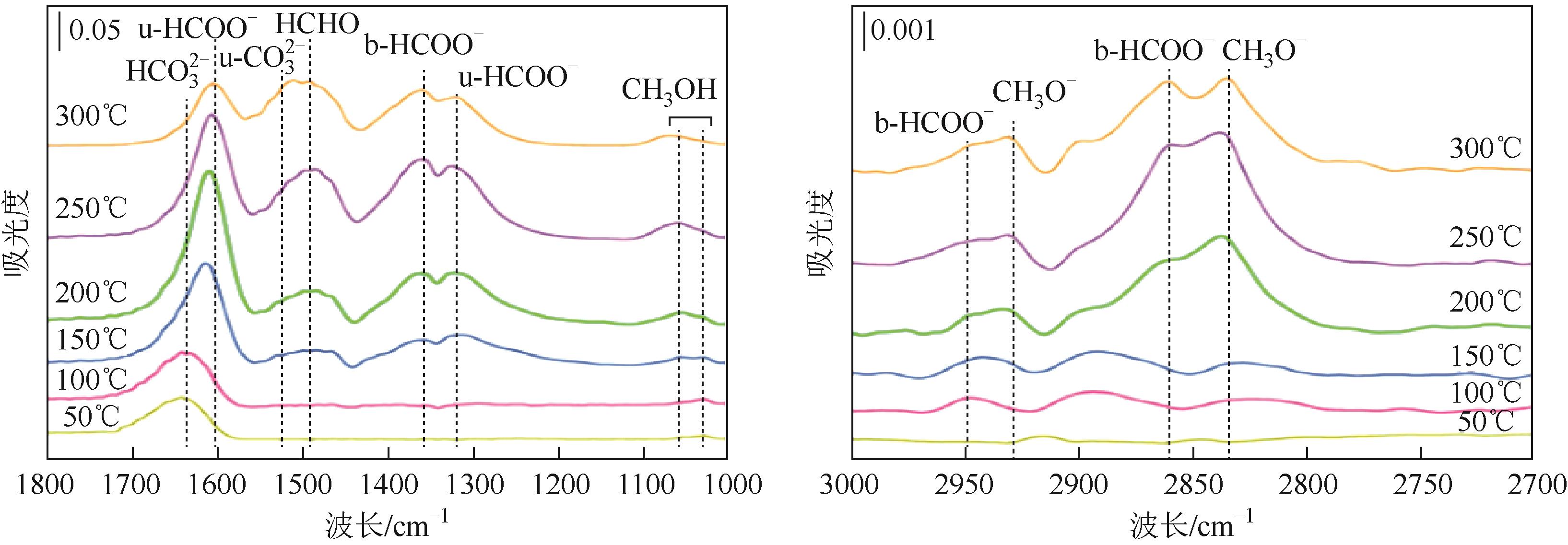
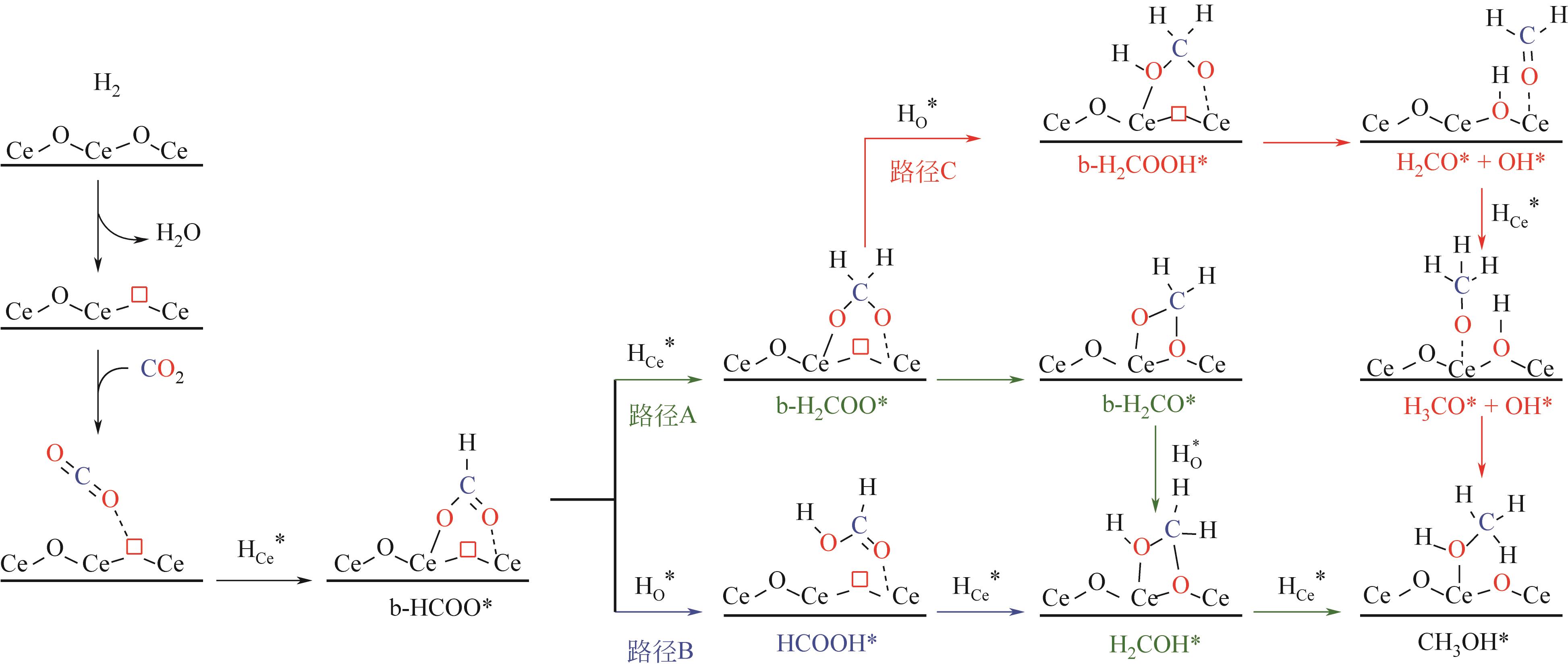
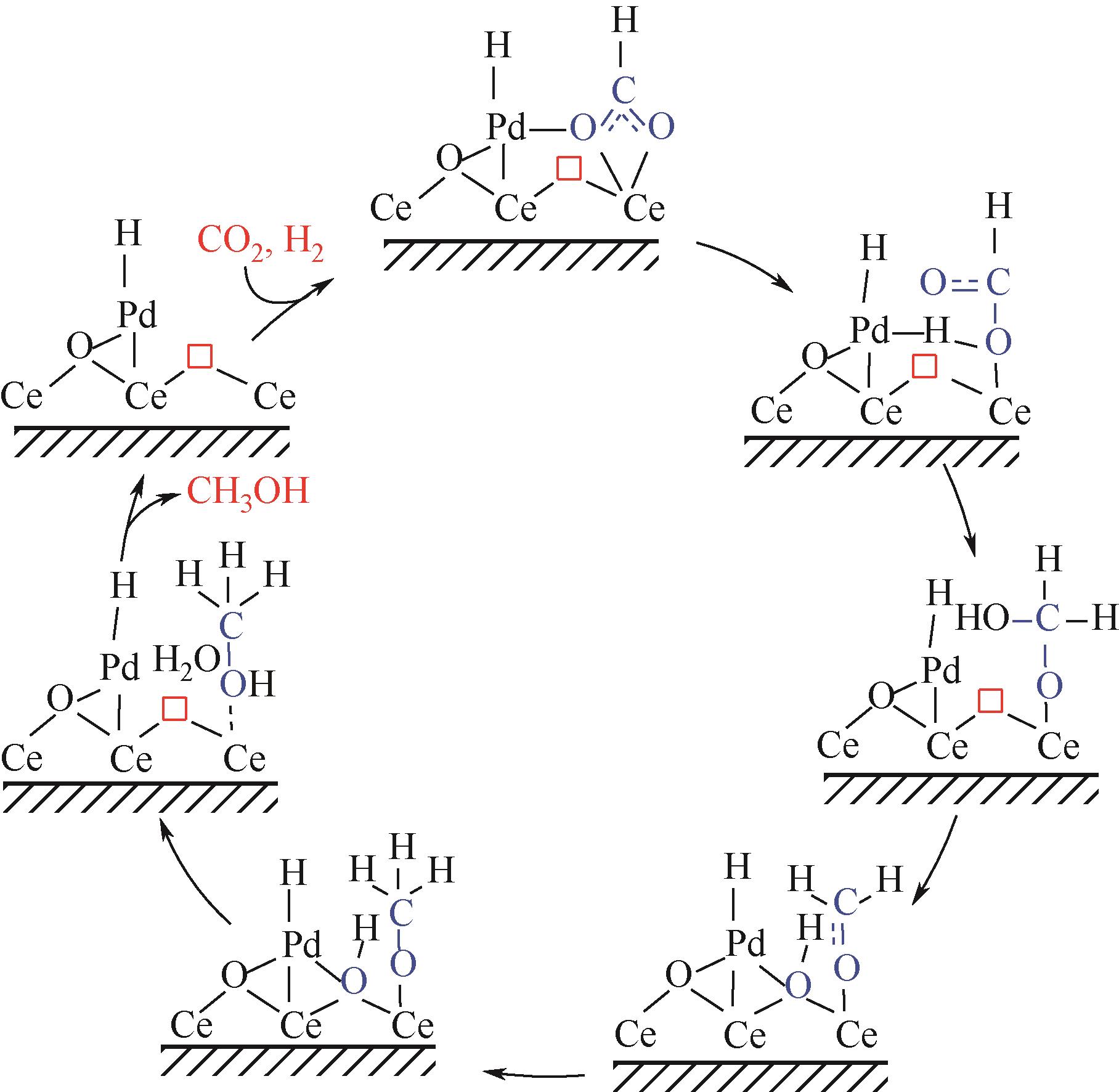
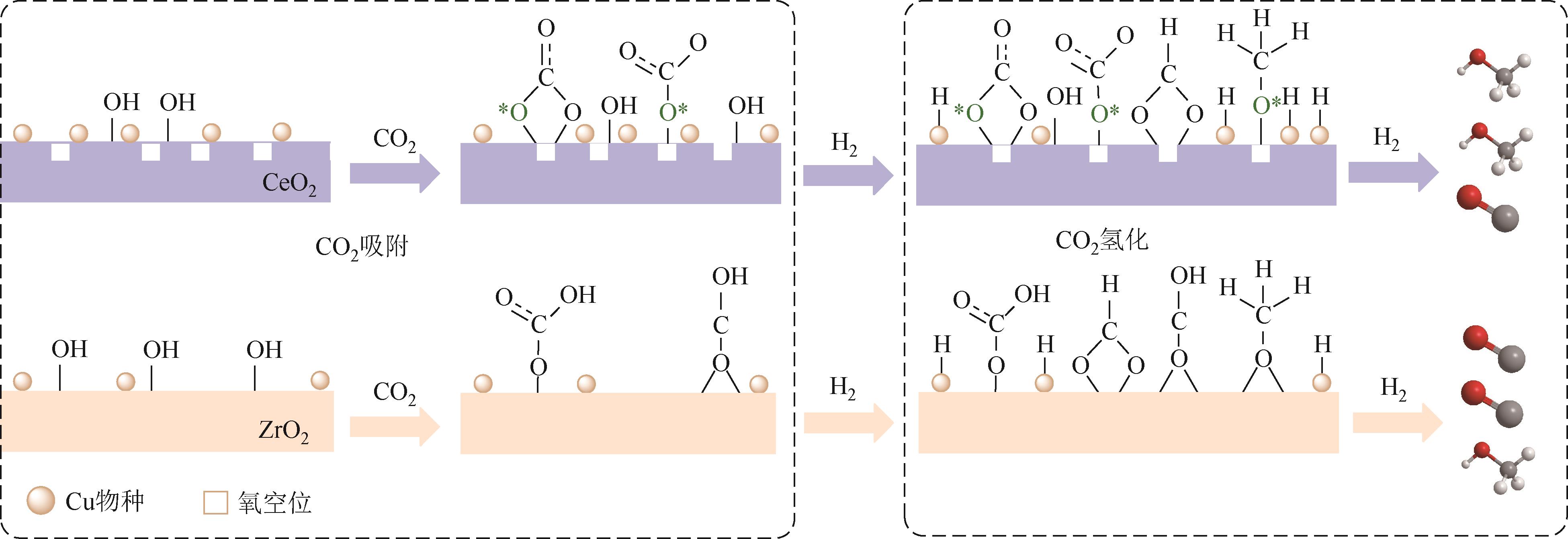
随着经济的快速发展,CO2排放导致的温室效应日益严峻,通过与绿氢反应将其转化为甲醇等有价值的化工产品是减少CO2排放、缓解全球气候变暖的有效途径。近年来,CeO2负载金属催化剂在CO2加氢制甲醇反应中得到了广泛关注。本文主要综述了CeO2表面氧空位和碱性位点、Ce3+/Ce4+可逆氧化还原能力、几何形貌等特性在CO2/H2吸附活化和甲醇形成过程中所起到的重要作用,比较了CeO2与ZrO2、ZnO以及复合金属氧化物等载体在反应中的差异,提出了CeO2载体催化剂在确认活性关键中间体、兼顾CO2转化率和甲醇选择性、提高催化活性与稳定性等方面的不足和挑战,以期为新型CO2加氢制甲醇催化剂的设计提供有益参考。
中图分类号:
引用本文
周运桃, 王洪星, 李新刚, 崔丽凤. CeO2载体在CO2加氢制甲醇中的应用和研究进展[J]. 化工进展, 2024, 43(5): 2723-2738.
ZHOU Yuntao, WANG Hongxing, LI Xingang, CUI Lifeng. Application and research progress of CeO2 support in CO2 hydrogenation to methanol[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2723-2738.
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
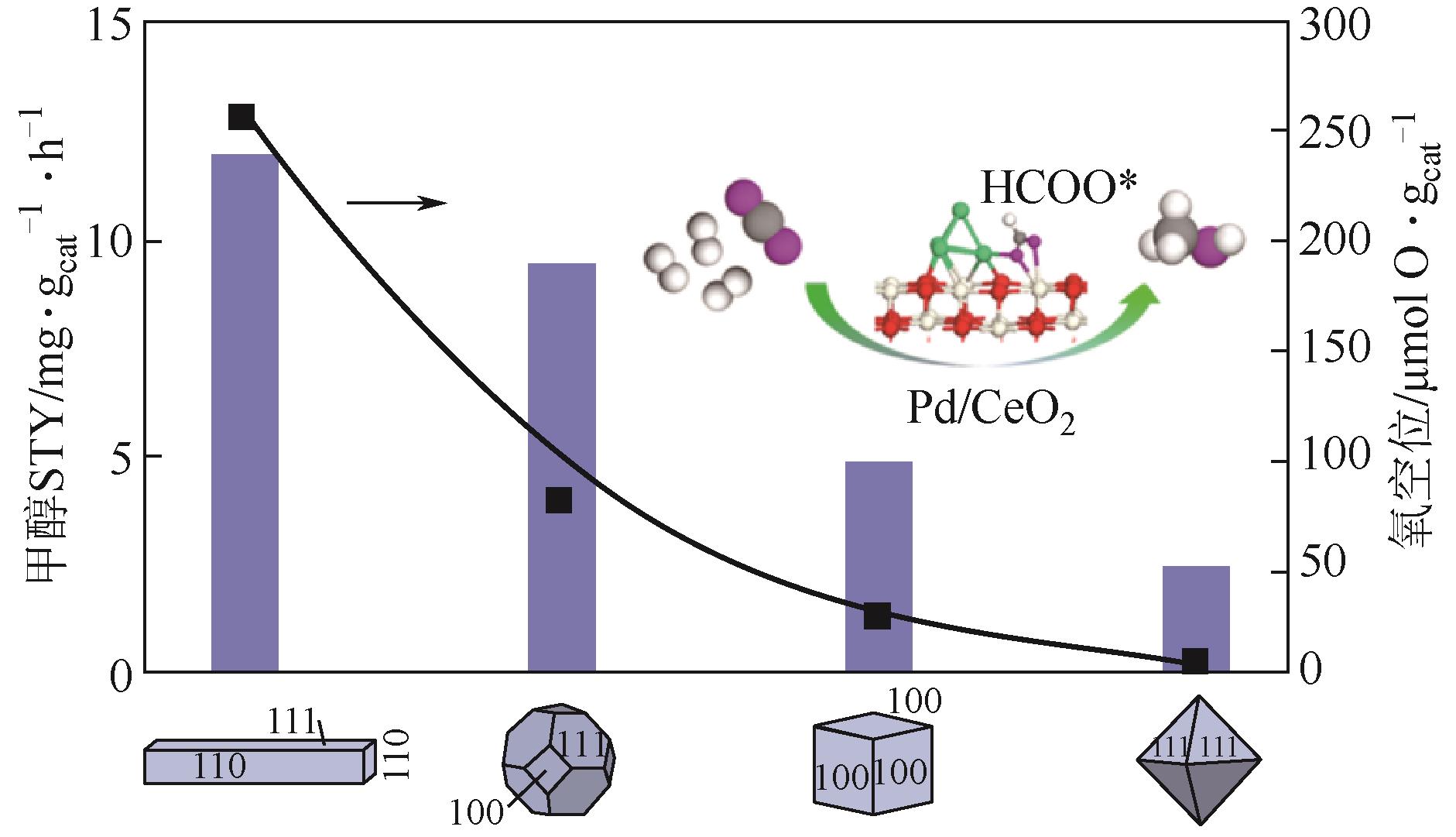
| 2Pd/CeO2-R[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.2① | 约30① | 12.0 |
| 2Pd/CeO2-P[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约29① | 9.5 |
| 2Pd/CeO2-C[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约25① | 5.0 |
| 2Pd/CeO2-O[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.3① | 约26① | 2.5 |
| Cu/CeO2-R[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约2.2① | 89.5 | 21.1 |
| Cu/CeO2-C[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约1.5① | 84 | 13.5 |
| Cu/CeO2-S[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约0.6① | 89 | 5.7 |
| 1Cu2Ni/CeO2-R[ | 3 | 260,3.0 | 6.0L/h | 18.3 | 73.3 | 230.2 |
| 1Cu2Ni/CeO2-S[ | 3 | 260,3.0 | 6.0L/h | 约17.5① | 约69.5① | 208.8 |
| 1Cu2Ni/CeO2-P[ | 3 | 260,3.0 | 6.0L/h | 约14.0① | 约68.5① | 164.6 |
| Cu/CeO2-SG[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 6.4 | 89.1 | 48.9 |
| Cu/CeO2-SCP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 5.9 | 84.6 | 42.8 |
| Cu/CeO2-CP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 3.8 | 80.5 | 26.2 |
| Cu-CF[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约9.8① | 约41.0① | 249.0 |
| Cu-CP[ | 3 | 260,3.0 | 18.0L·/(gcat·h) | 约9.0① | 约35.0① | 197.0 |
| Cu-IM[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约5.1① | 约42.5① | 143.0 |
| 5Ag/ZrCeO x-IM[ | 3 | 250,2.0 | 1.8L/h | 7 | 70 | 31.9 |
| 5Ag/ZrCeO x-CH[ | 3 | 250,2.0 | 1.8L/h | 8 | 60 | 30.9 |
表1 CeO2形貌对催化活性的影响
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 2Pd/CeO2-R[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.2① | 约30① | 12.0 |
| 2Pd/CeO2-P[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约29① | 9.5 |
| 2Pd/CeO2-C[ | 3 | 240,3.0 | 2.0L/(gcat·h) | — | 约25① | 5.0 |
| 2Pd/CeO2-O[ | 3 | 240,3.0 | 2.0L/(gcat·h) | 约6.3① | 约26① | 2.5 |
| Cu/CeO2-R[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约2.2① | 89.5 | 21.1 |
| Cu/CeO2-C[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约1.5① | 84 | 13.5 |
| Cu/CeO2-S[ | 3 | 240,2.0 | 3.0L/(gcat·h) | 约0.6① | 89 | 5.7 |
| 1Cu2Ni/CeO2-R[ | 3 | 260,3.0 | 6.0L/h | 18.3 | 73.3 | 230.2 |
| 1Cu2Ni/CeO2-S[ | 3 | 260,3.0 | 6.0L/h | 约17.5① | 约69.5① | 208.8 |
| 1Cu2Ni/CeO2-P[ | 3 | 260,3.0 | 6.0L/h | 约14.0① | 约68.5① | 164.6 |
| Cu/CeO2-SG[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 6.4 | 89.1 | 48.9 |
| Cu/CeO2-SCP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 5.9 | 84.6 | 42.8 |
| Cu/CeO2-CP[ | 3 | 240,3.0 | 2.4L/(gcat·h) | 3.8 | 80.5 | 26.2 |
| Cu-CF[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约9.8① | 约41.0① | 249.0 |
| Cu-CP[ | 3 | 260,3.0 | 18.0L·/(gcat·h) | 约9.0① | 约35.0① | 197.0 |
| Cu-IM[ | 3 | 260,3.0 | 18.0L/(gcat·h) | 约5.1① | 约42.5① | 143.0 |
| 5Ag/ZrCeO x-IM[ | 3 | 250,2.0 | 1.8L/h | 7 | 70 | 31.9 |
| 5Ag/ZrCeO x-CH[ | 3 | 250,2.0 | 1.8L/h | 8 | 60 | 30.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 1Pd-10Cu/CeO2[ | 3 | 250,3.0 | 3.0L/(gcat·h) | 16.1 | 26.7 | 28.3 |
| 5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 6.3 | 100 | 54.1 |
| 0.5Ca5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 7.7 | 100 | 66.1 |
| PdZn/CeO2[ | 3 | 220,2.0 | 2.4L/(gcat·h) | 14.1 | 94.5 | 114.3 |
| CuNi2/CeO2-NT[ | 3 | 260,3.0 | 6.0L/h | 17.8 | 78.8 | 579.9 |
表2 CeO2负载双金属催化剂
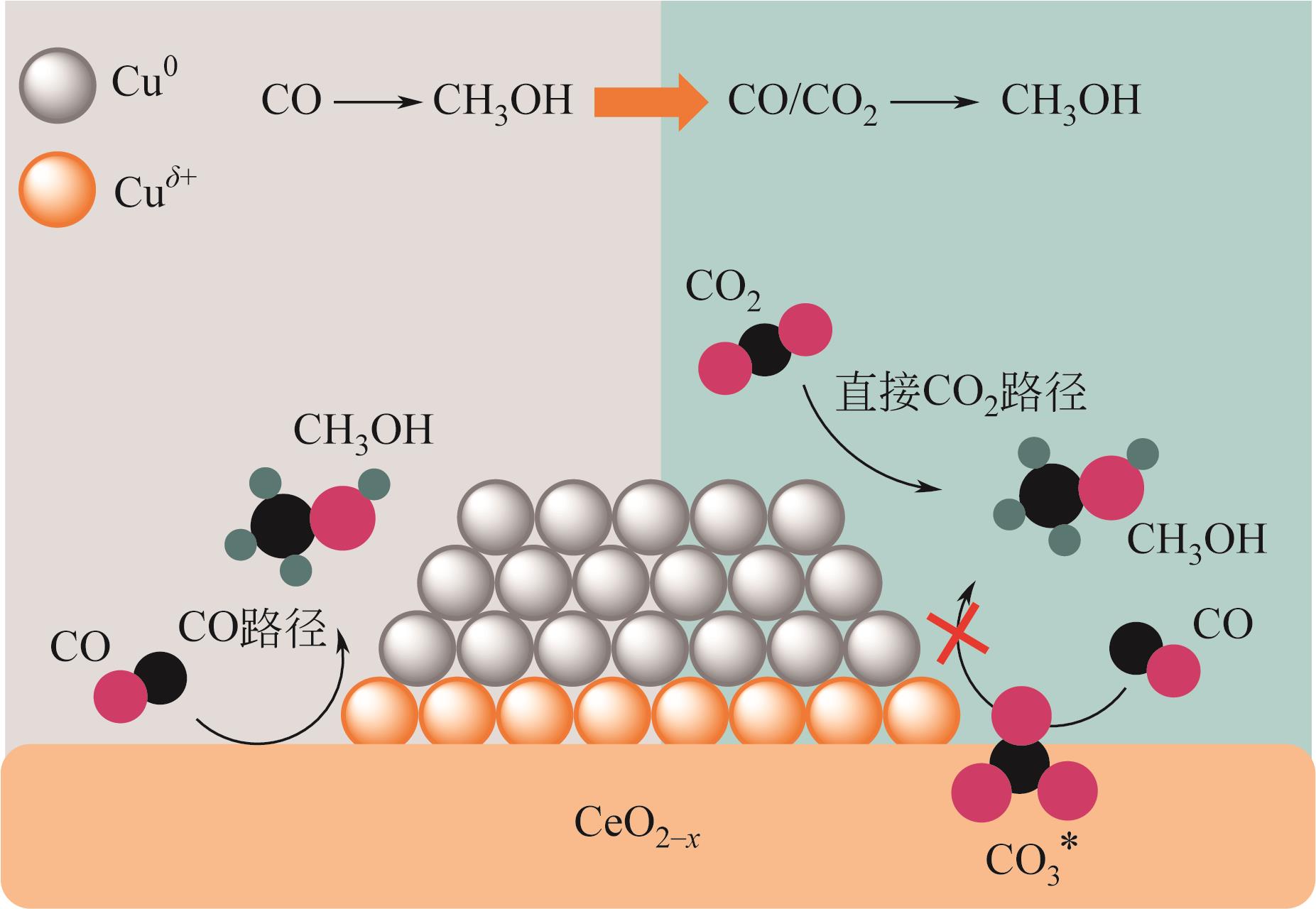
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| 1Pd-10Cu/CeO2[ | 3 | 250,3.0 | 3.0L/(gcat·h) | 16.1 | 26.7 | 28.3 |
| 5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 6.3 | 100 | 54.1 |
| 0.5Ca5Pd5Zn/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 7.7 | 100 | 66.1 |
| PdZn/CeO2[ | 3 | 220,2.0 | 2.4L/(gcat·h) | 14.1 | 94.5 | 114.3 |
| CuNi2/CeO2-NT[ | 3 | 260,3.0 | 6.0L/h | 17.8 | 78.8 | 579.9 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约2.5① | 91 | 19.5 |
| Cu/ZrO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.0① | 80 | 20.6 |
| Cu/ZnO[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.5① | 74 | 22.2 |
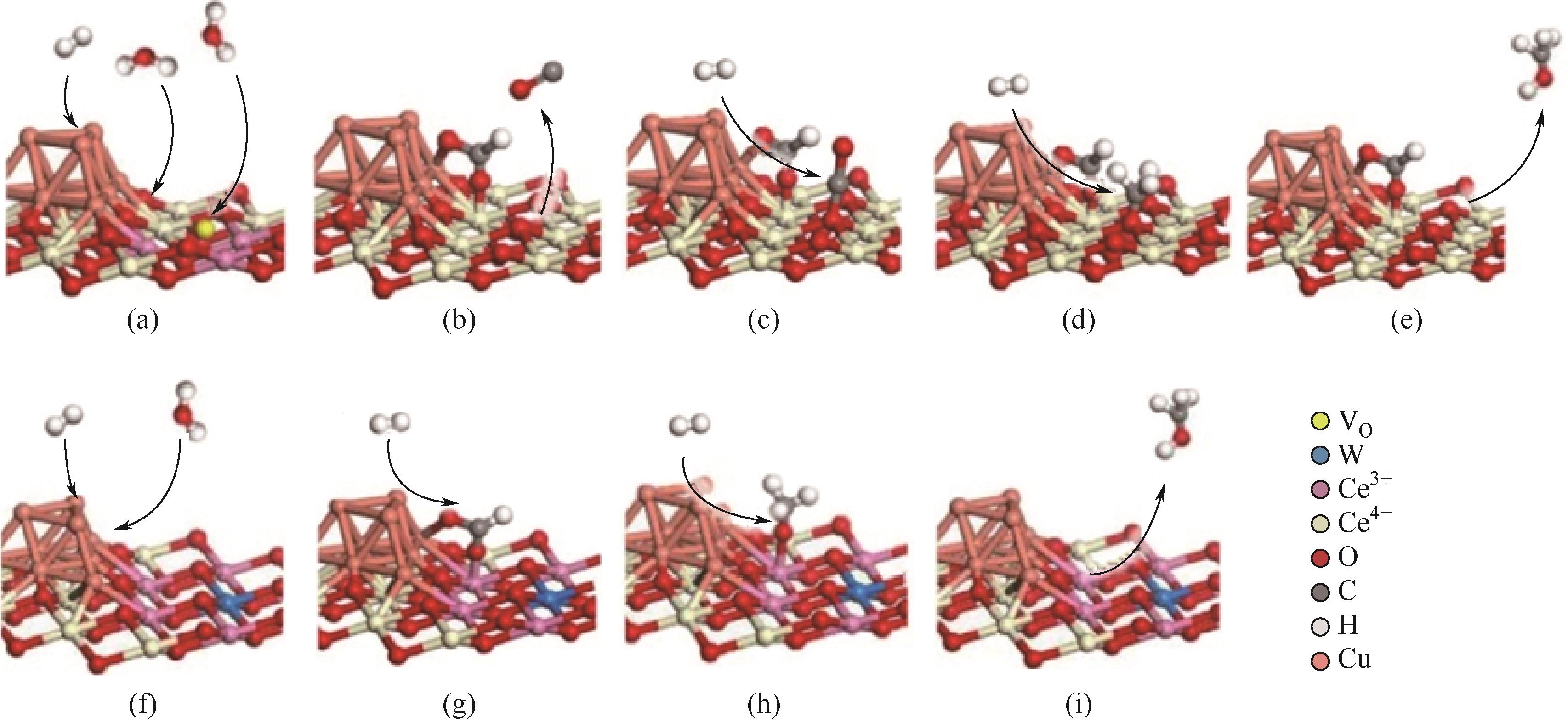
| Cu/CeO2[ | 3 | 220,3.0 | 10L/h | 4.5 | 约85① | 136.8 |
| Cu/ZrO2[ | 3 | 220,3.0 | 10L/h | 6.5 | 约77① | 179.0 |
| Cu/CeO2[ | 3 | 250,3.0 | 30L/(gcat·h) | 1.0 | 53 | 45.5 |
| Cu/SiO2[ | 3 | 250,3.0 | 60L/(gcat·h) | 1.1 | 16 | 30.2 |
| Au/CeO2[ | 9 | 225,0.1 | 20L/h | — | 62.2 | — |
| Au/ZnO[ | 9 | 225,0.1 | 20L/h | — | 88.9 | — |
| Ni5Ga3/SiO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 1.5 | 12.5 | 50.0 |
| Ni5Ga3/ZrO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 3.5 | 18.0 | 160.0 |
| Ni5Ga3/CeO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 6.1 | 3.4 | 54.0 |
表3 CO2加氢制甲醇催化剂载体效应
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/CeO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约2.5① | 91 | 19.5 |
| Cu/ZrO2[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.0① | 80 | 20.6 |
| Cu/ZnO[ | 3 | 220,3.0 | 2.4L/(gcat·h) | 约3.5① | 74 | 22.2 |
| Cu/CeO2[ | 3 | 220,3.0 | 10L/h | 4.5 | 约85① | 136.8 |
| Cu/ZrO2[ | 3 | 220,3.0 | 10L/h | 6.5 | 约77① | 179.0 |
| Cu/CeO2[ | 3 | 250,3.0 | 30L/(gcat·h) | 1.0 | 53 | 45.5 |
| Cu/SiO2[ | 3 | 250,3.0 | 60L/(gcat·h) | 1.1 | 16 | 30.2 |
| Au/CeO2[ | 9 | 225,0.1 | 20L/h | — | 62.2 | — |
| Au/ZnO[ | 9 | 225,0.1 | 20L/h | — | 88.9 | — |
| Ni5Ga3/SiO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 1.5 | 12.5 | 50.0 |
| Ni5Ga3/ZrO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 3.5 | 18.0 | 160.0 |
| Ni5Ga3/CeO2[ | 3 | 270,1.0 | 8.0L/(gcat·h) | 6.1 | 3.4 | 54.0 |
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/ZnO-CeO2[ | 3 | 280,3.0 | 12.0L/h | 15.6 | 64.5 | 431.7 |
| Cu/AlCeO-7[ | 3 | 260,3.0 | 14.4L/(gcat·h) | 约17① | 约45① | 381.3 |
| Cu0.3Zr0.3Ce0.7[ | 3 | 240,3.0 | 30.0L/(gcat·h) | 4.1 | 55.3 | 192.0 |
| Cu30Ce35Zr35O[ | 3 | 250,3.0 | 7.5L/(gcat·h) | 14.3 | 53.8 | 165.0 |
| 5-Cu-CeO2/ZrO2[ | 3 | 260,5.0 | 60.0L/(gcat·h) | 1.7 | 42 | 147.1 |
| CuO/Ce0.4Zr0.6O2[ | 3 | 280,3.0 | 10.0L/h | 13.2 | 71.8 | 338.9 |
| CuO/Mn0.2CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 14.2 | 82.3 | 250.0 |
| CuO/La0.25CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 15.7 | 83.3 | 281.0 |
| Cu/CeO2[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 1.3 | 51 | 40.4 |
| Cu/CeW0.25O x[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 13 | 87 | 394.7 |
表4 含CeO2复合氧化物催化剂活性
| 催化剂 | H2/CO2 | 温度/℃,压力/MPa | GHSV/WHSV | CO2转化率/% | 甲醇选择性/% | STY/g·kgcat-1·h-1 |
|---|---|---|---|---|---|---|
| Cu/ZnO-CeO2[ | 3 | 280,3.0 | 12.0L/h | 15.6 | 64.5 | 431.7 |
| Cu/AlCeO-7[ | 3 | 260,3.0 | 14.4L/(gcat·h) | 约17① | 约45① | 381.3 |
| Cu0.3Zr0.3Ce0.7[ | 3 | 240,3.0 | 30.0L/(gcat·h) | 4.1 | 55.3 | 192.0 |
| Cu30Ce35Zr35O[ | 3 | 250,3.0 | 7.5L/(gcat·h) | 14.3 | 53.8 | 165.0 |
| 5-Cu-CeO2/ZrO2[ | 3 | 260,5.0 | 60.0L/(gcat·h) | 1.7 | 42 | 147.1 |
| CuO/Ce0.4Zr0.6O2[ | 3 | 280,3.0 | 10.0L/h | 13.2 | 71.8 | 338.9 |
| CuO/Mn0.2CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 14.2 | 82.3 | 250.0 |
| CuO/La0.25CeO x[ | 3 | 260,1.5 | 6.0L/(gcat·h) | 15.7 | 83.3 | 281.0 |
| Cu/CeO2[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 1.3 | 51 | 40.4 |
| Cu/CeW0.25O x[ | 3 | 250,3.5 | 15.0L/(gcat·h) | 13 | 87 | 394.7 |
| 1 | FORSTER Piers M, SMITH Christopher J, WALSH Tristram, et al. Indicators of global climate change 2022: Annual update of large-scale indicators of the state of the climate system and human influence[J]. Earth System Science Data, 2023, 15(6): 2295-2327. |
| 2 | CHENG Lijing, ABRAHAM John, TRENBERTH Kevin E, et al. Another year of record heat for the oceans[J]. Advances in Atmospheric Sciences, 2023, 40(6): 963-974. |
| 3 | LAN Xin, TANS Pieter, KIRK Thoning. Trends in globally-averaged CO2 determined from NOAA Global Monitoring Laboratory measurements[EB/OL]. (2023-11-30) [2023-12-10]. . |
| 4 | LIN Qingyang, ZHANG Xiao, WANG Tao, et al. Technical perspective of carbon capture, utilization, and storage[J]. Engineering, 2022, 14: 27-32. |
| 5 | DE Sudipta, DOKANIA Abhay, RAMIREZ Adrian, et al. Advances in the design of heterogeneous catalysts and thermocatalytic processes for CO2 utilization[J]. ACS Catalysis, 2020, 10(23): 14147-14185. |
| 6 | KUMARAVEL Vignesh, BARTLETT John, PILLAI Suresh C. Photoelectrochemical conversion of carbon dioxide (CO2) into fuels and value-added products[J]. ACS Energy Letters, 2020, 5(2): 486-519. |
| 7 | GAO Peng, ZHANG Lina, LI Shenggang, et al. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Central Science, 2020, 6(10): 1657-1670. |
| 8 | KALIYAPERUMAL Alamelu, GUPTA Pooja, PRASAD Yadavalli Satya Sivaram, et al. Recent progress and perspective of the electrochemical conversion of carbon dioxide to alcohols[J]. ACS Engineering Au, 2023, 3(6): 403-425. |
| 9 | GANJI Parameswaram, CHOWDARI Ramesh Kumar, LIKOZAR Blaž. Photocatalytic reduction of carbon dioxide to methanol: Carbonaceous materials, kinetics, industrial feasibility, and future directions[J]. Energy & Fuels, 2023, 37(11): 7577-7602. |
| 10 | MINYUKOVA Tatyana P, DOKUCHITS Eugene V. Hydrogen for CO2 processing in heterogeneous catalytic reactions[J]. International Journal of Hydrogen Energy, 2023, 48(59): 22462-22483. |
| 11 | SHIH Choon Fong, ZHANG Tao, LI Jinghai, et al. Powering the future with liquid sunshine[J]. Joule, 2018, 2(10): 1925-1949. |
| 12 | DANG Shanshan, YANG Haiyan, GAO Peng, et al. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation[J]. Catalysis Today, 2019, 330: 61-75. |
| 13 | ZHONG Jiawei, YANG Xiaofeng, WU Zhilian, et al. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol[J]. Chemical Society Reviews, 2020, 49(5): 1385-1413. |
| 14 | ROY Soumyabrata, CHEREVOTAN Arjun, PETER Sebastian C. Thermochemical CO2 hydrogenation to single carbon products: Scientific and technological challenges[J]. ACS Energy Letters, 2018, 3(8): 1938-1966. |
| 15 | BAI Shaotao, DE SMET Gilles, LIAO Yuhe, et al. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions[J]. Chemical Society Reviews, 2021, 50(7): 4259-4298. |
| 16 | MURTHY Pradeep S, LIANG Weibin, JIANG Yijiao, et al. Cu-based nanocatalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2021, 35(10): 8558-8584. |
| 17 | VIEIRA Luiz H, RASTEIRO Letícia F, SANTANA Cássia S, et al. Noble metals in recent developments of heterogeneous catalysts for CO2 conversion processes[J]. ChemCatChem, 2023, 15(14): e202300493. |
| 18 | LIANG Binglian, MA Junguo, SU Xiong, et al. Investigation on deactivation of Cu/ZnO/Al2O3 catalyst for CO2 hydrogenation to methanol[J]. Industrial & Engineering Chemistry Research, 2019, 58(21): 9030-9037. |
| 19 | WANG Jijie, LI Guanna, LI Zelong, et al. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Science Advances, 2017, 3(10): e1701290. |
| 20 | YANG Chengsheng, PEI Chunlei, LUO Ran, et al. Strong electronic oxide-support interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol[J]. Journal of the American Chemical Society, 2020, 142(46): 19523-19531. |
| 21 | MARTIN Oliver, MARTÍN Antonio J, MONDELLI Cecilia, et al. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2016, 55(21): 6261-6265. |
| 22 | LI Huazheng, QIU Chenglong, REN Shoujie, et al. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels[J]. Science, 2020, 367(6478): 667-671. |
| 23 | SONOWAL Karanika, NANDAL Neha, BASYACH Purashri, et al. Photocatalytic reduction of CO2 to methanol using Zr(Ⅳ)-based MOF composite with g-C3N4 quantum dots under visible light irradiation[J]. Journal of CO2 Utilization, 2022, 57: 101905. |
| 24 | WANG Fei, WEI Min, EVANS David G, et al. CeO2-based heterogeneous catalysts toward catalytic conversion of CO2 [J]. Journal of Materials Chemistry A, 2016, 4(16): 5773-5783. |
| 25 | CHANG Kuan, ZHANG Haochen, CHENG Mu-jeng, et al. Application of ceria in CO2 conversion catalysis[J]. ACS Catalysis, 2020, 10(1): 613-631. |
| 26 | ZHANG Sai, TIAN Zhimin, MA Yuanyuan, et al. Adsorption of molecules on defective CeO2 for advanced catalysis[J]. ACS Catalysis, 2023, 13(7): 4629-4645. |
| 27 | GRACIANI Jesús, MUDIYANSELAGE Kumudu, XU Fang, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2 [J]. Science, 2014, 345(6196): 546-550. |
| 28 | HUANG Weixin, GAO Yuxian. Morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals[J]. Catalysis Science & Technology, 2014, 4(11): 3772-3784. |
| 29 | TROVARELLI Alessandro, LLORCA Jordi. Ceria catalysts at nanoscale: How do crystal shapes shape catalysis?[J]. ACS Catalysis, 2017, 7(7): 4716-4735. |
| 30 | MA Yuanyuan, GAO Wei, ZHANG Zhiyun, et al. Regulating the surface of nanoceria and its applications in heterogeneous catalysis[J]. Surface Science Reports, 2018, 73(1): 1-36. |
| 31 | LI Ping, CHEN Xiaoyin, LI Yongdan, et al. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control[J]. Catalysis Today, 2019, 327: 90-115. |
| 32 | HUANG Xiubing, ZHANG Kaiyue, PENG Baoxiang, et al. Ceria-based materials for thermocatalytic and photocatalytic organic synthesis[J]. ACS Catalysis, 2021, 11(15): 9618-9678. |
| 33 | VIVIER Laurence, DUPREZ Daniel. Ceria-based solid catalysts for organic chemistry[J]. ChemSusChem, 2010, 3(6): 654-678. |
| 34 | LI Ge, WANG Ping, HE Miao, et al. Cerium-based nanomaterials for photo/electrocatalysis[J]. Science China Chemistry, 2023, 66(8): 2204-2220. |
| 35 | CAI Jun, LI Danyang, JIANG Lei, et al. Review on CeO2-based photocatalysts for photocatalytic reduction of CO2: Progresses and perspectives[J]. Energy & Fuels, 2023, 37(7): 4878-4897. |
| 36 | WANG Jianda, XIAO Xiao, LIU Yong, et al. The application of CeO2-based materials in electrocatalysis[J]. Journal of Materials Chemistry A, 2019, 7(30): 17675-17702. |
| 37 | YU Jun, DU Xinjuan, LIU Hongzhi, et al. Mini review on active sites in Ce-based electrocatalysts for alkaline water splitting[J]. Energy & Fuels, 2021, 35(23): 19000-19011. |
| 38 | MONTINI Tiziano, MELCHIONNA Michele, MONAI Matteo, et al. Fundamentals and catalytic applications of CeO2-based materials[J]. Chemical Reviews, 2016, 116(10): 5987-6041. |
| 39 | SINGH Rajan, PANDEY Vaibhav, PANT Kamal Kishore. Promotional role of oxygen vacancy defects and Cu-Ce interfacial sites on the activity of Cu/CeO2 catalyst for CO2 hydrogenation to methanol[J]. ChemCatChem, 2022, 14(24): e202201053. |
| 40 | GAO Peng, LI Feng, ZHAO Ning, et al. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2013, 468: 442-452. |
| 41 | SINGH Rajan, TRIPATHI Komal, PANT Kamal Kishore. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation[J]. Fuel, 2021, 303: 121289. |
| 42 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction[J]. Journal of Energy Chemistry, 2020, 40: 22-30. |
| 43 | FAN Liping, ZHANG Jing, MA Kexin, et al. Ceria morphology-dependent Pd-CeO2 interaction and catalysis in CO2 hydrogenation into formate[J]. Journal of Catalysis, 2021, 397: 116-127. |
| 44 | XIE Yu, CHEN Jianjun, WU Xi, et al. Frustrated Lewis pairs boosting low-temperature CO2 methanation performance over Ni/CeO2 nanocatalysts[J]. ACS Catalysis, 2022, 12(17): 10587-10602. |
| 45 | ZHANG Sai, XIA Zhaoming, ZOU Yong, et al. Interfacial frustrated Lewis pairs of CeO2 activate CO2 for selective tandem transformation of olefins and CO2 into cyclic carbonates[J]. Journal of the American Chemical Society, 2019, 141(29): 11353-11357. |
| 46 | LIU Bin, LI Congming, ZHANG Guoqiang, et al. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods[J]. ACS Catalysis, 2018, 8(11): 10446-10456. |
| 47 | ZHANG Wei, MA Xuelu, XIAO Hai, et al. Mechanistic investigations on thermal hydrogenation of CO2 to methanol by nanostructured CeO2(100): The crystal-plane effect on catalytic reactivity[J]. The Journal of Physical Chemistry C, 2019, 123(18): 11763-11771. |
| 48 | KUMARI Neetu, HAIDER M ALI, AGARWAL Manish, et al. Role of reduced CeO2(110) surface for CO2 reduction to CO and methanol[J]. The Journal of Physical Chemistry C, 2016, 120(30): 16626-16635. |
| 49 | SCHMITT Rafael, NENNING Andreas, KRAYNIS Olga, et al. A review of defect structure and chemistry in ceria and its solid solutions[J]. Chemical Society Reviews, 2020, 49(2): 554-592. |
| 50 | GUO Chen, WEI Shuxian, ZHOU Sainan, et al. Initial reduction of CO2 on Pd-, Ru-, and Cu-doped CeO2(111) surfaces: Effects of surface modification on catalytic activity and selectivity[J]. ACS Applied Materials & Interfaces, 2017, 9(31): 26107-26117. |
| 51 | Marçal CAPDEVILA-CORTADA, Gianvito VILÉ, TESCHNER Detre, et al. Reactivity descriptors for ceria in catalysis[J]. Applied Catalysis B: Environmental, 2016, 197: 299-312. |
| 52 | ZHANG Yang, ZHAO Shuna, FENG Jing, et al. Unraveling the physical chemistry and materials science of CeO2-based nanostructures[J]. Chem., 2021, 7(8): 2022-2059. |
| 53 | JIANG Feng, WANG Shanshan, LIU Bing, et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts[J]. ACS Catalysis, 2020, 10(19): 11493-11509. |
| 54 | OUYANG Bi, TAN Weiling, LIU Bing. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation[J]. Catalysis Communications, 2017, 95: 36-39. |
| 55 | WEI Yuechang, ZHANG Yilin, ZHANG Peng, et al. Boosting the removal of diesel soot particles by the optimal exposed crystal facet of CeO2 in Au/CeO2 catalysts[J]. Environmental Science & Technology, 2020, 54(3): 2002-2011. |
| 56 | WANG Zheng, HUANG Zhenpeng, BROSNAHAN John T, et al. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion[J]. Environmental Science & Technology, 2019, 53(9): 5349-5358. |
| 57 | WERNER Kristin, WENG Xuefei, CALAZA Florencia, et al. Toward an understanding of selective alkyne hydrogenation on ceria: On the impact of O vacancies on H2 interaction with CeO2(111)[J]. Journal of the American Chemical Society, 2017, 139(48): 17608-17616. |
| 58 | WANG Zhiqiang, CHU Deren, ZHOU Hui, et al. Role of low-coordinated Ce in hydride formation and selective hydrogenation reactions on CeO2 surfaces[J]. ACS Catalysis, 2022, 12(1): 624-632. |
| 59 | WANG Zhiqiang, LIU Huihui, WU Xinping, et al. Hydride generation on the Cu-doped CeO2(111) surface and its role in CO2 hydrogenation reactions[J]. Catalysts, 2022, 12(9): 963. |
| 60 | LEE Jaeha, TIEU Peter, FINZEL Jordan, et al. How Pt influences H2 reactions on high surface-area Pt/CeO2 powder catalyst surfaces[J]. JACS Au, 2023, 3(8): 2299-2313. |
| 61 | AZHARI Noerma J, ERIKA Denanti, MARDIANA St, et al. Methanol synthesis from CO2: A mechanistic overview[J]. Results in Engineering, 2022, 16: 100711. |
| 62 | ZHAO Yafan, YANG Yong, MIMS Charles, et al. Insight into methanol synthesis from CO2 hydrogenation on Cu(111): Complex reaction network and the effects of H2O[J]. Journal of Catalysis, 2011, 281(2): 199-211. |
| 63 | LIU Lingna, YAO Hedan, JIANG Zhao, et al. Theoretical study of methanol synthesis from CO2 hydrogenation on PdCu3(111) surface[J]. Applied Surface Science, 2018, 451: 333-345. |
| 64 | NIE Xiaowa, JIANG Xiao, WANG Haozhi, et al. Mechanistic understanding of alloy effect and water promotion for Pd-Cu bimetallic catalysts in CO2 hydrogenation to methanol[J]. ACS Catalysis, 2018, 8(6): 4873-4892. |
| 65 | WU Wenlong, WANG Yanan, LUO Lei, et al. CO2 hydrogenation over copper/ZnO single-atom catalysts: Water-promoted transient synthesis of methanol[J]. Angewandte Chemie, 2022, 134(48): e202213024. |
| 66 | JIANG Lei, LI Kongzhai, PORTER William N, et al. Role of H2O in catalytic conversion of C1 molecules[J]. Journal of the American Chemical Society, 2024, 146(5): 2857-2875. |
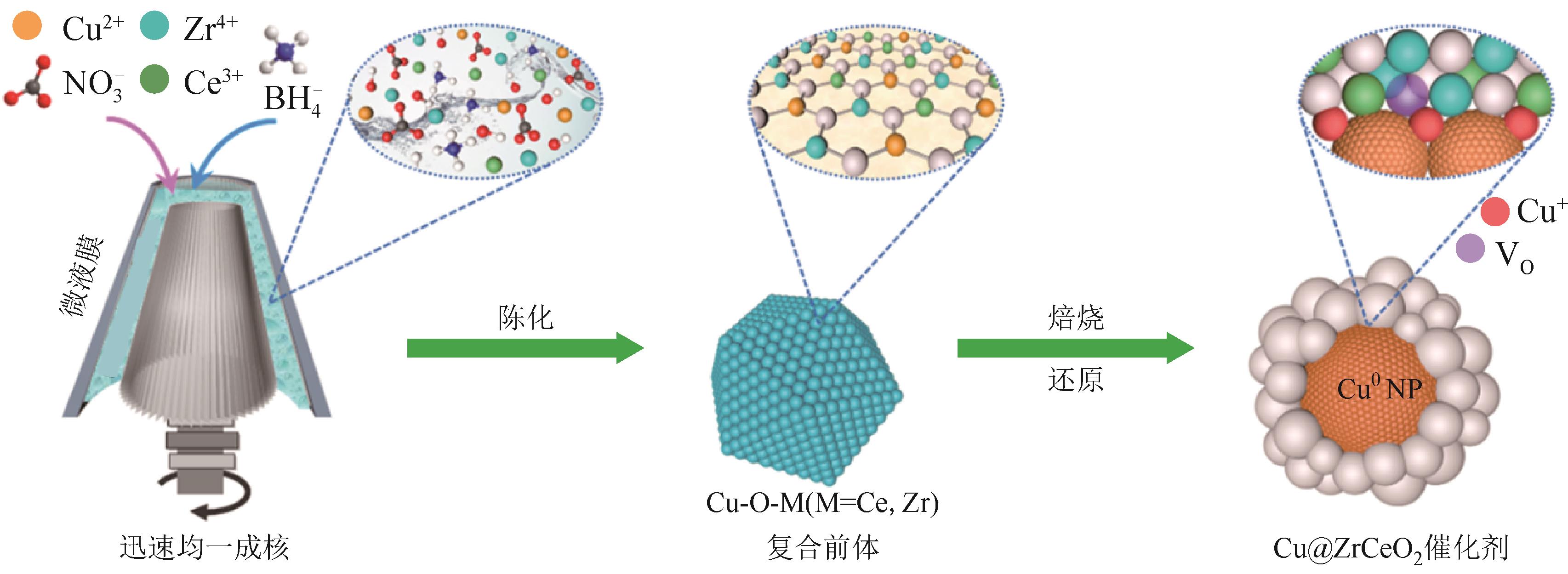
| 67 | WANG Hao, ZHANG Guangcheng, FAN Guoli, et al. Fabrication of Zr-Ce oxide solid solution surrounded Cu-based catalyst assisted by a microliquid film reactor for efficient CO2 hydrogenation to produce methanol[J]. Industrial & Engineering Chemistry Research, 2021, 60(45): 16188-16200. |
| 68 | NIE Mengdong, CUI Aixin, WU Man, et al. CuO/LaCeO x catalysts with enhanced metal-support interactions for CO2 methanolization[J]. Journal of CO2 Utilization, 2023, 75: 102579. |
| 69 | MALIK Ali Shan, ZAMAN Sharif F, AL-ZAHRANI Abdulrahim A, et al. Development of highly selective PdZn/CeO2 and Ca-doped PdZn/CeO2 catalysts for methanol synthesis from CO2 hydrogenation[J]. Applied Catalysis A: General, 2018, 560: 42-53. |
| 70 | REZVANI Azita, ABDEL-MAGEED Ali M, ISHIDA Tamao, et al. CO2 reduction to methanol on Au/CeO2 catalysts: Mechanistic insights from activation/deactivation and SSITKA measurements[J]. ACS Catalysis, 2020, 10(6): 3580-3594. |
| 71 | TAN Qingqing, SHI Zhisheng, WU Dongfang. CO2 hydrogenation over differently morphological CeO2-supported Cu-Ni catalysts[J]. International Journal of Energy Research, 2019, 43(10): 5392-5404. |
| 72 | PASUPULETY Nagaraju, DRISS Hafedh, RAFIQUI Mohammed Raoof A, et al. Methanol synthesis using CO2 and H2 on nano silver-ceria zirconia catalysts: Influence of preparation method[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(6): 3197-3204. |
| 73 | CHENG Zhuo, SHERMAN Brent J, Cynthia S LO. Carbon dioxide activation and dissociation on ceria(110): A density functional theory study[J]. The Journal of Chemical Physics, 2013, 138(1): 014702. |
| 74 | MURAVEV Valery, PARASTAEV Alexander, VAN DEN BOSCH Yannis, et al. Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts[J]. Science, 2023, 380(6650): 1174-1179. |
| 75 | CHOI Eun Jeong, LEE Yong Hee, LEE Dae-Won, et al. Hydrogenation of CO2 to methanol over Pd-Cu/CeO2 catalysts[J]. Molecular Catalysis, 2017, 434: 146-153. |
| 76 | OJELADE Opeyemi A, ZAMAN Sharif F, DAOUS Muhammad A, et al. Optimizing Pd: Zn molar ratio in PdZn/CeO2 for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2019, 584: 117185. |
| 77 | TAN Qingqing, SHI Zhisheng, WU Dongfang. CO2 hydrogenation to methanol over a highly active Cu-Ni/CeO2-nanotube catalyst[J]. Industrial & Engineering Chemistry Research, 2018, 57(31): 10148-10158. |
| 78 | WU Congyi, CHENG Danyang, WANG Meng, et al. Understanding and application of strong metal-support interactions in conversion of CO2 to methanol: A review[J]. Energy & Fuels, 2021, 35(23): 19012-19023. |
| 79 | ZHU Jiadong, SU Yaqiong, CHAI Jiachun, et al. Mechanism and nature of active sites for methanol synthesis from CO/CO2 on Cu/CeO2 [J]. ACS Catalysis, 2020, 10(19): 11532-11544. |
| 80 | VOURROS A, GARAGOUNIS I, KYRIAKOU V, et al. Carbon dioxide hydrogenation over supported Au nanoparticles: Effect of the support[J]. Journal of CO2 Utilization, 2017, 19: 247-256. |
| 81 | RASTEIRO Letícia F, DE SOUSA Rafael A, VIEIRA Luiz H, et al. Insights into the alloy-support synergistic effects for the CO2 hydrogenation towards methanol on oxide-supported Ni5Ga3 catalysts: An experimental and DFT study[J]. Applied Catalysis B: Environmental, 2022, 302: 120842. |
| 82 | YANG Xiaofang, KATTEL Shyam, SENANAYAKE Sanjaya D, et al. Low pressure CO2 hydrogenation to methanol over gold nanoparticles activated on a CeO x /TiO2 interface[J]. Journal of the American Chemical Society, 2015, 137(32): 10104-10107. |
| 83 | ABDEL-MAGEED Ali M, KLYUSHIN Alexander, REZVANI Azita, et al. Negative charging of Au nanoparticles during methanol synthesis from CO2/H2 on a Au/ZnO catalyst: Insights from operando IR and near-ambient-pressure XPS and XAS measurements[J]. Angewandte Chemie International Edition, 2019, 58(30): 10325-10329. |
| 84 | CHANG Shuai, NA Wei, ZHANG Jiaqi, et al. Effect of the Zn/Ce ratio in Cu/ZnO-CeO2 catalysts on CO2 hydrogenation for methanol synthesis[J]. New Journal of Chemistry, 2021, 45(48): 22814-22823. |
| 85 | LI Shaozhong, GUO Limin, ISHIHARA Tatsumi. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst[J]. Catalysis Today, 2020, 339: 352-361. |
| 86 | ZHANG Jingpeng, SUN Xiaohang, WU Congyi, et al. Engineering Cu+/CeZrO x interfaces to promote CO2 hydrogenation to methanol[J]. Journal of Energy Chemistry, 2023, 77: 45-53. |
| 87 | SHI Zhisheng, TAN Qingqing, WU Dongfang. Ternary copper-cerium-zirconium mixed metal oxide catalyst for direct CO2 hydrogenation to methanol[J]. Materials Chemistry and Physics, 2018, 219: 263-272. |
| 88 | ZABILSKIY Maxim, MA Kaibo, BECK Arik, et al. Methanol synthesis over Cu/CeO2-ZrO2 catalysts: The key role of multiple active components[J]. Catalysis Science & Technology, 2021, 11(1): 349-358. |
| 89 | WANG Weiwei, QU Zhenping, SONG Lixin, et al. An investigation of Zr/Ce ratio influencing the catalytic performance of CuO/Ce1- x Zr x O2 catalyst for CO2 hydrogenation to CH3OH[J]. Journal of Energy Chemistry, 2020, 47: 18-28. |
| 90 | NIE Mengdong, GUO Tuo, QIANG Fangyuan, et al. Effect of Mn content in CuO/MnCeO x catalysts on CO2 hydrogenation for methanol synthesis[J]. Reaction Chemistry & Engineering, 2023, 8(6): 1383-1394. |
| 91 | YAN Yong, WONG Roong Jien, MA Zhirui, et al. CO2 hydrogenation to methanol on tungsten-doped Cu/CeO2 catalysts[J]. Applied Catalysis B: Environmental, 2022, 306: 121098. |
| 92 | LI Shaozhong, WANG Yu, YANG Bin, et al. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol[J]. Applied Catalysis A: General, 2019, 571: 51-60. |
| 93 | ATTADA Yerrayya, VELISOJU Vijay Kumar, MOHAMED Hend Omar, et al. Dual experimental and computational approach to elucidate the effect of Ga on Cu/CeO2-ZrO2 catalyst for CO2 hydrogenation[J]. Journal of CO2 Utilization, 2022, 65: 102251. |
| 94 | MA Nana, CHENG Weiyi, WEI Changgeng, et al. Mechanism of methanol synthesis from CO2 on Cu/CeO2 and Cu/W-CeO2: A DFT investigation into the nature of W-doping[J]. Journal of Materials Chemistry A, 2024, 12(4): 2323-2334. |
| 95 | RODRIGUEZ José A, LIU Ping, GRACIANI Jesús, et al. Inverse oxide/metal catalysts in fundamental studies and practical applications: A perspective of recent developments[J]. The Journal of Physical Chemistry Letters, 2016, 7(13): 2627-2639. |
| 96 | SENANAYAKE Sanjaya D, STACCHIOLA Dario, RODRIGUEZ Jose A. Unique properties of ceria nanoparticles supported on metals: Novel inverse ceria/copper catalysts for CO oxidation and the water-gas shift reaction[J]. Accounts of Chemical Research, 2013, 46(8): 1702-1711. |
| 97 | KAPIAMBA Kashala Fabrice, OTOR Hope O, VIAMAJALA Sridhar, et al. Inverse oxide/metal catalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2022, 36(19): 11691-11711. |
| 98 | SENANAYAKE Sanjaya D, RAMÍREZ Pedro J, WALUYO Iradwikanari, et al. Hydrogenation of CO2 to methanol on CeO x /Cu(111) and ZnO/Cu(111) catalysts: Role of the metal-oxide interface and importance of Ce3+ sites[J]. The Journal of Physical Chemistry C, 2016, 120(3): 1778-1784. |
| 99 | MONCADA Jorge, CHEN Xiaobo, DENG Kaixi, et al. Structural and chemical evolution of an inverse CeO x /Cu catalyst under CO2 hydrogenation: Tunning oxide morphology to improve activity and selectivity[J]. ACS Catalysis, 2023, 13(23): 15248-15258. |
| 100 | LOU Yang, JIANG Feng, ZHU Wen, et al. CeO2 supported Pd dimers boosting CO2 hydrogenation to ethanol[J]. Applied Catalysis B: Environmental, 2021, 291: 120122. |
| 101 | ZHENG Ke, LI Yufeng, LIU Bing, et al. Ti-doped CeO2 stabilized single-atom rhodium catalyst for selective and stable CO2 hydrogenation to ethanol[J]. Angewandte Chemie International Edition, 2022, 61(44): e202210991. |
| 102 | CHEN Jie, ZHA Yajun, LIU Bing, et al. Rationally designed water enriched nano reactor for stable CO2 hydrogenation with near 100% ethanol selectivity over diatomic palladium active sites[J]. ACS Catalysis, 2023, 13(10): 7110-7121. |
| [1] | 冯勇强, 王洁茹, 王超娴, 李芳, 苏婉婷, 孙宇, 赵彬然. γ-Al2O3 负载的Ni、Fe、Cu对介质阻挡放电等离子体转化CO2/CH4的影响[J]. 化工进展, 2024, 43(5): 2705-2713. |
| [2] | 解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722. |
| [3] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [4] | 吴达, 蒋淑娇, 魏强, 袁胜华, 杨刚, 张成. 能源转型中渣油高效利用技术的研究进展[J]. 化工进展, 2024, 43(5): 2343-2353. |
| [5] | 江安迪, 丁雪兴, 王世鹏, 丁俊华, 力宁. 超临界CO2干气密封热动力学性能研究进展[J]. 化工进展, 2024, 43(5): 2354-2369. |
| [6] | 桂鑫, 陈汇勇, 白柏杨, 贾永梁, 马晓迅. Mo掺杂改性NiC/Al-MCM-41的芘催化加氢性能[J]. 化工进展, 2024, 43(5): 2386-2395. |
| [7] | 丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448. |
| [8] | 韩伟, 韩恒文, 程薇, 汤玮健. 碳中和目标驱动下生物质燃料技术研究进展[J]. 化工进展, 2024, 43(5): 2463-2474. |
| [9] | 黄坤, 许明, 吴秀娟, 裴思佳, 刘大伟, 马晓迅, 徐龙. 生物质活性炭的制备与微结构特性调控研究进展[J]. 化工进展, 2024, 43(5): 2475-2493. |
| [10] | 段翔, 田野, 董文威, 宋松, 李新刚. 苯酐合成的反应网络及催化反应机制研究现状与展望[J]. 化工进展, 2024, 43(5): 2587-2599. |
| [11] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [12] | 张金鹏, 屈婷, 荆洁颖, 李文英. 吸附强化水气变换制氢复合催化剂研究进展[J]. 化工进展, 2024, 43(5): 2629-2644. |
| [13] | 李娜, 赵婉彤, 凌丽霞, 王宝俊, 章日光. RhCu催化剂中限域环境调控合成气转化生成CH x 反应性能[J]. 化工进展, 2024, 43(5): 2684-2695. |
| [14] | 王冰, 王磊, 黄欣茹, 袁红鹏, 赖小娟, 李朋. 一种耐酸耐碱高强树脂的合成及性能[J]. 化工进展, 2024, 43(4): 1992-2000. |
| [15] | 廖昌建, 张可伟, 王晶, 曾翔宇, 金平, 刘志禹. 直接空气捕集二氧化碳技术研究进展[J]. 化工进展, 2024, 43(4): 2031-2048. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||