化工进展 ›› 2024, Vol. 43 ›› Issue (1): 455-464.DOI: 10.16085/j.issn.1000-6613.2023-0226
• 材料科学与技术 • 上一篇
CTAB改性Cu-BTC材料的合成及其吸附分离二甲苯异构体的性能
- 常州大学石油化工学院,江苏省绿色催化与技术重点实验室,江苏 常州 213164
-
收稿日期:2023-02-20修回日期:2023-05-09出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:陈乐 -
作者简介:陈乐(1981—),女,副教授,硕士生导师,研究方向为功能化吸附材料的合成及应用。E-mail:chenle@cczu.edu.cn。 -
基金资助:国家自然科学基金(11775037);江苏省高等学校自然科学研究重大项目(21KJA530001);江苏省“青蓝工程”
Synthesis of Cu-BTC modified by CTAB and its adsorption and separation of xylene isomers
CHEN Le( ), CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun
), CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun
- Laboratory of Green Catalysis and Technology of Jiangsu Province, College of Petrochemical Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
-
Received:2023-02-20Revised:2023-05-09Online:2024-01-20Published:2024-02-05 -
Contact:CHEN Le
摘要:
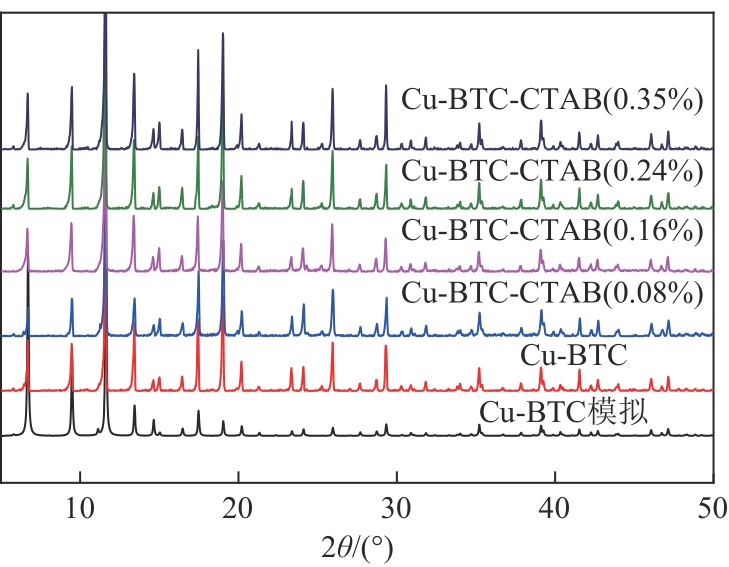
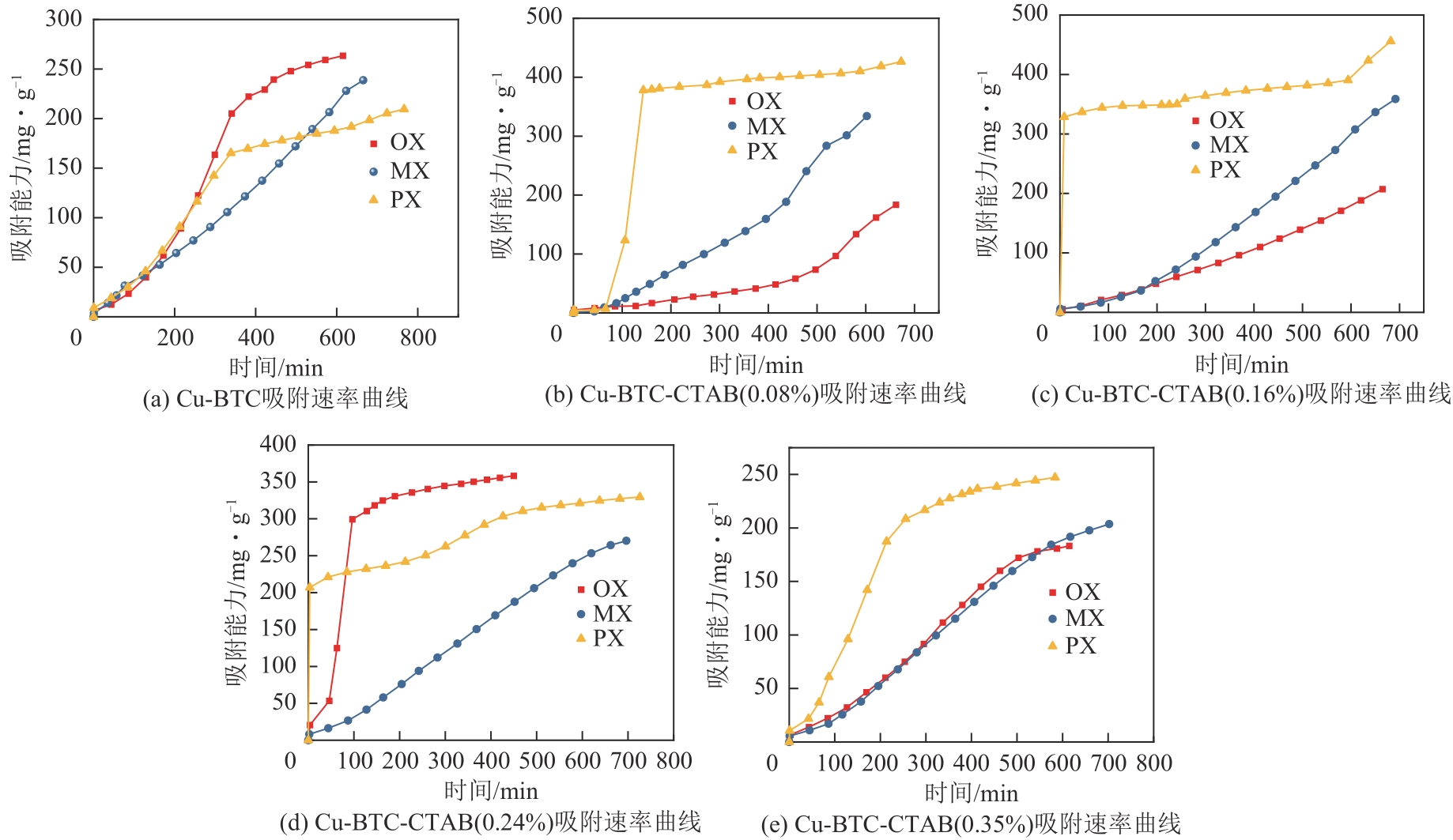
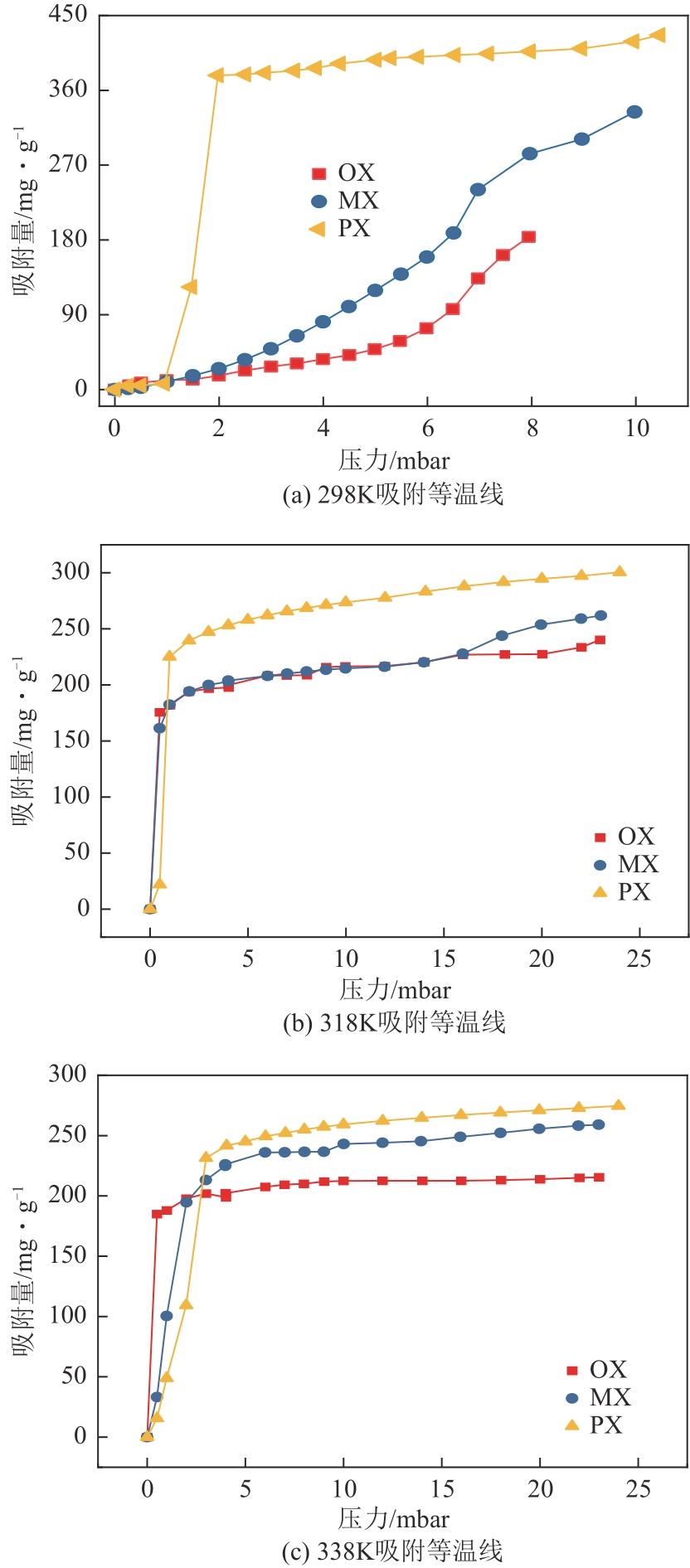
通过水热法合成Cu-BTC晶体的过程中添加十六烷基三甲基溴化铵(CTAB),通过调节CTAB的添加量对吸附材料的形貌和孔结构进行改性,改性后的Cu-BTC材料与原Cu-BTC相比,其对对二甲苯(PX)的静态吸附量明显提高,且高于间二甲苯(MX)和邻二甲苯(OX),从而提高了异构体的吸附选择性。研究了二甲苯异构体在Cu-BTC-CTAB材料上的静态吸附性能和吸附动力学性能。在298K、318K和338K温度下进行了一系列二甲苯有机蒸气吸附平衡实验,得到了静态吸附速率曲线和吸附等温线。改性材料中CTAB添加量为0.08%的Cu-BTC-CTAB样品对PX的吸附量和选择性最优。动力学研究表明,二甲苯异构体在Cu-BTC-CTAB上的吸附过程可以用一级动力学模型来描述。
中图分类号:
引用本文
陈乐, 种海玲, 张致慧, 何明阳, 陈群. CTAB改性Cu-BTC材料的合成及其吸附分离二甲苯异构体的性能[J]. 化工进展, 2024, 43(1): 455-464.
CHEN Le, CHONG Hailing, ZHANG Zhihui, HE Mingyang, CHEN Qun. Synthesis of Cu-BTC modified by CTAB and its adsorption and separation of xylene isomers[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 455-464.
| 样品 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 微孔孔容 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| Cu-BTC | 1139.6 | 0.439 | 0.399 | 2.07 |
| Cu-BTC-CTAB(0.08%) | 1140.2 | 0.506 | 0.393 | 2.38 |
| Cu-BTC-CTAB(0.16%) | 1052.0 | 0.484 | 0.361 | 2.40 |
| Cu-BTC-CTAB(0.24%) | 743.8 | 0.425 | 0.255 | 3.08 |
| Cu-BTC-CTAB(0.35%) | 576.8 | 0.523 | 0.196 | 4.78 |
表1 Cu-BTC-CTAB材料与Cu-BTC材料的孔结构参数
| 样品 | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 微孔孔容 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| Cu-BTC | 1139.6 | 0.439 | 0.399 | 2.07 |
| Cu-BTC-CTAB(0.08%) | 1140.2 | 0.506 | 0.393 | 2.38 |
| Cu-BTC-CTAB(0.16%) | 1052.0 | 0.484 | 0.361 | 2.40 |
| Cu-BTC-CTAB(0.24%) | 743.8 | 0.425 | 0.255 | 3.08 |
| Cu-BTC-CTAB(0.35%) | 576.8 | 0.523 | 0.196 | 4.78 |
| 吸附剂类型 | 吸附剂 | PX吸附量/mg·g-1 | 分离系数 | 参考文献 | |
|---|---|---|---|---|---|
| 堆叠效应的吸附 | MIL-125(Ti) | 150 | PX/MX=1.5 | PX/OX=1.6 | [ |
| Cu-(CDC) | — | PX/MX=7.0 | PX/OX=10 | [ | |
| MIL-47 | 483 | PX/MX=1.10 | PX/OX=0.60 | [ | |
| 分子筛效应的吸附 | NaYa | 110 | MX/PX=2.90 | MX/OX=3.16 | [ |
| NaY | 99.6 | MX/PX=2.62 | MX/OX=2.83 | [ | |
| MIL-53(Al) | 357.8 | OX/PX=3.50 | OX/MX=2.70 | [ | |
| OMSs的吸附 | HKUST-1 | 297 | OX/PX=1.08~1.21 | — | [ |
| CPO-27-Ni | 210 | OX/PX=3.3 | OX/MX=1.7 | [ | |
| 开门或呼吸效应的吸附 | ZIF-8 | 210 | MX/PX=3.1 | PX/OX=8.8 | [ |
| ZIF-76 | 260 | OX/PX=1.1 | [ | ||
| Mn-dhbq | — | PX/OX=37.9 | PX/MX=34.8 | [ | |
| Cu-BTC-CTAB(0.08%) | 426 | PX/OX=2.20 | — | 本工作 | |
表2 文献报道的吸附材料与本文改性材料对二甲苯的分离性能对比
| 吸附剂类型 | 吸附剂 | PX吸附量/mg·g-1 | 分离系数 | 参考文献 | |
|---|---|---|---|---|---|
| 堆叠效应的吸附 | MIL-125(Ti) | 150 | PX/MX=1.5 | PX/OX=1.6 | [ |
| Cu-(CDC) | — | PX/MX=7.0 | PX/OX=10 | [ | |
| MIL-47 | 483 | PX/MX=1.10 | PX/OX=0.60 | [ | |
| 分子筛效应的吸附 | NaYa | 110 | MX/PX=2.90 | MX/OX=3.16 | [ |
| NaY | 99.6 | MX/PX=2.62 | MX/OX=2.83 | [ | |
| MIL-53(Al) | 357.8 | OX/PX=3.50 | OX/MX=2.70 | [ | |
| OMSs的吸附 | HKUST-1 | 297 | OX/PX=1.08~1.21 | — | [ |
| CPO-27-Ni | 210 | OX/PX=3.3 | OX/MX=1.7 | [ | |
| 开门或呼吸效应的吸附 | ZIF-8 | 210 | MX/PX=3.1 | PX/OX=8.8 | [ |
| ZIF-76 | 260 | OX/PX=1.1 | [ | ||
| Mn-dhbq | — | PX/OX=37.9 | PX/MX=34.8 | [ | |
| Cu-BTC-CTAB(0.08%) | 426 | PX/OX=2.20 | — | 本工作 | |
| 样品 | 二甲苯 | 吸附量/mg·g-1 |
|---|---|---|
| Cu-BTC | OX | 263 |
| MX | 238 | |
| PX | 209 | |
| Cu-BTC-CTAB(0.08%) | OX | 184 |
| MX | 334 | |
| PX | 426 | |
| Cu-BTC-CTAB(0.16%) | OX | 207 |
| MX | 359 | |
| PX | 456 | |
| Cu-BTC-CTAB(0.24%) | OX | 358 |
| MX | 270 | |
| PX | 329 | |
| Cu-BTC-CTAB(0.35%) | OX | 183 |
| MX | 204 | |
| PX | 247 |
表3 298K下Cu-BTC、Cu-BTC-CTAB材料对OX、MX、PX的静态饱和吸附量
| 样品 | 二甲苯 | 吸附量/mg·g-1 |
|---|---|---|
| Cu-BTC | OX | 263 |
| MX | 238 | |
| PX | 209 | |
| Cu-BTC-CTAB(0.08%) | OX | 184 |
| MX | 334 | |
| PX | 426 | |
| Cu-BTC-CTAB(0.16%) | OX | 207 |
| MX | 359 | |
| PX | 456 | |
| Cu-BTC-CTAB(0.24%) | OX | 358 |
| MX | 270 | |
| PX | 329 | |
| Cu-BTC-CTAB(0.35%) | OX | 183 |
| MX | 204 | |
| PX | 247 |
| 温度/K | 二甲苯 | 一级动力学方程的非线性拟合 | 二级动力学方程的非线性拟合 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm/mg·g-1 | Qe/mg·g-1 | k1/min | R12 | Qe /mg·g-1 | k2/g·mg-1·min-1 | R22 | |||
| 298 | OX | 183.59 | -9.411 | 4.6×10-3 | 0.987 | 3380407 | 1.7×10-12 | 0.786 | |
| MX | 333.98 | 571488 | -8.4×10-7 | 0.939 | 380213 | 3.3×10-12 | 0.939 | ||
| PX | 426.45 | 432.8 | -0.7×10-2 | 0.862 | 552.35 | 1.2×10-5 | 0.836 | ||
| 318 | OX | 240.27 | 213.12 | -8.7×10-2 | 0.918 | 220.19 | 6.1×10-4 | 0.951 | |
| MX | 261.94 | 223.05 | -3.1×10-2 | 0.883 | 237.61 | 2.1×10-4 | 0.929 | ||
| PX | 300.52 | 278.10 | -2.6×10-2 | 0.972 | 306.23 | 1.3×10-4 | 0.990 | ||
| 338 | OX | 215.49 | 207.99 | -1.2×10-2 | 0.978 | 211.53 | 1.3×10-3 | 0.989 | |
| MX | 259.09 | 248.703 | -1.2×10-2 | 0.988 | 282.47 | 5.5×10-5 | 0.975 | ||
| PX | 127.43 | 274.810 | -1.0×10-2 | 0.941 | 324.16 | 3.9×10-5 | 0.916 | ||
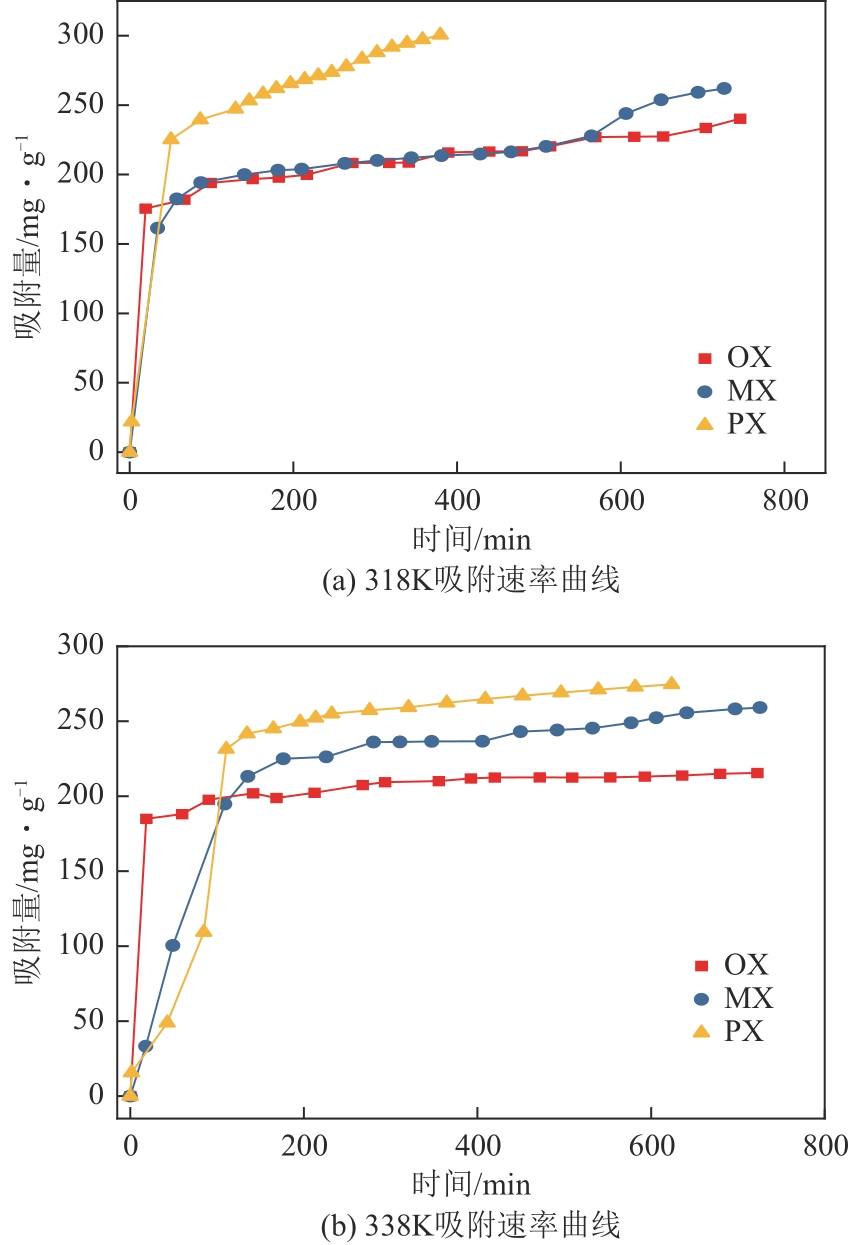
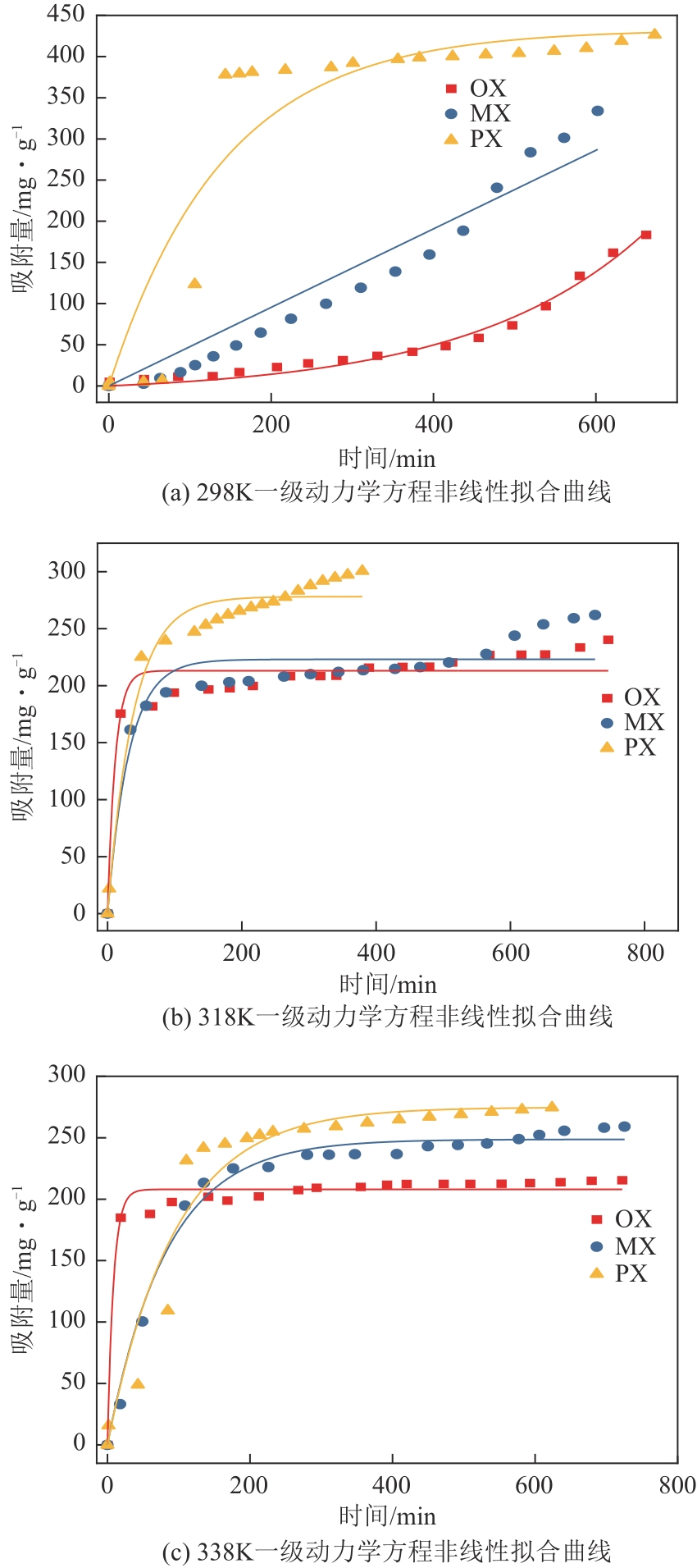
表4 Cu-BTC-CTAB(0.08%)材料吸附OX、MX、PX的静态吸附速率曲线的动力学方程的非线性拟合参数
| 温度/K | 二甲苯 | 一级动力学方程的非线性拟合 | 二级动力学方程的非线性拟合 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm/mg·g-1 | Qe/mg·g-1 | k1/min | R12 | Qe /mg·g-1 | k2/g·mg-1·min-1 | R22 | |||
| 298 | OX | 183.59 | -9.411 | 4.6×10-3 | 0.987 | 3380407 | 1.7×10-12 | 0.786 | |
| MX | 333.98 | 571488 | -8.4×10-7 | 0.939 | 380213 | 3.3×10-12 | 0.939 | ||
| PX | 426.45 | 432.8 | -0.7×10-2 | 0.862 | 552.35 | 1.2×10-5 | 0.836 | ||
| 318 | OX | 240.27 | 213.12 | -8.7×10-2 | 0.918 | 220.19 | 6.1×10-4 | 0.951 | |
| MX | 261.94 | 223.05 | -3.1×10-2 | 0.883 | 237.61 | 2.1×10-4 | 0.929 | ||
| PX | 300.52 | 278.10 | -2.6×10-2 | 0.972 | 306.23 | 1.3×10-4 | 0.990 | ||
| 338 | OX | 215.49 | 207.99 | -1.2×10-2 | 0.978 | 211.53 | 1.3×10-3 | 0.989 | |
| MX | 259.09 | 248.703 | -1.2×10-2 | 0.988 | 282.47 | 5.5×10-5 | 0.975 | ||
| PX | 127.43 | 274.810 | -1.0×10-2 | 0.941 | 324.16 | 3.9×10-5 | 0.916 | ||

图9 Cu-BTC-CTAB(0.08%)材料吸附OX、MX、PX的吸附速率曲线的拟合曲线k1 —— 一级吸附速率常数,(g·mg-1)/mink2 —— 二级吸附速率常数,(g·mg-1)/minQe —— 平衡吸附量,mg/gQm —— 最大吸附量,mg/gQt —— 某时刻对应的吸附量,mg/g
| 1 | ZHAO X, WANG Y X, LI D S, et al. Metal-organic frameworks for separation[J]. Advanced Materials, 2018, 30(37): e1705189. |
| 2 | TRENS P, BELARBI H, SHEPHERD C, et al. Adsorption and separation of xylene isomers vapors onto the chromium terephthalate-based porous material MIL-101(Cr): An experimental and computational study[J]. Microporous and Mesoporous Materials, 2014, 183: 17-22. |
| 3 | 张贺, 李国良, 张可刚, 等. 金属有机骨架材料在吸附分离研究中的应用进展[J]. 化学学报, 2017, 75(9): 841-859. |
| ZHANG He, LI Guoliang, ZHANG Kegang, et al. Advances of metal-organic frameworks in adsorption and separation applications[J]. Acta Chimica Sinica, 2017, 75(9): 841-859. | |
| 4 | NALAPARAJU A, JIANG J. Metal-organic frameworks: Metal-organic frameworks for liquid phase applications[J]. Advanced Science, 2021, 8(5): 2003143. |
| 5 | KAMONTHIP S, KANJANA M, PENWISA P, et al. Preparation of Cu-BTC/PVA fibers with antibacterial applications[J]. Fibers and Polymers, 2018, 19(7): 1373-1378. |
| 6 | ADHAM A, MARK F, ROB C, et al. Silica SOS@HKUST-1 composite microspheres as easily packed stationary phases for fast separation[J]. Journal of Materials Chemistry A, 2013, 1(10): 3276-3286. |
| 7 | ZHAO Q, ZHU L, LIN G H, et al. Controllable synthesis of porous Cu-BTC@polymer composite beads for iodine capture[J]. ACS Applied Materials Interfaces, 2019, 11(45): 42635-42645. |
| 8 | S-Y CHUI S, M-F LO S, CHARMANT J P H, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3] n [J]. Science, 1999, 283(5405): 1148-1150. |
| 9 | JANOS S, MARCO D, GUILLAUME C, et al. Well-studied Cu-BTC still serves surprises: Evidence for facile Cu2+/Cu+ interchange[J]. Physical Chemistry Chemical Physics, 2012, 14(13): 4383-4390. |
| 10 | HENDO C H, ARON W. Chemical principles underpinning the performance of the metal-organic framework HKUST-1[J]. Chemical Science, 2015, 6(7): 3674-3683. |
| 11 | LI L, LIU X L, GAO M, et al. The adsorption on magnetic hybrid Fe3O4/HKUST-1/GO of methylene blue from water solution[J]. Journal of Materials Chemistry A, 2014, 2(6): 1795-1801. |
| 12 | KUBO M, MORIYAMA R, SHIMADA M. Facile fabrication of HKUST-1 nanocomposites incorporating Fe3O4 and TiO2 nanoparticles by a spray-assisted synthetic process and their dye adsorption performances[J]. Microporous and Mesoporous Materials, 2019, 280: 227-235. |
| 13 | PERALTA D, BARTHELET K, PEREZ-PELLITERO J, et al. Adsorption and separation of xylene isomers: CPO-27-Ni vs HKUST-1 vs NaY[J]. The Journal of Physical Chemistry C, 2012, 116(41): 21844-21855. |
| 14 | GONZALEZ M I, KAPELEWSKI M T, BLOCH E D, et al. Separation of xylene isomers through multiple metal site interactions in metal-organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(9): 3412-3422. |
| 15 | YANG F, MU H, WANG C Q, et al. Morphological map of ZIF-8 crystals with five distinctive shapes: Feature of filler in mixed-matrix membranes on C3H6/C3H8 separation[J]. Chemistry of Materials, 2018, 30(10): 3467-3473. |
| 16 | YAN T X, LI X J, CHEN L, et al. Efficient adsorption separation of xylene isomers in zeolitic imidazolate framework-67@MCF hybrid materials[J]. Journal of Solid State Chemistry, 2022, 307: 122819. |
| 17 | JI G J, XIANG T, YAN T X, et al. Efficient adsorption separation of xylene isomers on Cu@Fe3O4 by appropriate activation methods[J]. Journal of Solid State Chemistry, 2022, 315: 123466. |
| 18 | YANG Y X, BAI P, GUO X H. Separation of xylene isomers: A review of recent advances in materials[J]. Industrial & Engineering Chemistry Research, 2017, 56(50): 14725-14753. |
| 19 | TORRES-KNOOP A, KRISHNA R, DUBBELDAM D. Separating xylene isomers by commensurate stacking of p-xylene within channels of MAF-X8[J]. Angewandte Chemie International Edition, 2014, 53(30): 7774-7778. |
| 20 | LANNOEYE J, VAN DE VOORDE B, BOZBIYIK B, et al. An aliphatic copper metal-organic framework as versatile shape selective adsorbent in liquid phase separations[J]. Microporous and Mesoporous Materials, 2016, 226: 292-298. |
| 21 | GEE J A, ZHANG K, BHATTACHARYYA S, et al. Computational identification and experimental evaluation of metal-organic frameworks for xylene enrichment[J]. The Journal of Physical Chemistry C, 2016, 120(22): 12075-12082. |
| 22 | RASOULI M, YAGHOBI N, CHITSAZAN S, et al. Effect of nanocrystalline zeolite Na-Y on meta-xylene separation[J]. Microporous and Mesoporous Materials, 2012, 152: 141-147. |
| 23 | RASOULI M, YAGHOBI N, CHITSAZAN S, et al. Adsorptive separation of meta-xylene from C8 aromatics[J]. Chemical Engineering Research and Design, 2012, 90(9): 1407-1415. |
| 24 | ALAERTS L, MAES M, GIEBELER L, et al. Selective adsorption and separation of ortho-substituted alkylaromatics with the microporous aluminum terephthalate MIL-53[J]. Journal of the American Chemical Society, 2008, 130(43): 14170-14178. |
| 25 | MUNCH A S, MERTENS F. HKUST-1 as an open metal site gas chromatographic stationary phase-capillary preparation, separation of small hydrocarbons and electron donating compounds, determination of thermodynamic data[J]. Journal of Materials Chemistry, 2012, 22(20): 10228-10234. |
| 26 | PERALTA D, BARTHELET K, PIRNGRUBER G, et al. Method for separating para-xylenes using an adsorbent from the family of ZIFs of sod structure: WO2013011210A1[P]. 2013. |
| 27 | PERALTA D, CHAPLAIS G, SIMON-MASSERON A, et al. Comparison of the behavior of metal-organic frameworks and zeolites for hydrocarbon separations[J]. Journal of the American Chemical Society, 2012, 134(19): 8115-8126. |
| 28 | LI L Y, GUO L D, OLSON D H, et al. Discrimination of xylene isomers in a stacked coordination polymer[J]. Science, 2022, 377(6603): 335-339. |
| [1] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [2] | 陈姝晖, 伍岳, 张文祥, 王闪闪, 马和平. 离子型有机多孔聚合物的制备及其烟气脱硫耦合脱碳性质[J]. 化工进展, 2023, 42(2): 1028-1038. |
| [3] | 武鲁明, 于海斌, 臧甲忠, 王亚权, 李滨, 孙振海. 多级孔硅铝酸盐纳米球的合成及其吸附分离多环芳烃[J]. 化工进展, 2023, 42(12): 6452-6460. |
| [4] | 伍岳, 李晓宇, 陶春珲, 张莹, 李印辉, 张文祥, 杨伯伦, 马和平. 离子改性的CON材料用于吸附分离NF3[J]. 化工进展, 2023, 42(11): 6076-6085. |
| [5] | 马蕾, 张飞飞, 宋志强, 杨江峰, 李立博, 李晋平. 金属有机骨架材料用于吸附分离CH4和N2的研究进展[J]. 化工进展, 2021, 40(9): 5107-5117. |
| [6] | 蒋博龙, 史顺杰, 蒋海林, 封鑫, 孙好芬. 金属有机框架材料吸附处理苯酚污水机理研究进展[J]. 化工进展, 2021, 40(8): 4525-4539. |
| [7] | 赵珂, 宁平, 李凯, 孙鑫, 宋辛, 王驰. Mn/Cu-BTC催化剂同时脱硫脱硝实验研究[J]. 化工进展, 2020, 39(5): 1784-1791. |
| [8] | 王俊, 韶晖, 解丹燕, 冷一欣, 黄春香. β-CD/Al2O3/ATP复合材料对乙腈/正丙醇的吸附分离性能的影响[J]. 化工进展, 2017, 36(09): 3407-3413. |
| [9] | 张敬升, 李东风. 炼厂干气的回收和利用技术概述[J]. 化工进展, 2015, 34(09): 3207-3215. |
| [10] | 盖月庭,顾昊辉,梁战桥,刘中勋,周震寰. 对二乙苯生产技术评述[J]. 化工进展, 2014, 33(03): 538-541. |
| [11] | 郝广平,李文翠,陆安慧. 纳米结构多孔固体在二氧化碳吸附分离中的应用[J]. 化工进展, 2012, 31(11): 2493-2510. |
| [12] | 李云东,易红宏,唐晓龙,李芬容,何 丹. 吸附剂特性对CO2/CH4吸附分离的影响分析[J]. 化工进展, 2012, 31(05): 974-980. |
| [13] | 柳能军,张 林,蔡荣锡,周志军,陈欢林. 膜法分离二甲苯异构体研究进展 [J]. 化工进展, 2007, 26(6): 804-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||