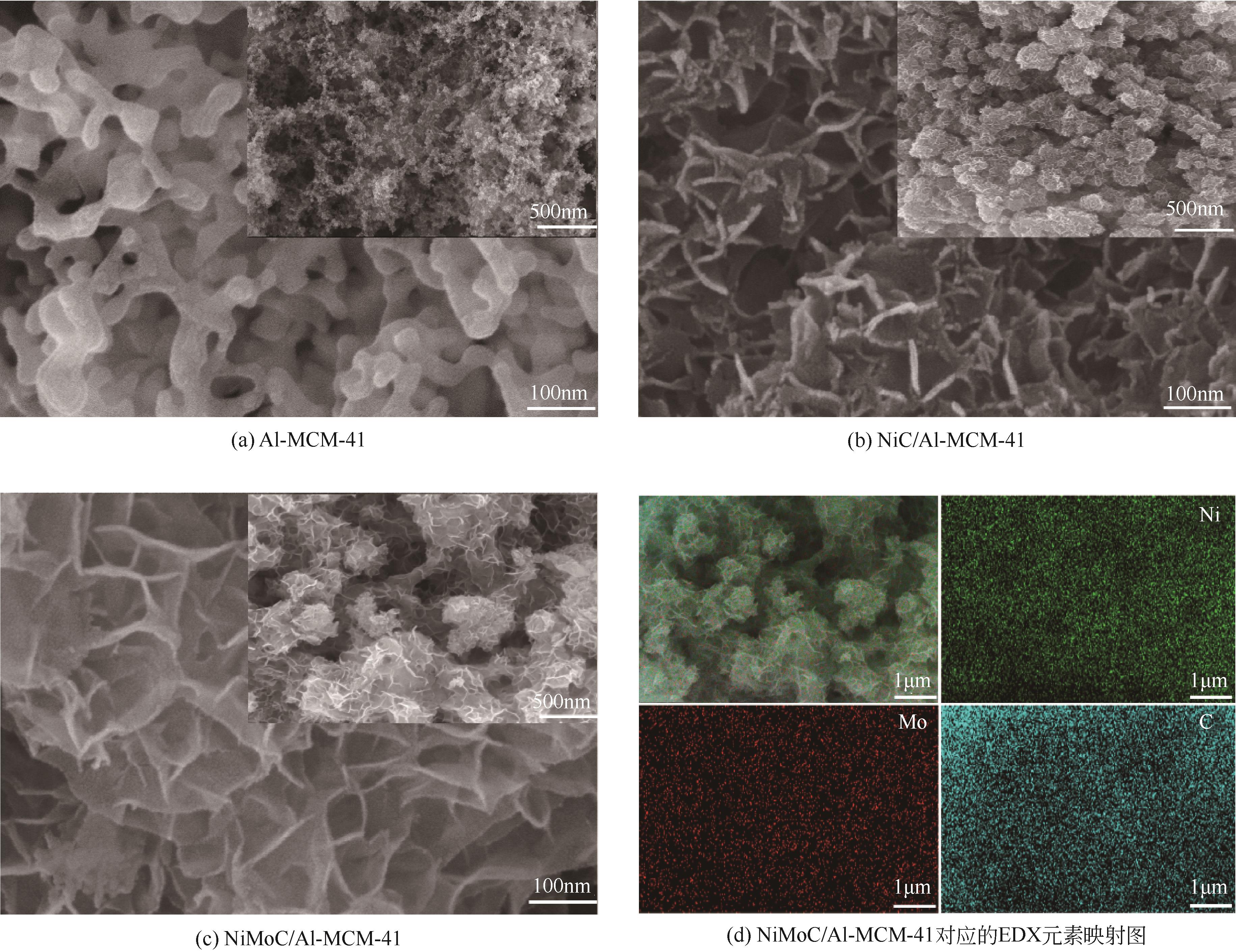
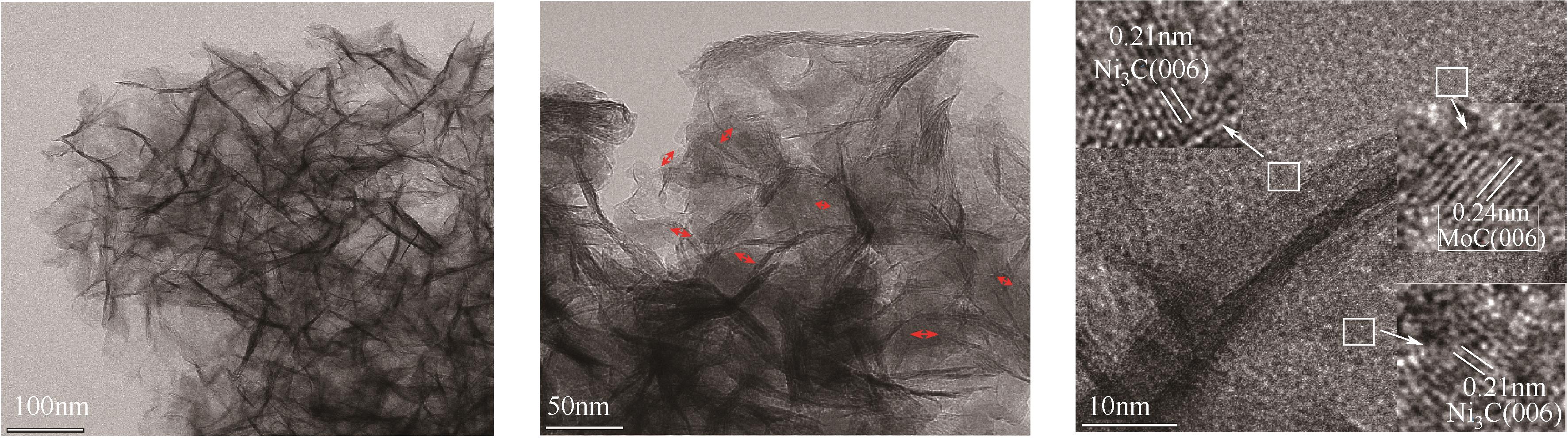
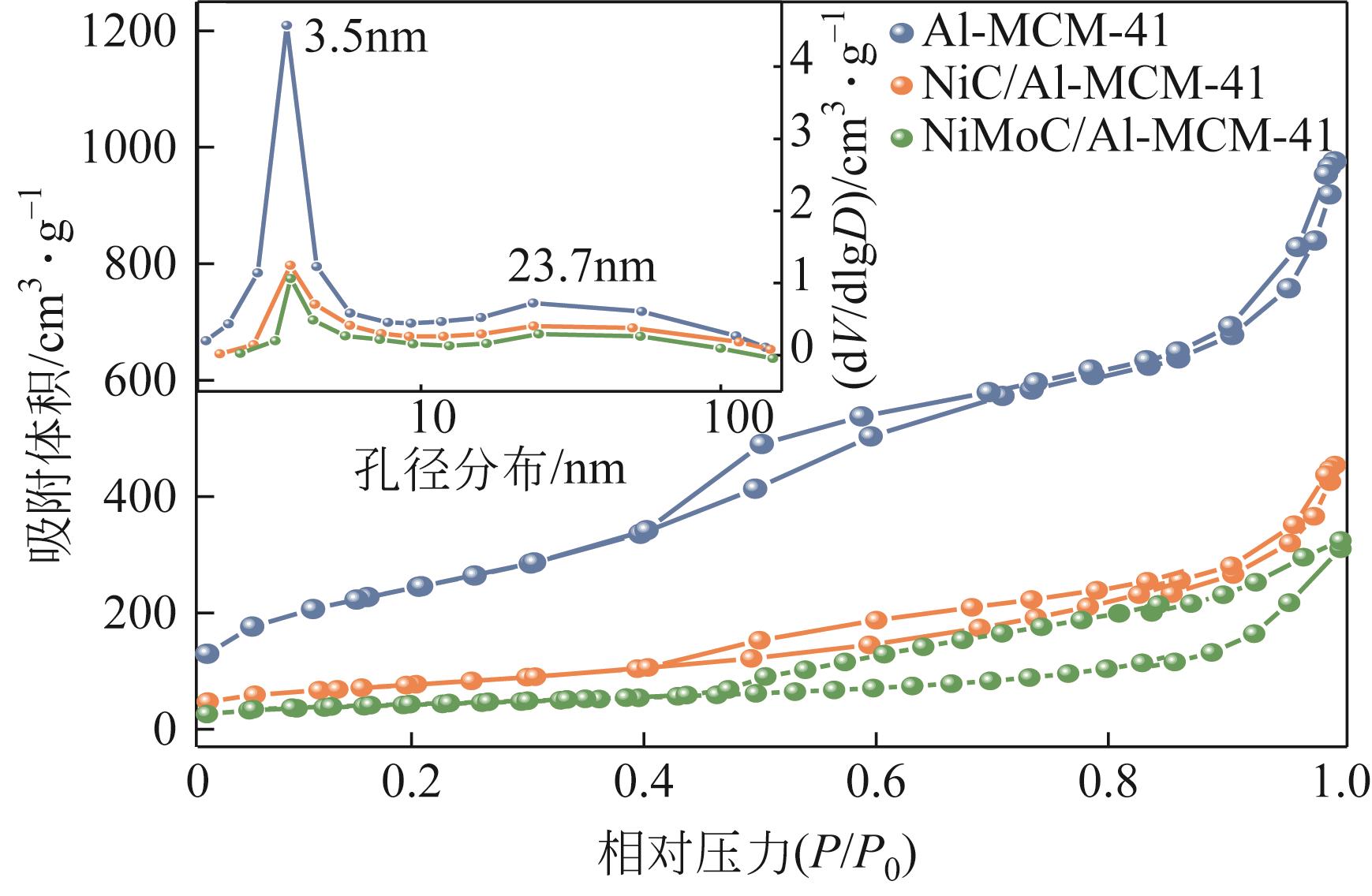
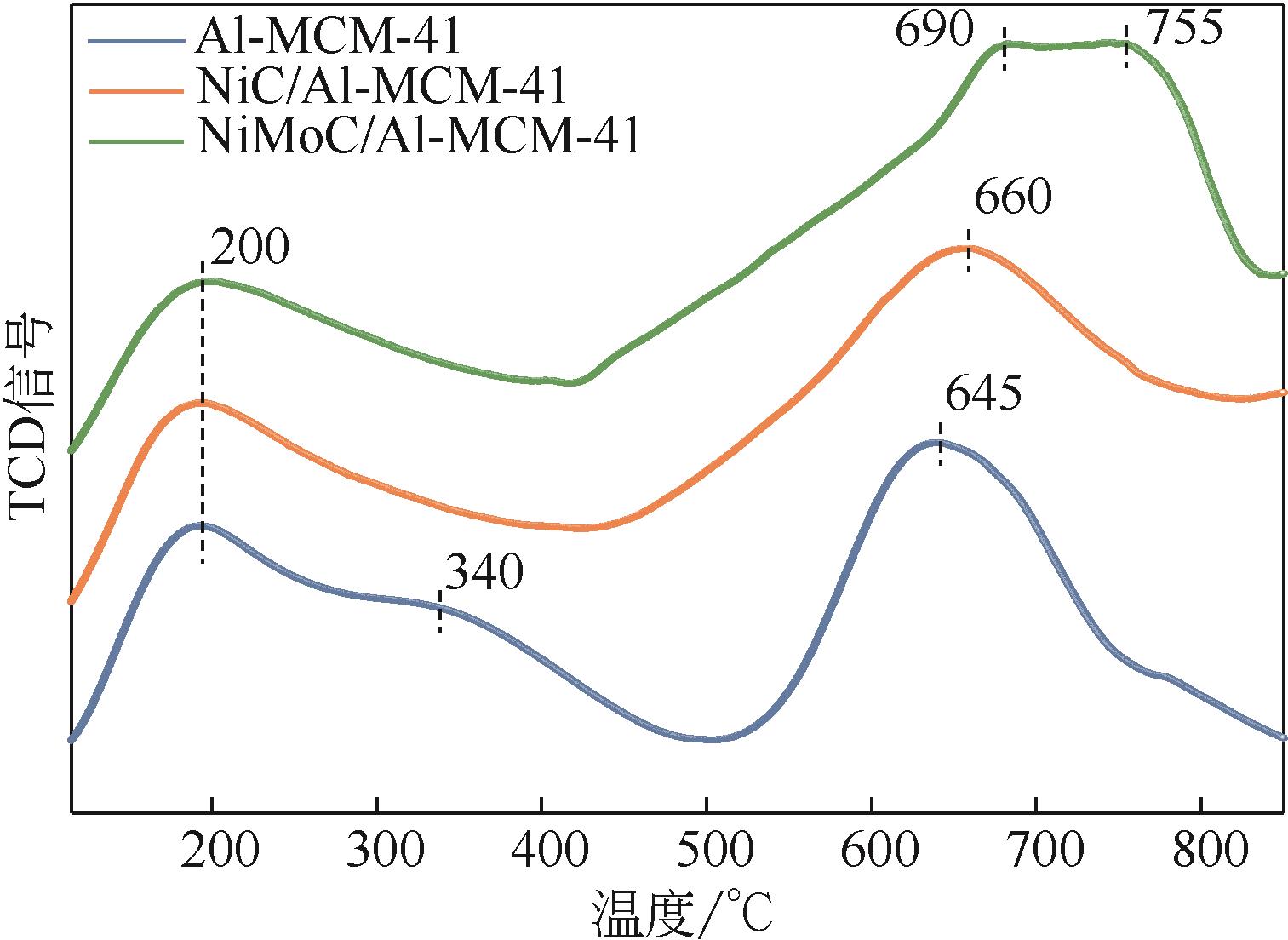
| 1 |
古丽娜尔·图尔逊, 李国峰. 煤焦油中芳烃化合物催化加氢机理研究进展[J]. 合成材料老化与应用, 2021, 50(3): 141-143.
|
|
GULNAR Tursen, LI Guofeng. Research progress in catalytic hydrogenation of aromatic compounds in coal tar[J]. Synthetic Materials Aging and Application, 2021, 50(3): 141-143.
|
| 2 |
FU Wenqian, ZHANG Lei, WU Dongfang, et al. Mesoporous zeolite-supported metal sulfide catalysts with high activities in the deep hydrogenation of phenanthrene[J]. Journal of Catalysis, 2015, 330: 423-433.
|
| 3 |
马雪飞, 李宗鸿, 肖植煌, 等. 有机液体储运氢技术经济分析与比较[J]. 现代化工, 2022, 42(6): 202-205, 210.
|
|
MA Xuefei, LI Zonghong, XIAO Zhihuang, et al. Techno-economic analysis and comparison of liquid organic hydrogen carrier system[J]. Modern Chemical Industry, 2022, 42(6): 202-205, 210.
|
| 4 |
LIU Daocheng, CHEN Yu, JING Jieying, et al. Synthesis of Ni/NiAlO x catalysts for hydrogenation saturation of phenanthrene[J]. Frontiers in Chemistry, 2021, 9: 757908.
|
| 5 |
ZHANG Minghui, SONG Qingyun, HE Zexing, et al. Tuning the mesopore-acid-metal balance in Pd/HY for efficient deep hydrogenation saturation of naphthalene[J]. International Journal of Hydrogen Energy, 2022, 47(48): 20881-20893.
|
| 6 |
WANG Enhua, HU Di, XIAO Chengkun, et al. Highly dispersed Pt on core-shell micro-mesoporous composites assembled by mordenite nanocrystals for selective hydrogenation of polycyclic aromatics[J]. Fuel, 2023, 331: 125852.
|
| 7 |
JACQUIN Mélanie, JONES Deborah J, Jacques ROZIÈRE, et al. Novel supported Rh, Pt, Ir and Ru mesoporous aluminosilicates as catalysts for the hydrogenation of naphthalene[J]. Applied Catalysis A: General, 2003, 251(1): 131-141.
|
| 8 |
HAO Jing, ZHANG Guofeng, ZHENG Yiteng, et al. Controlled synthesis of Ni3C/nitrogen-doped carbon nanoflakes for efficient oxygen evolution[J]. Electrochimica Acta, 2019, 320: 134631.
|
| 9 |
WANG Hao, CAO Yingjie, ZOU Guifu, et al. High-performance hydrogen evolution electrocatalyst derived from Ni3C nanoparticles embedded in a porous carbon network[J]. ACS Applied Materials & Interfaces, 2017, 9(1): 60-64.
|
| 10 |
ANDRÉ Rémi F, MEYNIEL Léna, CARENCO Sophie. Nickel carbide (Ni3C) nanoparticles for catalytic hydrogenation of model compounds in solvent[J]. Catalysis Science & Technology, 2022, 12(14): 4572-4583.
|
| 11 |
XIONG Kun, LI Li, ZHANG Li, et al. Ni-doped Mo2C nanowires supported on Ni foam as a binder-free electrode for enhancing the hydrogen evolution performance[J]. Journal of Materials Chemistry A, 2015, 3(5): 1863-1867.
|
| 12 |
OUYANG Ting, YE Yaqian, WU Chunyan, et al. Heterostructures composed of N-doped carbon nanotubes encapsulating cobalt and β-Mo2C nanoparticles as bifunctional electrodes for water splitting[J]. Angewandte Chemie International Edition, 2019, 58(15): 4923-4928.
|
| 13 |
SHEN Zhibing, FU Rao, ZHANG Shangli, et al. Selective hydrogenation of polycyclic aromatics to monocyclic aromatics over NiMoC/Hβ catalysts in a methane and hydrogen environment[J]. China Petroleum Processing and Petrochemical Technology, 2023, 25(2): 92-100.
|
| 14 |
JIANG Chenguang, WANG Yonggang, ZHANG Haiyong, et al. Effect of PDADMAC with different molecular weight regulating Hβ Properties on hydrogenation performance of NiMoC/Hβ catalysts[J]. Fuel Processing Technology, 2021, 213: 106704.
|
| 15 |
CHEN Wenbin, Francoise MAUGÉ, VAN GESTEL Jacob, et al. Effect of modification of the alumina acidity on the properties of supported Mo and CoMo sulfide catalysts[J]. Journal of Catalysis, 2013, 304: 47-62.
|
| 16 |
ITO Koki, KOGASAKA Yoshifumi, KUROKAWA Hideki, et al. Preliminary study on mechanism of naphthalene hydrogenation to form decalins via tetralin over Pt/TiO2 [J]. Fuel Processing Technology, 2002, 79(1): 77-80.
|
| 17 |
JIANG Chenguang, WANG Yonggang, ZHANG Haiyong, et al. Effect of initial Si/Al ratios on the performance of low crystallinity Hβ-x zeolite supported NiMo carbide catalysts for aromatics hydrogenation[J]. Catalysis Science & Technology, 2019, 9(18): 5031-5044.
|
| 18 |
USMAN Muhammad, LI Dan, RAZZAQ Rauf, et al. Novel MoP/HY catalyst for the selective conversion of naphthalene to tetralin[J]. Journal of Industrial and Engineering Chemistry, 2015, 23: 21-26.
|
| 19 |
TAGHVAEI Hamed, MOADDELI Ali, Ali KHALAFF-NEZHAD, et al. Catalytic hydrodeoxygenation of lignin pyrolytic-oil over Ni catalysts supported on spherical Al-MCM-41 nanoparticles: Effect of Si/Al ratio and Ni loading[J]. Fuel, 2021, 293: 120493.
|
| 20 |
KORRE Styliani C, KLEIN Michael T, QUANN Richard J. Polynuclear aromatic hydrocarbons hydrogenation. 1. Experimental reaction pathways and kinetics[J]. Industrial & Engineering Chemistry Research, 1995, 34(1): 101-117.
|
| 21 |
MENG Fanyu, ZHANG Shule, ZHANG Mingjia, et al. The mechanism of Ce-MCM-41 catalyzed peroxone reaction into ·OH and ·O 2 - radicals for enhanced NO oxidation[J]. Molecular Catalysis, 2022, 518: 112110.
|
| 22 |
QIN Jing, LI Baoshan, ZHANG Wen, et al. Synthesis, characterization and catalytic performance of well-ordered mesoporous Ni-MCM-41 with high nickel content[J]. Microporous and Mesoporous Materials, 2015, 208: 181-187.
|
| 23 |
HAN Guihong, ZHAO Jing, YANG Ze, et al. Facile hydrothermal synthesis and enhanced electrochemical properties of a layered NiSiO/RGO nanocomposite with an interesting dandelion-like structure[J]. Dalton Transactions, 2021, 50(39): 13756-13767.
|
| 24 |
HUANG Ting, SHEN Tao, GONG Mingxing, et al. Ultrafine Ni-B nanoparticles for efficient hydrogen evolution reaction[J]. Chinese Journal of Catalysis, 2019, 40(12): 1867-1873.
|
| 25 |
JIANG Jing, LIU Qiuxia, ZENG Chunmei, et al. Cobalt/molybdenum carbide@N-doped carbon as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions[J]. Journal of Materials Chemistry A, 2017, 5(32): 16929-16935.
|
| 26 |
HUANG Xiaoyu, ZHANG Wei, PENG Yaowei, et al. A multifunctional layered nickel silicate nanogenerator of synchronous oxygen self-supply and superoxide radical generation for hypoxic tumor therapy[J]. ACS Nano, 2022, 16(1): 974-983.
|
| 27 |
WANG Pengyan, QIN Rui, JI Pengxia, et al. Synergistic coupling of Ni nanoparticles with Ni3C nanosheets for highly efficient overall water splitting[J]. Small, 2020, 16(37): 2001642.
|
| 28 |
DOUKKALI M EI, PAUL S, DUMEIGNIL F. New insights in single-step hydrodeoxygenation of glycerol to propylene by coupling rational catalyst design with systematic analysis[J]. Applied Catalysis B: Environmental, 2023, 324: 122280.
|
| 29 |
SU Xiaoping, AN Pu, GAO Junwen, et al. Selective catalytic hydrogenation of naphthalene to tetralin over a Ni-Mo/Al2O3 catalyst[J]. Chinese Journal of Chemical Engineering, 2020, 28(10): 2566-2576.
|
| 30 |
HUANG Guan, SUN Zhichao, YU Zhiquan, et al. Supported Ni2P catalysts derived from nickel phyllosilicate with enhanced hydrodesulfurization performance[J]. Journal of Catalysis, 2023, 419: 37-48.
|
| 31 |
El‑AHWANY Omnia M, AWADALLAH Ahmed E, ABDEL-AZIM Samira M, et al. Chemical vapor deposition synthesis of high-quality Ni3C/GNPs composite material: Effect of growth time on the yield, morphology and adsorption behavior of metal ions[J]. Chemical Papers, 2022, 76(3): 1579-1592.
|
| 32 |
王悦燚, 王婧, 贾永梁, 等. 硫化钼催化剂用于催化多环芳烃芘的加氢反应[J]. 煤炭转化, 2023, 46(6): 1-11.
|
|
WANG Yueyi, WANG Jing, JIA Yongliang, et al. Molybdenum sulfide catalyst for hydrogenation of PAHs pyrene[J]. Coal Conversion, 2023, 46(6): 1-11.
|
| 33 |
LI Kang, ZHU Jianfeng, XU Zhanwei, et al. Tremella-like Mo and N codoped graphitic nanosheets by in situ carbonization of phthalocyanine for potassium-ion battery[J]. ACS Applied Materials & Interfaces, 2021, 13(26): 30583-30593.
|
| 34 |
LU Xinyu, GUO Haoquan, CHEN Jiajia, et al. Selective catalytic transfer hydrogenation of lignin to alkyl guaiacols over NiMo/Al-MCM-41[J]. ChemSusChem, 2022, 15(7): e202200099.
|
| 35 |
陈秀莹, 谢慧琳, 胡文斌, 等. MCM-41负载Pt-Al催化剂的制备及其表征[J]. 化工学报, 2018, 69(S1): 72-79.
|
|
CHEN Xiuying, XIE Huilin, HU Wenbin, et al. Preparation and characterization of MCM-41 supported Pt-Al catalysts[J]. CIESC Journal, 2018, 69(S1): 72-79.
|
| 36 |
GUO Haoquan, ZHAO Jiwu, CHEN Yu, et al. Mechanistic insights into hydrodeoxygenation of lignin derivatives over Ni single atoms supported on Mo2C[J]. ACS Catalysis, 2024, 14(2): 703-717.
|
| 37 |
CHEN Changzhou, LIU Peng, XIA Haihong, et al. Catalytic conversion of lignin to liquid fuels with an improved H/Ceff value over bimetallic NiMo-MOF-derived catalysts[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(41): 13937-13952.
|
| 38 |
CHEN Tingsheng, YANG Wenyi, DU Zhenyi, et al. Effects of mesopore introduction on the stability of zeolites for 4-iso-propylphenol dealkylation[J]. Catalysis Today, 2021, 371: 40-49.
|
 ), 陈汇勇1, 白柏杨2, 贾永梁1, 马晓迅1(
), 陈汇勇1, 白柏杨2, 贾永梁1, 马晓迅1( )
)
 ), CHEN Huiyong1, BAI Boyang2, JIA Yongliang1, MA Xiaoxun1(
), CHEN Huiyong1, BAI Boyang2, JIA Yongliang1, MA Xiaoxun1( )
)