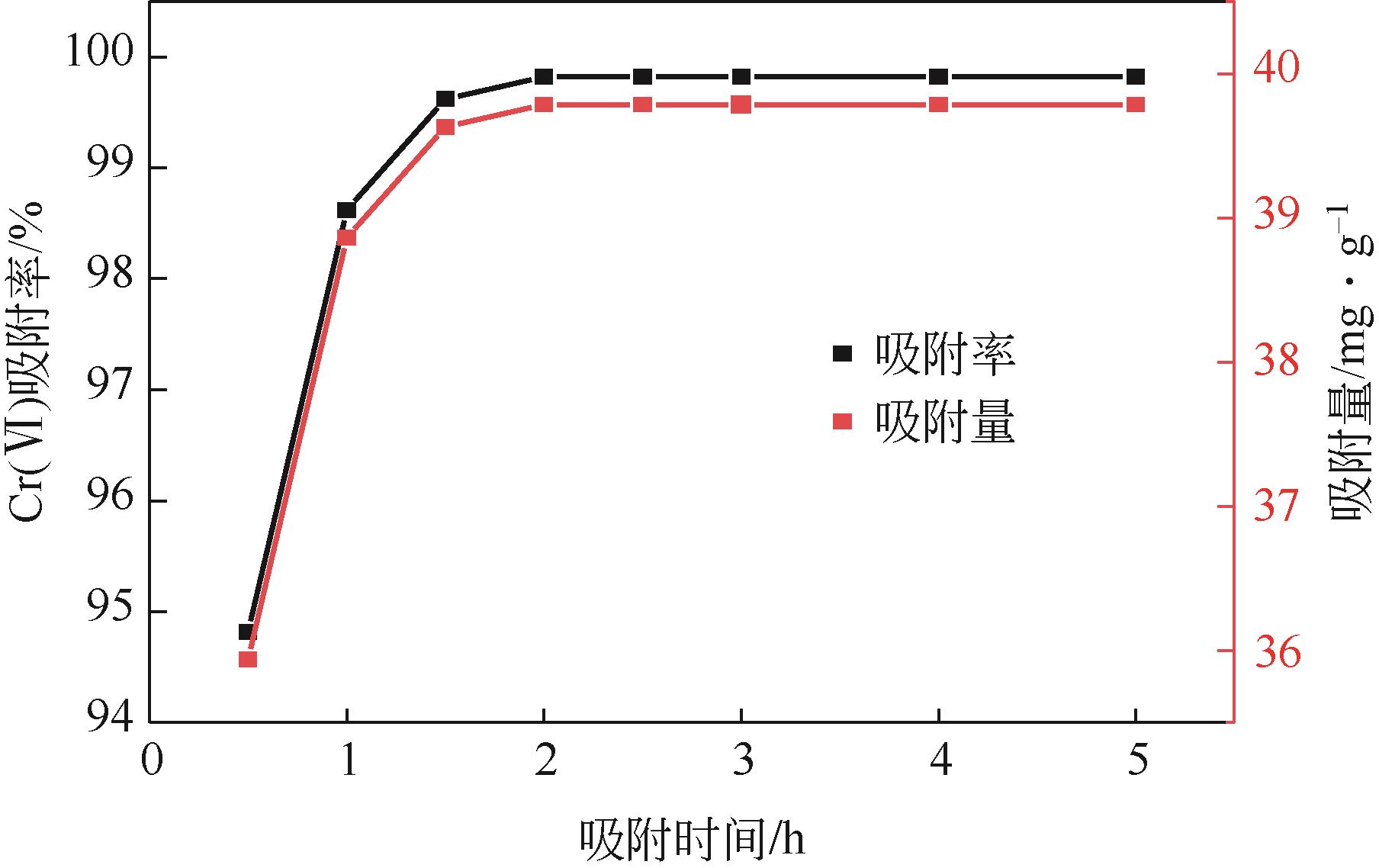
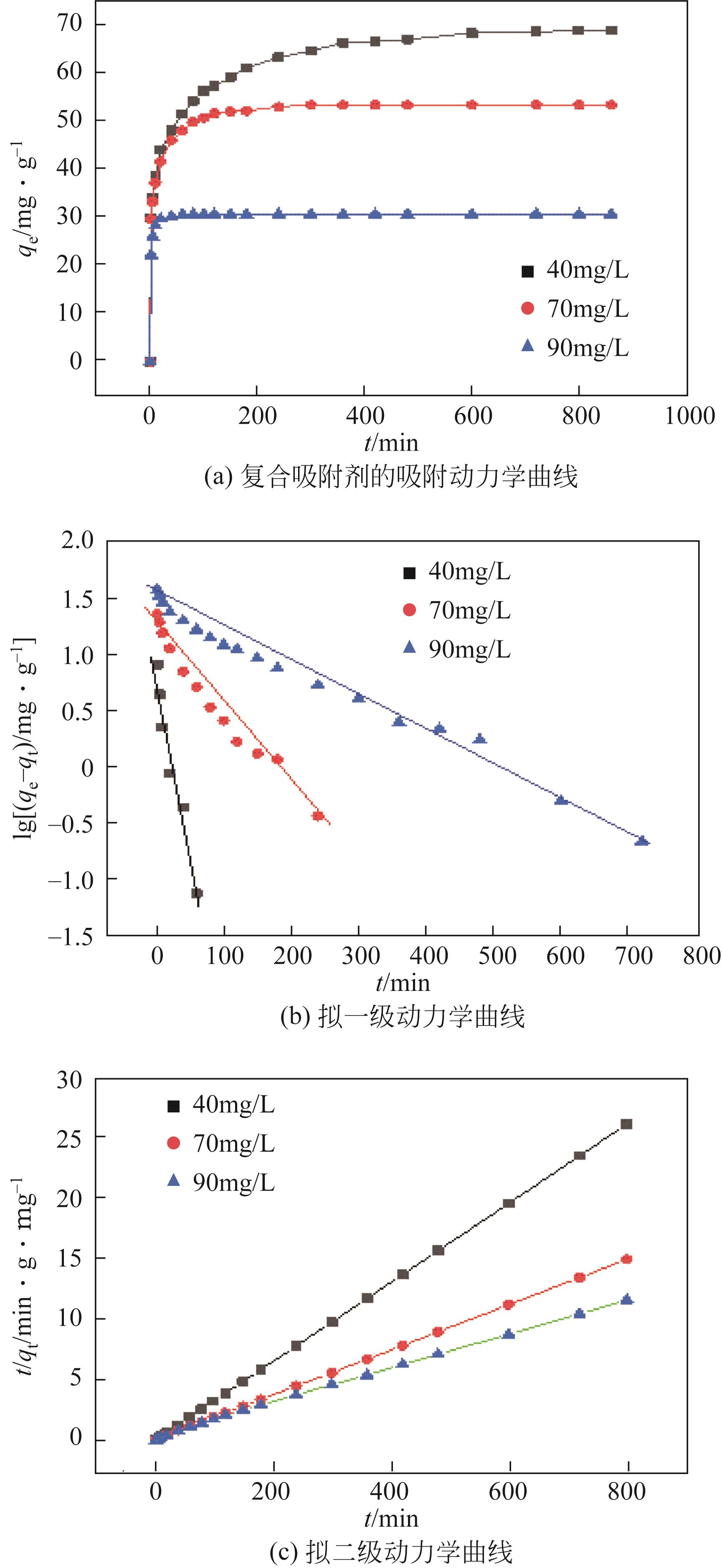
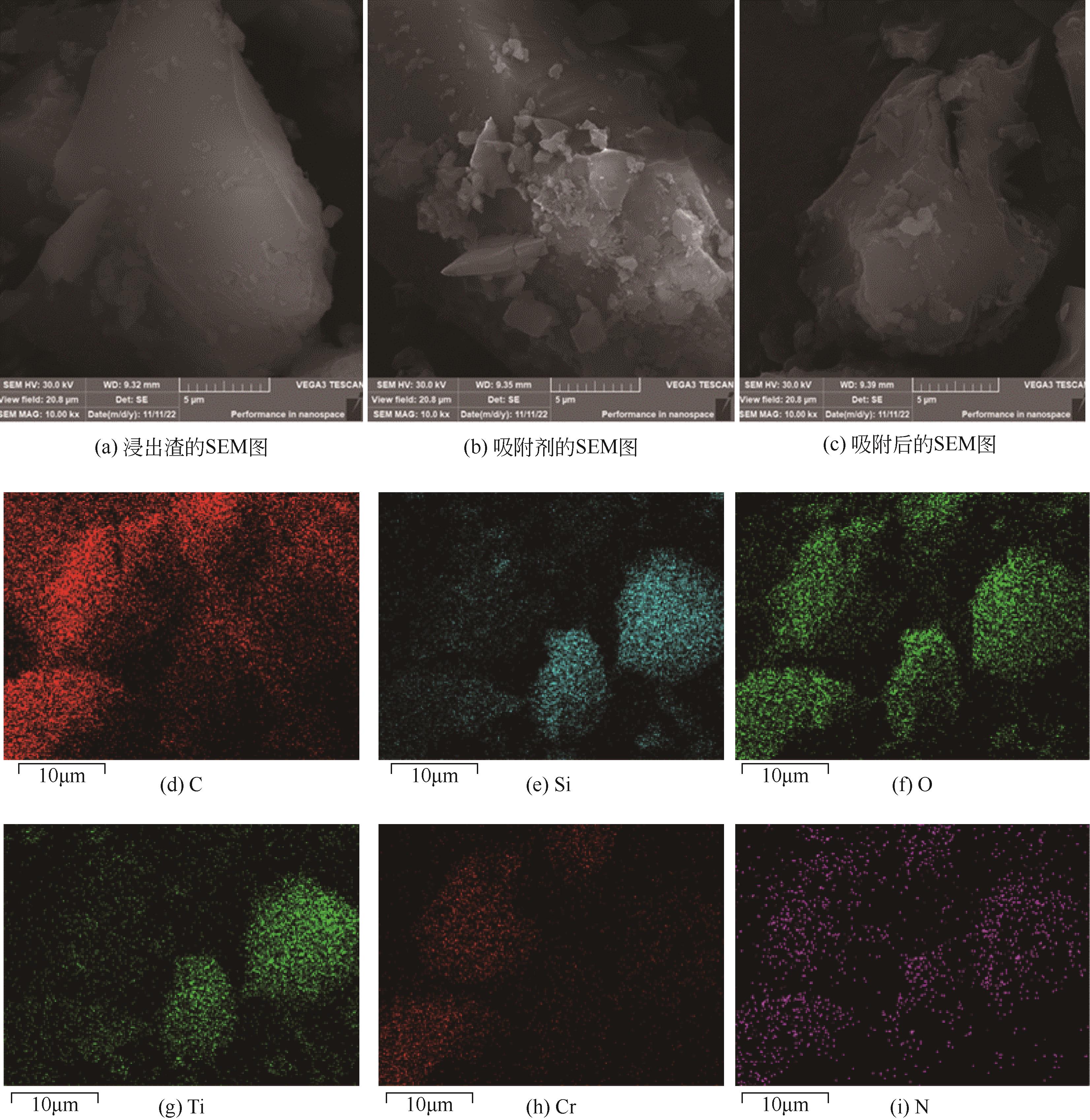
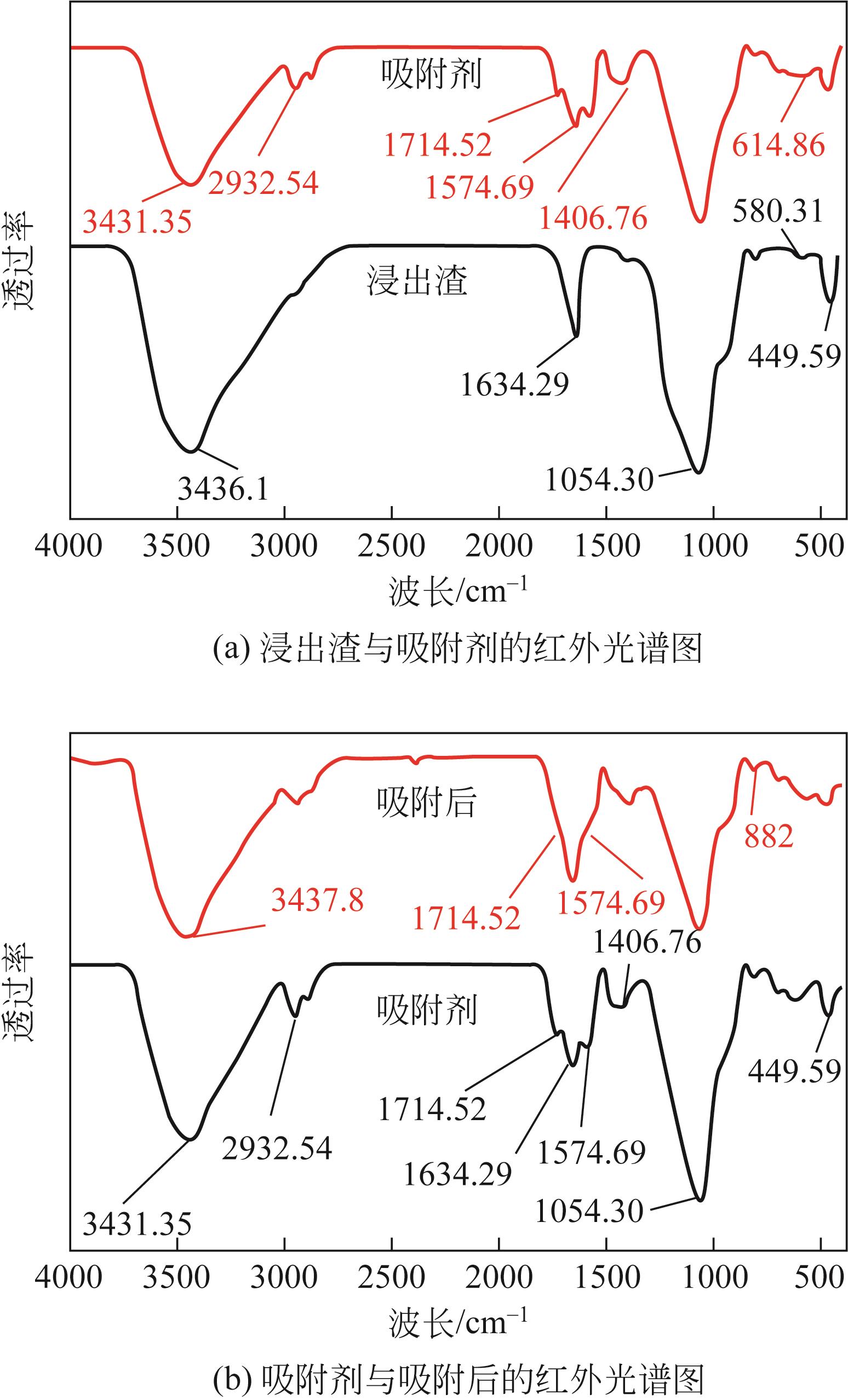
| 1 |
MEI Peng, LI Meng, ZHANG Qian, et al. Prediction model of drinking water source quality with potential industrial-agricultural pollution based on CNN-GRU-Attention[J]. Journal of Hydrology, 2022, 610: 127934.
|
| 2 |
ZUANE J D. Handbook of drinking water quality: Standards and controls[J]. Choice Reviews Online, 1991, 28(9): 28-5057.
|
| 3 |
DROSTE R L. Theory and practice of water and wastewater treatment[J]. Choice Reviews Online, 1997, 34(8): 34-4491.
|
| 4 |
VINOD Kumar, RIPU Daman Parihar, ANKET Sharma, et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses[J]. Chemosphere, 2019, 236: 124364.
|
| 5 |
AYDıN Yaşar Andelib, AKSOY Nuran Deveci. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics[J]. Chemical Engineering Journal, 2009, 151(1/2/3): 188-194.
|
| 6 |
MONDAL Supriyo Kumar, SAHA Prabirkumar. Separation of hexavalent chromium from industrial effluent through liquid membrane using environmentally benign solvent: A study of experimental optimization through response surface methodology[J]. Chemical Engineering Research and Design, 2018, 132: 564-583.
|
| 7 |
CHEN Zhongshan, WEI Benben, YANG Shanye, et al. Synthesis of PANI/AlOOH composite for Cr(Ⅵ) adsorption and reduction from aqueous solutions[J]. ChemistrySelect, 2019, 4(8): 2352-2362.
|
| 8 |
OLIVEIRA Helena. Chromium as an environmental pollutant: Insights on induced plant toxicity[J]. Journal of Botany, 2012, 2012: 375843.
|
| 9 |
KARTHIK Rathinam, MEENAKSHI Sankaran. Removal of hexavalent chromium ions using polyaniline/silica gel composite[J]. Journal of Water Process Engineering, 2014, 1: 37-45.
|
| 10 |
RAI M K, GIRI B S, NATH Y, et al. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from almond shell: Kinetics, equilibrium and thermodynamics study[J]. Journal of Water Supply: Research and Technology-Aqua, 2018, 67(8): 724-737.
|
| 11 |
JOBBY Renitta, Pamela JHA, YADAV Anoop Kumar, et al. Biosorption and biotransformation of hexavalent chromium[Cr(Ⅵ)]: A comprehensive review[J]. Chemosphere, 2018, 207: 255-266.
|
| 12 |
MOHAMED Laabd, ABDELAZIZ Imgharn, ABDELGHANI Hsini, et al. Efficient detoxification of Cr(Ⅵ)-containing effluents by sequential adsorption and reduction using a novel cysteine-doped PANi@faujasite composite: Experimental study supported by advanced statistical physics prediction[J]. Journal of Hazardous Materials, 2022, 422: 126857.
|
| 13 |
BEUKES J P, DU PREEZ S P, VAN ZYL P G, et al. Review of Cr(Ⅵ) environmental practices in the chromite mining and smelting industry—Relevance to development of the Ring of Fire, Canada[J]. Journal of Cleaner Production, 2017, 165: 874-889.
|
| 14 |
EDITION F. Guidelines for drinking-water quality[M]. World Health Organization, 2011.
|
| 15 |
GOLDER Animes K, CHANDA Ajoy K, SAMANTA Amar N, et al. Removal of Cr(Ⅵ) from aqueous solution: Electrocoagulation vs chemical coagulation[J]. Separation Science and Technology, 2007, 42(10): 2177-2193.
|
| 16 |
LI Yujiang, GAO Baoyu, WU Tao, et al. Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide[J]. Water Research, 2009, 43(12): 3067-3075.
|
| 17 |
BABU B V, GUPTA S. Adsorption of Cr(Ⅵ) using activated neem leaves: Kinetic studies[J]. Adsorption, 2008, 14(1): 85-92.
|
| 18 |
ZHANG Ailin, LI Xin, XING Jia, et al. Adsorption of potentially toxic elements in water by modified biochar: A review[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 104196.
|
| 19 |
QIU Jinli, LIU Fuqiang, CHENG Song, et al. Recyclable nanocomposite of flowerlike MoS2@Hybrid acid-doped PANI immobilized on porous PAN nanofibers for the efficient removal of Cr( Ⅵ )[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 447-456.
|
| 20 |
DENG Fang, LU Xiaoying, LUO Yingbo, et al. Novel visible-light-driven direct Z-scheme CdS/CuInS2 nanoplates for excellent photocatalytic degradation performance and highly-efficient Cr( Ⅵ ) reduction[J]. Chemical Engineering Journal, 2019, 361: 1451-1461.
|
| 21 |
LI Meirun, SONG Chi, WU Ying, et al. Novel Z-scheme visible-light photocatalyst based on CoFe2O4/BiOBr/Graphene composites for organic dye degradation and Cr( Ⅵ ) reduction[J]. Applied Surface Science, 2019, 478: 744-753.
|
| 22 |
JIAO Handong, TIAN Donghua, WANG Shuai, et al. Direct preparation of titanium alloys from Ti-bearing blast furnace slag[J]. Journal of the Electrochemical Society, 2017, 164(7): D511-D516.
|
| 23 |
王海波, 孙青竹, 张雪峰, 等. 磷酸三钠对高钛高炉渣制备微晶泡沫玻璃的影响[J]. 钢铁钒钛, 2021, 42(4): 57-61.
|
|
WANG Haibo, SUN Qingzhu, ZHANG Xuefeng, et al. Effect of Na3PO4·12H2O on foam glass-ceramics prepared from high titanium blast furnace slag[J]. Iron Steel Vanadium Titanium, 2021, 42(4): 57-61.
|
| 24 |
张宇鑫, 丁长坤, 岳程飞, 等. 壳聚糖微球的制备及其对甲基橙和Cr(Ⅵ)的吸附性能研究[J]. 天津化工, 2021, 35(4): 29-32.
|
|
ZHANG Yuxin, DING Changkun, YUE Chengfei, et al. Preparation of chitosan microspheres and study of adsorption performancefor methyl orange and Cr(Ⅵ)[J]. Tianjin Chemical Industry, 2021, 35(4): 29-32.
|
| 25 |
JIANG Chunlu, WANG Rui, CHEN Xing, et al. Preparation of chitosan modified fly ash under acid condition and its adsorption mechanism for Cr(Ⅵ)in water[J]. Journal of Central South University, 2021, 28(6): 1652-1664.
|
| 26 |
钟少锋, 吉婉丽, 刘晓云. 壳聚糖超细纤维的制备及其铬离子吸附性能研究[J]. 化学试剂, 2020, 42(3): 226-231.
|
|
ZHONG Shaofeng, JI Wanli, LIU Xiaoyun. Preparation of chitosan/poly(methacylic acid) superfine fiber mat and application in chromium ion removal[J]. Chemical Reagents, 2020, 42(3): 226-231.
|
| 27 |
周丽莎, 李若男, 卞雨洁, 等. TOCNF与磁性羧甲基壳聚糖纳米粒子复合物的制备及吸附Pb2+的特性[J]. 化工进展, 2022, 41(2): 901-910.
|
|
ZHOU Lisha, LI Ruonan, BIAN Yujie, et al. Preparation of TOCNF and magnetic carboxymethyl chitosan nanoparticles composite and adsorption properties of Pb2+ [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 901-910.
|
| 28 |
冯辉霞, 杨洋, 李全珍, 等. 硅藻土复合磁性壳聚糖对Cr(Ⅵ)离子吸附性能[J]. 精细化工, 2018, 35(4): 668-675.
|
|
FENG Huixia, YANG Yang, LI Quanzhen, et al. Adsorption of Cr(Ⅵ) ions on diatomite composite magnetic chitosan material[J]. Fine Chemicals, 2018, 35(4): 668-675.
|
| 29 |
张宇, 李倩, 任欢杰. 壳聚糖载铜改性及吸附Cr(Ⅵ)的试验研究[J]. 湿法冶金, 2019, 38(1): 35-38.
|
|
ZHANG Yu, LI Qian, REN Huanjie. Modifying of chitosan by supported Cu and adsorption of Cr(Ⅵ) in solution[J]. Hydrometallurgy of China, 2019, 38(1): 35-38.
|
| 30 |
王先利. 壳聚糖/有机酸复合物的制备及其除铬应用研究[D]. 东营: 中国石油大学(华东), 2017.
|
|
WANG Xianli. Study on preparation of chitosan/organic acid complex and its application in chromium removal[D]. Dongying: China University of Petroleum (Huadong), 2017.
|
| 31 |
WANG Xiangyu, DU Yi, MA Jun. Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole[J]. Applied Surface Science, 2016, 390: 50-59.
|
| 32 |
HSINI Abdelghani, BENAFQIR Mohamed, NACIRI Yassine, et al. Synthesis of an arginine-functionalized polyaniline@FeOOH composite with high removal performance of hexavalent chromium ions from water: Adsorption behavior, regeneration and process capability studies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 617: 126274.
|
| 33 |
李强. 壳聚糖/TiO2复合吸附剂的制备及吸附特性研究[D]. 北京: 北京化工大学, 2007.
|
|
LI Qiang. Preparation and adsorption characteristics of chitosan/TiO2 composite adsorbent[D]. Beijing: Beijing University of Chemical Technology, 2007.
|
| 34 |
HAN Shiqi, ZHOU Xuelei, XIE Honghao, et al. Chitosan-based composite microspheres for treatment of hexavalent chromium and EBBR from aqueous solution[J]. Chemosphere, 2022, 305: 135486.
|
| 35 |
NESIC Aleksandra R, VELICKOVIC Sava J, ANTONOVIC Dusan G. Characterization of chitosan/montmorillonite membranes as adsorbents for Bezactiv Orange V-3R dye[J]. Journal of Hazardous Materials, 2012, 209/210: 256-263.
|
| 36 |
HALDORAI Yuvaraj, SHIM Jae Jin. Novel chitosan-TiO2 nanohybrid: Preparation, characterization, antibacterial, and photocatalytic properties[J]. Polymer Composites, 2014, 35(2): 327-333.
|
| 37 |
LI Qiang, SU Haijia, TAN Tianwei. Synthesis of ion-imprinted chitosan-TiO2 adsorbent and its multi-functional performances[J]. Biochemical Engineering Journal, 2008, 38(2): 212-218.
|
| 38 |
Ting LYU, MA Ronggang, KE Ke, et al. Synthesis of Gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium[J]. Chemical Engineering Journal, 2021, 408: 127327.
|
| 39 |
夏远涛. 黑曲霉菌丝体-壳聚糖复合吸附剂的制备及对Cr(Ⅵ)的吸附机理研究[D]. 南宁: 广西民族大学, 2019.
|
|
XIA Yuantao. Preparation of Aspergillus Niger mycelium-chitosan composite adsorbent and its adsorption mechanism for Cr( Ⅵ )[D]. Nanning: Guangxi University for Nationalities, 2019.
|
| 40 |
CHEN Na, CAO Shiyu, ZHANG Lin, et al. Structural dependent Cr(Ⅵ) adsorption and reduction of biochar: Hydrochar versus pyrochar[J]. Science of the Total Environment, 2021, 783: 147084.
|
| 41 |
HUANG Xiaopeng, HOU Xiaojing, SONG Fahui, et al. Facet-dependent Cr( Ⅵ ) adsorption of hematite nanocrystals[J]. Environmental Science & Technology, 2016, 50(4): 1964-1972.
|
| 42 |
CHEN Lifeng, LIANG Haiwei, LU Yang, et al. Synthesis of an attapulgite Clay@Carbon nanocomposite adsorbent by a hydrothermal carbonization process and their application in the removal of toxic metal ions from water[J]. Langmuir, 2011, 27(14): 8998-9004.
|
| 43 |
BHAUMIK Madhumita, MCCRINDLE Rob I, MAITY Arjun, et al. Polyaniline nanofibers as highly effective re-usable adsorbent for removal of reactive black 5 from aqueous solutions[J]. Journal of Colloid and Interface Science, 2016, 466: 442-451.
|
| 44 |
SAHNOUN Sousna, BOUTAHALA Mokhtar. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies[J]. International Journal of Biological Macromolecules, 2018, 114: 1345-1353.
|
 ), TIAN Yue, SU Yi(
), TIAN Yue, SU Yi( )
)