化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2436-2448.DOI: 10.16085/j.issn.1000-6613.2023-2068
• 化石能源的清洁高效转化利用 • 上一篇
基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂
丁思佳1( ), 蒋淑娇1, 杨占林1(
), 蒋淑娇1, 杨占林1( ), 彭绍忠1, 蒋乾民2
), 彭绍忠1, 蒋乾民2
- 1.中石化(大连)石油化工研究院,辽宁 大连 116045
2.中国石油大学(北京)化学工程与环境学院,北京 102249
-
收稿日期:2023-11-28修回日期:2024-03-14出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:杨占林 -
作者简介:丁思佳(1987—),男,博士,副研究员,研究方向为催化加氢理论和加氢精制催化剂研发。E-mail:dingsijia.fshy@sinopec.com。
Design of heavy oil hydrodenitrogenation catalysts based on hydrogenation performance determined by structure of nitrogen compounds
DING Sijia1( ), JIANG Shujiao1, YANG Zhanlin1(
), JIANG Shujiao1, YANG Zhanlin1( ), PENG Shaozhong1, JIANG Qianmin2
), PENG Shaozhong1, JIANG Qianmin2
- 1.Sinopec (Dalian) Petrochemical Research Institute, Dalian 116045, Liaoning, China
2.College of Chemical Engineering and Environment, China University of Petroleum, Beijing 102249, China
-
Received:2023-11-28Revised:2024-03-14Online:2024-05-15Published:2024-06-15 -
Contact:YANG Zhanlin
摘要:
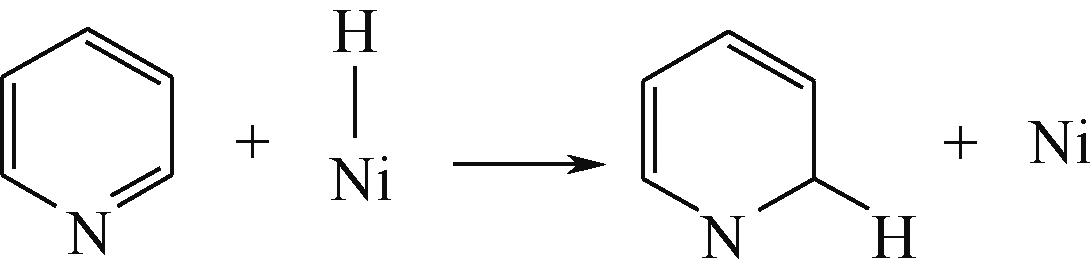
氮化物分子的结构随着油品馏程增加呈规律性变化,氮化物结构与加氢行为之间的关系是重油加氢脱氮催化剂设计的重要指导理论。本文采用量子化学理论计算的方法研究碱性氮化物和非碱性氮化物的理化性质与反应行为随氮化物结构的变化规律。研究发现,随着氮化物中共轭芳环数目的增加:氮化物吸附能力增强,且吸附过程中电荷转移能力也随之增强,氮化物的平躺吸附逐渐占优势;C—N键通过低活化能的消去路径断裂的难度增加,只能通过高活化能的取代反应路径实现断裂,这就要求与氮化物相邻芳环在C—N键断裂前必须充分得到加氢饱和。高氢解能力催化剂加氢产物中剩余氮化物以含有多环芳烃的氮化物为主,而高加氢饱和能力催化剂加氢产物剩余氮化物中含有更多的双环氮化物,加氢实验结果说明了具有高氢解能力的加氢催化剂更有利于轻质油品中氮化物的脱除,而具有高加氢饱和能力的催化剂更有利于重质油品中氮化物的加氢脱除。
中图分类号:
引用本文
丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448.
DING Sijia, JIANG Shujiao, YANG Zhanlin, PENG Shaozhong, JIANG Qianmin. Design of heavy oil hydrodenitrogenation catalysts based on hydrogenation performance determined by structure of nitrogen compounds[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2436-2448.
| 计算项目/方法 | 参数 |
|---|---|
| 电子赝势 | 超平滑(ultrasoft)电子赝势 |
| 能量截断 | 10.0eV |
| 电子自洽收敛阶(SCF) | 1.0×10-6 |
| 体系能量收敛阶 | 2.0×10-5Hartree (Ha) |
| 体系残余内应力 | 0.05Ha/Å |
| 色散力矫正方法 | Grimme 06[ |
| 独立交换参数s6 | 1.00 |
| 阻尼衰减参数d | 20.0 |
表1 计算方法和参数选择
| 计算项目/方法 | 参数 |
|---|---|
| 电子赝势 | 超平滑(ultrasoft)电子赝势 |
| 能量截断 | 10.0eV |
| 电子自洽收敛阶(SCF) | 1.0×10-6 |
| 体系能量收敛阶 | 2.0×10-5Hartree (Ha) |
| 体系残余内应力 | 0.05Ha/Å |
| 色散力矫正方法 | Grimme 06[ |
| 独立交换参数s6 | 1.00 |
| 阻尼衰减参数d | 20.0 |
| 原料 | 50%馏程温度 /℃ | 95%馏程温度 /℃ | 密度 /g·cm-3 | 硫含量 /μg·g-1 | 氮含量 /μg·g-1 | 链烷烃 质量分数/% | 环烷烃 质量分数/% | 单环芳烃 质量分数/% | 二环及以上芳烃 质量分数/% | 总环芳烃 质量分数/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 常三线柴油 | 309.9 | 360.7 | 855.6 | 10170 | 352 | 40.8 | 28.6 | 15.1 | 14.3 | 29.4 |
| 减二线蜡油 | 445.3 | 525.3 | 906.5 | 14220 | 1094 | 25.4 | 27.9 | 17.3 | 29.5 | 46.8 |
| 减三线蜡油 | 464.3 | 576.9 | 926.3 | 18960 | 1566 | 34.0 | 13.7 | 19.2 | 31.2 | 50.4 |
表2 加氢评价实验油品性质
| 原料 | 50%馏程温度 /℃ | 95%馏程温度 /℃ | 密度 /g·cm-3 | 硫含量 /μg·g-1 | 氮含量 /μg·g-1 | 链烷烃 质量分数/% | 环烷烃 质量分数/% | 单环芳烃 质量分数/% | 二环及以上芳烃 质量分数/% | 总环芳烃 质量分数/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 常三线柴油 | 309.9 | 360.7 | 855.6 | 10170 | 352 | 40.8 | 28.6 | 15.1 | 14.3 | 29.4 |
| 减二线蜡油 | 445.3 | 525.3 | 906.5 | 14220 | 1094 | 25.4 | 27.9 | 17.3 | 29.5 | 46.8 |
| 减三线蜡油 | 464.3 | 576.9 | 926.3 | 18960 | 1566 | 34.0 | 13.7 | 19.2 | 31.2 | 50.4 |
| 碱性氮化物 | 氮原子 电荷/e | 孤对电子 轨道形貌 | 轨道能级 /eV | π-电子 轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|---|---|
 | -0.205 | 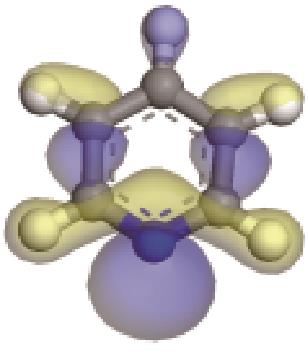 | -6.016 | 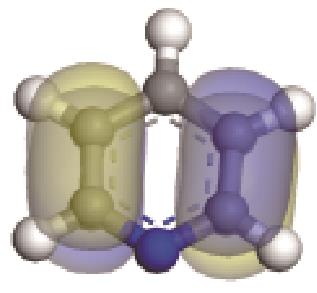 | -6.68 | 5.318 | 13.69 | 10.95 |
 | -0.202 | 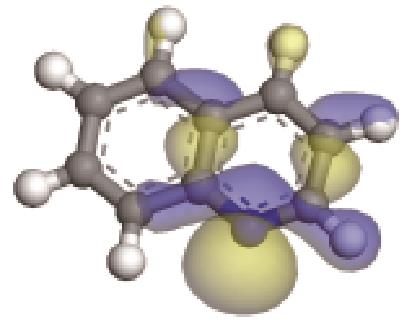 | -6.040 | 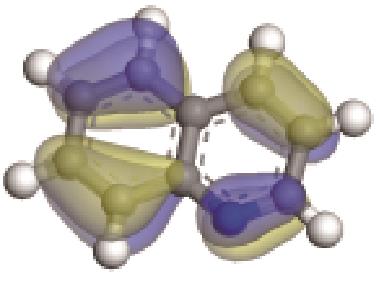 | -5.944 | 4.454 | 25.56 | 23.91 |
 | -0.206 | 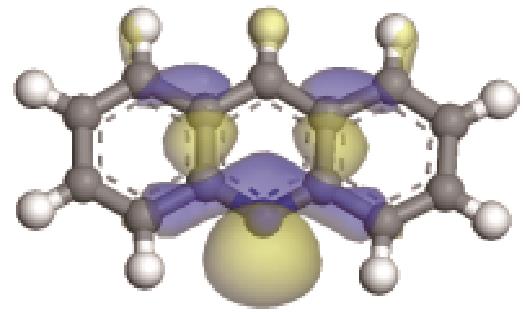 | -6.039 | 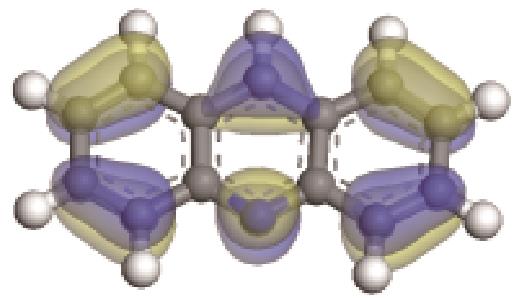 | -5.409 | 2.904 | 40.54 | 42.16 |
 | -0.206 | 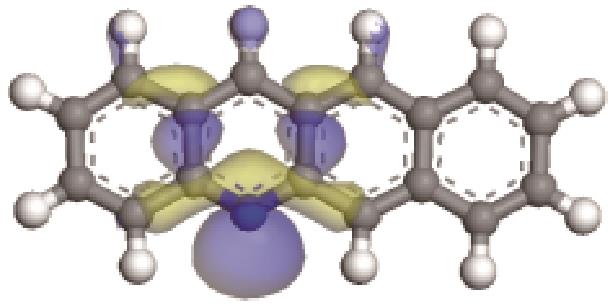 | -6.030 | 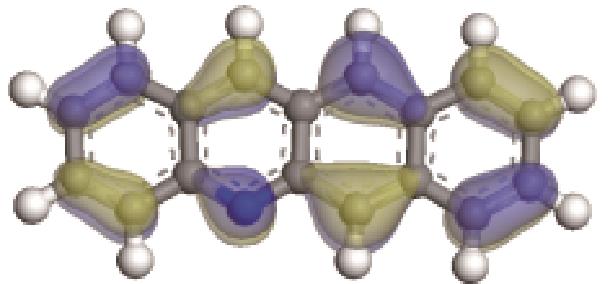 | -4.987 | 2.962 | 58.65 | 68.32 |
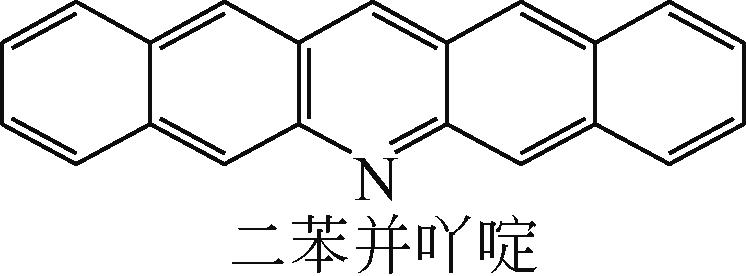 | -0.207 | 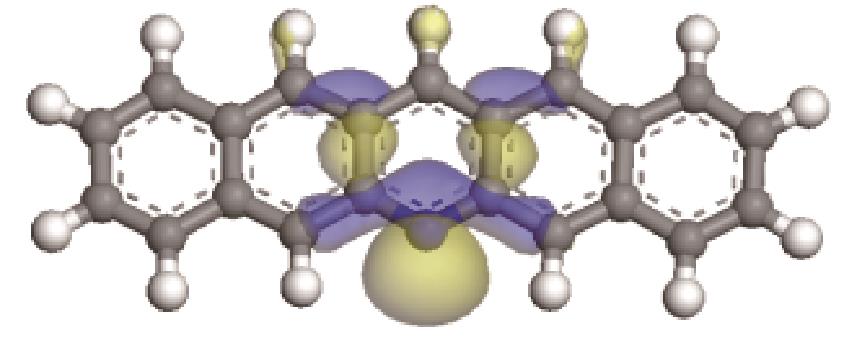 | -6.016 | 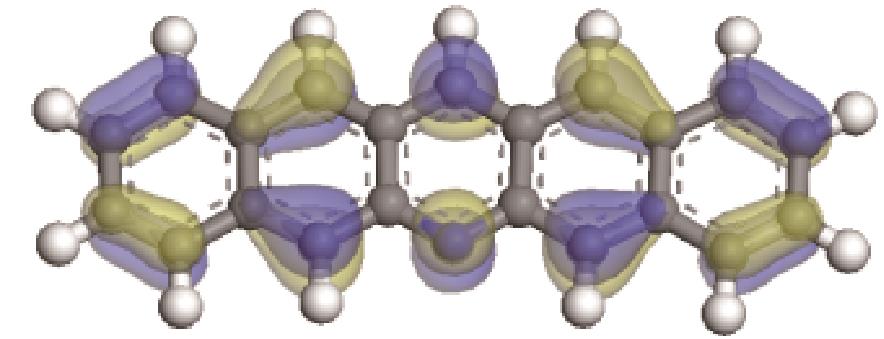 | -4.74 | 2.634 | 77.26 | 91.11 |
表3 碱性氮化物理化性质与结构关系
| 碱性氮化物 | 氮原子 电荷/e | 孤对电子 轨道形貌 | 轨道能级 /eV | π-电子 轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|---|---|
 | -0.205 |  | -6.016 |  | -6.68 | 5.318 | 13.69 | 10.95 |
 | -0.202 |  | -6.040 |  | -5.944 | 4.454 | 25.56 | 23.91 |
 | -0.206 |  | -6.039 |  | -5.409 | 2.904 | 40.54 | 42.16 |
 | -0.206 |  | -6.030 |  | -4.987 | 2.962 | 58.65 | 68.32 |
 | -0.207 |  | -6.016 |  | -4.74 | 2.634 | 77.26 | 91.11 |
| 非碱性氮化物 | 氮原子Mulliken Charge /e | π-电子轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|
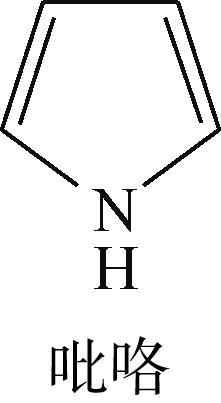 | -0.150 | 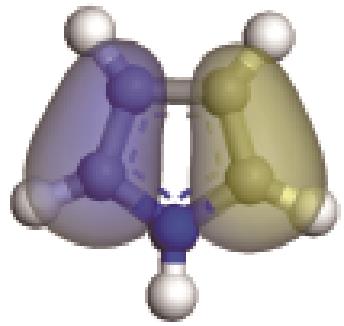 | -5.081 | 6.278 | 11.10 | 8.26 |
 | -0.196 | 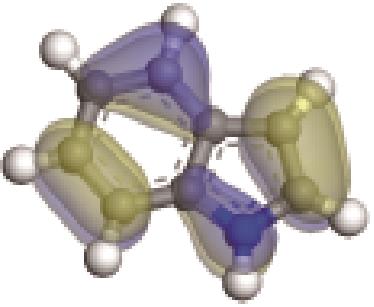 | -5.065 | 7.285 | 22.26 | 20.01 |
 | -0.229 | 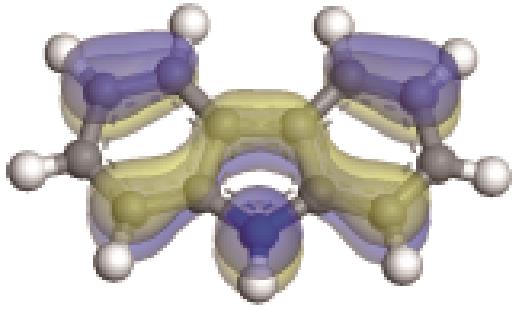 | -5.129 | 5.326 | 35.18 | 33.94 |
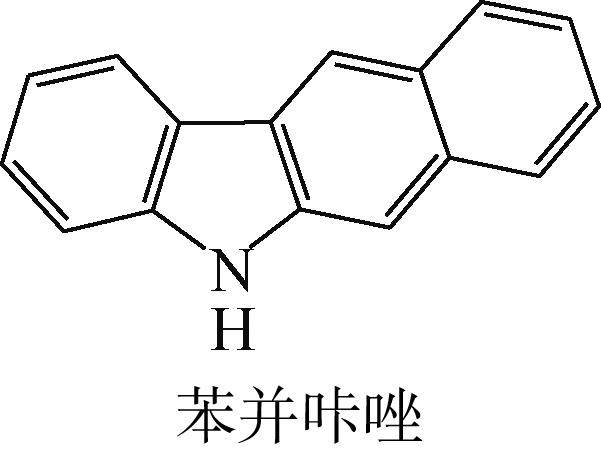 | -0.233 | 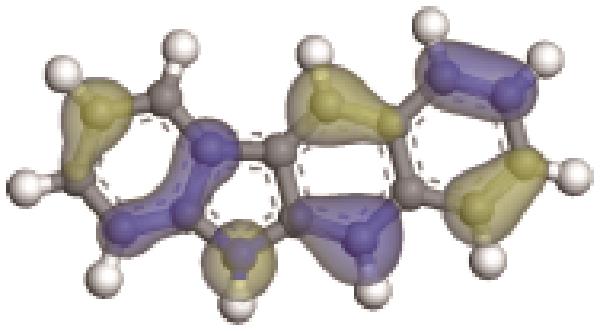 | -4.287 | 5.830 | 51.26 | 54.52 |
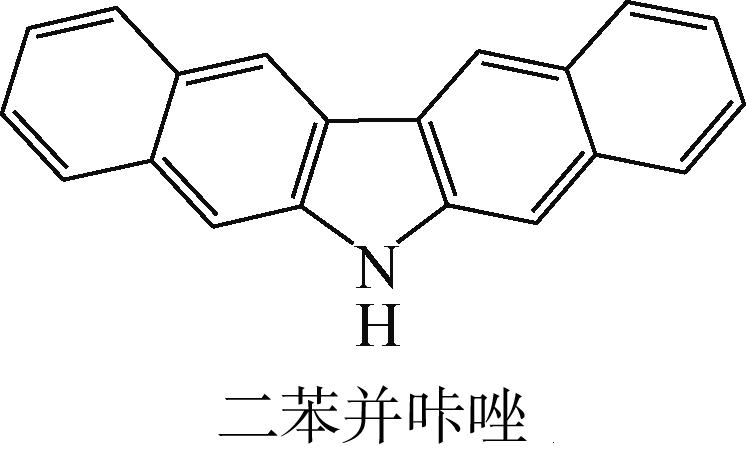 | -0.235 | 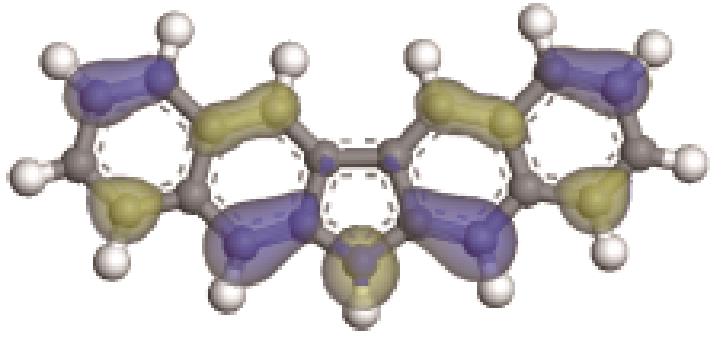 | -4.816 | 4.905 | 69.60 | 82.71 |
表4 非碱性氮化物理化性质与结构关系
| 非碱性氮化物 | 氮原子Mulliken Charge /e | π-电子轨道形貌 | 轨道能级 /eV | 偶极矩μ /10-30·C·m | 极化率 /10-40·C·m2·V-1 | 各向异性 /10-40·C·m2·V-1 |
|---|---|---|---|---|---|---|
 | -0.150 |  | -5.081 | 6.278 | 11.10 | 8.26 |
 | -0.196 |  | -5.065 | 7.285 | 22.26 | 20.01 |
 | -0.229 |  | -5.129 | 5.326 | 35.18 | 33.94 |
 | -0.233 |  | -4.287 | 5.830 | 51.26 | 54.52 |
 | -0.235 |  | -4.816 | 4.905 | 69.60 | 82.71 |
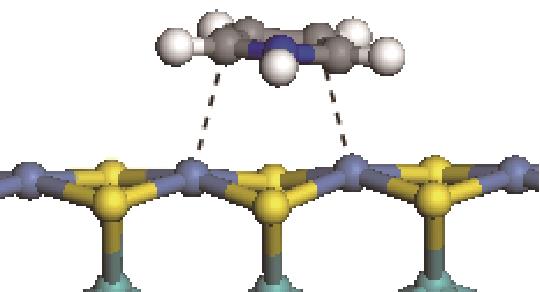
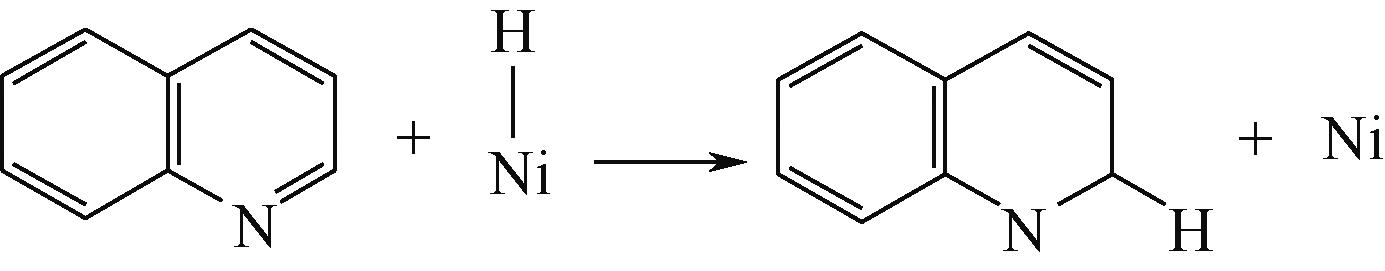
| 碱性氮化物 | 吸附方式 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|---|
 | N-Ni | 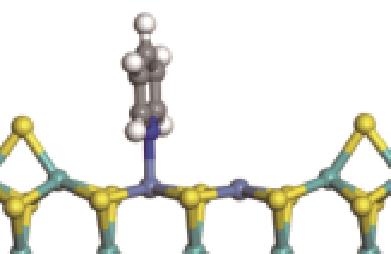 | -100.69 | +0.252 |
| N-Mo | 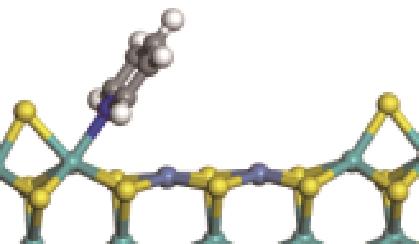 | -117.07 | +0.258 | |
| π-Ni | 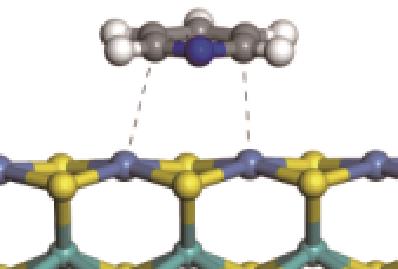 | -48.72 | +0.018 | |
 | N-Ni | 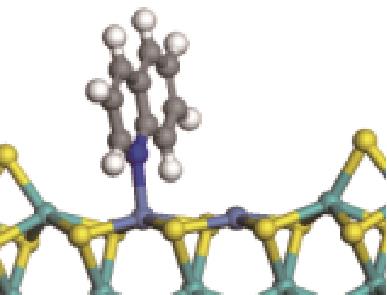 | -118.51 | +0.262 |
| N-Mo | 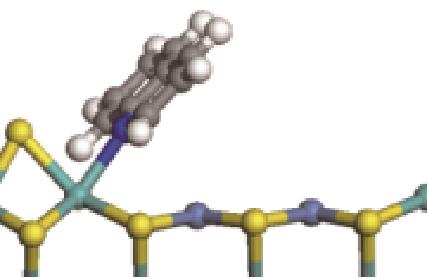 | -145.78 | +0.293 | |
| π-M | 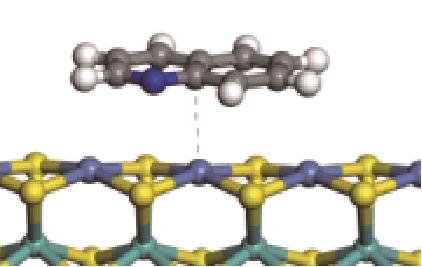 | -92.42 | +0.023 | |
 | N-Ni | 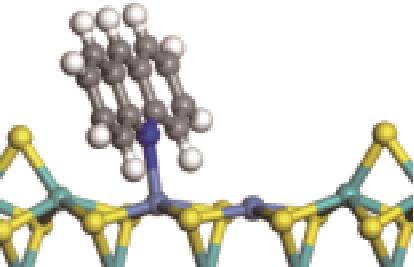 | -112.80 | +0.227 |
| N-Mo | 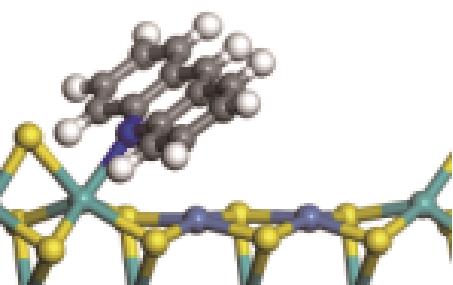 | -154.10 | +0.311 | |
| π-M |  | -112.84 | +0.026 | |
 | N-Ni | 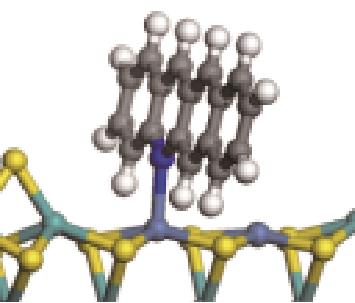 | -122.20 | +0.262 |
| N-Mo |  | -171.91 | +0.326 | |
| π-M | 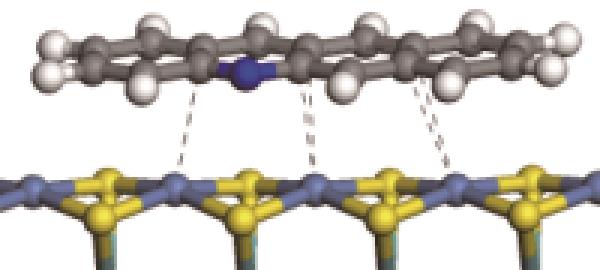 | -135.30 | +0.028 | |
 | N-Ni | 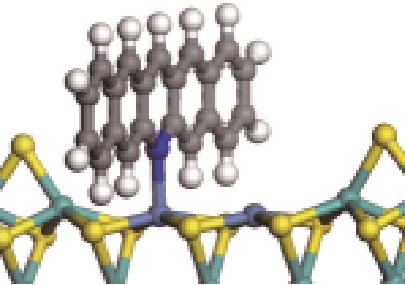 | -124.20 | +0.309 |
| N-Mo | 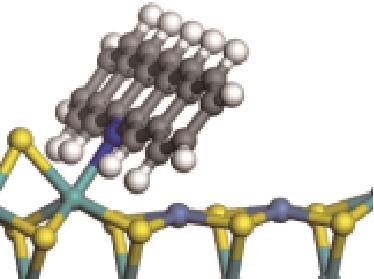 | -172.32 | +0.313 | |
| π-M | 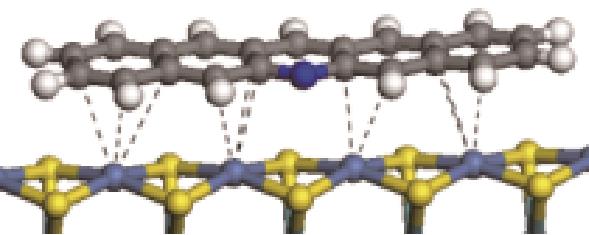 | -164.39 | +0.085 |
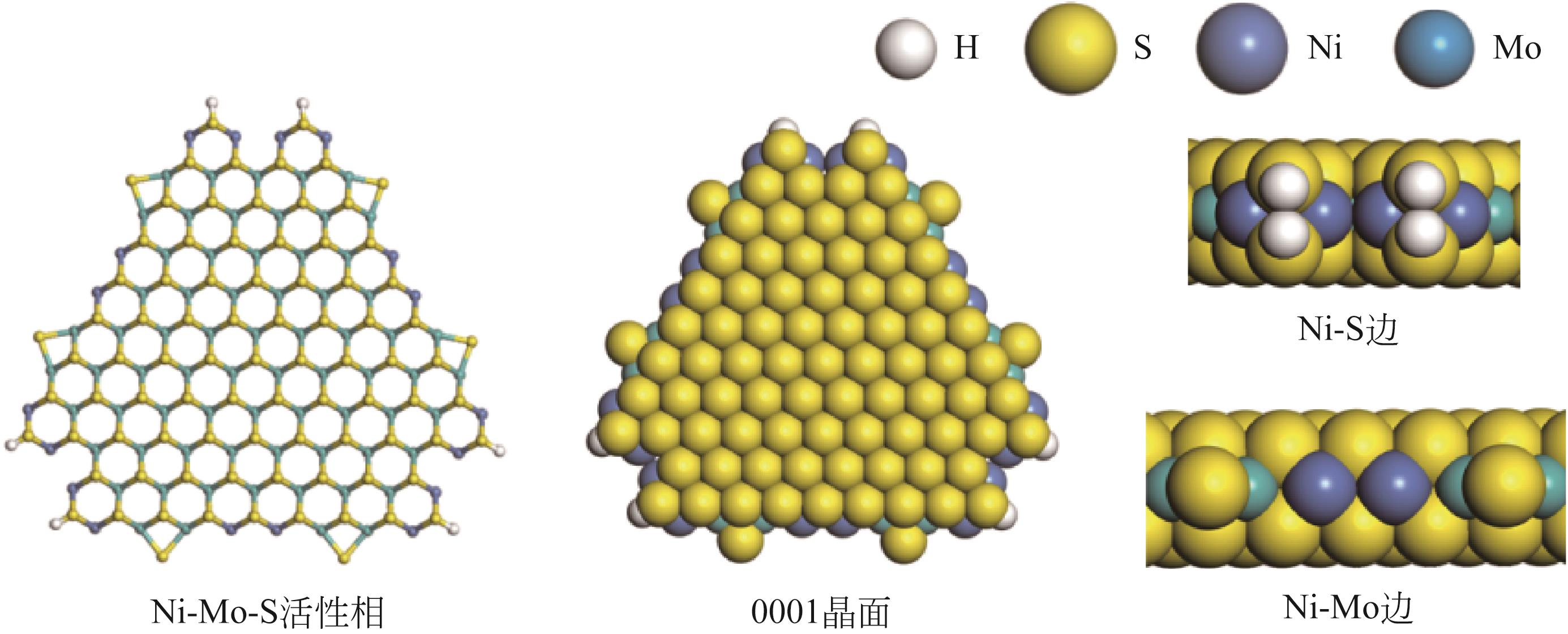
表5 碱性氮化物分子在Ni-Mo-S活性相的吸附
| 碱性氮化物 | 吸附方式 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|---|
 | N-Ni |  | -100.69 | +0.252 |
| N-Mo |  | -117.07 | +0.258 | |
| π-Ni |  | -48.72 | +0.018 | |
 | N-Ni |  | -118.51 | +0.262 |
| N-Mo |  | -145.78 | +0.293 | |
| π-M |  | -92.42 | +0.023 | |
 | N-Ni |  | -112.80 | +0.227 |
| N-Mo |  | -154.10 | +0.311 | |
| π-M |  | -112.84 | +0.026 | |
 | N-Ni |  | -122.20 | +0.262 |
| N-Mo |  | -171.91 | +0.326 | |
| π-M |  | -135.30 | +0.028 | |
 | N-Ni |  | -124.20 | +0.309 |
| N-Mo |  | -172.32 | +0.313 | |
| π-M |  | -164.39 | +0.085 |
| 非碱性氮化物 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|
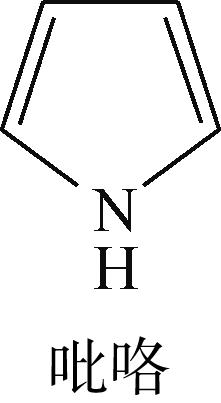 |  | -82.68 | +0.032 |
 | 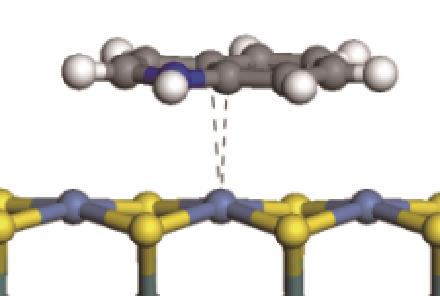 | -81.14 | +0.058 |
 | 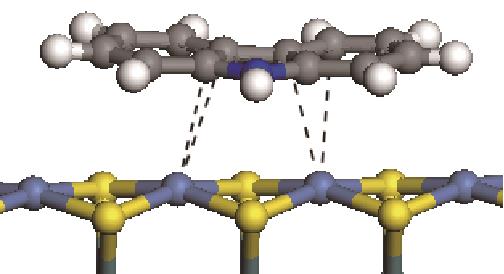 | -114.26 | +0.084 |
 | 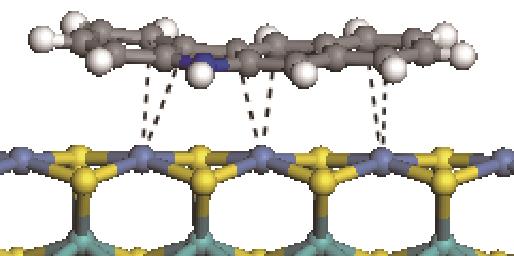 | -121.35 | +0.102 |
 | 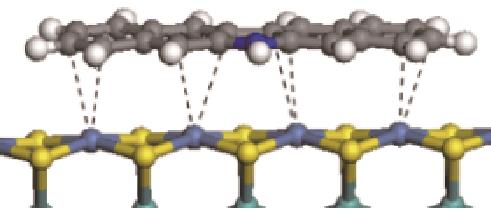 | -186.60 | +0.136 |
表6 非碱性氮化物分子在Ni-Mo-S活性相的吸附
| 非碱性氮化物 | 吸附形貌 | 结合能/kJ·mol-1 | 氮化物电荷转移量/e |
|---|---|---|---|
 |  | -82.68 | +0.032 |
 |  | -81.14 | +0.058 |
 |  | -114.26 | +0.084 |
 |  | -121.35 | +0.102 |
 |  | -186.60 | +0.136 |
| 加氢前状态 | 加氢过渡态 | 加氢后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
 | ||||
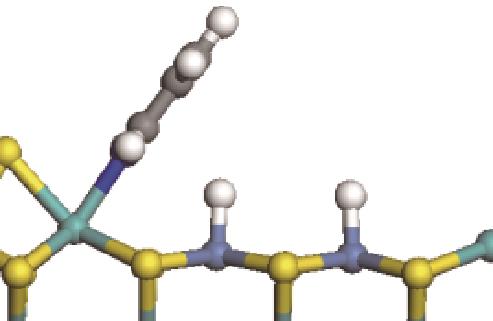 | 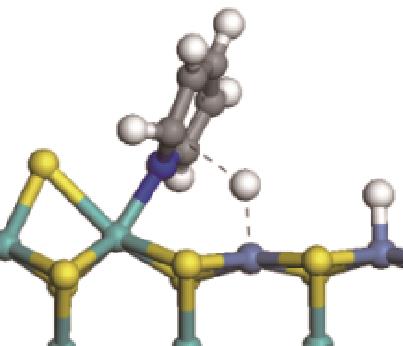 | 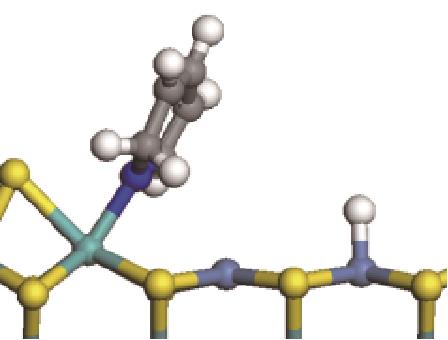 | -11.82 | +79.51 |
 | ||||
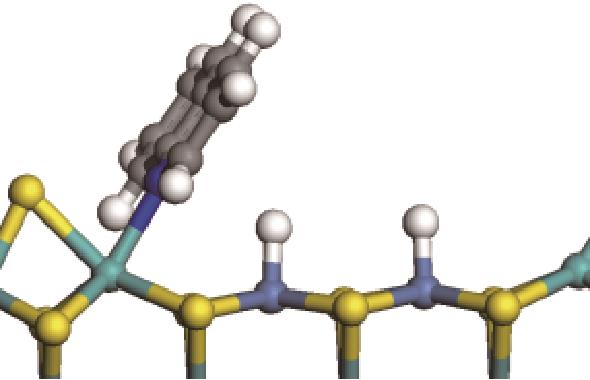 |  | 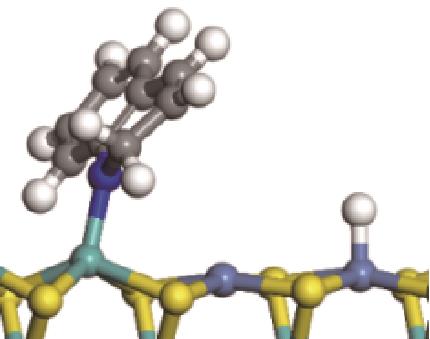 | -18.10 | +86.94 |
 | ||||
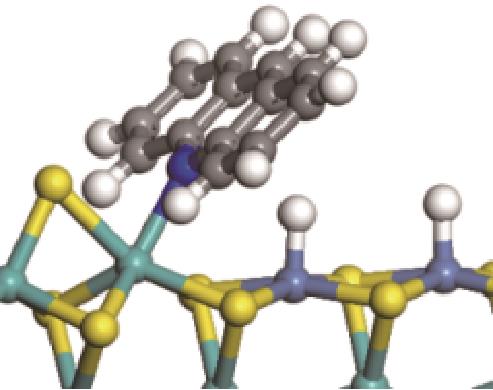 | 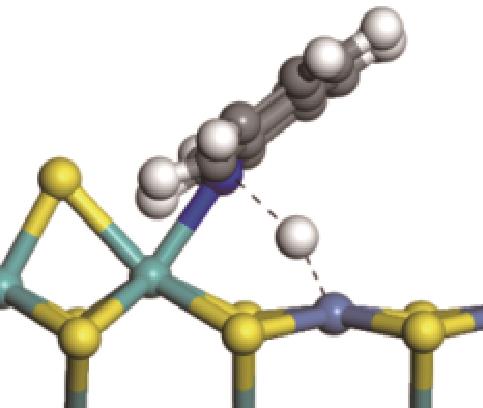 |  | +59.87 | +146.88 |
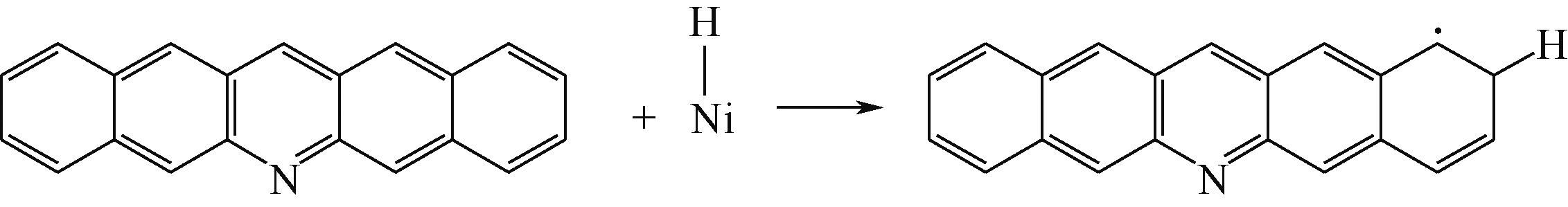 | ||||
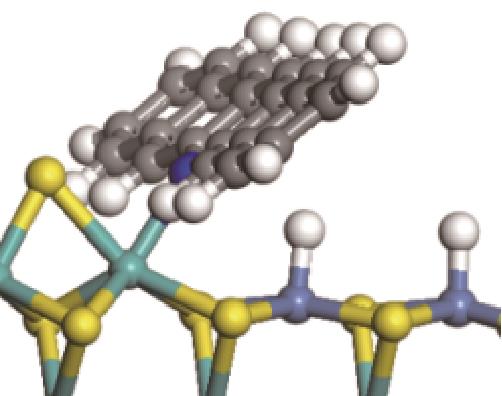 | 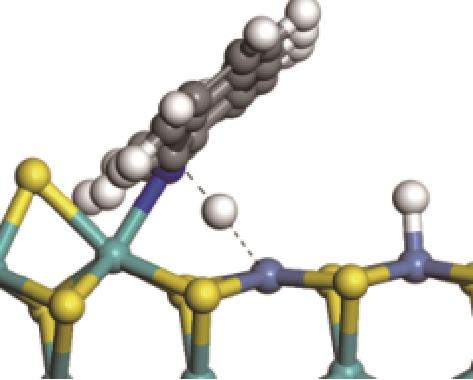 | 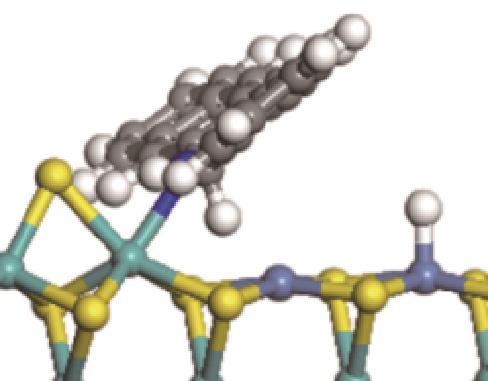 | +45.04 | +139.99 |
表7 碱性氮化物分子的加氢饱和
| 加氢前状态 | 加氢过渡态 | 加氢后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
 | ||||
 |  |  | -11.82 | +79.51 |
 | ||||
 |  |  | -18.10 | +86.94 |
 | ||||
 |  |  | +59.87 | +146.88 |
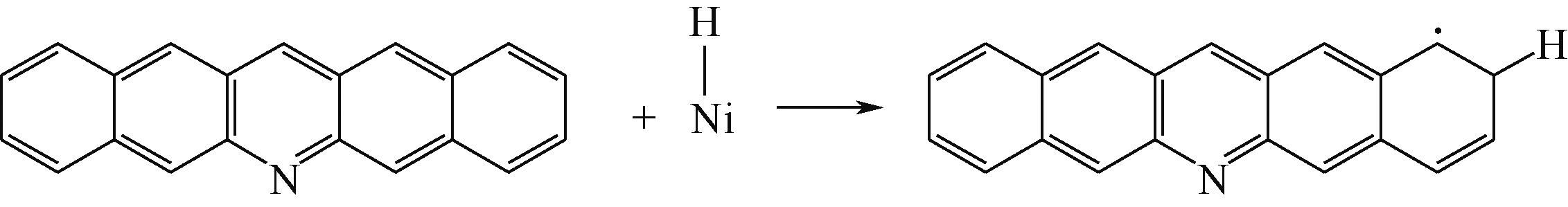 | ||||
 |  |  | +45.04 | +139.99 |
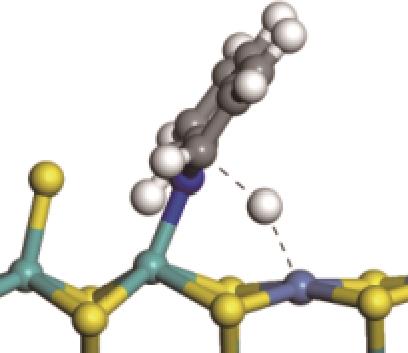
| 氢解前状态 | 加氢过渡态 | 氢解后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
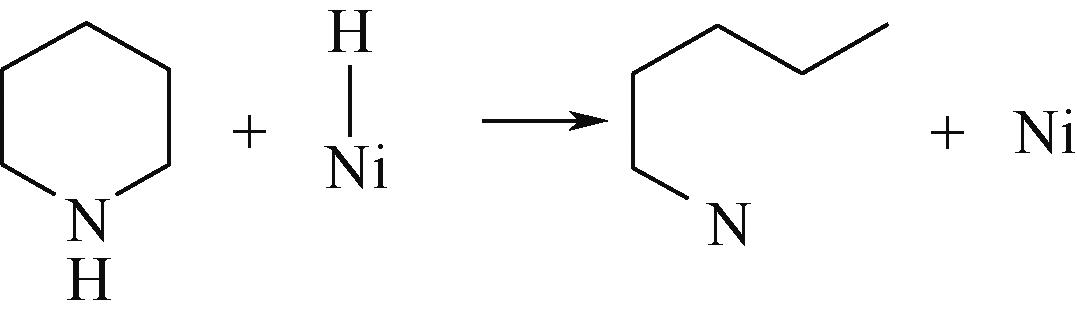 | ||||
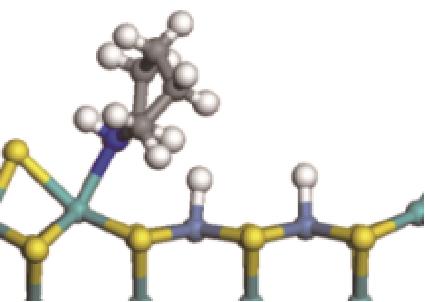 | 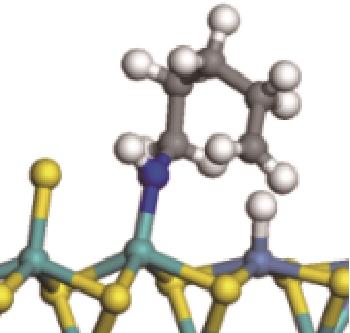 | 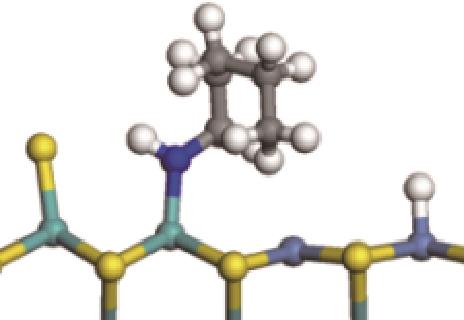 | -118.64 | +197.10 |
 | ||||
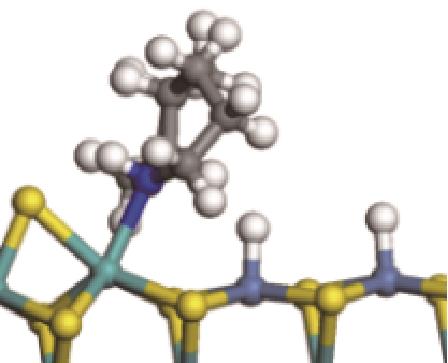 | 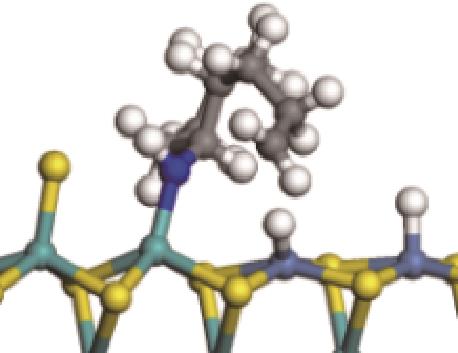 |  | -144.93 | +223.89 |
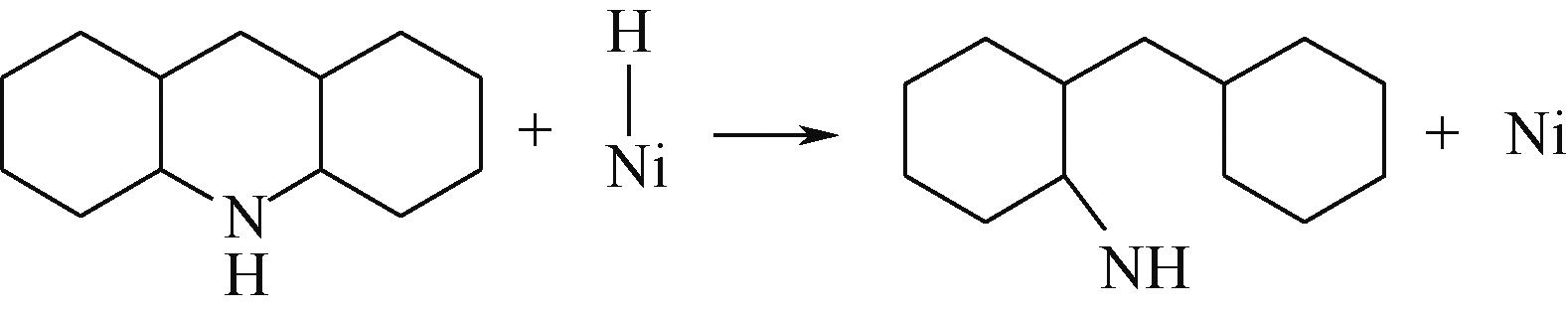 | ||||
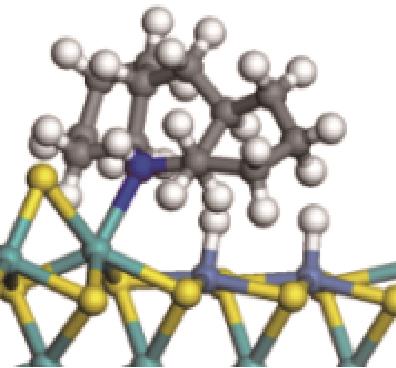 | 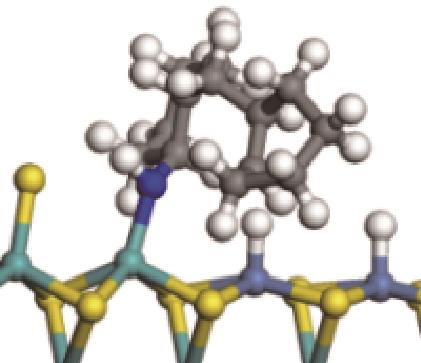 | 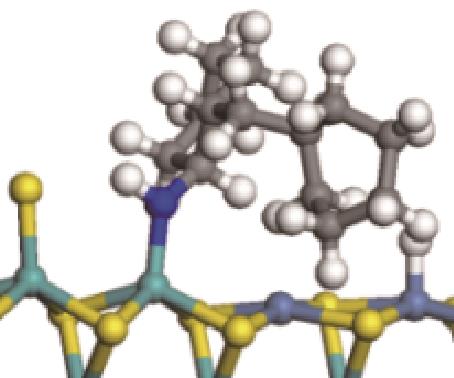 | -99.14 | +206.77 |
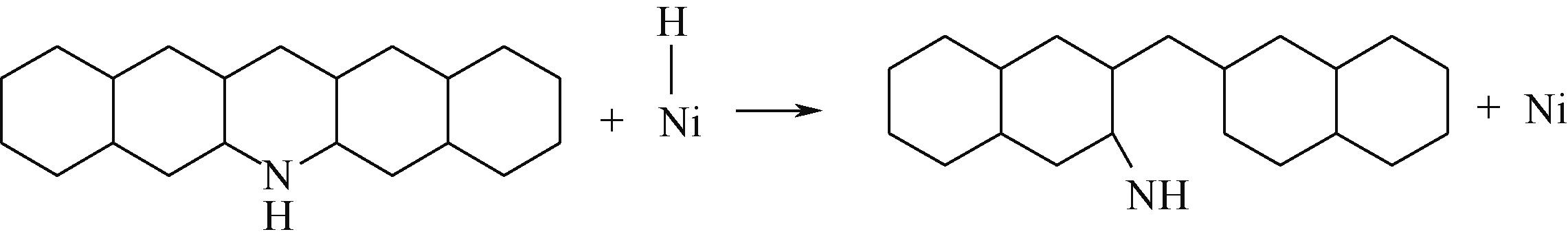 | ||||
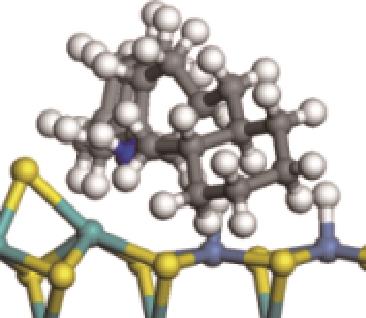 | 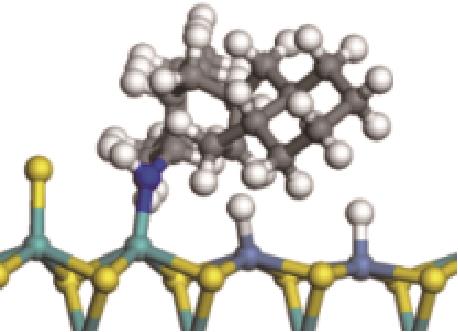 | 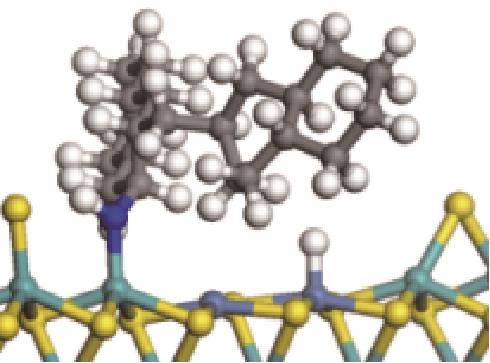 | -84.72 | +223.82 |
表8 C—N键的加氢断裂过程(SN2路径)
| 氢解前状态 | 加氢过渡态 | 氢解后状态 | 反应能/kJ·mol-1 | 活化能/kJ·mol-1 |
|---|---|---|---|---|
 | ||||
 |  |  | -118.64 | +197.10 |
 | ||||
 |  |  | -144.93 | +223.89 |
 | ||||
 |  |  | -99.14 | +206.77 |
 | ||||
 |  |  | -84.72 | +223.82 |
| 催化剂 | 活性相平均堆垛层数 | 活性相平均长度/nm |
|---|---|---|
| CS-1 | 1.3 | 6.2 |
| CS-2 | 2.9 | 7.9 |
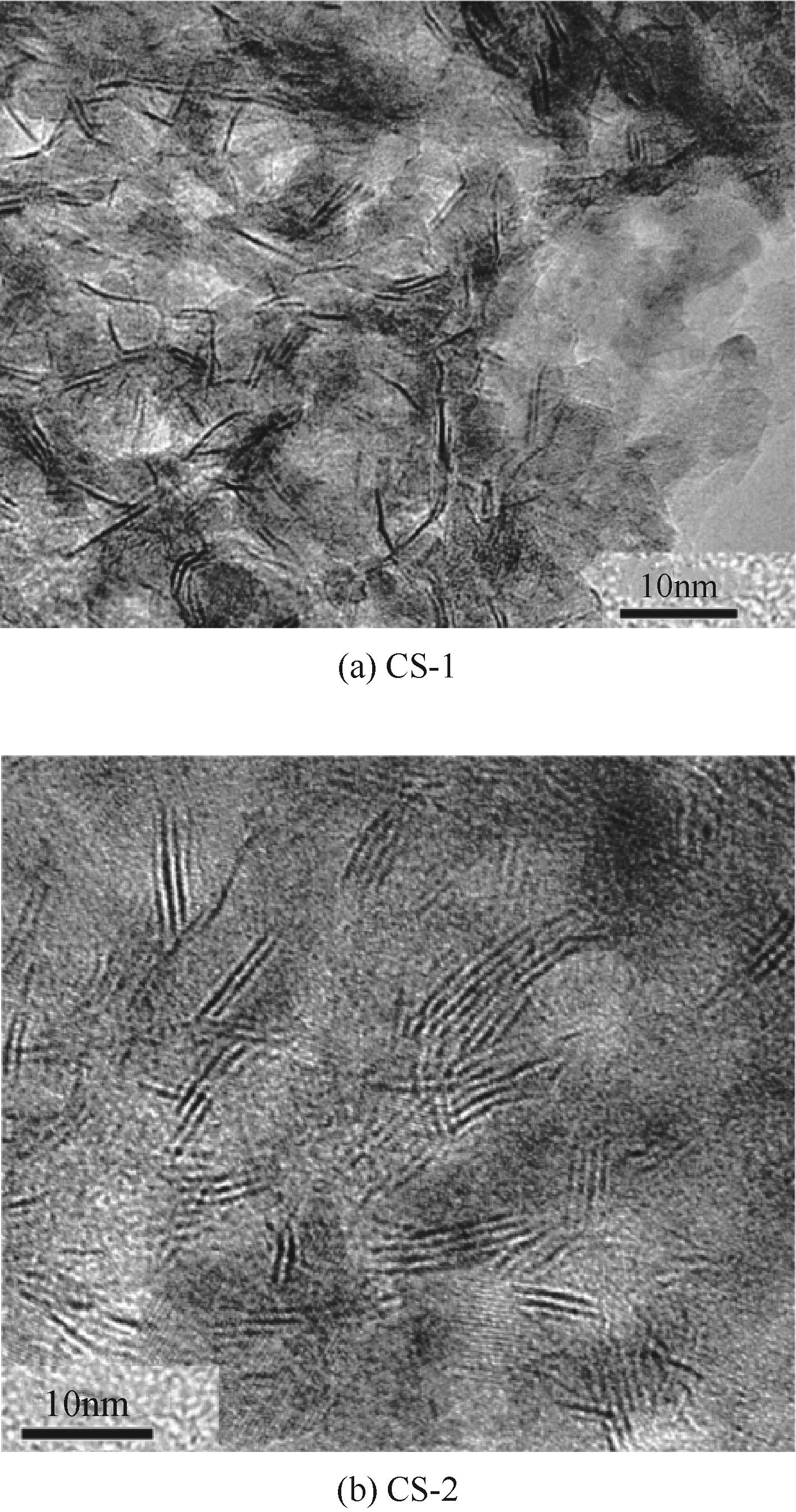
表10 催化剂活性相TEM表征结果
| 催化剂 | 活性相平均堆垛层数 | 活性相平均长度/nm |
|---|---|---|
| CS-1 | 1.3 | 6.2 |
| CS-2 | 2.9 | 7.9 |
| 催化剂 | 反应物 | 反应温度 /℃ | 反应压力 /MPa | 液时空速 /mL·mL-1·h-1 | 转化率 /% |
|---|---|---|---|---|---|
| CS-1 | 1-甲基萘 | 300 | 5.0 | 4.0 | 81.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 94.0 | |
| CS-2 | 1-甲基萘 | 300 | 5.0 | 4.0 | 95.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 75.7 |
表11 CS-1和CS-2催化剂的加氢选择性测试
| 催化剂 | 反应物 | 反应温度 /℃ | 反应压力 /MPa | 液时空速 /mL·mL-1·h-1 | 转化率 /% |
|---|---|---|---|---|---|
| CS-1 | 1-甲基萘 | 300 | 5.0 | 4.0 | 81.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 94.0 | |
| CS-2 | 1-甲基萘 | 300 | 5.0 | 4.0 | 95.3 |
| 二苯并噻吩 | 320 | 4.0 | 4.0 | 75.7 |
| 原料 | 催化剂 | 评价条件 | 产物性质 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
反应压力 /MPa | 反应温度 /℃ | 液时空速 /mL·mL-1·h-1 | 氢油比 /mL·mL-1 | 氮含量 /μg·g-1 | 单环芳烃 质量分数/% | 双环及以上芳烃 质量分数/% | 总芳烃 质量分数/% | |||
| 常三线柴油 | CS-1 | 6.0 | 350 | 2.0 | 500∶1 | 10.3 | 15.8 | 4.4 | 20.2 | |
| CS-2 | 19.6 | 14.7 | 3.5 | 18.2 | ||||||
| 减二线蜡油 | CS-1 | 14.5 | 365 | 1.0 | 1000∶1 | 5.9 | 22.8 | 4.5 | 27.3 | |
| CS-2 | 6.3 | 20.2 | 3.6 | 23.8 | ||||||
| 减三线蜡油 | CS-1 | 16.5 | 380 | 1.0 | 1200∶1 | 11.3 | 20.2 | 3.9 | 24.1 | |
| CS-2 | 3.8 | 14.8 | 3.2 | 18.0 | ||||||
表12 CS-1和CS-2对三种油品的加氢催化实验
| 原料 | 催化剂 | 评价条件 | 产物性质 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
反应压力 /MPa | 反应温度 /℃ | 液时空速 /mL·mL-1·h-1 | 氢油比 /mL·mL-1 | 氮含量 /μg·g-1 | 单环芳烃 质量分数/% | 双环及以上芳烃 质量分数/% | 总芳烃 质量分数/% | |||
| 常三线柴油 | CS-1 | 6.0 | 350 | 2.0 | 500∶1 | 10.3 | 15.8 | 4.4 | 20.2 | |
| CS-2 | 19.6 | 14.7 | 3.5 | 18.2 | ||||||
| 减二线蜡油 | CS-1 | 14.5 | 365 | 1.0 | 1000∶1 | 5.9 | 22.8 | 4.5 | 27.3 | |
| CS-2 | 6.3 | 20.2 | 3.6 | 23.8 | ||||||
| 减三线蜡油 | CS-1 | 16.5 | 380 | 1.0 | 1200∶1 | 11.3 | 20.2 | 3.9 | 24.1 | |
| CS-2 | 3.8 | 14.8 | 3.2 | 18.0 | ||||||
| 油品名称 | 氮化物类型 | 单芳环氮化物质量分数/% | 双芳环氮化物质量分数/% | 三芳环氮化物质量分数/% | 四芳环及以上氮化物质量分数/% |
|---|---|---|---|---|---|
| 原料 | 碱氮 | 26.3 | 36.8 | 30.7 | 6.2 |
| 非碱氮 | 0.8 | 38.4 | 46.7 | 14.1 | |
| CS-1加氢产物 | 碱氮 | 0.2 | 6.4 | 86.5 | 6.9 |
| 非碱氮 | 0.9 | 6.3 | 81.3 | 11.5 | |
| CS-2加氢产物 | 碱氮 | 0.4 | 34.1 | 54.8 | 10.7 |
| 非碱氮 | 0.1 | 12.7 | 71.9 | 6.3 |
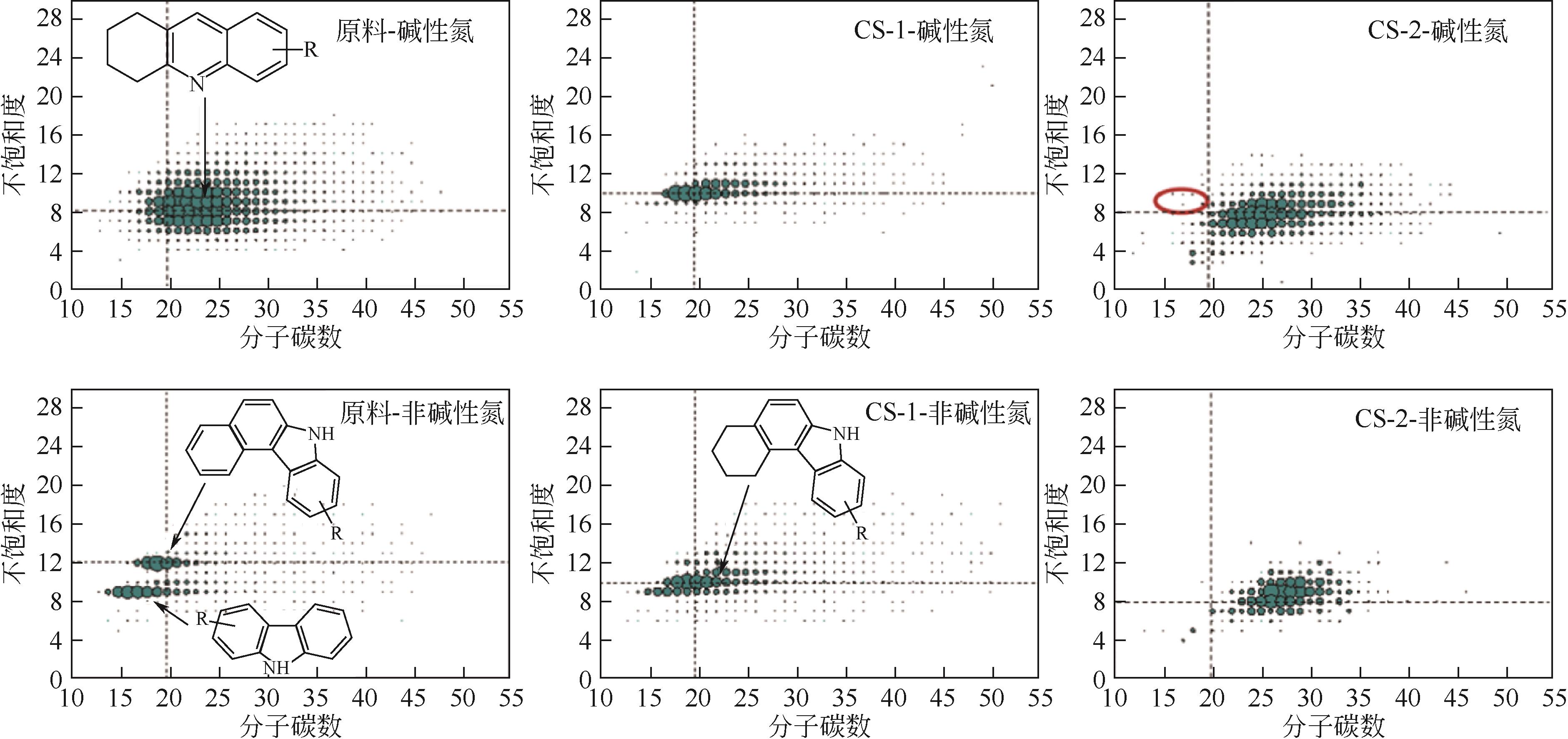
表13 VGO加氢前后氮化物结构统计
| 油品名称 | 氮化物类型 | 单芳环氮化物质量分数/% | 双芳环氮化物质量分数/% | 三芳环氮化物质量分数/% | 四芳环及以上氮化物质量分数/% |
|---|---|---|---|---|---|
| 原料 | 碱氮 | 26.3 | 36.8 | 30.7 | 6.2 |
| 非碱氮 | 0.8 | 38.4 | 46.7 | 14.1 | |
| CS-1加氢产物 | 碱氮 | 0.2 | 6.4 | 86.5 | 6.9 |
| 非碱氮 | 0.9 | 6.3 | 81.3 | 11.5 | |
| CS-2加氢产物 | 碱氮 | 0.4 | 34.1 | 54.8 | 10.7 |
| 非碱氮 | 0.1 | 12.7 | 71.9 | 6.3 |
| 1 | 王福江, 张毓莹, 龙湘云, 等. 催化裂化柴油馏分加氢精制提高十六烷值研究[J]. 石油炼制与化工, 2013, 44(10): 27-31. |
| WANG Fujiang, ZHANG Yuying, LONG Xiangyun, et al. Study on cetane number improvement of lco by hydrotreating[J]. Petroleum Processing and Petrochemicals, 2013, 44(10): 27-31. | |
| 2 | 柴永明, 相春娥, 孔会清, 等. 馏分油浆态床加氢处理研究 Ⅰ: 催化剂制备方法[J]. 燃料化学学报, 2008, 36(6): 720-725. |
| CHAI Yongming, XIANG Chune, KONG Huiqing, et al. A study on slurry-bed hydrotreating process of distillate oilⅠ: Catalyst preparation[J]. Journal of Fuel Chemistry and Technology, 2008, 36(6): 720-725. | |
| 3 | 王继锋, 梁相程, 温德荣, 等. 重质馏分油加氢精制催化剂的研制、生产和应用[J]. 石油化工高等学校学报, 2001, 14(1): 42-47. |
| WANG Jifeng, LIANG Xiangcheng, WEN Derong, et al. The research, manufacture and application of heavy distillate hydrorefining catalyst[J]. Journal of Petrochemical Universities, 2001, 14(1): 42-47. | |
| 4 | 高玉兰, 曹凤兰, 周勇. FH-5A加氢精制催化剂的研制[J]. 石油炼制与化工, 2002, 33(1): 18-21. |
| GAO Yulan, CAO Fenglan, ZHOU Yong. Preparation of hydrorefining catalyst FH-5A[J]. Petroleum Processing and Petrochemicals, 2002, 33(1): 18-21. | |
| 5 | 刘文勇, 田然, 陈世安, 等. 新型重质馏分油加氢精制催化剂的研制[J]. 化工进展, 2009, 28(3): 401-405. |
| LIU Wenyong, TIAN Ran, CHEN Shian, et al. Study on new catalysts of hydrorefining for VGO[J]. Chemical Industry and Engineering Progress, 2009, 28(3): 401-405. | |
| 6 | 朱全力, 赵旭涛, 赵振兴, 等. 加氢脱硫催化剂与反应机理的研究进展[J]. 分子催化, 2006, 20(4): 372-383. |
| ZHU Quanli, ZHAO Xutao, ZHAO Zhenxing, et al. Research progress of hydrodesulfurization catalyst and reaction mechanism[J]. Journal of Molecular Catalysis, 2006, 20(4): 372-383. | |
| 7 | 刘大鹏, 李永丹. 加氢脱氮催化研究的新进展[J]. 化学进展, 2006, 18(4): 417-428. |
| LIU Dapeng, LI Yongdan. Advances in catalytic hydrodenitrogenation[J]. Progress in Chemistry, 2006, 18(4): 417-428. | |
| 8 | 杨骏, 陈满英, 任杰. Mo、W对Ni/Al2O3催化剂加氢脱氧性能的影响[J]. 化工进展, 2005, 24(12): 1386-1389. |
| YANG Jun, CHEN Manying, REN Jie. Effect of Mo, W addition on performance of Ni/Al2O3 catalyst for hydrodeoxygenation[J]. Chemical Industry and Engineering Progress, 2005, 24(12): 1386-1389. | |
| 9 | 张孔远, 燕京, 吕才山. 重油加氢脱金属催化剂的性能及沉积金属的分布研究[J]. 石油炼制与化工, 2004, 35(8): 30-33. |
| ZHANG Kongyuan, YAN Jing, Caishan LYU. The performance of heavy oilhydrodemetallization catalystand its deposited metal distribution[J]. Petroleum Processing and Petrochemicals, 2004, 35(8): 30-33. | |
| 10 | 相春娥, 柴永明, 柳云骐, 等. NiMoS/γ-Al2O3上二苯并噻吩加氢脱硫和喹啉加氢脱氮反应的相互影响[J]. 催化学报, 2008, 29(7): 595-601. |
| XIANG Chune, CHAI Yongming, LIU Yunqi, et al. Mutual influence of hydrodesulfurization of dibenzothiophene and hydrodenitrogenation of quinoline over NiMoS/γ-Al2O3 catalyst[J]. Chinese Journal of Catalysis, 2008, 29 (7): 595-601. | |
| 11 | 张奇, 刘春艳, 梁长海, 等. NiWS/γ-Al2O3催化剂上吲哚加氢脱氮反应: H2S和喹啉的影响[J]. 燃料化学学报, 2011, 39(5): 361-366. |
| ZHANG Qi, LIU Chunyan, LIANG Changhai, et al. Hydrodenitrogenation of indole over NiWS/γ-Al2O3 catalyst: Effects of H2S and quinoline[J]. Journal of Fuel Chemistry and Technology, 2011, 39(5): 361-366. | |
| 12 | 相春娥, 柴永明, 柳云骐, 等. 二苯并噻吩对吲哚加氢脱氮反应的影响[C]//第十一届全国青年催化学术会议论文集(下). 青岛, 2007: 276-277. |
| XIANG Chun’e, CHAI Yongming, LIU Yunqi, et al. Effect of dibenzothiophene on the denitrification reaction of indole hydrogenation[C]// Proceedings of the 11th National Youth Catalytic Academic Conference (Part Ⅱ). Qingdao, 2007: 276-277. | |
| 13 | ADAMSKI G, DYREK K, KOTARBA A, et al. Kinetic model of indole HDN over molybdenum carbide: Influence of potassium on early and late denitrogenation pathways[J]. Catalysis Today, 2004, 90(1/2): 115-119. |
| 14 | 李贵贤, 曹彦伟, 李梦晨, 等. 煤焦油加氢脱氮反应网络及催化剂研究进展[J]. 化工进展, 2015, 34(5): 1283-1290. |
| LI Guixian, CAO Yanwei, LI Mengchen, et al. Research progress in hydrodenitrogenation reaction network and its catalysts for coal-tar[J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1283-1290. | |
| 15 | 韩姝娜, 周同娜, 柴永明, 等. 存在4, 6-DMDBT时喹啉、吲哚和咔唑在Ni-Mo加氢催化剂上加氢脱氮自抑制作用[J]. 石油学报(石油加工), 2010, 26(3): 351-356. |
| HAN Shuna, ZHOU Tongna, CHAI Yongming, et al. Self-inhibition for HDN of quinoline, indole and carbazole over Ni-Mo catalyst in the presence of 4,6-DMDBT[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2010, 26(3): 351-356. | |
| 16 | 彭全铸, 赵琰. 重质馏分油性质及其加氢处理[J]. 工业催化, 1997, 5(2): 18-27. |
| PENG Quanzhu, ZHAO Yan. Properties of heavy distillates and their hydrotreatment[J]. Gongye Cuihua (Industrial Catalysis), 1997, 5(2): 18-27. | |
| 17 | 王宝俊, 张玉贵, 谢克昌. 量子化学计算在煤的结构与反应性研究中的应用[J]. 化工学报, 2003, 54(4): 477-488. |
| WANG Baojun, ZHANG Yugui, XIE Kechang. Application of quantum chemistry calculation to investigation on coal structure and reactivity[J]. Journal of Chemical Industry and Engineering (China), 2003, 54(4): 477-488. | |
| 18 | 付立海, 李秀梅, 刘博, 等. 两种铜配位聚合物的晶体结构和量子化学计算[J]. 无机化学学报, 2022, 38(11): 2249-2258. |
| FU Lihai, LI Xiumei, LIU Bo, et al. Two copper coordination polymers: Crystal structure and quantum chemistry calculation[J]. Chinese Journal of Inorganic Chemistry, 2022, 38(11): 2249-2258. | |
| 19 | 丁思佳, 彭绍忠, 闫作杰, 等. 量子化学计算在催化加氢研究中的应用[J]. 当代化工, 2021, 50(4): 938-943. |
| DING Sijia, PENG Shaozhong, YAN Zuojie, et al. Application of quantum chemical calculation in catalytic hydrogenation[J]. Contemporary Chemical Industry, 2021, 50(4): 938-943. | |
| 20 | DING Sijia, JIANG Shujiao, ZHOU Yasong, et al. Catalytic characteristics of active corner sites in CoMoS nanostructure hydrodesulfurization—A mechanism study based on DFT calculations[J]. Journal of Catalysis, 2017, 345: 24-38. |
| 21 | DING Sijia, ZHOU Yasong, WEI Qiang, et al. Substituent effects of 4,6-DMDBT on direct hydrodesulfurization routes catalyzed by Ni-Mo-S active nanocluster—A theoretical study[J]. Catalysis Today, 2018, 305: 28-39. |
| 22 | DING Sijia, LI Anqi, JIANG Shujiao, et al. Niobium modification effects on hydrodesulfurization of 4,6-DMDBT catalyzed on Ni-Mo-S active sites: A combination of experiments and theoretical study[J]. Fuel, 2019, 237: 429-441. |
| 23 | DING Sijia, JIANG Shujiao, WANG Jifeng, et al. Effects of the Ni-Mo ratio on olefin selective hydrogenation catalyzed on Ni-Mo-S active sites: A theoretical study by DFT calculation[J]. Fuel, 2020, 277: 118136. |
| 24 | HUMBERT S, IZZET G, RAYBAUD P. Competitive adsorption of nitrogen and sulphur compounds on a multisite model of NiMoS catalyst: A theoretical study[J]. Journal of Catalysis, 2016, 333: 78-93. |
| 25 | INADA Yasuji, ORITA Hideo. Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: Evidence of small basis set superposition error compared to Gaussian basis sets[J]. Journal of Computational Chemistry, 2008, 29(2): 225-232. |
| 26 | DELLEY B, ELLIS D E. Efficient and accurate expansion methods for molecules in local density models[J]. The Journal of Chemical Physics, 1982, 76(4): 1949-1960. |
| 27 | CHIGO ANOTA Ernesto, COCOLETZI Gregorio H. GGA-based analysis of the metformin adsorption on BN nanotubes[J]. Physica E: Low-Dimensional Systems and Nanostructures, 2014, 56: 134-140. |
| 28 | GRIMME Stefan. Semiempirical GGA-type density functional constructed with a long-range dispersion correction[J]. Journal of Computational Chemistry, 2006, 27(15): 1787-1799. |
| 29 | GRIMME Stefan. Density functional theory with London dispersion corrections[J]. WIREs Computational Molecular Science, 2011, 1(2): 211-228. |
| 30 | GRIMME Stefan, ANTONY Jens, EHRLICH Stephan, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| [1] | 吴达, 蒋淑娇, 魏强, 袁胜华, 杨刚, 张成. 能源转型中渣油高效利用技术的研究进展[J]. 化工进展, 2024, 43(5): 2343-2353. |
| [2] | 桂鑫, 陈汇勇, 白柏杨, 贾永梁, 马晓迅. Mo掺杂改性NiC/Al-MCM-41的芘催化加氢性能[J]. 化工进展, 2024, 43(5): 2386-2395. |
| [3] | 王冰, 王磊, 黄欣茹, 袁红鹏, 赖小娟, 李朋. 一种耐酸耐碱高强树脂的合成及性能[J]. 化工进展, 2024, 43(4): 1992-2000. |
| [4] | 刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741. |
| [5] | 王红妍, 马子然, 李歌, 马静, 赵春林, 周佳丽, 王磊, 彭胜攀. 燃煤耦合可再生燃料烟气多污染物协同催化脱除研究进展[J]. 化工进展, 2024, 43(4): 1783-1795. |
| [6] | 陈家一, 高帷韬, 阴亚楠, 王诚, 欧阳鸿武, 毛宗强. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| [7] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [8] | 刘雨蓉, 王兴宝, 李文英. 分子筛负载Pt催化剂酸性位点调控及对蒽深度加氢性能的影响[J]. 化工进展, 2024, 43(4): 1832-1839. |
| [9] | 郭潇东, 毛玉娇, 刘相洋, 邱丽, 于峰, 闫晓亮. Ni/Sm2O3-CeO2/Al2O3催化剂氧空位对二氧化碳低温甲烷化的影响[J]. 化工进展, 2024, 43(4): 1840-1850. |
| [10] | 高飞, 刘志松, 潘珂珂, 刘敏敏, 代斌, 但建明, 于锋. 蛭石基FeCeO x 催化剂及CO选择性催化还原NO[J]. 化工进展, 2024, 43(4): 1851-1862. |
| [11] | 刘方旺, 韩艺, 张佳佳, 步红红, 王兴鹏, 于传峰, 刘猛帅. CO2与环氧化物耦合制备环状碳酸酯的多相催化体系研究进展[J]. 化工进展, 2024, 43(3): 1252-1265. |
| [12] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [13] | 谷星朋, 马红钦, 刘嘉豪. 雷尼镍的磷量子点改性及其催化加氢脱硫性能[J]. 化工进展, 2024, 43(3): 1293-1301. |
| [14] | 张书铭, 刘化章. 基于BP神经网络模型优化Fe1-x O基氨合成催化剂[J]. 化工进展, 2024, 43(3): 1302-1308. |
| [15] | 陈风, 王宣德, 黄伟, 王晓东, 王琰. HZSM-22的粒径调控及Pt/HZSM-22的正十二烷加氢异构催化性能[J]. 化工进展, 2024, 43(3): 1309-1317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||