化工进展 ›› 2024, Vol. 43 ›› Issue (4): 1953-1961.DOI: 10.16085/j.issn.1000-6613.2023-0555
• 材料科学与技术 • 上一篇
锌掺杂型钛系铯离子筛的制备及其吸附性能
- 青岛科技大学化工学院,山东 青岛 266100
-
收稿日期:2023-04-07修回日期:2023-05-11出版日期:2024-04-15发布日期:2024-05-13 -
通讯作者:董殿权 -
作者简介:王德斌(2000—),男,硕士研究生,研究方向为化工材料与分离工程。E-mail:4021010072@mails.qust.edu.cn。 -
基金资助:山东省重点研发计划(2019GGX102034)
Preparation and adsorption properties of zinc-doped titanium-based cesium ion sieves
WANG Debin( ), LIN Mengyu, YANG Xue, DONG Dianquan(
), LIN Mengyu, YANG Xue, DONG Dianquan( )
)
- College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266100, Shandong, China
-
Received:2023-04-07Revised:2023-05-11Online:2024-04-15Published:2024-05-13 -
Contact:DONG Dianquan
摘要:
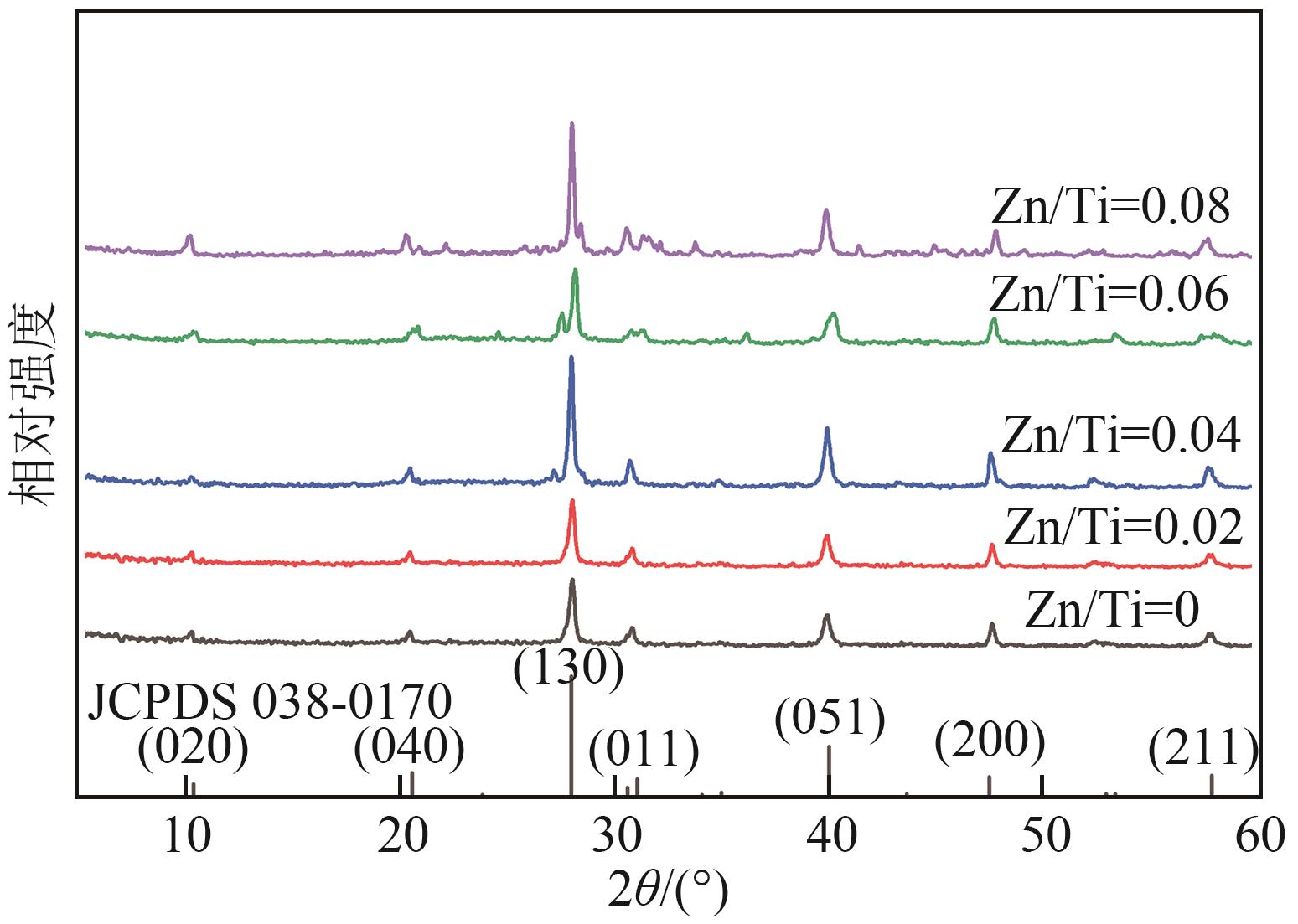
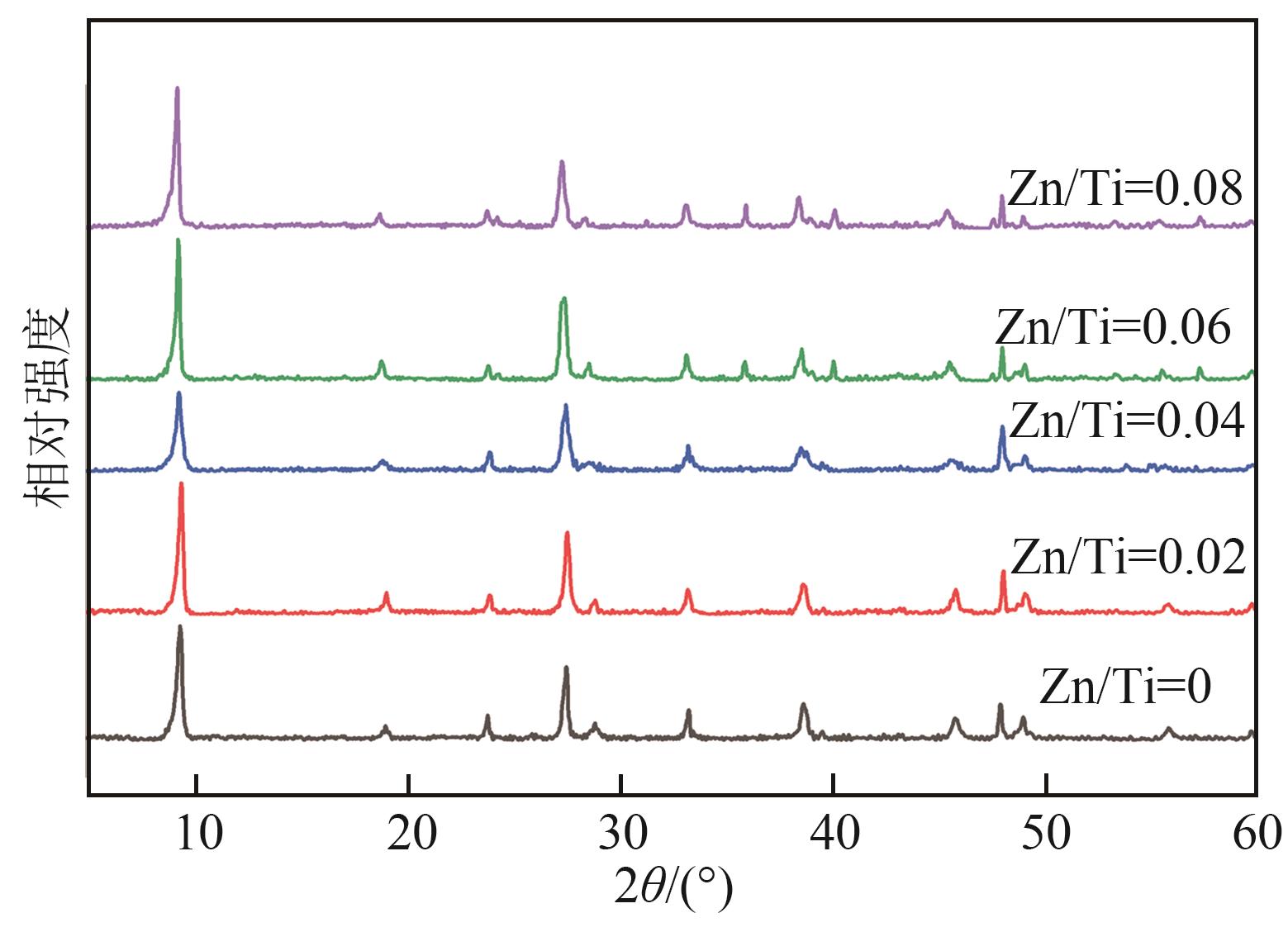
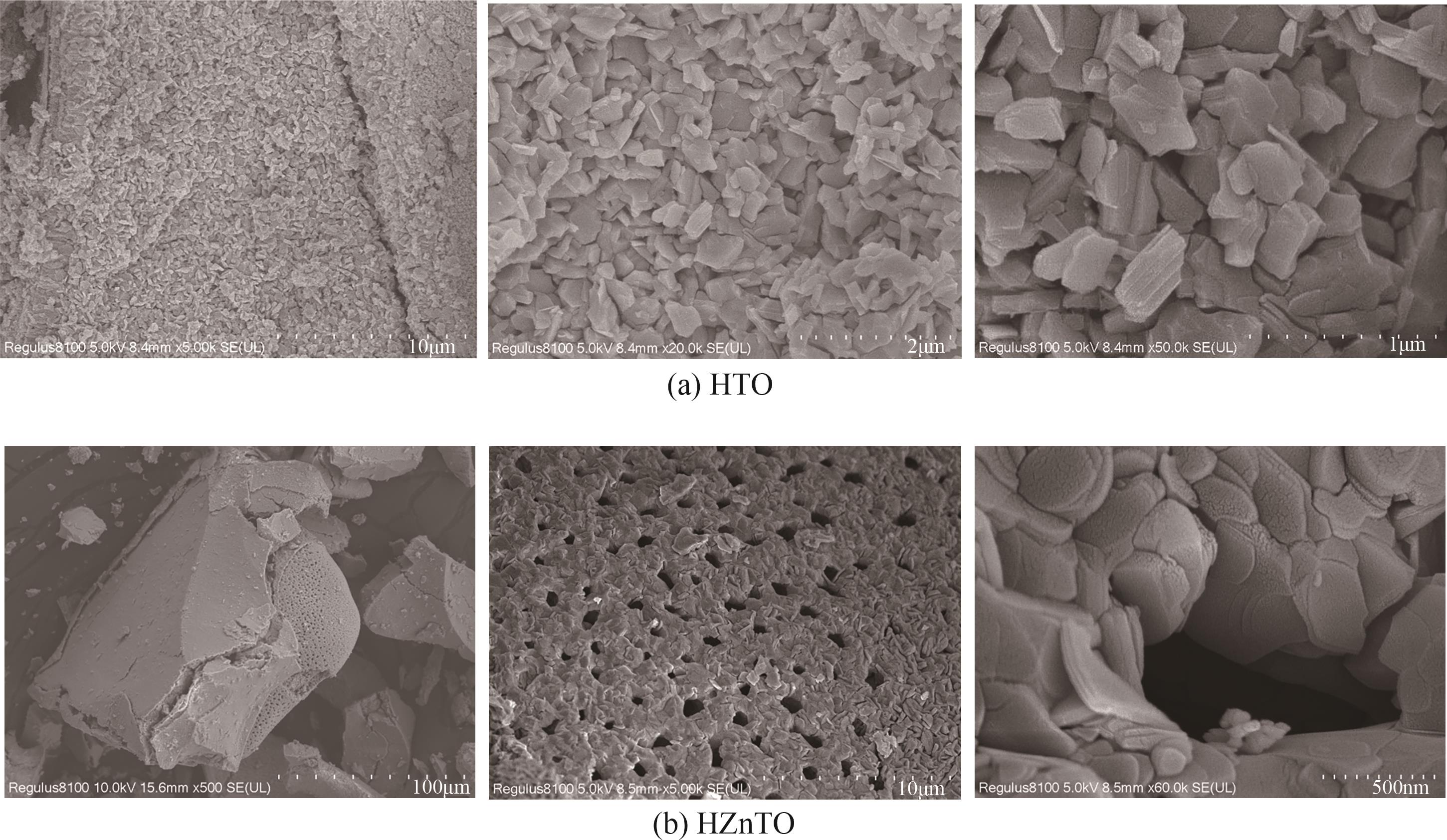
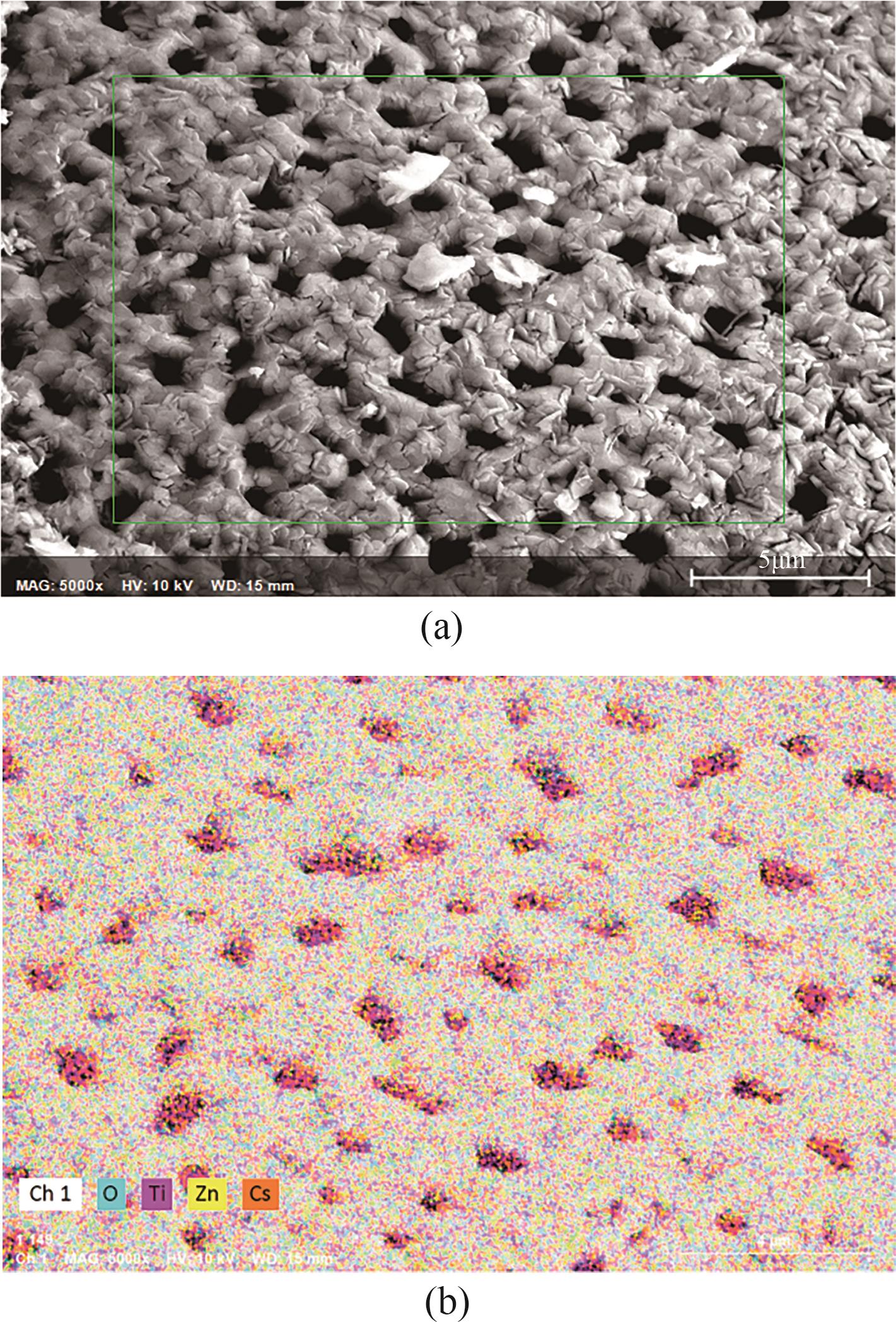
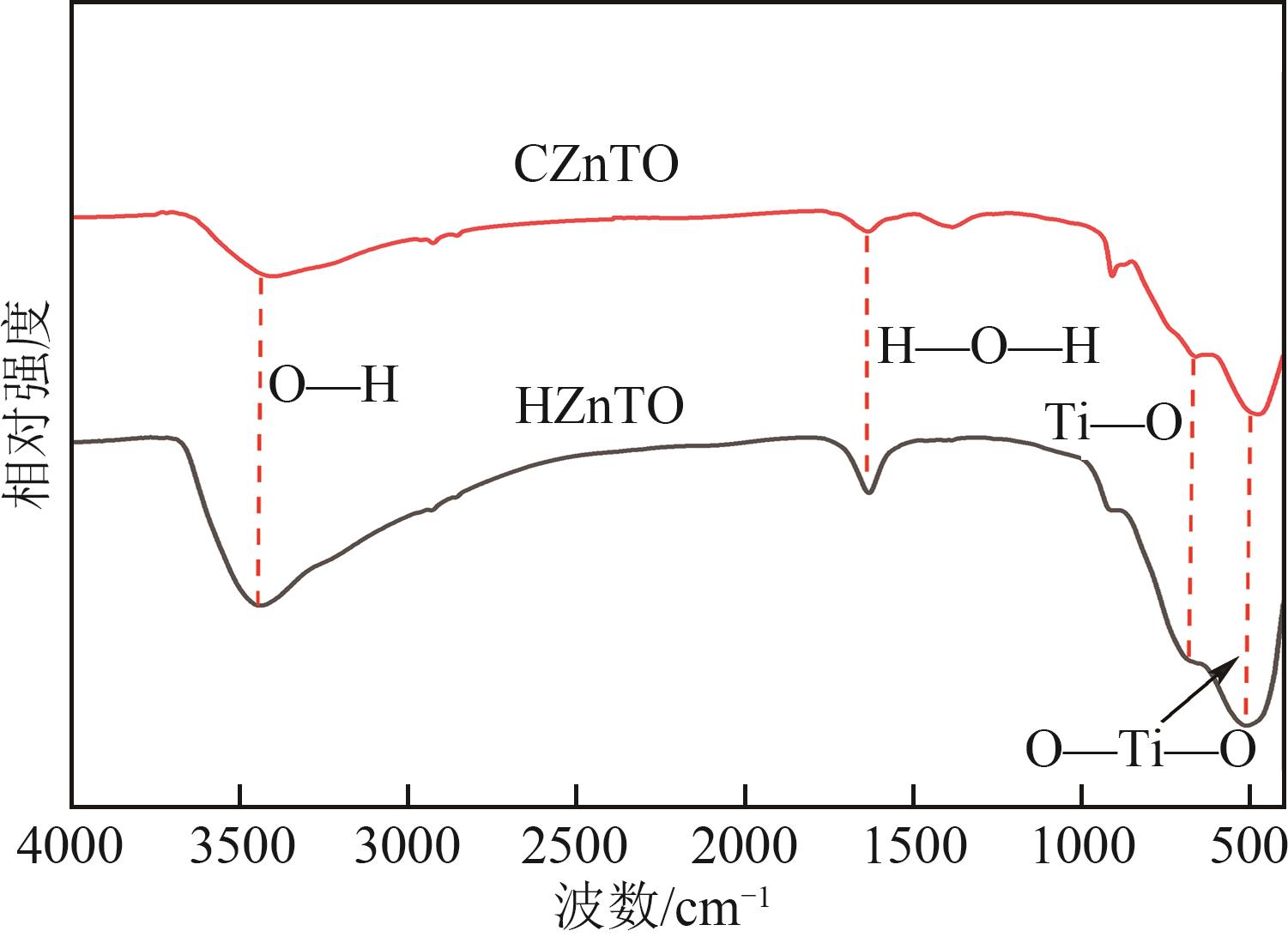
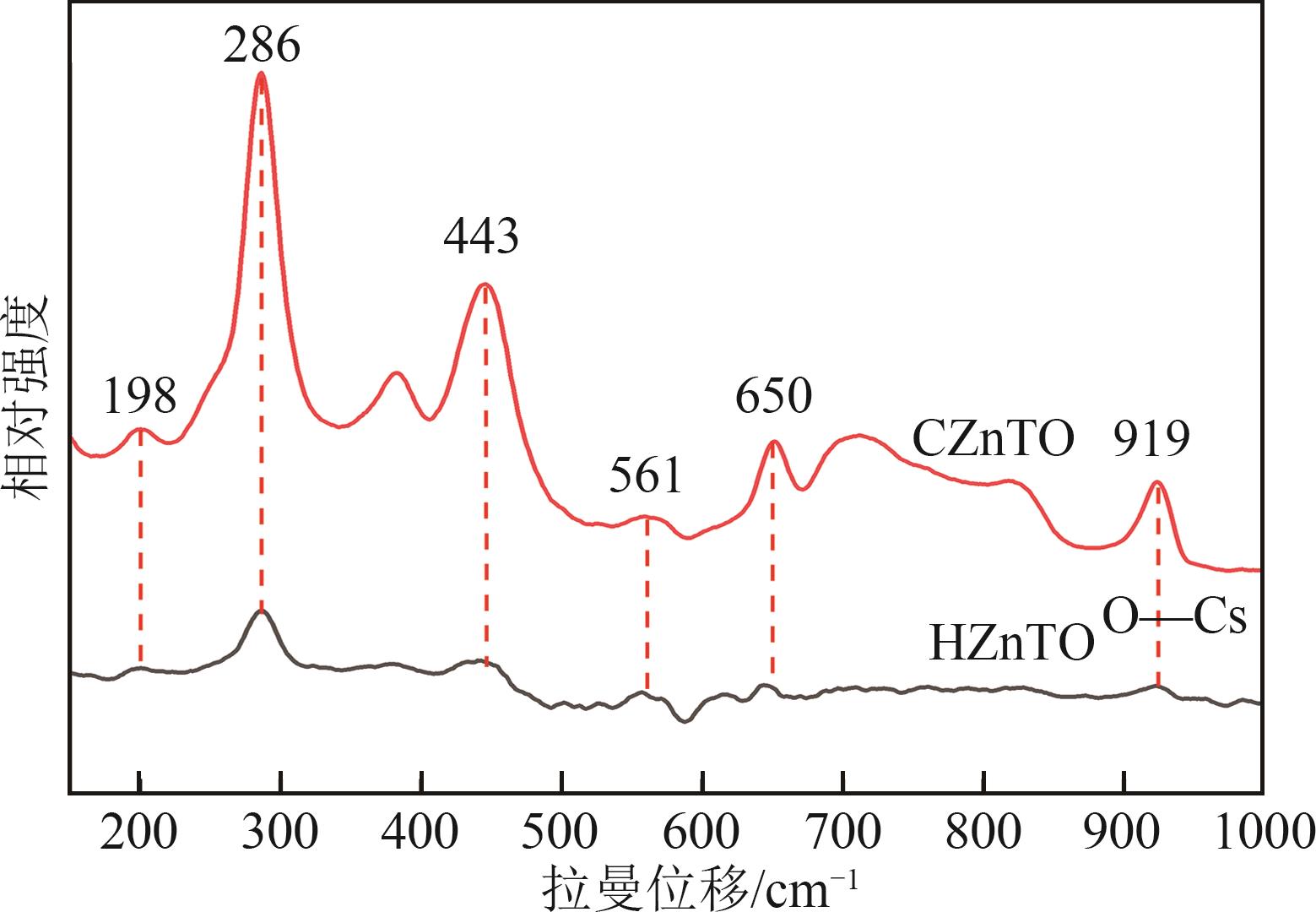
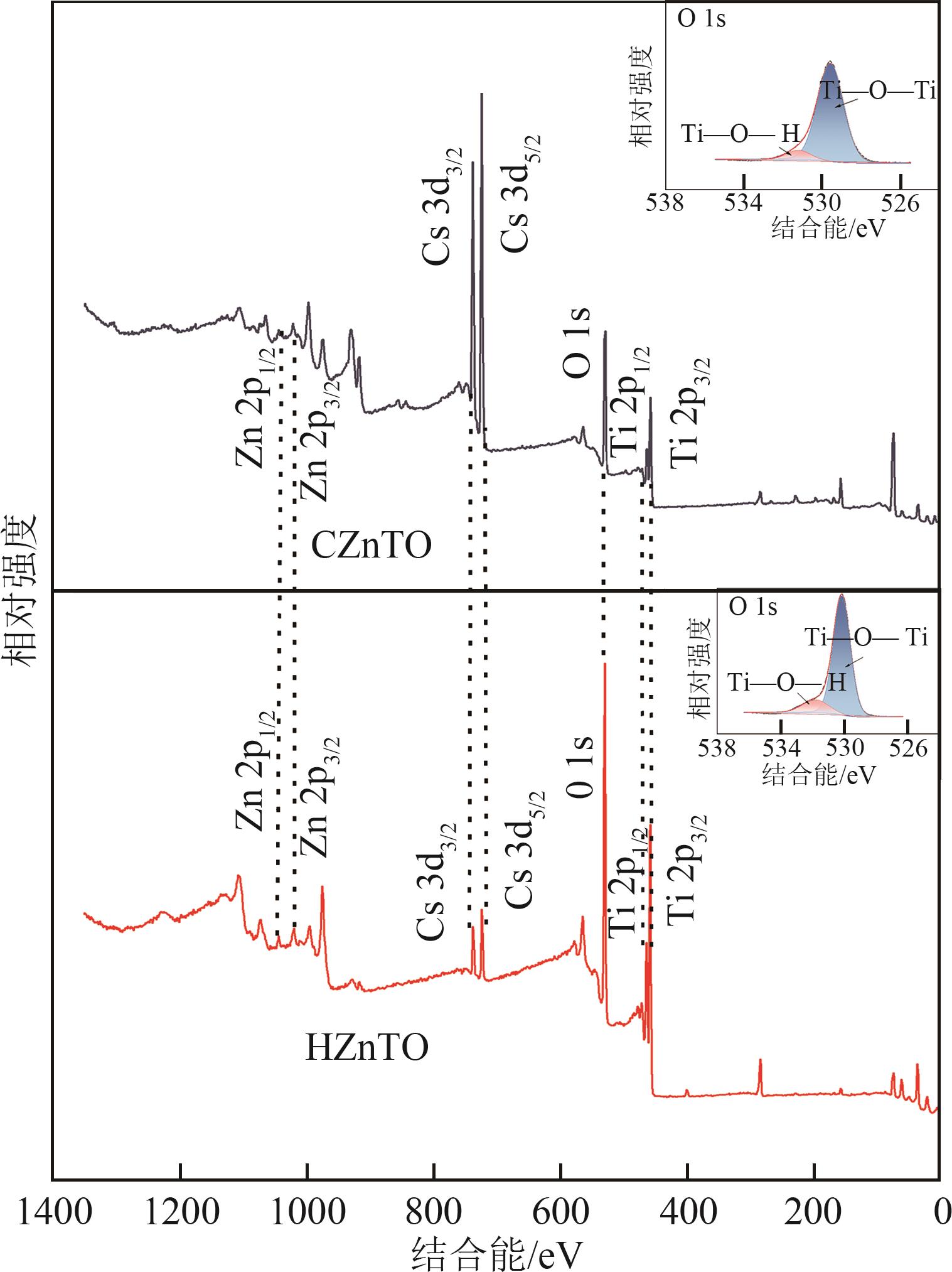
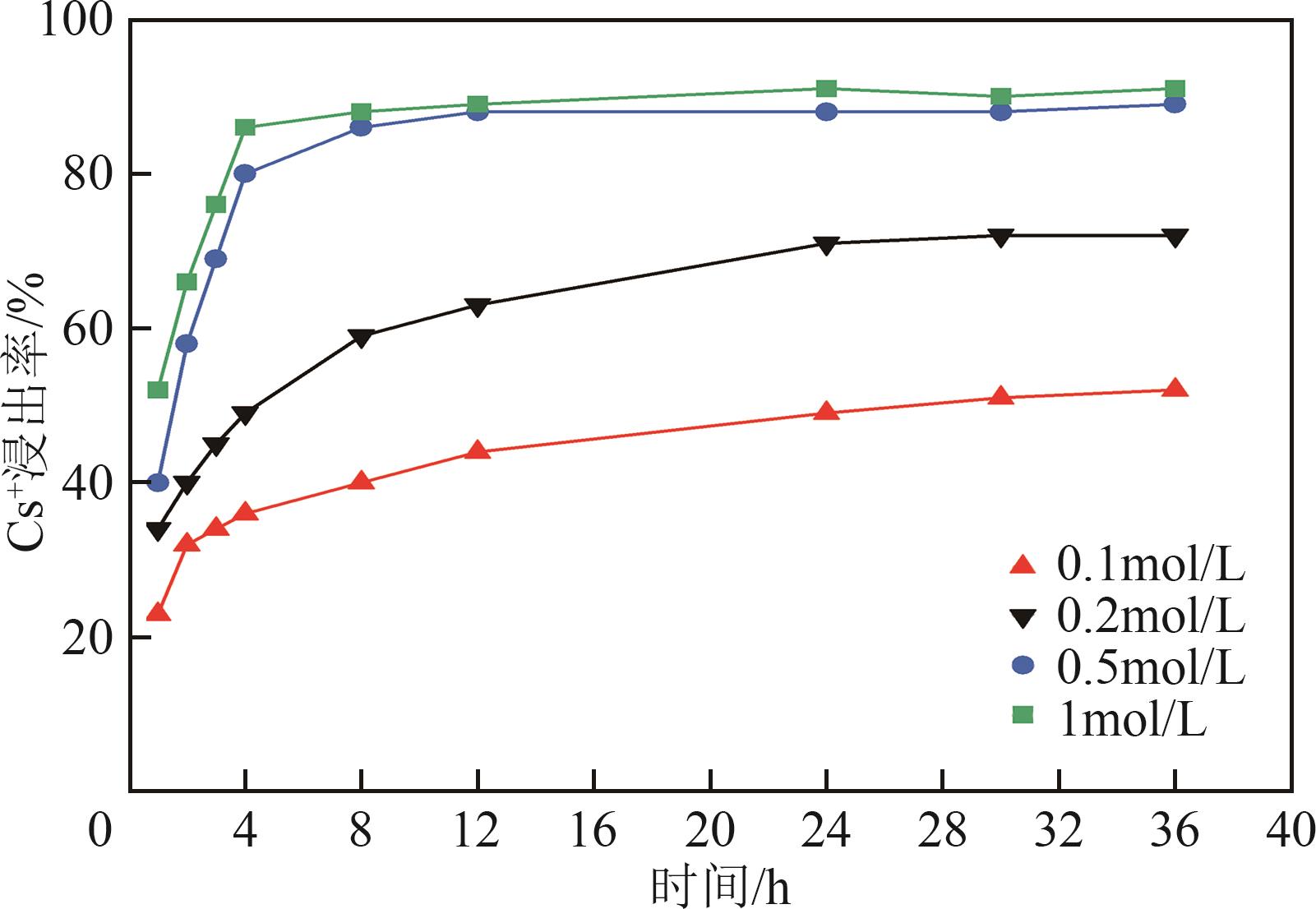
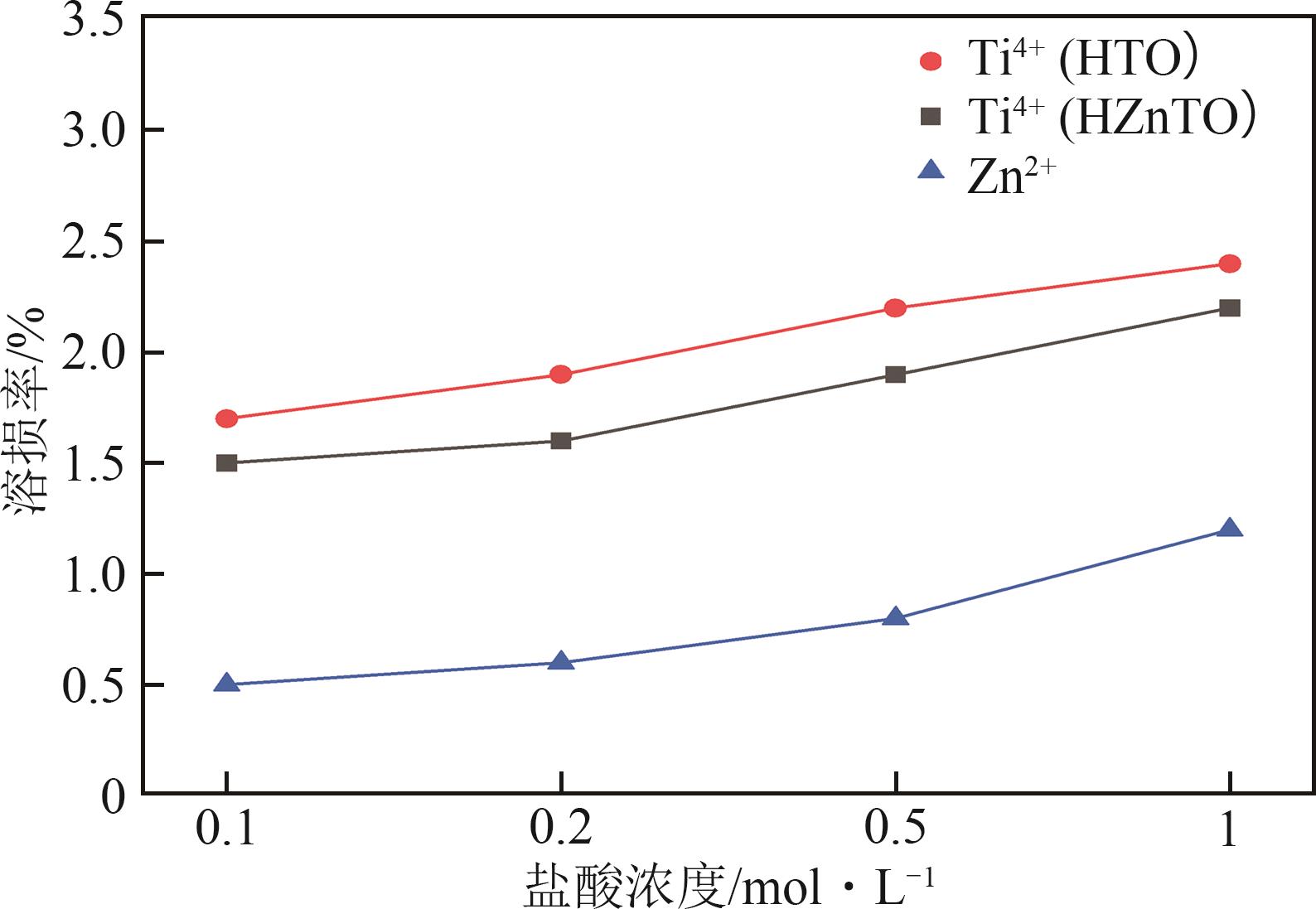
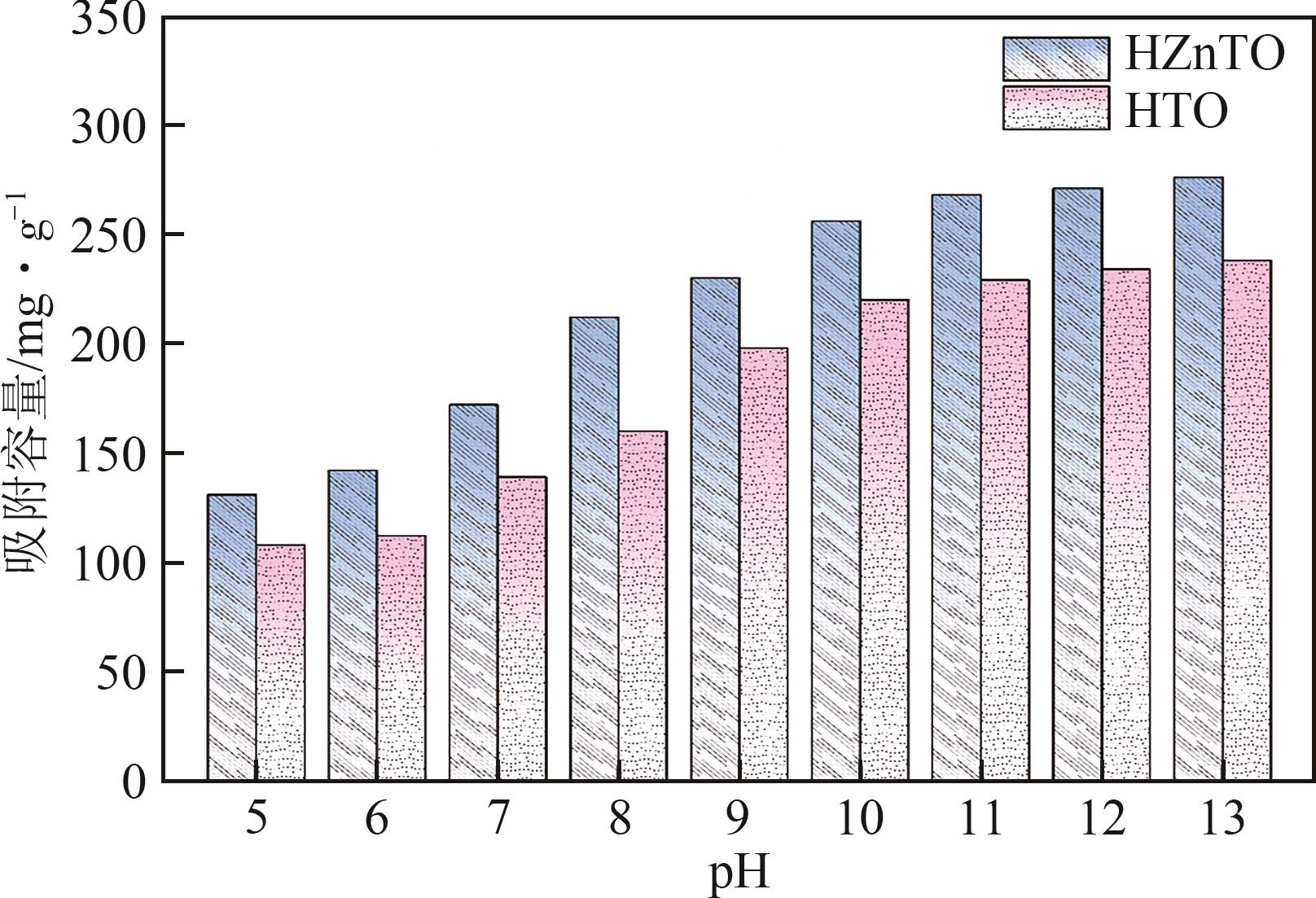
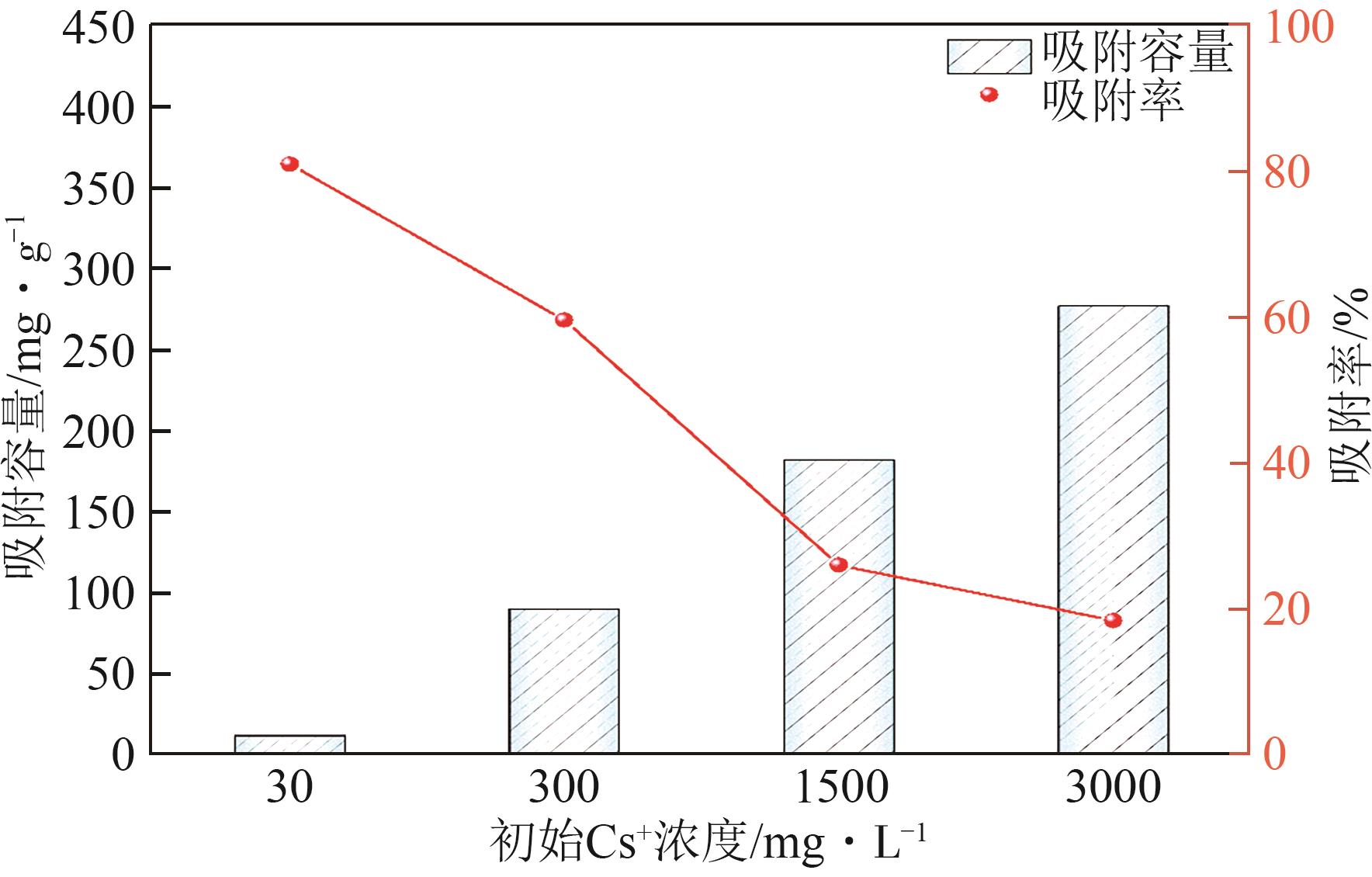
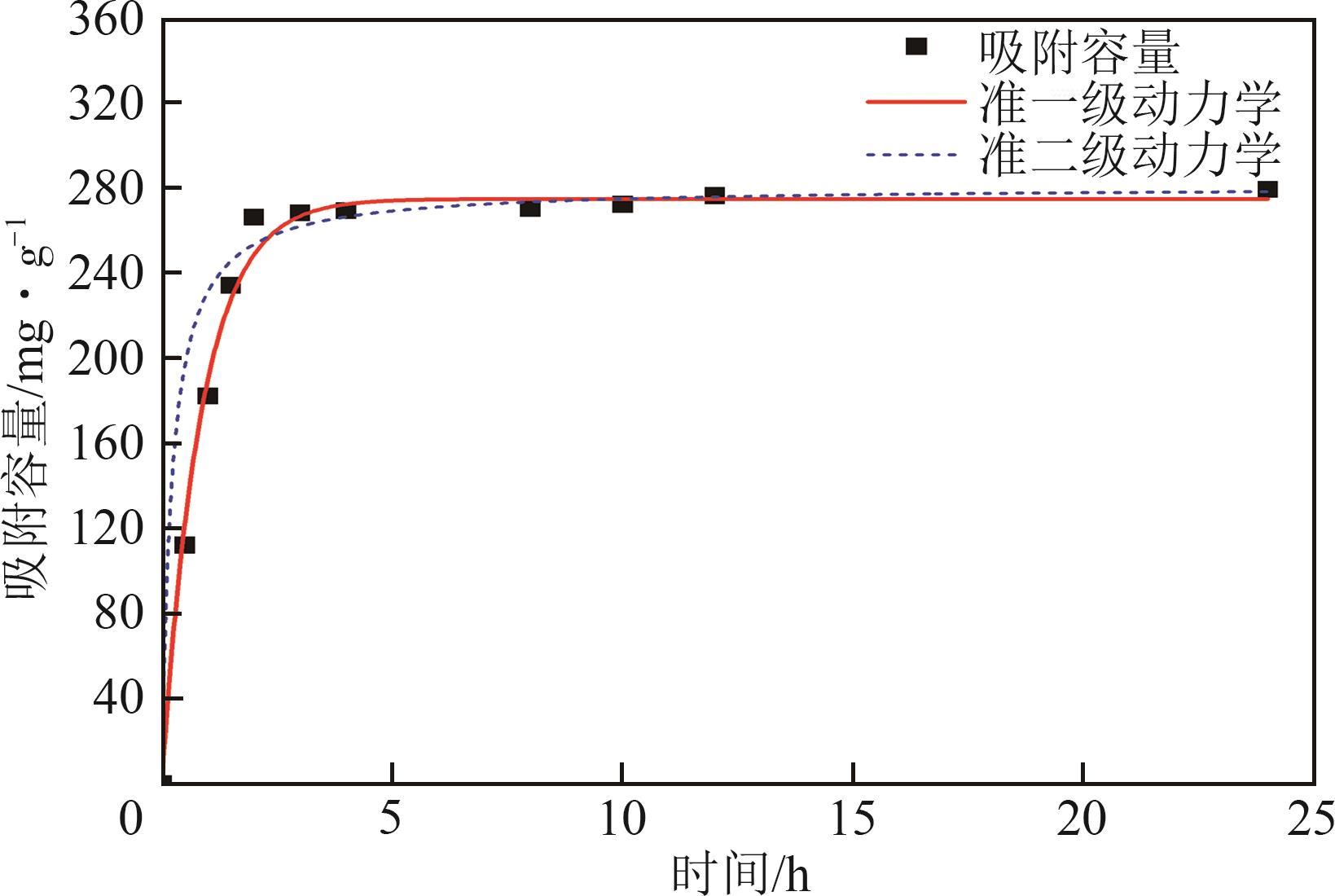
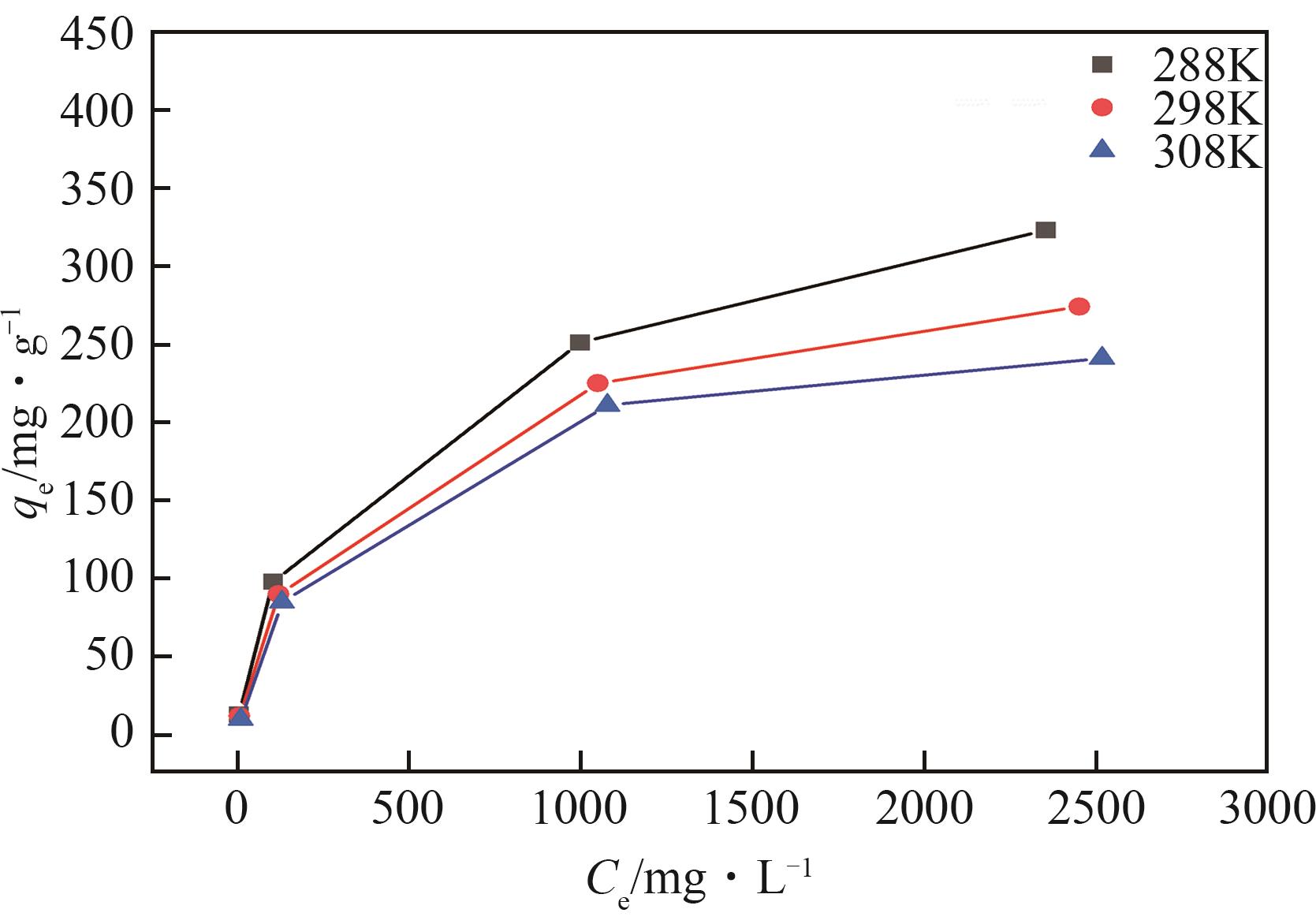
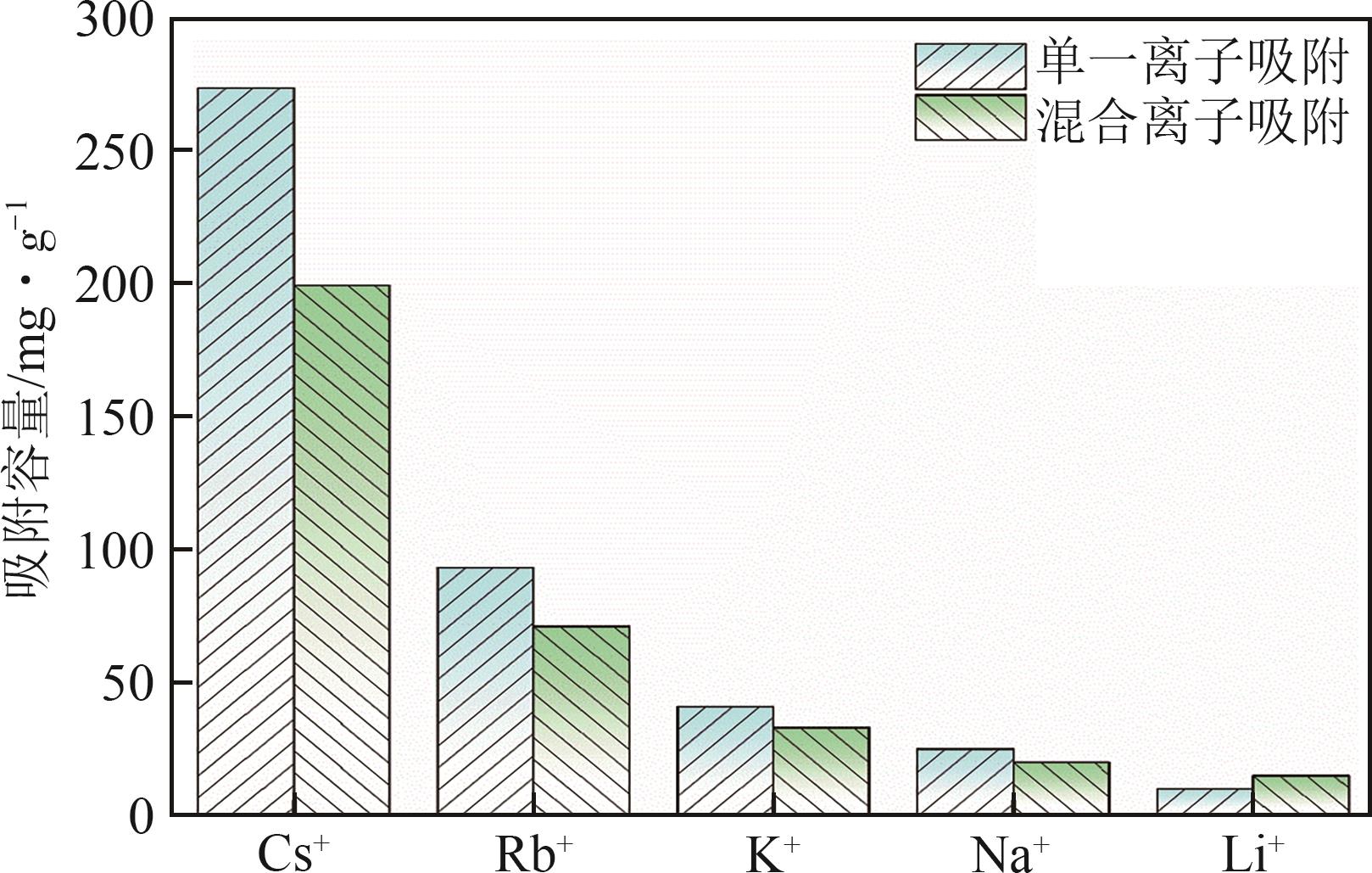
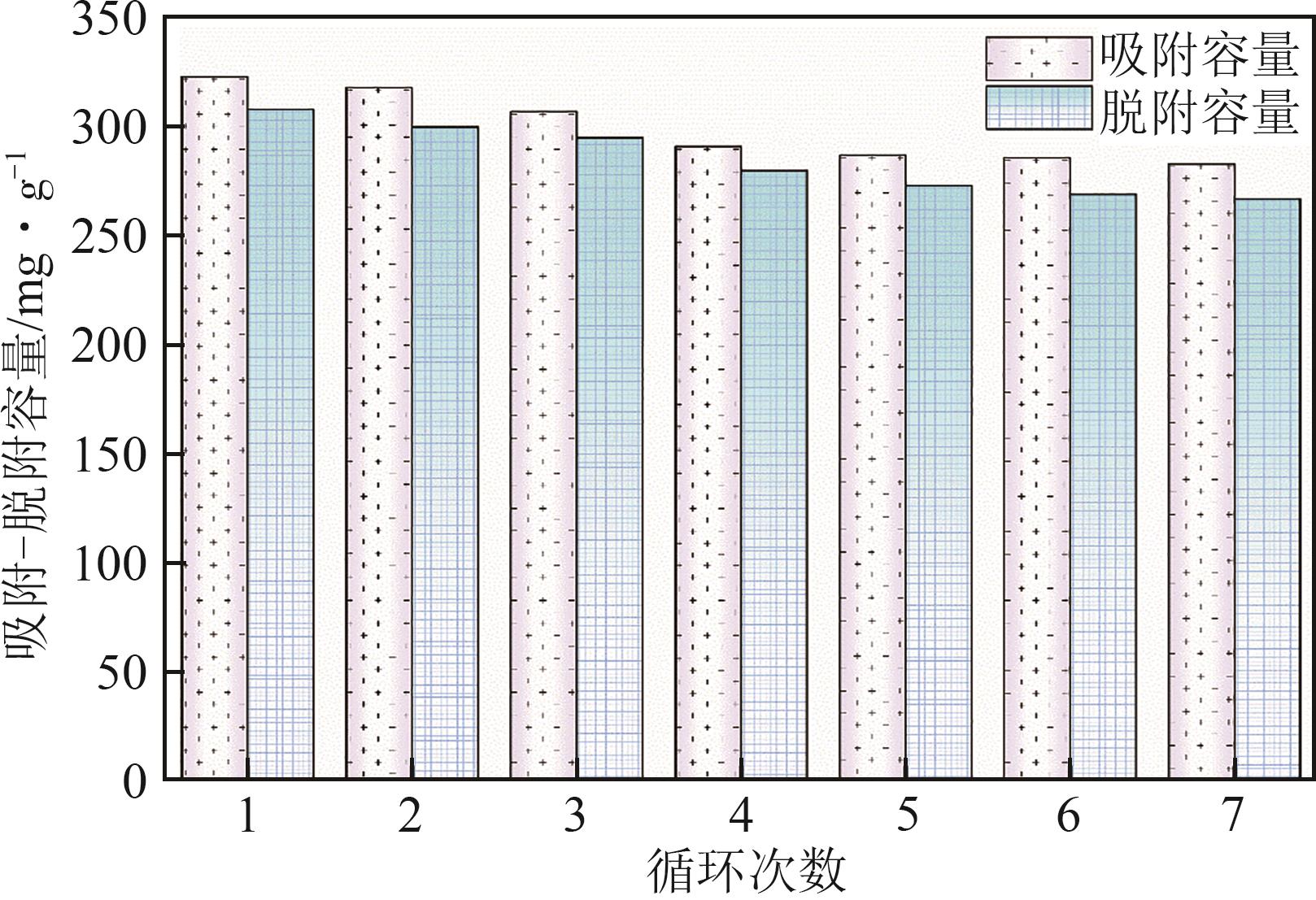
钛系离子筛型吸附剂在盐湖卤水提铯、处理放射性核废水等方面具有很好的应用前景。本文基于改进型溶胶-凝胶技术,将微量Zn2+掺入Cs2Ti6O13的Ti—O晶格中以改善晶胞结构,制备出锌掺杂型钛酸盐前体材料(CZnTO)。前体经过酸处理形成锌掺杂型质子化钛酸盐(HZnTO),通过X射线衍射仪、扫描电子显微镜与能谱仪、傅里叶变换红外光谱、拉曼光谱和X射线光电子能谱等表征手段对制备的HZnTO进行表征,证明微量Zn2+的掺入未破坏原有层状结构。通过吸附实验,考察了水相pH、初始Cs+浓度、吸附时间和温度等因素对HZnTO吸附行为的影响。研究表明,在288K、pH=11且初始Cs+浓度为3000mg/L的最优条件下,吸附2h左右,HZnTO即可达到饱和吸附容量323mg/g。在初始Cs+浓度为30mg/L的低浓度溶液下,HZnTO依然能保持80%以上的吸附率。HZnTO对Cs+的吸附符合准二级动力学方程和Langmuir吸附等温式,说明Cs+在HZnTO上的吸附为化学吸附,以单分子层吸附为主。选择性吸附实验和循环吸附-脱附实验表明,HZnTO对于Cs+的选择性远高于其他离子,循环吸附7次后,HZnTO的吸附容量依然能达到初次循环的87.6%,表现出良好的稳定性。
中图分类号:
引用本文
王德斌, 林梦雨, 杨雪, 董殿权. 锌掺杂型钛系铯离子筛的制备及其吸附性能[J]. 化工进展, 2024, 43(4): 1953-1961.
WANG Debin, LIN Mengyu, YANG Xue, DONG Dianquan. Preparation and adsorption properties of zinc-doped titanium-based cesium ion sieves[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1953-1961.
| 溶液浓度 /mg·L-1 | 准一级动力学 | 准二级动力学 | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | K1 | R2 | qm/mg·g-1 | K2 | R2 | |
| 3000 | 274.498 | 1.172 | 0.981 | 280.430 | 0.01657 | 0.999 |
表1 准一级、准二级动力学相关参数
| 溶液浓度 /mg·L-1 | 准一级动力学 | 准二级动力学 | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | K1 | R2 | qm/mg·g-1 | K2 | R2 | |
| 3000 | 274.498 | 1.172 | 0.981 | 280.430 | 0.01657 | 0.999 |
| 温度/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | 1/n | R2 | |
| 288 | 344.82 | 0.0045 | 0.993 | 7.26 | 0.508 | 0.977 |
| 298 | 295.82 | 0.0043 | 0.987 | 5.43 | 0.527 | 0.964 |
| 308 | 266.30 | 0.0037 | 0.982 | 3.32 | 0.582 | 0.929 |
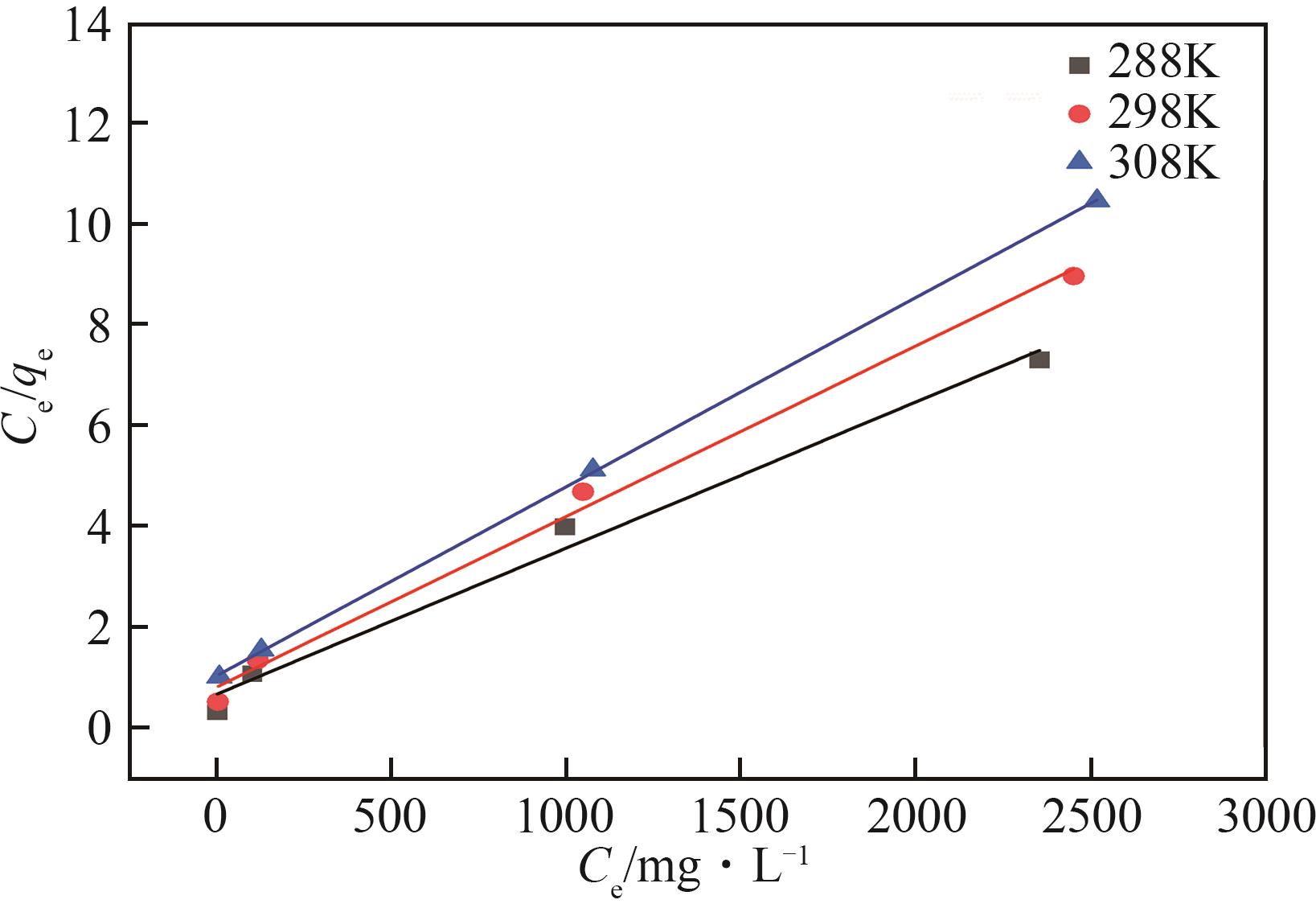
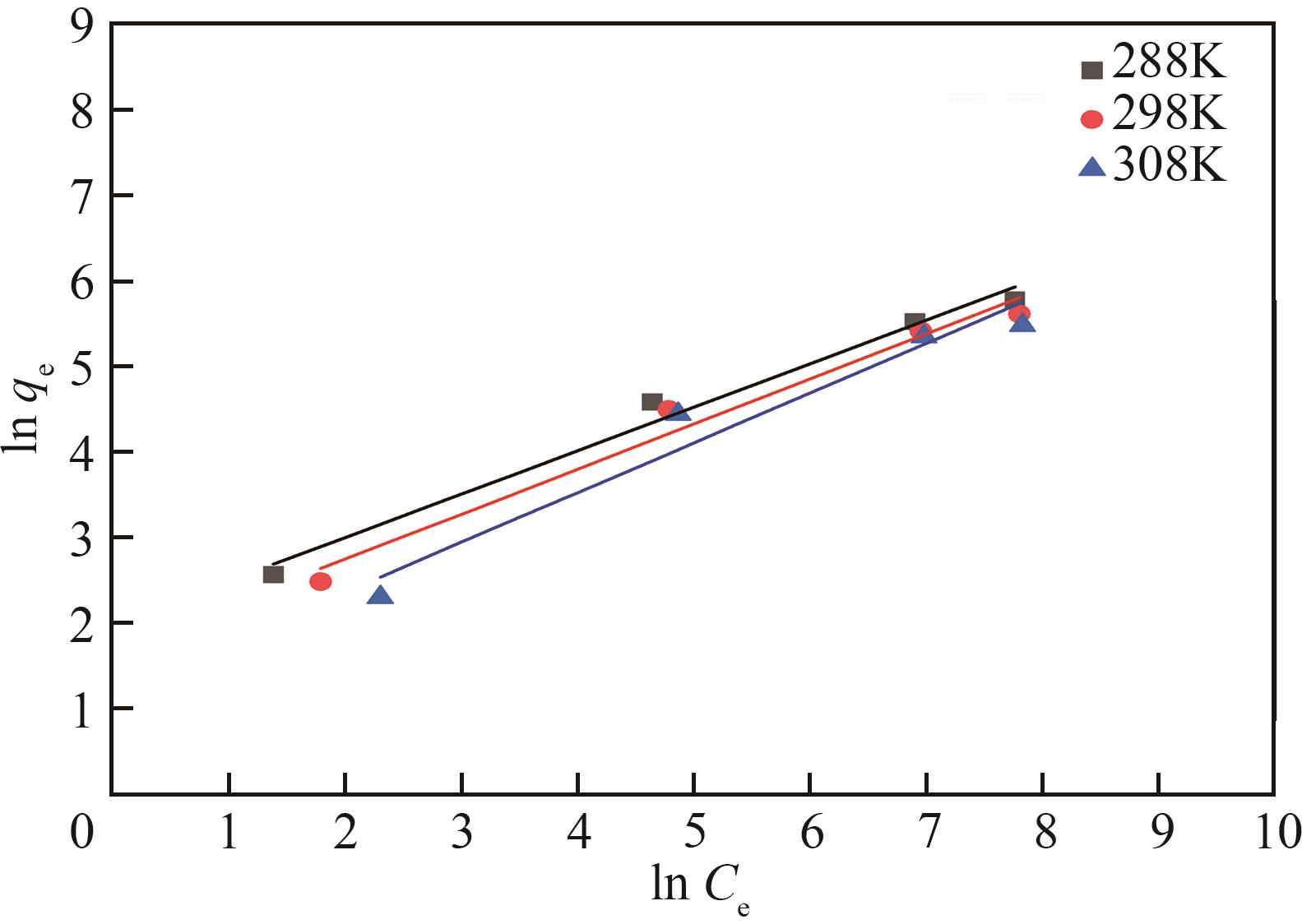
表2 Langmuir模型和Freundlich模型的相关参数
| 温度/K | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | KL | R2 | KF | 1/n | R2 | |
| 288 | 344.82 | 0.0045 | 0.993 | 7.26 | 0.508 | 0.977 |
| 298 | 295.82 | 0.0043 | 0.987 | 5.43 | 0.527 | 0.964 |
| 308 | 266.30 | 0.0037 | 0.982 | 3.32 | 0.582 | 0.929 |
| 1 | LIU Ronghuan, ZHANG Jianqiang, ZHOU Hai, et al. Solution‐processed high-quality cesium lead bromine perovskite photodetectors with high detectivity for application in visible light communication[J]. Advanced Optical Materials, 2020, 8(8): 1901735. |
| 2 | JENA Ajay Kumar, ISHII Ayumi, GUO Zhanlin, et al. Cesium acetate-induced interfacial compositional change and graded band level in MAPbI3 perovskite solar cells[J]. ACS Applied Materials & Interfaces, 2020, 12(30): 33631-33637. |
| 3 | PILARSKI Martin, MARSCHALL Roland, GROSS Silvia, et al. Layered cesium copper titanate for photocatalytic hydrogen production[J]. Applied Catalysis B: Environmental, 2018, 227: 349-355. |
| 4 | CHIANG Tzu Hsuan, ZHOU Zhengxian, HSU Jia-Wei. The photocatalytic performance of cesium tungsten oxide particles under visible-light irradiation and preparation using a glycothermal process[J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 95: 393-404. |
| 5 | ZENGOTITA F E, EMERSON H P, STANLEY F E, et al. Potential for biocolloid transport of cesium at high ionic strength[J]. Chemosphere, 2019, 235: 1059-1065. |
| 6 | DAZA Enrique A, MISRA Santosh K, SCHWARTZ-DUVAL Aaron S, et al. Nano-cesium for anti-cancer properties: An investigation into cesium induced metabolic interference[J]. ACS Applied Materials & Interfaces, 2016, 8(40): 26600-26612. |
| 7 | XIE Weibin, WANG Qing, HE Xuan, et al. An optically pumped atomic clock based on a continuous slow cesium beam[J]. Frontiers in Physics, 2022, 10: 1073854. |
| 8 | CHIROV A A, BELYAKOVA N G. Change in the transparency of thin cesium films on the glass surface of optical devices of spacecraft[J]. Journal of Surface Investigation X-Ray, Synchrotron and Neutron Techniques, 2013, 7(6): 1207-1211. |
| 9 | MUSTAFAEV A S, POLISHCHUK V A, TSYGANOV A B, et al. Effects of graphite intercalation with cesium in a thermionic converter[J]. Russian Journal of Physical Chemistry B, 2017, 11(1): 118-120. |
| 10 | 张晓伟, 辛天宇, 刘佳兴, 等. 全球铯矿资源现状及其综合利用技术分析[J]. 矿产保护与利用, 2021, 41(5): 7-11. |
| ZHANG Xiaowei, XIN Tianyu, LIU Jiaxing, et al. Analysis on the current situation of global cesium resources and its comprehensive utilization technology[J]. Conservation and Utilization of Mineral Resources, 2021, 41(5): 7-11. | |
| 11 | 郭秀红, 郑绵平, 刘喜方, 等. 西藏盐湖卤水铯资源及其开发利用前景[J]. 盐业与化工, 2008,37(3): 8-13. |
| GUO Xiuhong, ZHENG Mianping, LIU Xifang, et al. Saline cesium resource and prospect of its exploitation and utilization in Tibet[J]. Journal of Salt and Chemical Industry, 2008,37(3): 8-13. | |
| 12 | 宋维君, 杨世忠, 牟伯中. 盐湖卤水中铷、铯的检测方法[J]. 无机盐工业, 2014, 46(11): 55-58. |
| SONG Weijun, YANG Shizhong, MU Bozhong, et al. Determination methods for rubidium and cesium in salt lake brine[J]. Inorganic Chemicals Industry, 2014, 46(11): 55-58. | |
| 13 | GAO Li, MA Guihua, ZHENG Youxiong, et al. Research trends on separation and extraction of rare alkali metal from salt lake brine: Rubidium and cesium[J]. Solvent Extraction and Ion Exchange, 2020, 38(7): 753-776. |
| 14 | LEHTO J, PUUKKO E, JAAKKOLA T. Separation of cesium from a nuclear waste solution by precipitation with sodium hexanitrocobaltate and tungstophosphoric acid[J]. Radiochimica Acta, 1985, 38(1): 53-56. |
| 15 | 宝阿敏, 钱志强, 郑红, 等. 铷、铯的分离提取方法及其研究进展[J]. 应用化工, 2017, 46(7): 1377-1382. |
| BAO Amin, QIAN Zhiqiang, ZHENG Hong, et al. Progress of separation and extraction methods for rare alkali metals rubidium and cesium[J]. Applied Chemical Industry, 2017, 46(7): 1377-1382. | |
| 16 | WANG Jianlong, ZHUANG Shuting. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes[J]. Nuclear Engineering and Technology, 2020, 52(2): 328-336. |
| 17 | SHI Zhen, DU Xuemin, WANG Shiqiang, et al. Application status of rubidium, cesium and research situation of its separation from brine with solvent extraction[J]. IOP Conference Series: Materials Science and Engineering, 2017, 274: 012081. |
| 18 | 张振国, 张铭栋, 顾平, 等. 沸石材料吸附水中放射性锶和铯的研究进展[J]. 化工进展, 2019, 38(4): 1984-1995. |
| ZHANG Zhenguo, ZHANG Mingdong, GU Ping, et al. Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials[J]. Chemical Industry and Engineering Progress, 2019, 38(4): 1984-1995. | |
| 19 | LEE Ha Young, KIM Hu Sik, JEONG Hae-Kwon, et al. Selective removal of radioactive cesium from nuclear waste by zeolites: On the origin of cesium selectivity revealed by systematic crystallographic studies[J]. The Journal of Physical Chemistry C, 2017, 121(19): 10594-10608. |
| 20 | YANG Jiayi, LUO Xuegang, YAN Tingsong, et al. Recovery of cesium from saline lake brine with potassium cobalt hexacyanoferrate-modified chrome-tanned leather scrap adsorbent[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 537: 268-280. |
| 21 | 王启龙. 多孔性硅基磷钼酸铵吸附剂的合成及其对铯的吸附研究[D]. 上海: 上海交通大学, 2014. |
| WANG Qilong. Study on the synthesis of AMP loaded porous silica and its adsorption behavior for cesium[D]. Shanghai: Shanghai Jiao Tong University, 2014. | |
| 22 | 肖哲. 花状纳米磷酸钛对铀(Ⅵ)、钍(Ⅳ)、铕(Ⅲ)和铯(Ⅰ)吸附性能研究[D]. 南昌: 东华理工大学, 2019. |
| XIAO Zhe. Study on adsorption properties of flower-like nano-titanium phosphate for U(Ⅵ), Th(Ⅳ), Eu(Ⅲ) and Cs(Ⅰ)[D]. Nanchang: East China University of Technology, 2019 | |
| 23 | PARK Bumjun, GHOREISHIAN Seyed Majid, KIM Yeonho, et al. Dual-functional micro-adsorbents: Application for simultaneous adsorption of cesium and strontium[J]. Chemosphere, 2021, 263: 128266. |
| 24 | 刘银凤, 贺帅, 耿万磊, 等. 铯离子筛的制备及其对铯的吸附交换性能的研究[J]. 化工新型材料, 2021, 49(11): 207-210, 216. |
| LIU Yinfeng, HE Shuai, GENG Wanlei, et al. Study of preparation and ion exchange of cesium ion sieve[J]. New Chemical Materials, 2021, 49(11): 207-210, 216. | |
| 25 | GENG Wanlei, WANG Debin, LIU Yinfeng, et al. Preparation of layered titanate nanosheets and the application for Cs+ adsorption from wastewater, effluents and a simulated brine[J]. Hydrometallurgy, 2023, 216: 105999. |
| 26 | JANG Seongwan, KIM Taewoo, PARK Kang. Fabrication of crumpled ball-like nickel doped palladium-iron oxide hybrid nanoparticles with controlled morphology as effective catalyst for suzuki-miyaura coupling reaction[J]. Catalysts, 2017, 7(9): 247. |
| 27 | SEHATI S, ENTEZARI M H. Ultrasound facilitates the synthesis of potassium hexatitanate and co-intercalation with PbS-CdS nanoparticles[J]. Ultrasonics Sonochemistry, 2016, 32: 348-356. |
| 28 | DONG Xiaoping, OSADA Minoru, UEDA Hidekazu, et al. Synthesis of Mn-substituted titania nanosheets and ferromagnetic thin films with controlled doping[J]. Chemistry of Materials, 2009, 21(19): 4366-4373. |
| 29 | MALUANGNONT Tosapol, WUTTITHAM Boonyawat, HONGKLAI Panisa, et al. An unusually acidic and thermally stable cesium titanate Cs x Ti2– y M y O4 (x=0.67 or 0.70; M=vacancy or Zn)[J]. Inorganic Chemistry, 2019, 58(10): 6885-6892. |
| 30 | PU Xinghong, DU Xinhe, JING Peng, et al. Interface and defect engineering enable fast and high-efficiency Li extraction of metatitanic acid adsorbent[J]. Chemical Engineering Journal, 2021, 425:130550. |
| 31 | ZHANG Peng, WANG Lin, YUAN Liyong, et al. Sorption of Eu(Ⅲ) on MXene-derived titanate structures: The effect of nano-confined space[J]. Chemical Engineering Journal, 2019, 370: 1200-1209. |
| 32 | NOSHEEN Shaneela, GALASSO Francis S, SUIB Steven L. Role of Ti—O bonds in phase transitions of TiO2 [J]. Langmuir, 2009, 25(13): 7623-7630. |
| [1] | 祝妍妮, 王维, 孙闫晨昊, 魏岗, 张大为. 基于单液滴蒸发的离心喷雾干燥数值模拟[J]. 化工进展, 2024, 43(4): 1700-1710. |
| [2] | 何发超, 刘海龙, 李昌烽, 王军锋. 非均匀电场对高黏流体中气泡分散特性的影响[J]. 化工进展, 2024, 43(4): 1774-1782. |
| [3] | 金彬浩, 朱小倩, 柯天, 张治国, 鲍宗必, 任其龙, 苏宝根, 杨启炜. 芳香烃/环烷烃吸附分离材料研究进展[J]. 化工进展, 2024, 43(4): 1863-1881. |
| [4] | 杨东晓, 熊启钊, 王毅, 陈杨, 李立博, 李晋平. 多级孔MOF的制备及其吸附分离应用研究进展[J]. 化工进展, 2024, 43(4): 1882-1896. |
| [5] | 周逸寰, 解强, 周红阳, 梁鼎成, 刘金昌. 基于分子模拟的多孔炭材料结构模型构建方法研究进展[J]. 化工进展, 2024, 43(3): 1535-1551. |
| [6] | 董晓涵, 田月, 苏毅. 含钛高炉渣制备复合吸附剂及其铬吸附性能[J]. 化工进展, 2024, 43(3): 1552-1564. |
| [7] | 陈林林, 于飞, 马杰. 木头基纤维素/石墨烯分离膜制备及污染物分离性能[J]. 化工进展, 2024, 43(3): 1584-1592. |
| [8] | 柴多生, 高峰, 吴友兵, 孙昕, 郝然, 杨宇, 焦翔飞. γ-Al2O3/CuO-ACF电吸附除盐的影响因素及反应动力学[J]. 化工进展, 2024, 43(3): 1637-1647. |
| [9] | 黄梦, 孙志高, 徐文超, 张焕然, 杨扬. 内酯型槐糖脂促进HCFC-141b水合物生成[J]. 化工进展, 2024, 43(3): 1199-1205. |
| [10] | 彭程, 徐漪琳, 石钰婧, 张玟, 李宇涛, 王皓冉, 张卫, 占绣萍. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| [11] | 郭迎春, 梁晓怿. 柠檬酸改性球形活性炭对氨气吸附性能的影响[J]. 化工进展, 2024, 43(2): 1082-1088. |
| [12] | 于松民, 金洪波, 杨明虎, 余海峰, 江浩. 氟掺杂改性LiMn0.5Fe0.5PO4正极材料及其电化学性能[J]. 化工进展, 2024, 43(1): 302-309. |
| [13] | 陈乐, 种海玲, 张致慧, 何明阳, 陈群. CTAB改性Cu-BTC材料的合成及其吸附分离二甲苯异构体的性能[J]. 化工进展, 2024, 43(1): 455-464. |
| [14] | 杨成功, 黄蓉, 王冬娥, 田志坚. 氮掺杂二硫化钼纳米催化剂的电催化析氢性能[J]. 化工进展, 2024, 43(1): 465-472. |
| [15] | 王棵旭, 张香平, 王红岩, 柏䶮, 王慧. 电流响应催化剂及其强化典型反应的研究进展[J]. 化工进展, 2024, 43(1): 49-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||