化工进展 ›› 2022, Vol. 41 ›› Issue (5): 2623-2635.DOI: 10.16085/j.issn.1000-6613.2021-1282
生物炭/凹凸棒土的制备及对磺胺嘧啶的吸附
- 广西大学化学与化工学院,广西 南宁 530004
-
收稿日期:2021-06-18修回日期:2021-10-11出版日期:2022-05-05发布日期:2022-05-24 -
通讯作者:李志礼 -
作者简介:陈茂(1995—),男,硕士研究生。E-mail:895285424 @qq.com -
基金资助:国家自然科学基金(21978057);广西自然科学基金(2018GXNSFAA294101);广西石油化工资源加工与过程强化技术重点实验室项目(2020Z004);广西百人计划
Preparation of biocarbon/attapulgite and sulfadiazine adsorption
CHEN Mao( ), ZHANG Xing, XIE Wei, CHEN Guanghui, LI Zhili(
), ZHANG Xing, XIE Wei, CHEN Guanghui, LI Zhili( )
)
- College of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China
-
Received:2021-06-18Revised:2021-10-11Online:2022-05-05Published:2022-05-24 -
Contact:LI Zhili
摘要:
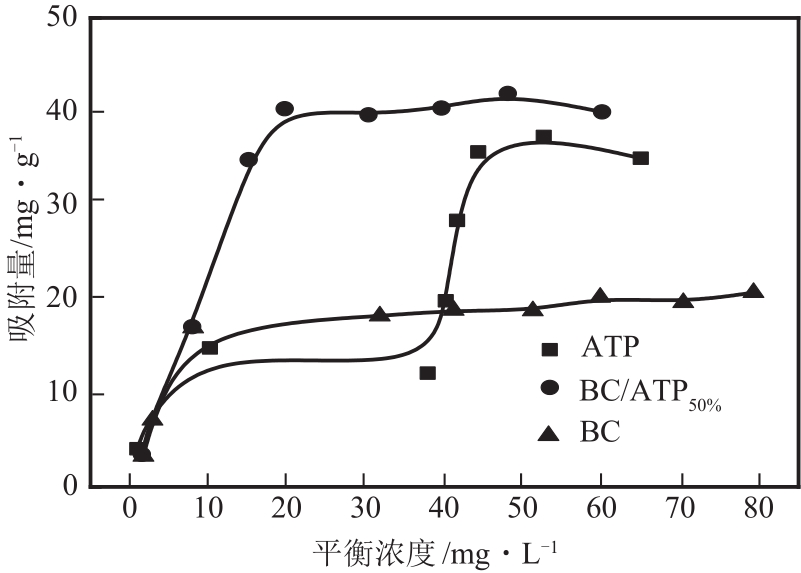
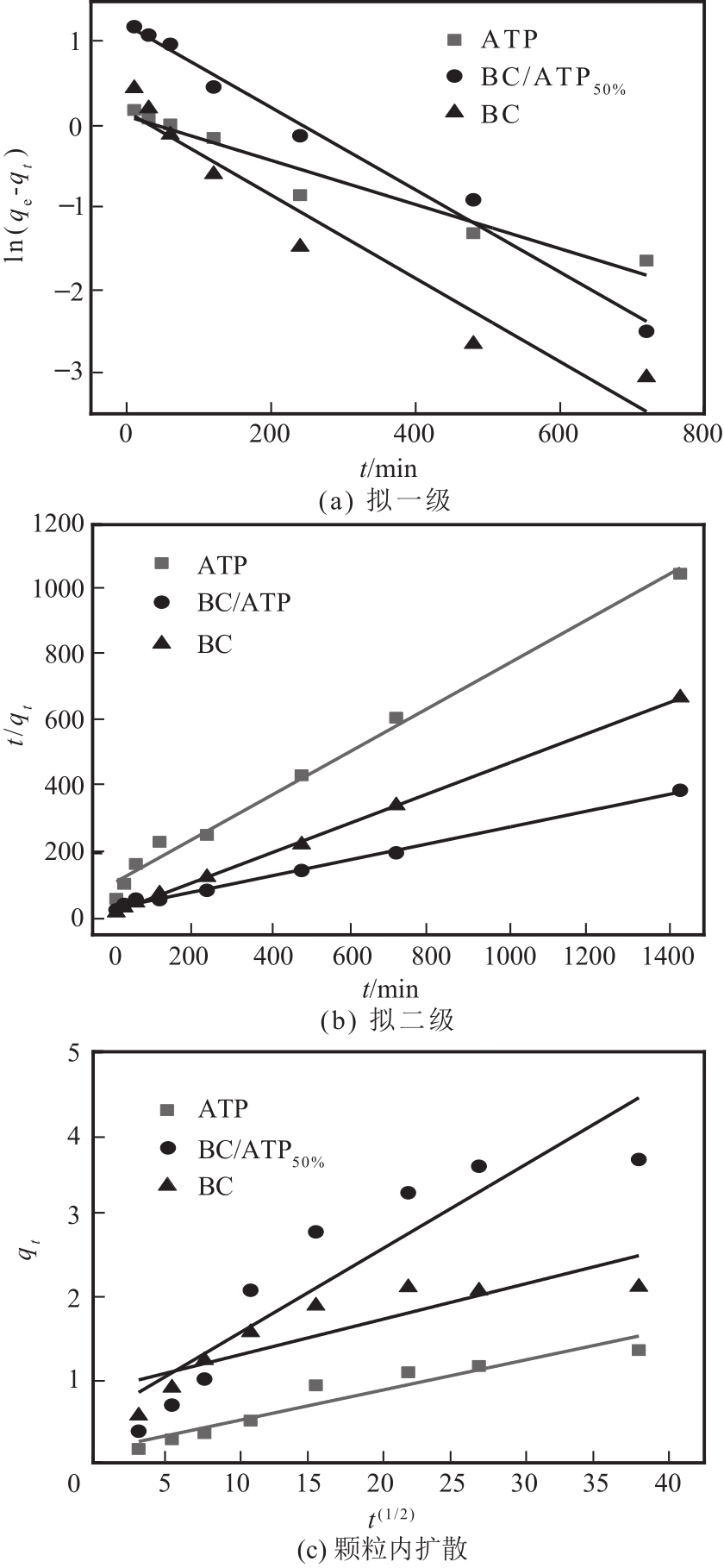
利用凹凸棒土(ATP)和碱性木质素(AL)慢速限氧热解制备生物炭/凹凸棒土(BC/ATP)吸附水中的磺胺嘧啶(SDZ),研究原料比例和热解温度对产品组分含量和吸附效果的影响,并探讨初始pH、BC/ATP投加量、吸附时间和SDZ初始浓度等因素对去除率的影响。分别采用拟一级、拟二级和颗粒内扩散方程拟合吸附过程动力学,用Langmuir和Freundlich方程拟合等温吸附线。通过扫描电镜、傅里叶红外光谱、X射线衍射、拉曼光谱和比表面积测定分析BC/ATP的表面形貌、孔结构和官能团。结果表明ATP能有效促进热解过程中挥发性中间产物二次热解,提高BC得率,改善BC/ATP吸附性能,并通过ATP的金属离子作用扩宽BC/ATP的pH敏感度。吸附动力学均符合拟二级动力学模型,且由颗粒内扩散模型拟合说明该扩散行为不是限制吸附速率的唯一因素,等温吸附线更符合Langmuir等温吸附模型,0<RL<1,为优惠吸附,说明吸附过程易于进行,最大吸附量为109.53mg/g。不同pH条件下吸附机理可分为两部分:①在酸性和中性条件下,主要依靠BC/ATP的上BC表面负电荷与SDZ静电作用;②碱性条件下主要依靠ATP表面金属离子与SDZ氢键的金属阳离子桥接作用。
中图分类号:
引用本文
陈茂, 张鑫, 谢伟, 陈广辉, 李志礼. 生物炭/凹凸棒土的制备及对磺胺嘧啶的吸附[J]. 化工进展, 2022, 41(5): 2623-2635.
CHEN Mao, ZHANG Xing, XIE Wei, CHEN Guanghui, LI Zhili. Preparation of biocarbon/attapulgite and sulfadiazine adsorption[J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2623-2635.
| 热解温度/℃ | 原料 | 灰分质量分数/% | 挥发分质量分数/% | BC得率/% | pH |
|---|---|---|---|---|---|
| 600 | AL/ATP50% | 8.63 | 11.98 | 68.80 | 8.13 |
| AL | 41.98 | 38.79 | 19.23 | 10.33 | |
| 800 | AL/ATP50% | 10.43 | 17.74 | 49.05 | 8.58 |
| AL | 45.25 | 41.32 | 13.43 | 11.04 | |
| 1000 | AL/ATP50% | 11.81 | 23.97 | 36.43 | 9.05 |
| AL | 45.68 | 44.77 | 9.55 | 11.58 |
表1 不同热解温度下BC得率及主要理化性质
| 热解温度/℃ | 原料 | 灰分质量分数/% | 挥发分质量分数/% | BC得率/% | pH |
|---|---|---|---|---|---|
| 600 | AL/ATP50% | 8.63 | 11.98 | 68.80 | 8.13 |
| AL | 41.98 | 38.79 | 19.23 | 10.33 | |
| 800 | AL/ATP50% | 10.43 | 17.74 | 49.05 | 8.58 |
| AL | 45.25 | 41.32 | 13.43 | 11.04 | |
| 1000 | AL/ATP50% | 11.81 | 23.97 | 36.43 | 9.05 |
| AL | 45.68 | 44.77 | 9.55 | 11.58 |
| 材料 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| ATP | 35.3105 | 0.0345 | 13.5411 |
| BC/ATP90% | 58.6890 | 0.0485 | 8.8842 |
| BC/ATP50% | 78.9582 | 0.1267 | 11.4652 |
| BC/ATP10% | 52.7816 | 0.0326 | 7.1564 |
| BC | 17.5881 | 0.0427 | 9.7015 |
表2 BC/ATP和BC、ATP物理参数
| 材料 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| ATP | 35.3105 | 0.0345 | 13.5411 |
| BC/ATP90% | 58.6890 | 0.0485 | 8.8842 |
| BC/ATP50% | 78.9582 | 0.1267 | 11.4652 |
| BC/ATP10% | 52.7816 | 0.0326 | 7.1564 |
| BC | 17.5881 | 0.0427 | 9.7015 |
| 吸附剂 | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qm/mg·g-1 | RL | R2 | Kf/L·mg-1 | n | R2 | ||
| ATP | 0.1790 | 27.4801 | 0.1569 | 0.9719 | 4.4478 | 2.0650 | 0.9228 | |
| BC/ATP50% | 0.0227 | 109.5291 | 0.5952 | 0.9815 | 3.7240 | 1.4858 | 0.9036 | |
| BC | 0.0884 | 27.2628 | 0.2738 | 0.9387 | 4.1692 | 2.5214 | 0.8132 | |
表3 BC/ATP吸附SDZ的等温吸附方程拟合参数
| 吸附剂 | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qm/mg·g-1 | RL | R2 | Kf/L·mg-1 | n | R2 | ||
| ATP | 0.1790 | 27.4801 | 0.1569 | 0.9719 | 4.4478 | 2.0650 | 0.9228 | |
| BC/ATP50% | 0.0227 | 109.5291 | 0.5952 | 0.9815 | 3.7240 | 1.4858 | 0.9036 | |
| BC | 0.0884 | 27.2628 | 0.2738 | 0.9387 | 4.1692 | 2.5214 | 0.8132 | |
| 材料 | 拟一级 | 拟二级 | ||||||
|---|---|---|---|---|---|---|---|---|
| qe/mg·g-1 | K1/min-1 | R2 | qe/mg·g-1 | K2/g·mg-1·min-1 | R2 | |||
| ATP | 1.1157 | 0.0027 | 0.9484 | 1.4961 | 0.0042 | 0.9908 | ||
| BC/ATP50% | 3.3599 | 0.0050 | 0.9870 | 4.0568 | 0.0021 | 0.9963 | ||
| BC | 1.1767 | 0.1650 | 0.9418 | 2.2026 | 0.0123 | 0.9996 | ||
表4 BC/ATP动力学拟一级和拟二级拟合参数
| 材料 | 拟一级 | 拟二级 | ||||||
|---|---|---|---|---|---|---|---|---|
| qe/mg·g-1 | K1/min-1 | R2 | qe/mg·g-1 | K2/g·mg-1·min-1 | R2 | |||
| ATP | 1.1157 | 0.0027 | 0.9484 | 1.4961 | 0.0042 | 0.9908 | ||
| BC/ATP50% | 3.3599 | 0.0050 | 0.9870 | 4.0568 | 0.0021 | 0.9963 | ||
| BC | 1.1767 | 0.1650 | 0.9418 | 2.2026 | 0.0123 | 0.9996 | ||
| 复合材料 | C1 | Kd1/mg·g-1·min-1/2 | R2 | C2 | Kd2/mg·g-1·min-1/2 | R2 | C3 | Kd3/ mg·g-1·min-1/2 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| ATP | 0.0344 | 0.0436 | 0.9956 | 0.6707 | 0.0187 | 0.9910 | — | — | — |
| BC/ATP50% | 0.0533 | 0.1383 | 0.9999 | 1.0227 | 0.1060 | 0.9555 | 3.4085 | 0.0074 | 0.9999 |
| BC | 0.1070 | 0.1472 | 0.1070 | 1.0906 | 0.0488 | 0.9590 | 1.9794 | 0.0042 | 0.9999 |
表5 BC/ATP吸附SDZ的吸附颗粒内扩散动力学方程拟合参数
| 复合材料 | C1 | Kd1/mg·g-1·min-1/2 | R2 | C2 | Kd2/mg·g-1·min-1/2 | R2 | C3 | Kd3/ mg·g-1·min-1/2 | R2 |
|---|---|---|---|---|---|---|---|---|---|
| ATP | 0.0344 | 0.0436 | 0.9956 | 0.6707 | 0.0187 | 0.9910 | — | — | — |
| BC/ATP50% | 0.0533 | 0.1383 | 0.9999 | 1.0227 | 0.1060 | 0.9555 | 3.4085 | 0.0074 | 0.9999 |
| BC | 0.1070 | 0.1472 | 0.1070 | 1.0906 | 0.0488 | 0.9590 | 1.9794 | 0.0042 | 0.9999 |
| 1 | 王路光, 朱晓磊, 王靖飞, 等. 环境水体中的残留抗生素及其潜在风险[J]. 工业水处理, 2009, 29(5): 10-14. |
| WANG Luguang, ZHU Xiaolei, WANG Jingfei, et al. Antibiotic residual in environmental water body and its potential risks[J]. Industrial Water Treatment, 2009, 29(5): 10-14. | |
| 2 | 张申平, 王艺蒙, 葛宇, 等. 基于孔材料的多元复合光催化剂降解抗生素[J]. 化工进展, 2021, 40(6): 3287-3299. |
| ZHANG Shenping, WANG Yimeng, GE Yu, et al. Degradation of antibiotics by porous composite photocatalyst[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3287-3299. | |
| 3 | MARTÍNEZ-CARBALLO E, GONZÁLEZ-BARREIRO C, SCHARF S, et al. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria[J]. Environmental Pollution, 2007, 148(2): 570-579. |
| 4 | LIU B Y, NIE X P, LIU W Q, et al. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole on photosynthetic apparatus in Selenastrum capricornutum [J]. Ecotoxicology and Environmental Safety, 2011, 74(4): 1027-1035. |
| 5 | SACHDEVA S, PALUR R V, SUDHAKAR K U, et al. E. coli group 1 capsular polysaccharide exportation nanomachinary as a plausible antivirulence target in the perspective of emerging antimicrobial resistance[J]. Frontiers in Microbiology, 2017, 8: 70. |
| 6 | KANAKARAJU D, GLASS B D, OELGEMÖLLER M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review[J]. Journal of Environmental Management, 2018, 219: 189-207. |
| 7 | ISLAS-ESPINOZA M, REID B J, WEXLER M, et al. Soil bacterial consortia and previous exposure enhance the biodegradation of sulfonamides from pig manure[J]. Microbial Ecology, 2012, 64(1): 140-151. |
| 8 | 王健, 贲伟伟, 强志民, 等. 我国养猪业废弃物中四环素类、磺胺类抗生素及相关抗性基因污染研究进展[J]. 生态毒理学报, 2015, 10(5): 2-10. |
| WANG Jian, Weiwei BEN, QIANG Zhimin, et al. Contamination of tetracyclines, sulfonamides and corresponding resistance genes in the waste from Chinese pig industry[J]. Asian Journal of Ecotoxicology, 2015, 10(5): 2-10. | |
| 9 | ZHANG H B, LUO Y M, WU L H, et al. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming[J]. Environmental Science and Pollution Research, 2015, 22(8): 5908-5918. |
| 10 | ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance[J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. |
| 11 | LUO Y, XU L, RYSZ M, et al. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China[J]. Environmental Science & Technology, 2011, 45(5): 1827-1833. |
| 12 | WANG W, FANG J J, CHEN H, et al. Rice-husk-derived mesoporous 0D/2D C3N4 isotype heterojunction with improved quantum effect for photodegradation of tetracycline antibiotics[J]. Ceramics International, 2019, 45(2): 2234-2240. |
| 13 | DOLAR D, GROS M, RODRIGUEZ-MOZAZ S, et al. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO[J]. Journal of Hazardous Materials, 2012, 239/240: 64-69. |
| 14 | TRAN N H, CHEN H J, REINHARD M, et al. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes[J]. Water Research, 2016, 104: 461-472. |
| 15 | PREMARATHNA K S D, RAJAPAKSHA A U, ADASSORIYA N, et al. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media[J]. Journal of Environmental Management, 2019, 238: 315-322. |
| 16 | ALAHABADI A, HOSSEINI-BANDEGHARAEI A, MOUSSAVI G, et al. Comparing adsorption properties of NH4Cl-modified activated carbon towards chlortetracycline antibiotic with those of commercial activated carbon[J]. Journal of Molecular Liquids, 2017, 232: 367-381. |
| 17 | 吴艳. 石墨烯纳米复合材料的合成及其吸附与光催化性能研究[D]. 广州: 华南理工大学, 2016. |
| WU Yan. Synthesis of graphene nanocomposites and their adsorption and photocatalytic behaviors study[D]. Guangzhou: South China University of Technology, 2016. | |
| 18 | ZHANG P Z, LI Y F, CAO Y Y, et al. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures[J]. Bioresource Technology, 2019, 285: 121348. |
| 19 | MANDAL S, SARKAR B, BOLAN N, et al. Designing advanced biochar products for maximizing greenhouse gas mitigation potential[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(17): 1367-1401. |
| 20 | 李蕊宁, 王兆炜, 郭家磊, 等. 酸碱改性生物炭对水中磺胺噻唑的吸附性能研究[J]. 环境科学学报, 2017, 37(11): 4119-4128. |
| LI Ruining, WANG Zhaowei, GUO Jialei, et al. Adsorption characteristics of sulfathiazole in aqueous solution by acid/alkali modified biochars[J]. Acta Scientiae Circumstantiae, 2017, 37(11): 4119-4128. | |
| 21 | 赵迎新, 麻泽浩, 杨知凡, 等. 污泥生物炭催化高级氧化过程进展[J]. 化工进展, 2021, 40(7): 3984-3994. |
| ZHAO Yingxin, MA Zehao, YANG Zhifan, et al. Progress of advanced oxidation process catalyzed by sludge biochar[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3984-3994. | |
| 22 | 李江遐, 吴林春, 张军, 等. 生物炭修复土壤重金属污染的研究进展[J]. 生态环境学报, 2015, 24(12): 2075-2081. |
| LI Jiangxia, WU Linchun, ZHANG Jun, et al. Research progresses in remediation of heavy metal contaminated soils by biochar[J]. Ecology and Environmental Sciences, 2015, 24(12): 2075-2081. | |
| 23 | 孙彤, 李梦瑶, 吕学红, 等. 玉米秸秆生物炭对水中戊唑醇和稻瘟酰胺的吸附特性研究[J]. 山东农业科学, 2019, 51(6): 117-124. |
| SUN Tong, LI Mengyao, Xuehong LYU, et al. Adsorption characteristics of corn straw biochar on tebuconazole and fenoxanil from aqueous solution[J]. Shandong Agricultural Sciences, 2019, 51(6): 117-124. | |
| 24 | YAO Y, ZHANG Y, GAO B, et al. Removal of sulfamethoxazole (SMX) and sulfapyridine (SPY) from aqueous solutions by biochars derived from anaerobically digested bagasse[J]. Environmental Science and Pollution Research, 2018, 25(26): 25659-25667. |
| 25 | 陈心想, 耿增超. 生物质炭在农业上的应用[J]. 西北农林科技大学学报(自然科学版), 2013, 41(2): 167-174. |
| CHEN Xinxiang, GENG Zengchao. Application of biochar in agriculture[J]. Journal of Northwest A & F University (Natural Science Edition), 2013, 41(2): 167-174. | |
| 26 | PARK K M, NAM H G, LEE K B, et al. Adsorption behaviors of sugars and sulfuric acid on activated porous carbon[J]. Journal of Industrial and Engineering Chemistry, 2016, 34: 21-26. |
| 27 | YUE L M, XIA Q Z, WANG L W, et al. CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell[J]. Journal of Colloid and Interface Science, 2018, 511: 259-267. |
| 28 | LIDEN A G, BERRUTI F, SCOTT D S. A kinetic model for the production of liquids from the flash pyrolysis of biomass[J]. Chemical Engineering Communications, 1988, 65(1): 207-221. |
| 29 | BRADBURY A G W, SAKAI Y, SHAFIZADEH F. A kinetic model for pyrolysis of cellulose[J]. Journal of Applied Polymer Science, 1979, 23(11): 3271-3280. |
| 30 | RUTKOWSKI P. Pyrolytic behavior of cellulose in presence of montmorillonite K10 as catalyst[J]. Journal of Analytical and Applied Pyrolysis, 2012, 98: 115-122. |
| 31 | SHEN Z T, ZHANG Y H, MCMILLAN O, et al. Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk[J]. Environmental Science and Pollution Research, 2017, 24(14): 12809-12819. |
| 32 | ARAVINDHAN R, RAGHAVA RAO J, UNNI NAIR B. Preparation and characterization of activated carbon from marine macro-algal biomass[J]. Journal of Hazardous Materials, 2009, 162(2/3): 688-694. |
| 33 | HERATH I, KUMARATHILAKA P, AL-WABEL M I, et al. Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar[J]. Microporous and Mesoporous Materials, 2016, 225: 280-288. |
| 34 | MAYAKADUWA S S, KUMARATHILAKA P, HERATH I, et al. Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal[J]. Chemosphere, 2016, 144: 2516-2521. |
| 35 | RODRIGUEZ CORREA C, HEHR T, VOGLHUBER-SLAVINSKY A, et al. Pyrolysis vs. hydrothermal carbonization: understanding the effect of biomass structural components and inorganic compounds on the char properties[J]. Journal of Analytical and Applied Pyrolysis, 2019, 140: 137-147. |
| 36 | WANG H H, WANG X, CUI Y S, et al. Slow pyrolysis polygeneration of bamboo (Phyllostachys pubescens): product yield prediction and biochar formation mechanism[J]. Bioresource Technology, 2018, 263: 444-449. |
| 37 | TANG J, MU B, ZONG L, et al. One-step synthesis of magnetic attapulgite/carbon supported NiFe-LDHs by hydrothermal process of spent bleaching earth for pollutants removal[J]. Journal of Cleaner Production, 2018, 172: 673-685. |
| 38 | WU Z B, ZHONG H, YUAN X Z, et al. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater[J]. Water Research, 2014, 67: 330-344. |
| 39 | 高丽慧. 黏土矿物催化生物质热解机理及生物炭吸附特性研究[D]. 徐州: 中国矿业大学, 2019. |
| GAO Lihui. Study on catalytic pyrolysis mechanism of clay minerals to biomass and adsorption characteristics of biochar[D]. Xuzhou: China University of Mining and Technology, 2019. | |
| 40 | AI L H, LI L L. Efficient removal of organic dyes from aqueous solution with ecofriendly biomass-derived carbon@montmorillonite nanocomposites by one-step hydrothermal process[J]. Chemical Engineering Journal, 2013, 223: 688-695. |
| 41 | MU B, WANG A Q. One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support[J]. Journal of Materials Chemistry A, 2015, 3(1): 281-289. |
| 42 | UDDIN M K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade[J]. Chemical Engineering Journal, 2017, 308: 438-462. |
| 43 | 张枝焕, 高先志, 方朝亮. 黏土矿物对干酪根热解产物的影响及其作用机理[J]. 石油大学学报(自然科学版), 1995, 19(5): 11-17. |
| ZHANG Zhihuan, GAO Xianzhi, FANG Zhaoliang. Effect of clay minerals on kerogen pyrolysis and the reaction mechanism[J]. Journal of the University of Petroleum, China, 1995, 19(5): 11-17 | |
| 44 | 肖金凯, 荣天君. 黏土矿物在催化裂化催化剂中的应用[J]. 高校地质学报, 2000, 6(2): 282-286. |
| XIAO Jinkai, RONG Tianjun. Application of clay minerals in catalytic cracking catalysts[J]. Geological Journal of China Universitiesf, 2000, 6(2): 282-286. | |
| 45 | 尤凌聪, 汪玉瑛, 刘玉学, 等. 生物炭-凹凸棒土复合材料对水稻土锌镉的钝化及土壤养分和酶活性的影响研究[J]. 核农学报, 2021, 35(7): 1717-1723. |
| YOU Lingcong, WANG Yuying, LIU Yuxue, et al. Effects of biochar-attapulgite composite on zinc and cadmium passivation of paddy soil and soil nutrient and enzyme activities[J]. Journal of Nuclear Agricultural Sciences, 2021, 35(7): 1717-1723. | |
| 46 | 曾小康. 油茶壳炭/凹凸棒土复合材料的制备及其吸附水体中Cr(Ⅵ)的性能研究[D]. 长沙: 长沙理工大学, 2020. |
| ZENG Xiaokang. Preparation of camellia oleifera shell biochar/attapulgite composite material and the adsorption performance on Cr(Ⅵ) in water[D]. Changsha: Changsha University of Science & Technology, 2020. | |
| 47 | 王海英, 韩洪晶, 宋华, 等. 木质素及其模型化合物热解研究进展[J]. 化工进展, 2019, 38(7): 3088-3096. |
| WANG Haiying, HAN Hongjing, SONG Hua, et al. Progress in pyrolysis of lignin and its model compounds[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3088-3096. | |
| 48 | BRITT P F, BUCHANAN A C III, THOMAS K B III, et al. Pyrolysis mechanisms of lignin: surface-immobilized model compound investigation of acid-catalyzed and free-radical reaction pathways[J]. Journal of Analytical and Applied Pyrolysis, 1995, 33: 1-19. |
| 49 | ZHANG G X, LIU X T, SUN K, et al. Competitive sorption of metsulfuron-methyl and tetracycline on corn straw biochars[J]. Journal of Environmental Quality, 2012, 41(6): 1906-1915. |
| 50 | NUNN T R, HOWARD J B, LONGWELL J P, et al. Product compositions and kinetics in the rapid pyrolysis of milled wood lignin[J]. Industrial & Engineering Chemistry Process Design and Development, 1985, 24(3): 844-852. |
| 51 | YUAN J H, XU R K, ZHANG H. The forms of alkalis in the biochar produced from crop residues at different temperatures[J]. Bioresource Technology, 2011, 102(3): 3488-3497. |
| 52 | 王佳楠, 羿颖, 边勇军, 等. 羟甲基化木质素/纤维素气凝胶粒子的制备、表征及吸附性能[J]. 生物质化学工程, 2020, 54(1): 16-22. |
| WANG Jianan, YI Ying, BIAN Yongjun, et al. Preparation, characterization and adsorption performance of hydroxymethylated lignin/cellulose aerogel particles[J]. Biomass Chemical Engineering, 2020, 54(1): 16-22. | |
| 53 | MANDAL S, PU S Y, SHANGGUAN L X, et al. Synergistic construction of green tea biochar supported nZVI for immobilization of lead in soil: a mechanistic investigation[J]. Environment International, 2020, 135: 105374. |
| 54 | KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. |
| 55 | LOU J Q, XU X, GAO Y F, et al. Preparation of magnetic activated carbon from waste rice husk for the determination of tetracycline antibiotics in water samples[J]. RSC Advances, 2016, 6(113): 112166-112174. |
| 56 | 李美兰. 三种典型抗生素在凹凸棒土中的吸附行为研究[D]. 南京: 南京大学, 2012. |
| LI Meilan. The study of adsorption behavior of three typical antibiotics on attapulgite[D]. Nanjing: Nanjing University, 2012. | |
| 57 | 朱青. 改性生物炭对水中磺胺嘧啶的去除试验研究[D]. 济南: 山东师范大学, 2018. |
| ZHU Qing. Experimental study on the removal of sulfadiazine from water by modified biochar[D]. Jinan: Shandong Normal University, 2018. | |
| 58 | WU M, PAN B, ZHANG D, et al. The sorption of organic contaminants on biochars derived from sediments with high organic carbon content[J]. Chemosphere, 2013, 90(2): 782-788. |
| 59 | SRINIVASAN P, SARMAH A K. Characterisation of agricultural waste-derived biochars and their sorption potential for sulfamethoxazole in pasture soil: a spectroscopic investigation[J]. Science of the Total Environment, 2015, 502: 471-480. |
| 60 | SUN B B, LIAN F, BAO Q L, et al. Impact of low molecular weight organic acids (LMWOAs) on biochar micropores and sorption properties for sulfamethoxazole[J]. Environmental Pollution, 2016, 214: 142-148. |
| 61 | TZENG T W, LIU Y T, DENG Y, et al. Removal of sulfamethazine antibiotics using cow manure-based carbon adsorbents[J]. International Journal of Environmental Science and Technology, 2016, 13(3): 973-984. |
| 62 | POURETEDAL H R, SADEGH N. Effective removal of amoxicillin, cephalexin, tetracycline and penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood[J]. Journal of Water Process Engineering, 2014, 1: 64-73. |
| 63 | ZHENG H, WANG Z Y, ZHAO J, et al. Sorption of antibiotic sulfamethoxazole varies with biochars produced at different temperatures[J]. Environmental Pollution, 2013, 181: 60-67. |
| 64 | ZHU X D, LIU Y C, ZHOU C, et al. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline[J]. Carbon, 2014, 77: 627-636. |
| 65 | PREMARATHNA K S D, RAJAPAKSHA A U, SARKAR B, et al. Biochar-based engineered composites for sorptive decontamination of water: a review[J]. Chemical Engineering Journal, 2019, 372: 536-550. |
| 66 | PEI Z G, YANG S, LI L Y, et al. Effects of copper and aluminum on the adsorption of sulfathiazole and tylosin on peat and soil[J]. Environmental Pollution, 2014, 184: 579-585. |
| 67 | AVISAR D, PRIMOR O, GOZLAN I, et al. Sorption of sulfonamides and tetracyclines to montmorillonite clay[J]. Water, Air, & Soil Pollution, 2010, 209(1/2/3/4): 439-450. |
| 68 | 侯少芹, 王海增, 孙金香, 等. “氧化镁/活性炭”新型吸附剂的制备及其对Cr(Ⅵ)的吸附研究[J]. 环境工程学报, 2009, 3(12): 2133-2137. |
| HOU Shaoqin, WANG Haizeng, SUN Jinxiang, et al. Preparation of “MgO/AC” and its adsorption properties of Cr(Ⅵ)[J]. Chinese Journal of Environmental Engineering, 2009, 3(12): 2133-2137. | |
| 69 | GHORAI S, SINHAMAHPATRA A, SARKAR A, et al. Novel biodegradable nanocomposite based on XG-g-PAM/SiO2: application of an efficient adsorbent for Pb2+ ions from aqueous solution[J]. Bioresource Technology, 2012, 119: 181-190. |
| 70 | SUN P Z, LI Y X, MENG T, et al. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine[J]. Water Research, 2018, 147: 91-100. |
| 71 | HU H, ZHANG X, WANG T, et al. Bamboo (Acidosasa longiligula) shoot shell biochar: its potential application to isolation of uranium(Ⅵ) from aqueous solution[J]. Journal of Radioanalytical and Nuclear Chemistry, 2018, 316(1): 349-362. |
| 72 | ZHANG Z Q, XIA S Q, ZHAO J F, et al. Characterization and flocculation mechanism of high efficiency microbial flocculant TJ-F1 from Proteus mirabilis [J]. Colloids and Surfaces B: Biointerfaces, 2010, 75(1): 247-251. |
| 73 | 汤睿, 张寒冰, 陆彩妹, 等. 有机磁性膨润土对环丙沙星和四环素的吸附性能[J]. 化工进展, 2021, 40(11): 6231-6241. |
| TANG R, ZHANG H B, LU C M, et al. Adsorption properties of organic magnetic bentonite for ciprofloxacin and tetracycline[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6231-6241. | |
| 74 | 孟庆梅, 孟迪, 张艳丽, 等. 榴莲壳生物炭对磺胺嘧啶的吸附性能[J]. 化工进展, 2020, 39(11): 4651-4659. |
| MENG Qingmei, MENG Di, ZHANG Yanli, et al. Adsorption characteristics of biochar prepared by durian shell on sulfadiazine[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4651-4659. | |
| 75 | SUMALINOG D A G, CAPAREDA S C, DE LUNA M D G. Evaluation of the effectiveness and mechanisms of acetaminophen and methylene blue dye adsorption on activated biochar derived from municipal solid wastes[J]. Journal of Environmental Management, 2018, 210: 255-262. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [3] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [4] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [7] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [8] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [9] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [10] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [11] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [12] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [13] | 王浩然, 殷全玉, 方明, 侯建林, 李军, 何斌, 张明月. 近临界水处理废弃烟梗工艺优化[J]. 化工进展, 2023, 42(9): 5019-5027. |
| [14] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [15] | 郑梦启, 王成业, 汪炎, 王伟, 袁守军, 胡真虎, 何春华, 王杰, 梅红. 菌藻共生技术在工业废水零排放中的应用与展望[J]. 化工进展, 2023, 42(8): 4424-4431. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||