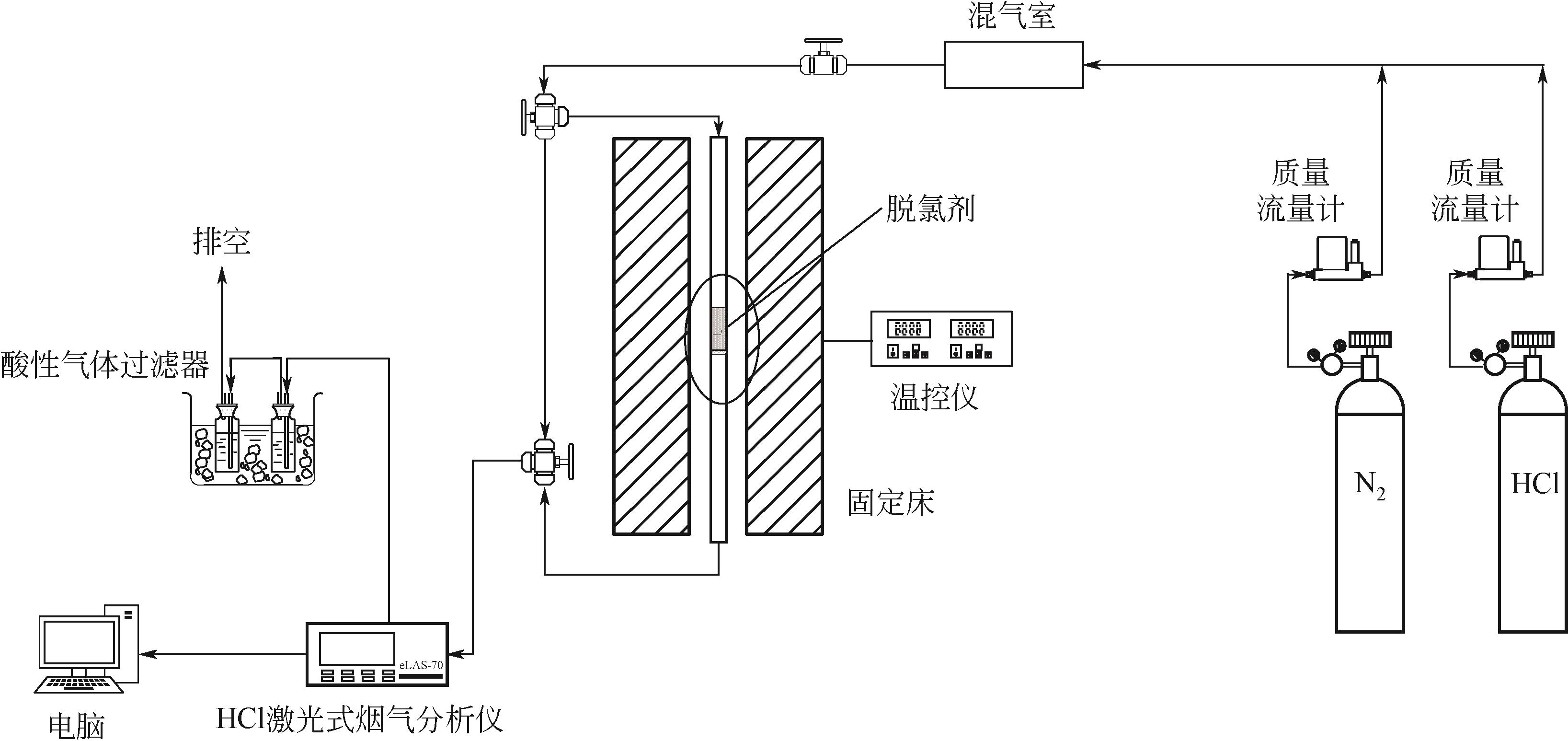
化工进展 ›› 2023, Vol. 42 ›› Issue (11): 6053-6063.DOI: 10.16085/j.issn.1000-6613.2022-2362
• 资源与环境化工 • 上一篇
乙醇改性钙基脱氯剂实验及动力学分析
王雨晴1( ), 段钰锋1(
), 段钰锋1( ), 王睿1, 刘晓硕1, 申镇2
), 王睿1, 刘晓硕1, 申镇2
- 1.能源热转换及其过程测控教育部重点实验室,东南大学能源与环境学院,江苏 南京 210096
2.清华大学能源与动力工程系,北京 100084
-
收稿日期:2022-12-27修回日期:2023-02-18出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:段钰锋 -
作者简介:王雨晴(1997—),女,硕士研究生,研究方向为烟气脱氯。E-mail:220200399@seu.edu.cn。 -
基金资助:国家自然科学基金(51876039)
Experimental and kinetics analysis of ethanol-hydrated calcium-based adsorbents
WANG Yuqing1( ), DUAN Yufeng1(
), DUAN Yufeng1( ), WANG Rui1, LIU Xiaoshuo1, SHEN Zhen2
), WANG Rui1, LIU Xiaoshuo1, SHEN Zhen2
- 1.Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
2.Department of Energy and Power Engineering, Tsinghua University, Beijing 100084, China
-
Received:2022-12-27Revised:2023-02-18Online:2023-11-20Published:2023-12-15 -
Contact:DUAN Yufeng
摘要:
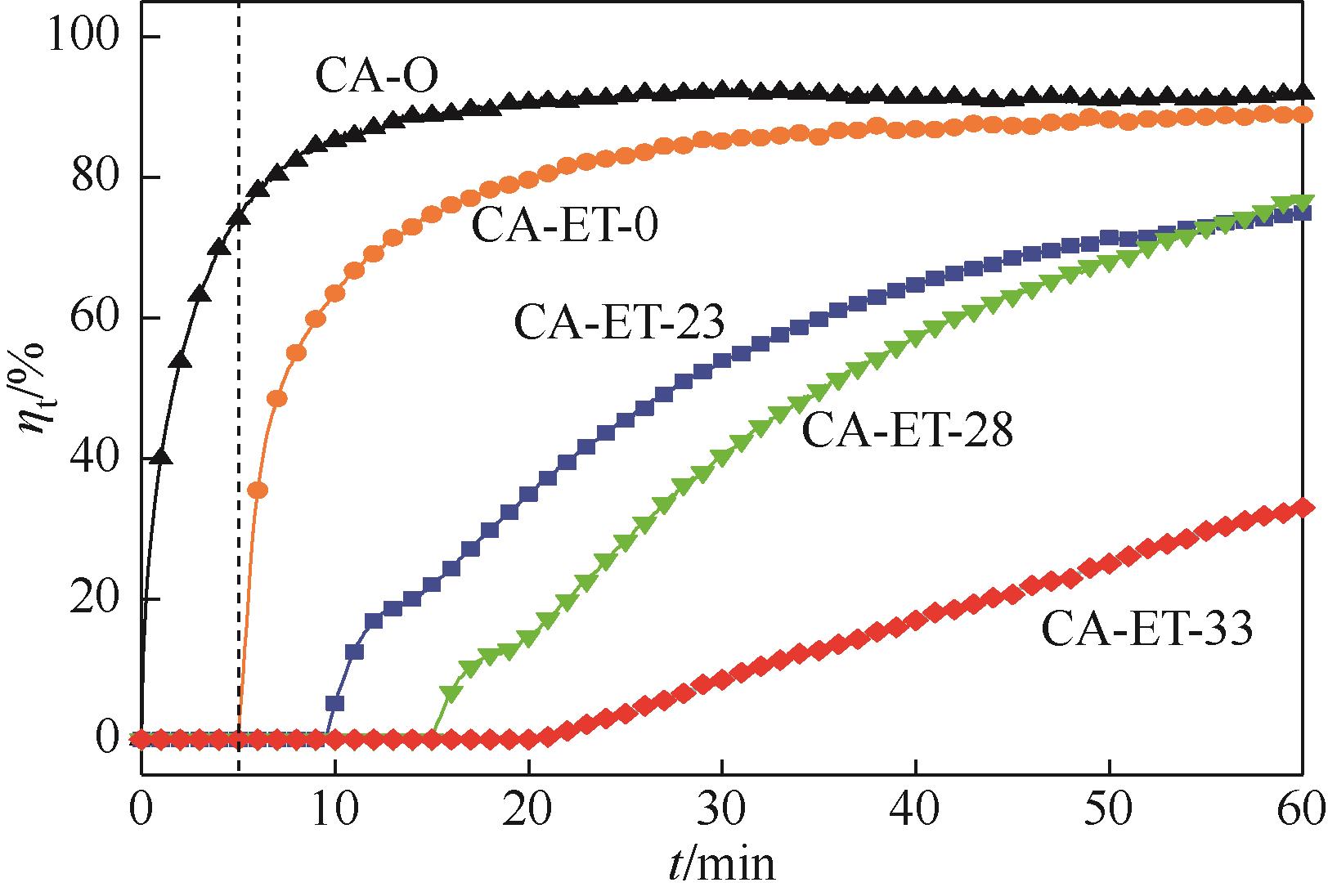
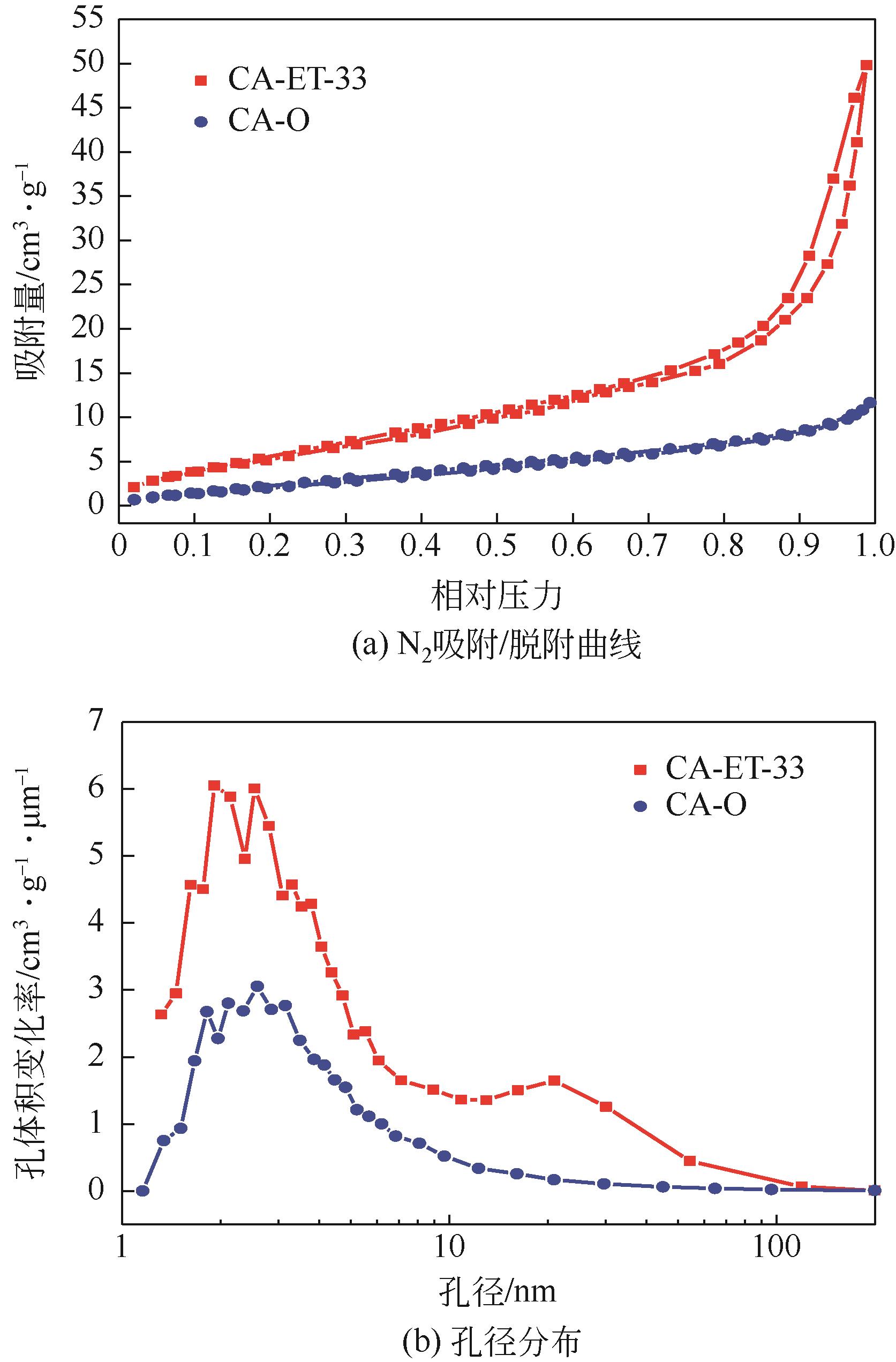
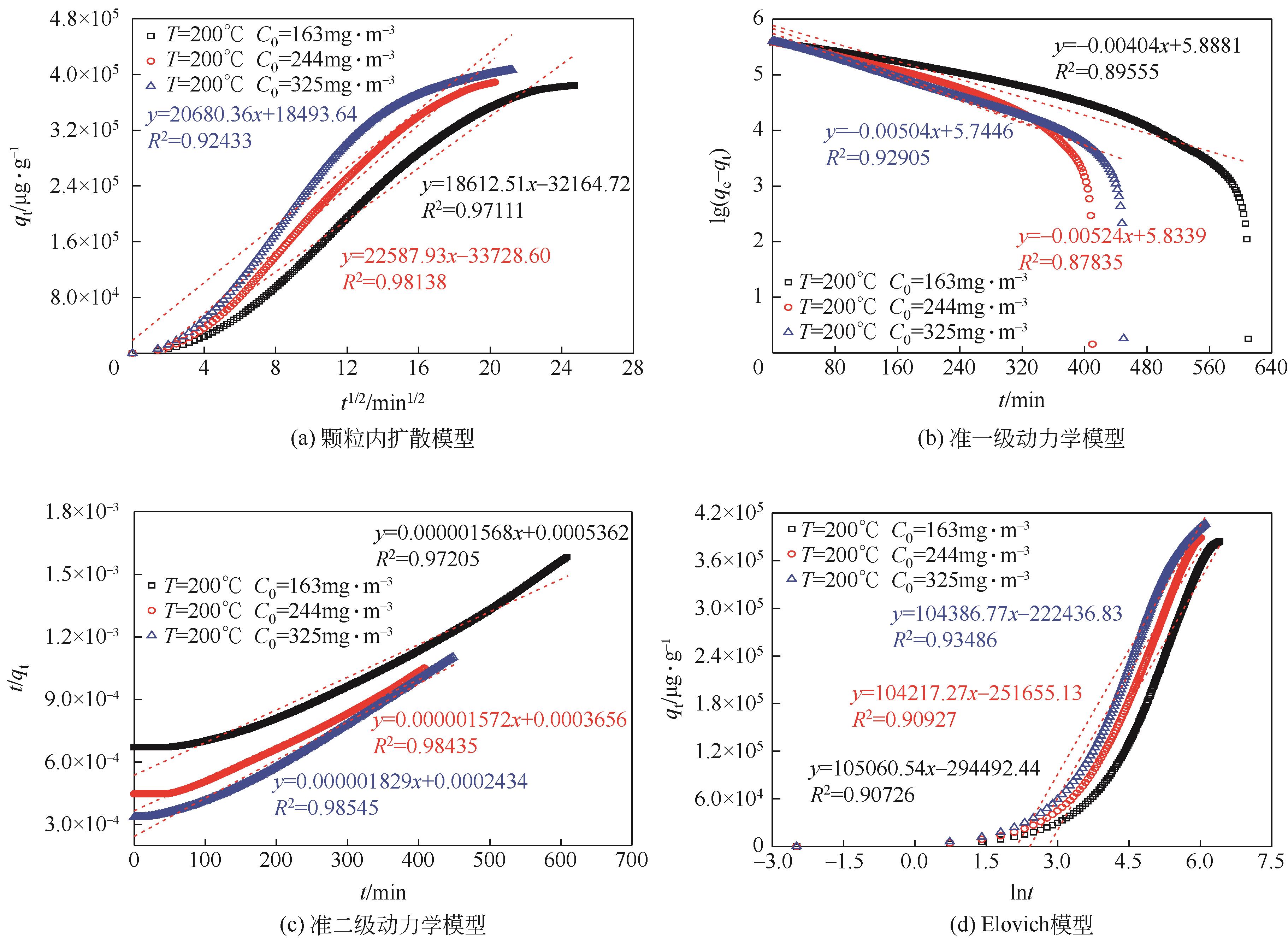
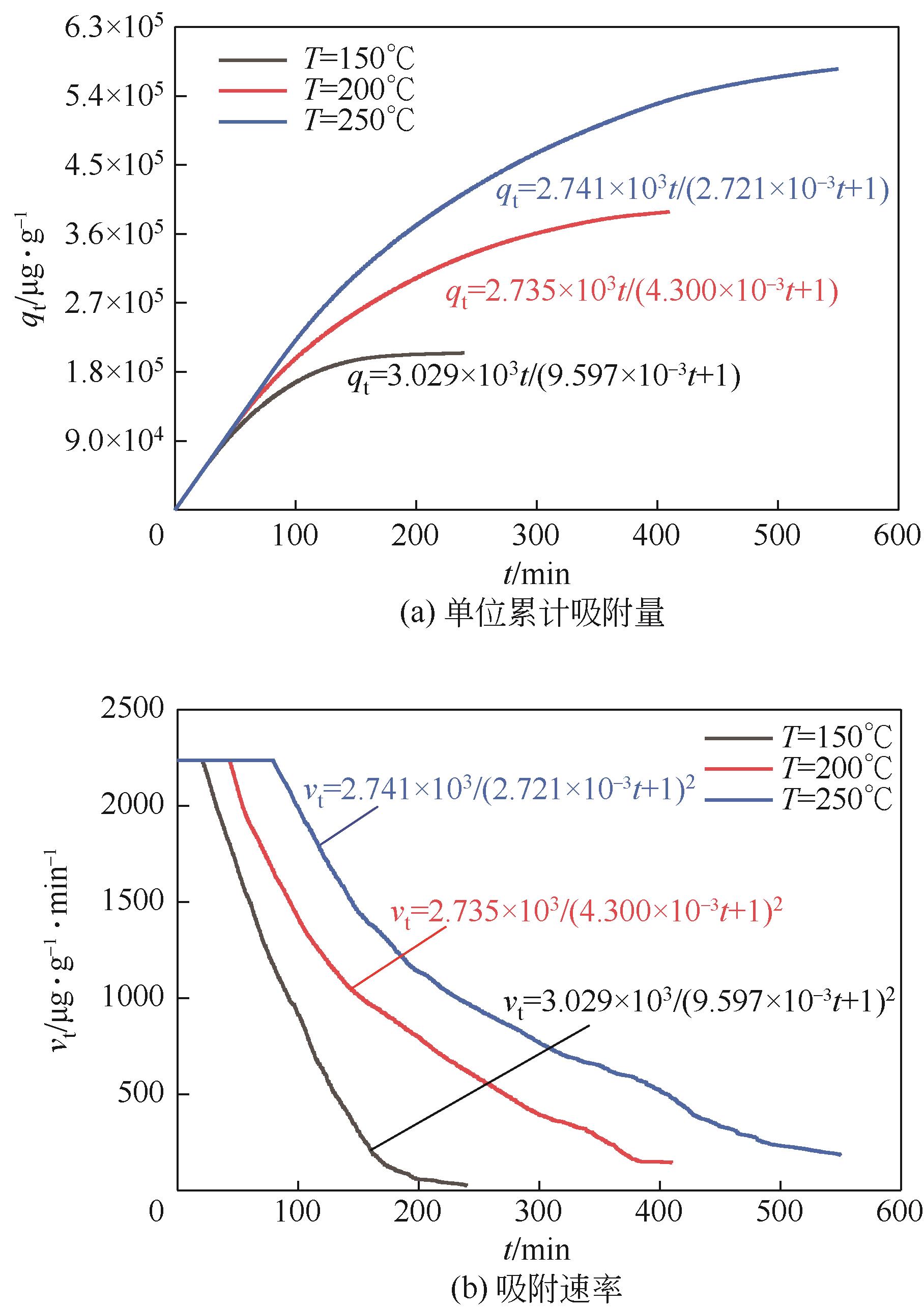
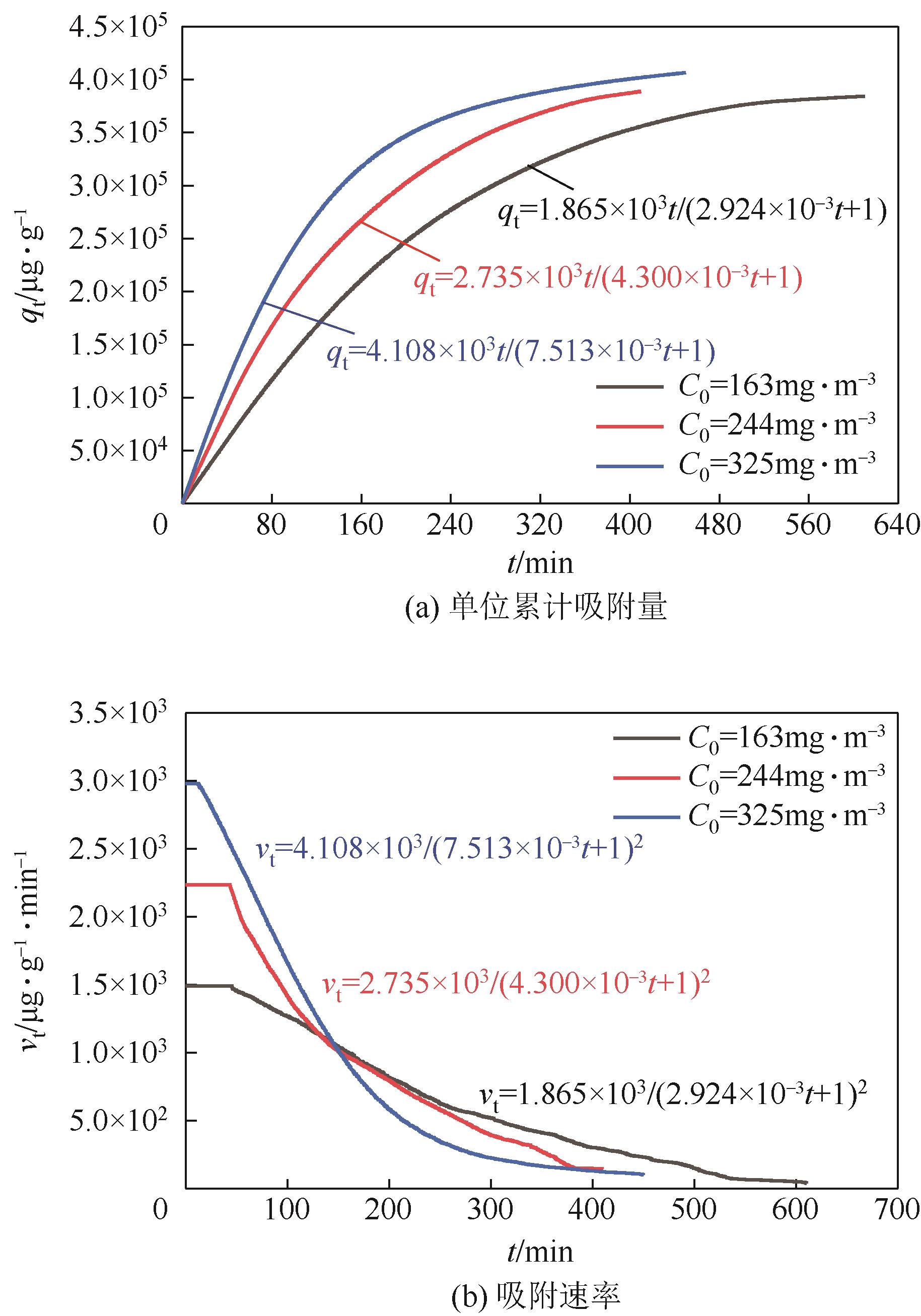
利用乙醇溶液对分析纯氧化钙进行消化改性,制备HCl脱氯剂。通过固定床实验,考察不同反应温度和初始HCl浓度下该脱氯剂对HCl的吸附性能,利用四种反应动力学模型对实验数据进行拟合分析。结果表明:乙醇有机物分子的覆盖和动态沉积过程,使脱氯剂CA-ET-33的表面呈现为多孔隙、大比表面积的结构,同时大大增加了2~10nm孔占比,促进了其对HCl的吸附脱除。表观反应动力学模型对实验结果的拟合效果从优到差依次为:准二级吸附动力学模型、Elovich模型、准一级动力学模型、颗粒内扩散模型。准二级动力学模型可准确描述脱氯剂对HCl的吸附机理,吸附以化学吸附为主。温度升高为化学吸附提供了足够的活化能,HCl浓度的升高增大了从气相到固相表面的传质驱动力。脱氯剂的累计吸附量随温度的升高而增大,达到平衡所消耗的时间随HCl浓度的增加而缩短。确定了一个通用的动力学方程,可用于预测钙基脱氯剂对HCl的吸附过程。
中图分类号:
引用本文
王雨晴, 段钰锋, 王睿, 刘晓硕, 申镇. 乙醇改性钙基脱氯剂实验及动力学分析[J]. 化工进展, 2023, 42(11): 6053-6063.
WANG Yuqing, DUAN Yufeng, WANG Rui, LIU Xiaoshuo, SHEN Zhen. Experimental and kinetics analysis of ethanol-hydrated calcium-based adsorbents[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6053-6063.
| 样品 | 比表面积/m2·g-1 | 微孔容积/ cm3·g-1 | 总孔容积/ cm3·g-1 | 平均孔径/nm |
|---|---|---|---|---|
| CA-O | 10.083 | 1.074×10-3 | 2.090×10-2 | 7.142 |
| CA-ET-33 | 23.376 | 3.353×10-3 | 8.314×10-2 | 13.190 |
表1 脱氯剂改性前后的孔结构参数
| 样品 | 比表面积/m2·g-1 | 微孔容积/ cm3·g-1 | 总孔容积/ cm3·g-1 | 平均孔径/nm |
|---|---|---|---|---|
| CA-O | 10.083 | 1.074×10-3 | 2.090×10-2 | 7.142 |
| CA-ET-33 | 23.376 | 3.353×10-3 | 8.314×10-2 | 13.190 |
| T/℃ | C0 /mg·m-3 | 颗粒内扩散模型 | 准一级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| kid | c | R2 | qe | k1 | R2 | ||
| 150 | 163 | 14062.88 | -19751.55 | 0.96843 | 407511.62 | 0.019944 | 0.89911 |
| 150 | 244 | 15567.01 | -6790.77 | 0.93919 | 436455.53 | 0.028051 | 0.94356 |
| 150 | 325 | 15963.22 | 2641.96 | 0.9453 | 286800.56 | 0.021786 | 0.90545 |
| 200 | 163 | 18612.51 | -32164.72 | 0.97111 | 772876.32 | 0.009304 | 0.89555 |
| 200 | 244 | 22587.93 | -33728.60 | 0.98138 | 682213.01 | 0.012068 | 0.87835 |
| 200 | 325 | 20680.36 | 18493.64 | 0.92433 | 555430.85 | 0.011607 | 0.92905 |
| 250 | 163 | 28047.48 | -105723.07 | 0.98373 | 1085500.43 | 0.0084981 | 0.87935 |
| 250 | 244 | 29312.14 | -63623.26 | 0.98361 | 1003275.02 | 0.0087053 | 0.87738 |
| 250 | 325 | 30364.12 | -32319.20 | 0.97125 | 1005101.68 | 0.0079223 | 0.89946 |
| T/℃ | C0 /mg·m-3 | 准二级动力学模型 | Elovich 模型 | ||||
| qe | k2 | R2 | α | β | R2 | ||
| 150 | 163 | 344734.86 | 1.59×10-8 | 0.95326 | 6591.04 | 1.82×10-5 | 0.90259 |
| 150 | 244 | 315608.75 | 3.04×10-8 | 0.96060 | 9761.29 | 1.77×10-5 | 0.91557 |
| 150 | 325 | 296611.80 | 4.54×10-8 | 0.98650 | 12320.43 | 1.81×10-5 | 0.93628 |
| 200 | 163 | 637893.42 | 4.58×10-9 | 0.97205 | 6369.13 | 9.52×10-6 | 0.90726 |
| 200 | 244 | 636108.04 | 6.76×10-9 | 0.98435 | 9316.26 | 9.60×10-6 | 0.90927 |
| 200 | 325 | 546815.62 | 1.37×10-8 | 0.98545 | 12393.99 | 9.58×10-6 | 0.93486 |
| 250 | 163 | 1348901.45 | 9.53×10-10 | 0.98306 | 7005.67 | 6.63×10-6 | 0.90085 |
| 250 | 244 | 1007604.39 | 2.70×10-9 | 0.97305 | 9713.18 | 6.43×10-6 | 0.90056 |
| 250 | 325 | 978129.03 | 3.74×10-9 | 0.99000 | 11643.79 | 5.85×10-6 | 0.91846 |
表2 四种模型在CA-ET-33上的HCl吸附动力学参数
| T/℃ | C0 /mg·m-3 | 颗粒内扩散模型 | 准一级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| kid | c | R2 | qe | k1 | R2 | ||
| 150 | 163 | 14062.88 | -19751.55 | 0.96843 | 407511.62 | 0.019944 | 0.89911 |
| 150 | 244 | 15567.01 | -6790.77 | 0.93919 | 436455.53 | 0.028051 | 0.94356 |
| 150 | 325 | 15963.22 | 2641.96 | 0.9453 | 286800.56 | 0.021786 | 0.90545 |
| 200 | 163 | 18612.51 | -32164.72 | 0.97111 | 772876.32 | 0.009304 | 0.89555 |
| 200 | 244 | 22587.93 | -33728.60 | 0.98138 | 682213.01 | 0.012068 | 0.87835 |
| 200 | 325 | 20680.36 | 18493.64 | 0.92433 | 555430.85 | 0.011607 | 0.92905 |
| 250 | 163 | 28047.48 | -105723.07 | 0.98373 | 1085500.43 | 0.0084981 | 0.87935 |
| 250 | 244 | 29312.14 | -63623.26 | 0.98361 | 1003275.02 | 0.0087053 | 0.87738 |
| 250 | 325 | 30364.12 | -32319.20 | 0.97125 | 1005101.68 | 0.0079223 | 0.89946 |
| T/℃ | C0 /mg·m-3 | 准二级动力学模型 | Elovich 模型 | ||||
| qe | k2 | R2 | α | β | R2 | ||
| 150 | 163 | 344734.86 | 1.59×10-8 | 0.95326 | 6591.04 | 1.82×10-5 | 0.90259 |
| 150 | 244 | 315608.75 | 3.04×10-8 | 0.96060 | 9761.29 | 1.77×10-5 | 0.91557 |
| 150 | 325 | 296611.80 | 4.54×10-8 | 0.98650 | 12320.43 | 1.81×10-5 | 0.93628 |
| 200 | 163 | 637893.42 | 4.58×10-9 | 0.97205 | 6369.13 | 9.52×10-6 | 0.90726 |
| 200 | 244 | 636108.04 | 6.76×10-9 | 0.98435 | 9316.26 | 9.60×10-6 | 0.90927 |
| 200 | 325 | 546815.62 | 1.37×10-8 | 0.98545 | 12393.99 | 9.58×10-6 | 0.93486 |
| 250 | 163 | 1348901.45 | 9.53×10-10 | 0.98306 | 7005.67 | 6.63×10-6 | 0.90085 |
| 250 | 244 | 1007604.39 | 2.70×10-9 | 0.97305 | 9713.18 | 6.43×10-6 | 0.90056 |
| 250 | 325 | 978129.03 | 3.74×10-9 | 0.99000 | 11643.79 | 5.85×10-6 | 0.91846 |
| 序号 | T/℃ | C0/mg·m-3 | k2(实验值) | k2(计算值) | 误差/% |
|---|---|---|---|---|---|
| 1 | 105 | 146 | 1.898×10-7 | 1.817×10-7 | 4.433 |
| 2 | 140 | 439 | 2.000×10-9 | 1.849×10-9 | 8.164 |
| 3 | 225 | 496 | 2.332×10-9 | 2.164×10-9 | 7.755 |
表3 通用的吸附动力学常数(k2)的验证
| 序号 | T/℃ | C0/mg·m-3 | k2(实验值) | k2(计算值) | 误差/% |
|---|---|---|---|---|---|
| 1 | 105 | 146 | 1.898×10-7 | 1.817×10-7 | 4.433 |
| 2 | 140 | 439 | 2.000×10-9 | 1.849×10-9 | 8.164 |
| 3 | 225 | 496 | 2.332×10-9 | 2.164×10-9 | 7.755 |
| 1 | 周吉光, 张举钢, 丁欣, 等. 油气资源供给能力约束下未来中国煤炭资源开采总量控制指标测度[J]. 河北地质大学学报, 2020, 43(6): 101-112. |
| ZHOU Jiguang, ZHANG Jugang, DING Xin, et al. Measurement of the total control amount of China’s coal resource exploitation in the future under the constraints of oil and gas resource supply capacity[J]. Journal of Hebei GEO University, 2020, 43(6): 101-112. | |
| 2 | 张天诏. 浅谈我国能源利用现状及发展趋势[J]. 国土与自然资源研究, 2021(5): 76-78. |
| ZHANG Tianzhao. Discussion on the current situation and development trend of energy utilization in China[J]. Territory & Natural Resources Study, 2021(5): 76-78. | |
| 3 | ZHANG Lei, WANG Shuxiao, MENG Yang, et al. Influence of mercury and chlorine content of coal on mercury emissions from coal-fired power plants in China[J]. Environmental Science & Technology, 2012, 46(11): 6385-6392. |
| 4 | ROWE Christopher L, HOPKINS William A, CONGDON Justin D. Ecotoxicological implications of aquatic disposal of coal combustion residues in the United States: A review[J]. Environmental Monitoring and Assessment, 2002, 80(3): 207-276. |
| 5 | TSUBOUCHI Naoto, MOCHIZUKI Yuuki, WANG Yanhui, et al. Fate of the chlorine in coal in the heating process[J]. ISIJ International, 2018, 58(2): 227-235. |
| 6 | 笪春年, 汪海, 徐波, 等. 燃煤电厂煤中氯的迁移和释放特征[J]. 环境化学, 2020, 39(10): 2833-2839. |
| Chunnian DA, WANG Hai, XU Bo, et al. The migration and release characteristics of chlorine during coal combustion in coal-fired power plants[J]. Environmental Chemistry, 2020, 39(10): 2833-2839. | |
| 7 | WANG Guangmeng, LUO Zixue, ZHANG Junying, et al. Modes of occurrence of fluorine by extraction and SEM method in a coal-fired power plant from Inner Mongolia, China[J]. Minerals, 2015, 5(4): 863-869. |
| 8 | VASSILEV S V, ESKENAZY G M, VASSILEVA C G. Contents, modes of occurrence and behaviour of chlorine and bromine in combustion wastes from coal-fired power stations[J]. Fuel, 2000, 79(8): 923-938. |
| 9 | 蒋旭光, 李琦, 李香排, 等. 燃煤过程中钙基及镁基吸收剂对HCl吸收作用的试验研究[J]. 煤炭学报, 2003, 28(6): 626-630. |
| JIANG Xuguang, LI Qi, LI Xiangpai, et al. Chloride of emission control by calcium-based and magnesium-based sorbents during coal combustion[J]. Journal of China Coal Society, 2003, 28(6): 626-630. | |
| 10 | 李水清, 池涌, 李威武, 等. 固定床PVC燃烧脱氯的机理和试验[J]. 环境科学, 2001, 22(2): 95-100. |
| LI Shuiqing, CHI Yong, LI Weiwu, et al. Experimental and mechanism analyses on HCl emission control during PVC combustion in fixed beds[J]. Chinese Journal of Enviromental Science, 2001, 22(2): 95-100. | |
| 11 | 卿山, 王华, 何屏, 等. 医疗废物焚烧过程中脱氯机理和试验[J]. 环境工程, 2007, 25(3): 66-70, 5. |
| QING Shan, WANG Hua, HE Bing|Ping), et al. Dechloridization mechanism and experiment in incineration process of medical wastes[J]. Environmental Engineering, 2007, 25(3): 66-70, 5. | |
| 12 | CAO Jun, ZHONG Wenqi, JIN Baosheng, et al. Treatment of hydrochloric acid in flue gas from municipal solid waste incineration with Ca-Mg-Al mixed oxides at medium-high temperatures[J]. Energy & Fuels, 2014, 28(6): 4112-4117. |
| 13 | Gargiulo Nicola, Peluso Antonio, Aprea Paolo, et al. Use of a metal organic framework for the adsorptive removal of gaseous HCl: A new approach for a challenging task[J]. ACS Applied Materials & Interfaces, 2018, 10(17): 14271-14275. |
| 14 | 张衍国, 贾金岩, 李清海, 等. 微波活化对Ca(OH)2孔隙结构及脱氯性能的影响[J]. 环境工程学报, 2007, 1(10): 136-140. |
| ZHANG Yanguo, JIA Jinyan, LI Qinghai, et al. Influence of microwave activation on pore structure and dechlorination porformance of Ca(OH)2 [J]. Chinese Journal of Environmental Engineering, 2007, 1(10): 136-140. | |
| 15 | 林瑜, 陈德珍, 魏魏芃, 等. 改性CaCO3代替Ca(OH)2高温净化烟气中HCl气体的研究[J]. 同济大学学报(自然科学版), 2006, 34(10): 1389-1393. |
| LIN Yu, CHEN Dezhen, WEI Peng, et al. Investigation of gaseous HCl high temperature scrubbing from incineration flue gas with modified CaCO3 alternative to Ca(OH)2 [J]. Journal of Tongji University (Natural Science), 2006, 34(10): 1389-1393. | |
| 16 | 陈德珍, 张鹤声. 中低温下钠碱对氢氧化钙干式吸收HCl气体的改良[J]. 工程热物理学报, 1999, 20(4): 520-524. |
| CHEN Dezhen, ZHANG Hesheng. Modification of calcium hydroxides with sodium alkali for enhancing hcl removxl under moderate temperatures[J]. Journal of Engineering Thermophysics, 1999, 20(4): 520-524. | |
| 17 | 陈德珍, 张鹤声, 何于涛, 等. 改性消石灰吸收剂用于干法脱除HCl的研究[J]. 工程热物理学报, 2000, 21(3): 388-392. |
| CHEN Dezhen, ZHANG Hesheng, HE Yutao, et al. Research of modified limes reacting with gaseous HCl[J]. Journal of Engineering Thermophysics, 2000, 21(3): 388-392. | |
| 18 | 黄建平, 陈广伟, 秦大川. 垃圾电站HCl气体高温脱除实验研究[J]. 热力发电, 2014, 43(10): 85-89, 99. |
| HUANG Jianping, CHEN Guangwei, QIN Dachuan. Experimental study on HCl removal from waste incineration stations at high temperatures[J]. Thermal Power Generation, 2014, 43(10): 85-89, 99. | |
| 19 | 万旦. 高温下氧化钙脱除氯化氢研究[D]. 武汉: 华中科技大学, 2013. |
| WAN Dan. Study of HCI absorption by CaO at high temperature[D]. Wuhan: Huazhong University of Science and Technology, 2013. | |
| 20 | MURA G, LALLAI A. On the kinetics of dry reaction between calcium oxide and gas hydrochloric acid[J]. Chemical Engineering Science, 1992, 47(9/10/11): 2407-2411. |
| 21 | WEINELL Claus E, JENSEN Peter I, Kim DAM-JOHANSEN, et al. Hydrogen chloride reaction with lime and limestone: Kinetics and sorption capacity[J]. Industrial & Engineering Chemistry Research, 1992, 31(1): 164-171. |
| 22 | GULLETT Brian K, JOZEWICZ Wojciech, STEFANSKI Leonard A. Reaction kinetics of calcium-based sorbents with hydrogen chloride[J]. Industrial & Engineering Chemistry Research, 1992, 31(11): 2437-2446. |
| 23 | WANG Wuyin, YE Zhicheng, BJERLE Ingemar. The kinetics of the reaction of hydrogen chloride with fresh and spent Ca-based desulfurization sorbents[J]. Fuel, 1996, 75(2): 207-212. |
| 24 | 李晓航, 滕阳, 彭皓, 等. CuCl2改性飞灰吸附气相零价汞[J]. 化工环保, 2020, 40(3): 271-278. |
| LI Xiaohang, TENG Yang, PENG Hao, et al. Adsorption of gaseous elemental mercury on CuCl2-modified fly ash[J]. Environmental Protection of Chemical Industry, 2020, 40(3): 271-278. | |
| 25 | 何佳豪, 崔梦祺, 季雷, 等. SO2在成型活性炭表面吸附脱附性能实验研究[J]. 江苏科技大学学报(自然科学版), 2021, 35(1): 51-57. |
| HE Jiahao, CUI Mengqi, JI Lei, et al. Experimental investigation of adsorption and desorption of SO2 over molded activated carbon surface[J]. Journal of Jiangsu University of Science and Technology (Natural Science Edition), 2021, 35(1): 51-57. | |
| 26 | LAFI Ridha, CHARRADI Khaled, DJEBBI Mohamed Amine, et al. Adsorption study of Congo red dye from aqueous solution to Mg-Al-layered double hydroxide[J]. Advanced Powder Technology, 2016, 27(1): 232-237. |
| 27 | 曹俊. 城市生活垃圾焚烧烟气的中高温脱氯研究[D]. 南京: 东南大学, 2016. |
| CAO Jun. Study on removal of HCl in municipal solid waste incineration flue gas at medium-high temperature[D]. Nanjing: Southeast University, 2016. | |
| 28 | ZHONG Longchun, LI Wenhan, ZHANG Yongsheng, et al. Kinetic studies of mercury adsorption in activated carbon modified by iodine steam vapor deposition method[J]. Fuel, 2017, 188: 343-351. |
| 29 | 杜迎春. 数值法解催化剂粒内模型及内扩散有效因子[J]. 计算机与应用化学, 2000, 17(3): 243-246. |
| DU Yingchun. Numerical solution for the internal reaction-diffusion equations and effectiveness factor[J]. Computers and Applied Chemistry, 2000, 17(3): 243-246. | |
| 30 | 钟隆春. 沥青基活性炭汞吸附剂的开发与机理研究[D]. 北京: 华北电力大学(北京), 2018. |
| ZHONG Longchun. Development and mechanism study of mercury removal with activated carbon adsorbent from asphalt[D]. Beijing: North China Electric Power University(Beijing), 2018. | |
| 31 | PÉREZ-MARÍN A B, Meseguer ZAPATA V, ORTUÑO J F, et al. Removal of cadmium from aqueous solutions by adsorption onto orange waste[J]. Journal of Hazardous Materials, 2007, 139(1): 122-131. |
| 32 | POLYZOPOULOS N A, KERAMIDAS V Z, PAVLATOU ATHENA. On the limitations of the simplified Elovich equation in describing the kinetics of phosphate sorption and release from soils[J]. Journal of Soil Science, 1986, 37(1): 81-87. |
| 33 | 郝志飞, 张印民, 张永锋, 等. 湿法改性制备高比表面积氢氧化钙及表征[J]. 无机盐工业, 2015, 47(12): 19-21. |
| HAO Zhifei, ZHANG Yinmin, ZHANG Yongfeng, et al. Wet modified preparation and characterization of calcium hydroxide with high specific surface area[J]. Inorganic Chemicals Industry, 2015, 47(12): 19-21. | |
| 34 | 沈辉. 氧化钙基吸附剂的制备及其CO2吸附性能研究[D]. 天津: 天津大学, 2013. |
| SHEN Hui. Reserch on the preparation and CO2 capture performance of CaO based adsorbents[D]. Tianjin: Tianjin University, 2013. | |
| 35 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional)[J]. Pure and Applied Chemistry, 1982, 54(11): 2201-2218. |
| 36 | SKODRAS G, DIAMANTOPOULOU Ir, ZABANIOTOU A, et al. Enhanced mercury adsorption in activated carbons from biomass materials and waste tires[J]. Fuel Processing Technology, 2007, 88(8): 749-758. |
| 37 | 李锐, 夏明桂, 邓国伟, 等. 共沉淀法制备钙基脱氯剂及其脱氯性能研究[J]. 武汉纺织大学学报, 2017, 30(6): 59-65. |
| LI Rui, XIA Minggui, DENG Guowei, et al. Preparation and performance of Ca-baseddechlorination adsorbent by co-precipition process[J]. Journal of Wuhan Textile University, 2017, 30(6): 59-65. | |
| 38 | 迟莹. 养生时间对钙基脱氯剂孔隙结构及脱氯性能的影响[J]. 辽宁化工, 2021, 50(11): 1635-1637, 1641. |
| CHI Ying. Effect of curing time on pore structure and dechlorination performance of calcium-based dechlorinating agent[J]. Liaoning Chemical Industry, 2021, 50(11): 1635-1637, 1641. | |
| 39 | 张波, 胡宾生, 贵永亮, 等. 孔隙结构对高炉炉顶煤气脱氯剂性能的影响[J]. 现代化工, 2016, 36(3): 137-140. |
| ZHANG Bo, HU Binsheng, GUI Yongliang, et al. Effect of pore structure on the performance of blast furnace top gas dechlorination agent[J]. Modern Chemical Industry, 2016, 36(3): 137-140. | |
| 40 | 黄霄. 文丘里式循环流化床生石灰干法消化装置研发及数值模拟[D]. 南京: 南京师范大学, 2020. |
| HUANG Xiao. Development and numerical simulation of venturi circulating fluidized bed quicklime dry digestion device[D]. Nanjing: Nanjing Normal University, 2020. | |
| 41 | 李慧芝. 氢氧化钙的表面改性及其应用研究[D]. 广州: 广东工业大学, 2008. |
| LI Huizhi. Research on superficial modification of calcium hydroxide and its application[D]. Guangzhou: Guangdong University of Technology, 2008. | |
| 42 | KUNDU Sanghamitra, GUPTA A K. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization[J]. Chemical Engineering Journal, 2006, 122(1/2): 93-106. |
| 43 | SKODRAS G, DIAMANTOPOULOU Ir, PANTOLEONTOS G, et al. Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor[J]. Journal of Hazardous Materials, 2008, 158(1): 1-13. |
| 44 | Rodrigo SERNA-GUERRERO, SAYARI Abdelhamid. Modeling adsorption of CO2 on amine-functionalized mesoporous silica. 2: Kinetics and breakthrough curves[J]. Chemical Engineering Journal, 2010, 161(1/2): 182-190. |
| 45 | 王恺. 垃圾焚烧中高温烟气脱除氯化氢的研究[D]. 南京: 东南大学, 2014. |
| WANG Kai. Study on hydrogen chloride removal from high temperature flue gas in garbage incineration[D]. Nanjing: Southeast University, 2014. | |
| 46 | 胡俊鹏. 氧化钙高温脱除生活垃圾热处理过程中含氯酸性气体的实验研究[D]. 杭州: 浙江大学, 2021. |
| HU Junpeng. Experimental research on the removal of chlorine-containing acid gas from municipal solid waste thermal treatment by calcium oxide at high temperature[D]. Hangzhou: Zhejiang University, 2021. | |
| 47 | 王统伟. 改性类水滑石吸附剂的制备及脱除氯化氢性能研究[D]. 南京: 东南大学, 2021. |
| WANG Tongwei. Research on preparation and hcl removal performance of modified hydrotalcite-like adsorbent[D]. Nanjing: Southeast University, 2021. | |
| 48 | 豆斌林, 高晋生, 鲁军, 等. 高温煤气中HCl气体与脱氯剂的反应动力学[J]. 华东理工大学学报, 2000, 26(4): 372-375. |
| DOU Binlin, GAO Jinsheng, LU Jun, et al. Reaction kinetics of sorbent for chloride removal with HCl in a high-temperature coal gas[J]. Journal of East China University of Science and Technology, 2000, 26(4): 372-375. | |
| 49 | 邱华禹, 陈冬梅, 何智, 等. 基于钙基吸收剂的旋风烟道内垃圾焚烧烟气脱氯特性研究[J]. 工程热物理学报, 2022, 43(3): 712-719. |
| QIU Huayu, CHEN Dongmei, HE Zhi, et al. Study on dechlorination characteristics of waste incineration flue gas in cyclone flue based on Ca-based sorbents[J]. Journal of Engineering Thermophysics, 2022, 43(3): 712-719. |
| [1] | 况文娟,考宏涛,任 斌,郭 涛,李爱莉. 钙基吸收剂循环吸收CO2技术的研究进展 [J]. 化工进展, 2011, 30(6): 1356-. |
| [2] | 花 磊,杨福胜,孟翔宇,鲍泽威,张早校. 金属氢化物氢化/脱氢反应动力学模型研究进展 [J]. 化工进展, 2010, 29(3): 413-. |
| [3] | 黄莉莉;何小荣;邱 彤;陈丙珍;李吉春;田 亮;王 燕. 拔头油热裂解制乙烯反应动力学模型及模拟 [J]. 化工进展, 2007, 26(1): 105-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||