| 1 |
王君妍,白云,马国强,等. 重质油-固体系分离与资源化回收研究进展[J]. 化工进展, 2019, 38(1): 649-663.
|
|
WAN Junyan, BAI Yun, MA Guoqiang, et al. Recent advances in separation and recovery of oil from heavy oil-solid systems[J].Chemical Industry and Engineering Progress, 2019, 38(1): 649-663.
|
| 2 |
SANTOS R G, LOH W, BANNWART A C, et al. An overview of heavy oil properties and its recovery and transportation methods[J]. Brazilian Journal of Chemical Engineering, 2014, 31(3): 571-590.
|
| 3 |
HEATH G A, MASNADI M S, EL-HOUJEIRI H M, et al. Global carbon intensity of crude oil production[J]. Science, 2018, 361(6405): 851-853.
|
| 4 |
MASLIYAH J, ZHOU Z J, XU Z H, et al. Understanding water-based bitumen extraction from athabasca oil sands[J]. The Canadian Journal of Chemical Engineering, 2004, 82(4): 628-654.
|
| 5 |
LIN F, STOYANOV S R, XU Y M. Recent advances in nonaqueous extraction of bitumen from mineable oil sands: a review[J]. Organic Process Research & Development, 2017, 21(4): 492-510.
|
| 6 |
LI X G, YANG Z Q, SUI H, et al. A hybrid process for oil-solid separation by a novel multifunctional switchable solvent[J]. Fuel, 2018, 221: 303-310.
|
| 7 |
SUI H, MA G Q, HE L, et al. Recovery of heavy hydrocarbons from Indonesian carbonate asphalt rocks (I): Solvent extraction, particle sedimentation, and solvent recycling[J]. Energy & Fuels, 2016, 30(11): 9242-9249.
|
| 8 |
GONG Z Q, DU A X, WANG Z B, et al. Analysis on integrated thermal treatment of oil sludge by Aspen Plus[J]. Waste Manage, 2019, 87: 512-524.
|
| 9 |
MA Y, LI S Y. The pyrolysis, extraction and kinetics of Buton oil sand bitumen[J]. Fuel Processing Technology, 2012, 100: 11-15.
|
| 10 |
DING M S, ZHANG Y, LIU J, et al. Application of microbial enhanced oil recovery technology in water-based bitumen extraction from weathered oil sands[J]. American Institute of Chemical Engineers, 2014, 60(8): 2985-2993.
|
| 11 |
HE L, LIN F, LI X G, et al. Interfacial sciences in unconventional petroleum production: from fundamentals to applications[J]. Chemical Society Reviews, 2015, 44(15): 5446-5494.
|
| 12 |
LI X G, HE L, WU G Z, et al. Operational parameters, evaluation methods, and fundamental mechanisms: aspects of nonaqueous extraction of bitumen from oil sands[J]. Energy & Fuels, 2012, 26(6): 3553-3563.
|
| 13 |
NIKAKHTARI H, PAL K, WOLF S, et al. Solvent removal from cyclohexane-extracted oil sands gangue[J]. The Canadian Journal of Chemical Engineering, 2016, 94(3): 408-414.
|
| 14 |
PANDA S, PAL K, MERZARA S, et al. Transport and removal of a solvent in porous media in the presence of bitumen, a highly viscous solute[J]. Chemical Engineering Science, 2017, 165: 229-239.
|
| 15 |
RENAUD R, PAL K, WEIß T, et al. Vacuum drying of cyclohexane from solvent-extracted oil sands gangue[J]. The Canadian Journal of Chemical Engineering, 2017, 95(3): 459-466.
|
| 16 |
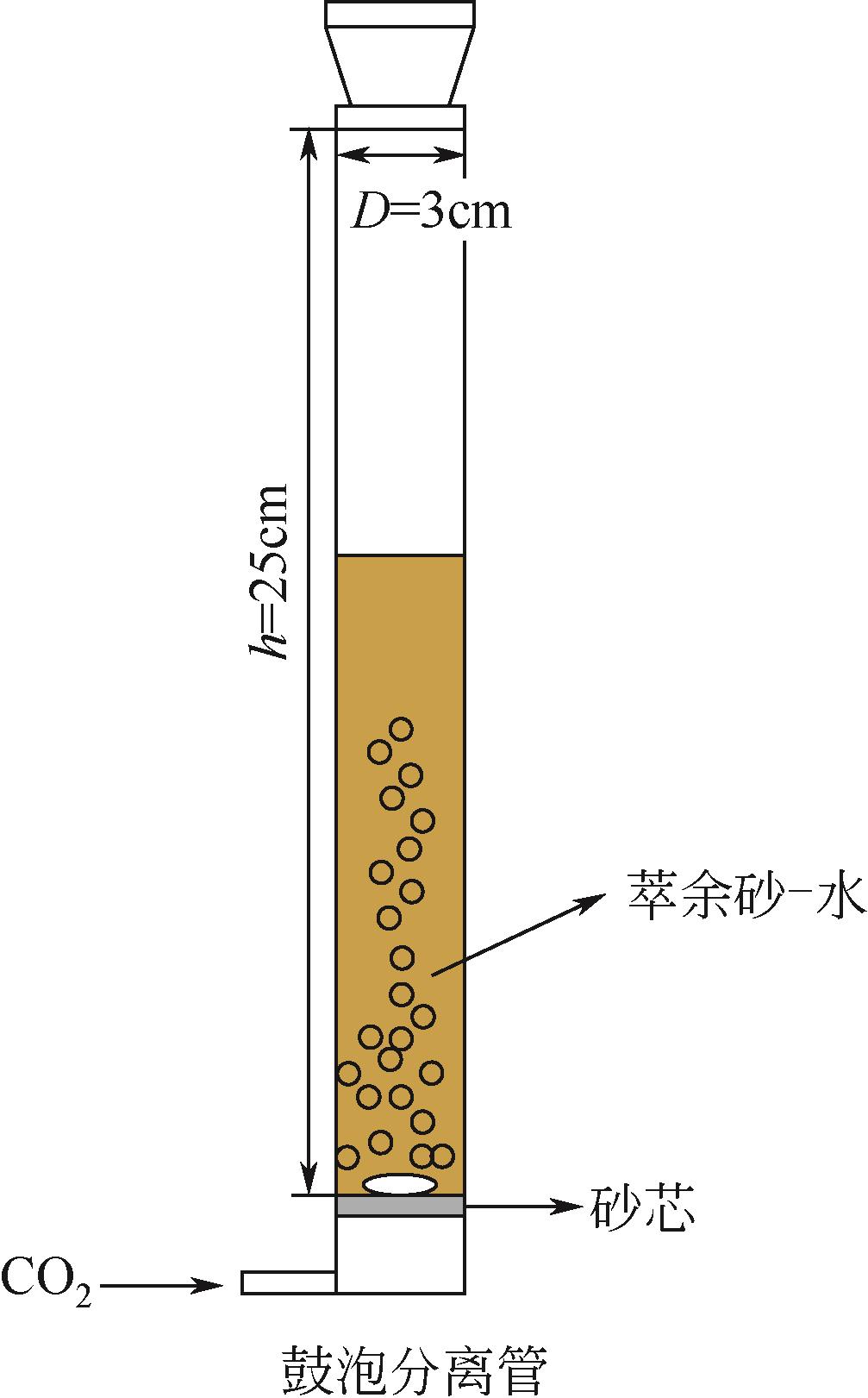
WANG J Y, LI X G, MA G Q, et al. Removal of residual solvent from solvent-extracted unconventional oil ores gangue by gas bubbling[J]. Separation and Purification Technology, 2021, 254: 117551.
|
| 17 |
NAJAFI A S, DRELICH J, YEUNG A, et al. A novel method of measuring electrophoretic mobility of gas bubbles[J]. Journal of Colloid and Interface Science, 2007, 308(2): 344-350.
|
| 18 |
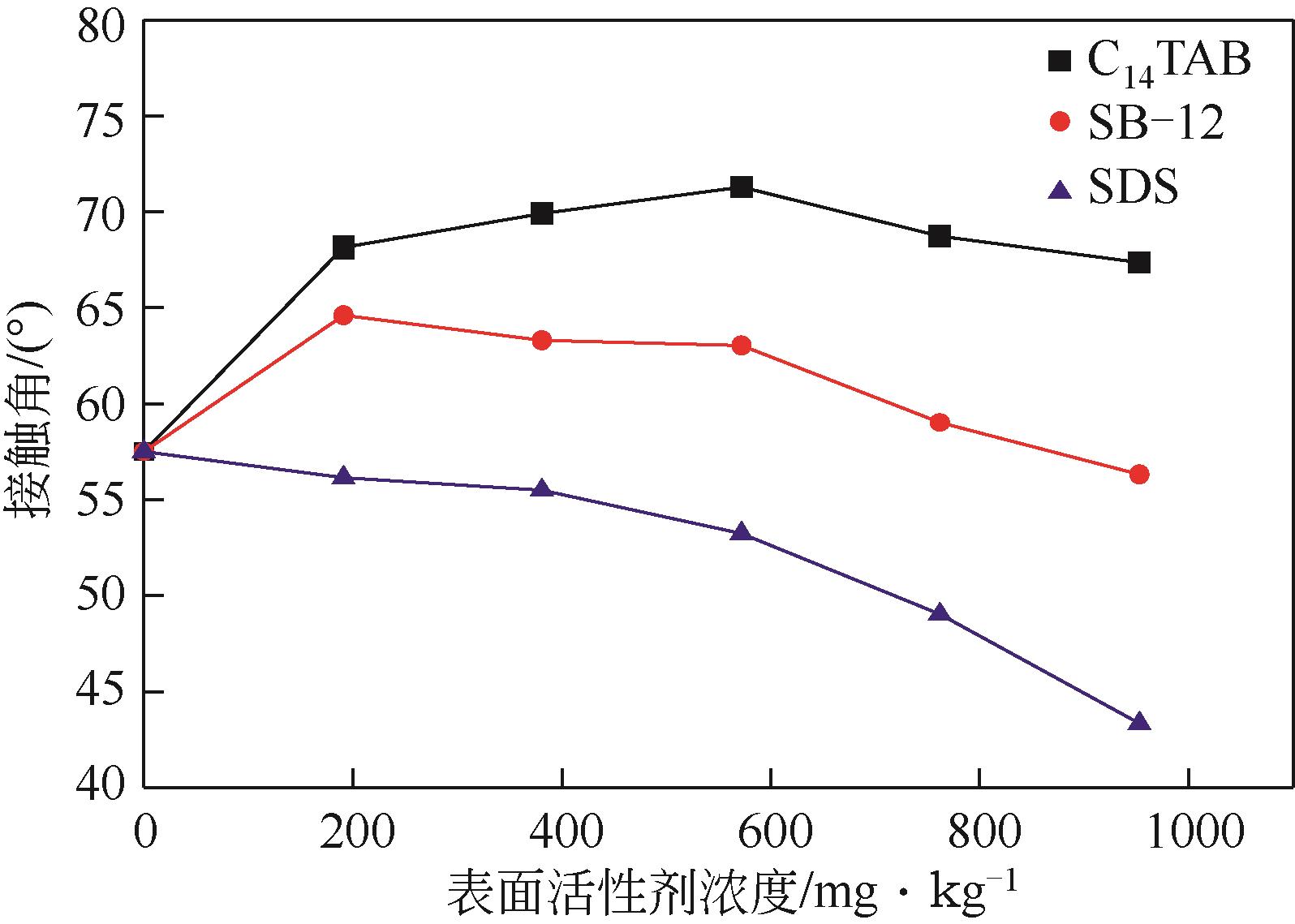
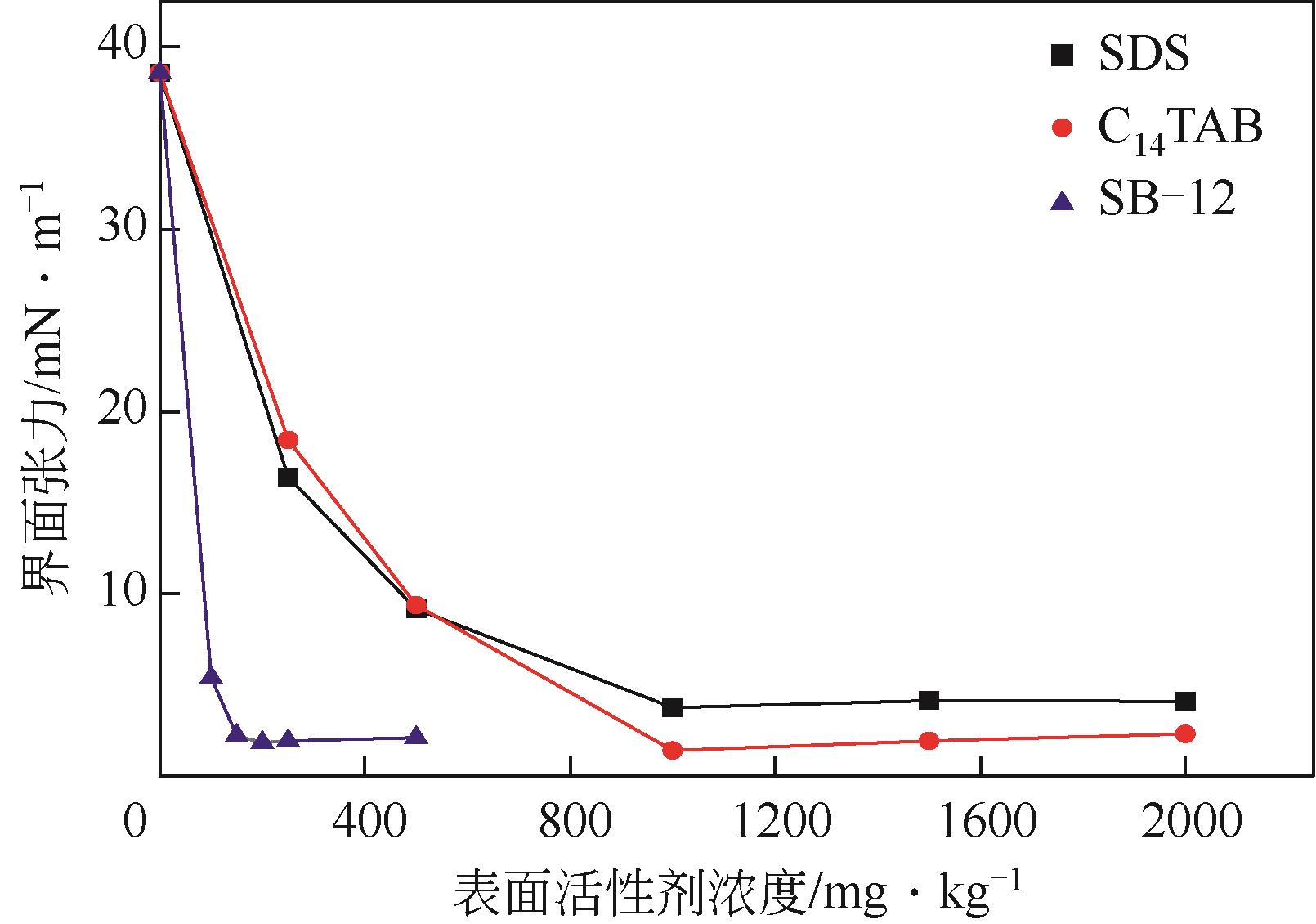
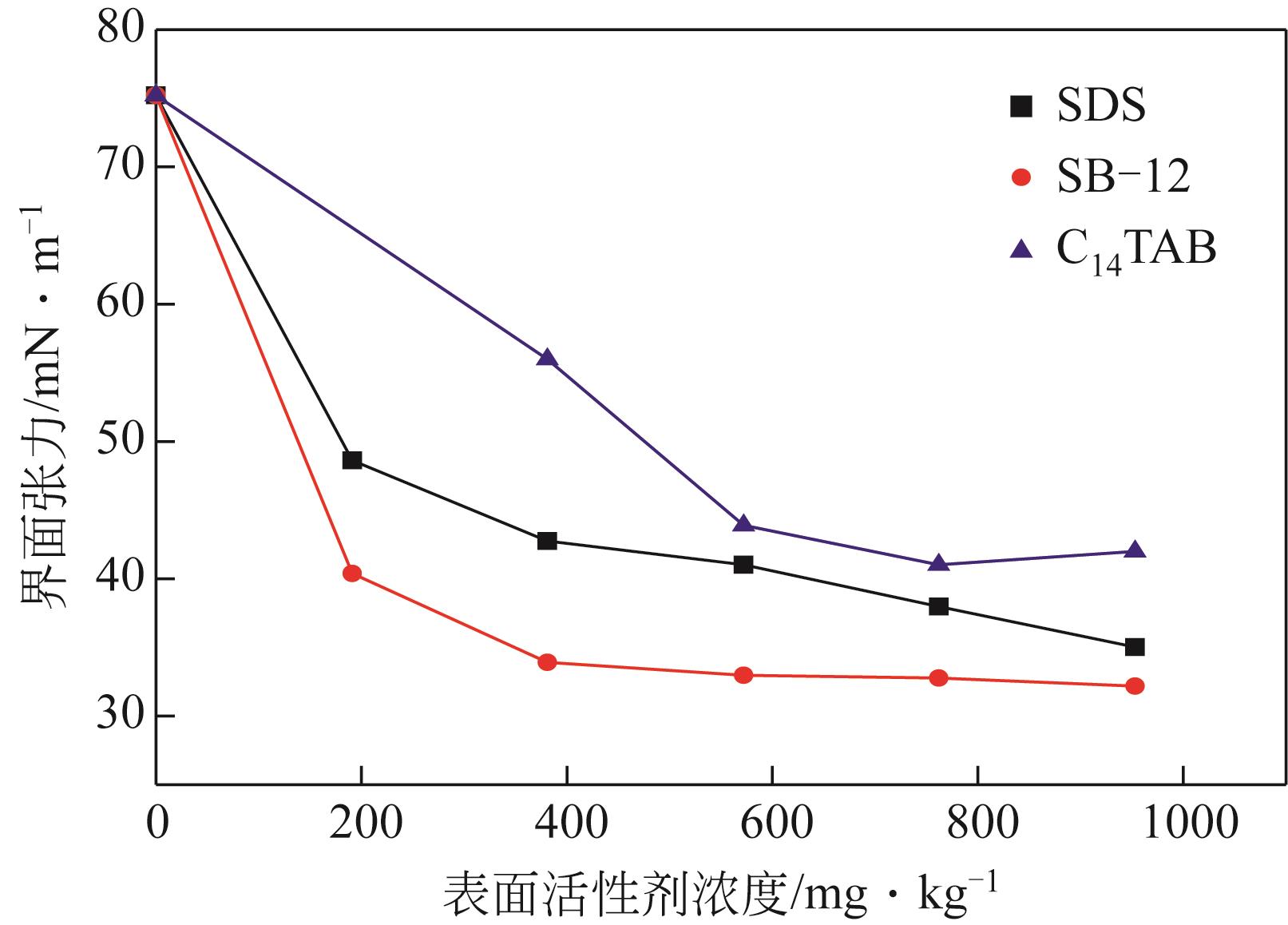
蒋平, 张贵才, 葛际江, 等. 表面活性剂剥离固体表面原油机理[J]. 石油学报(石油加工), 2008, 24(2): 222-226.
|
|
JIANG Ping, ZHANG Guicai, GE Jijiang, et al. The mechanism of oil displacement from solid surface by surfactant[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2008, 24(2): 222-226
|
| 19 |
HARKINS W D, Films FELDMAN A.. the spreading of liquids and the spreading coefficient[J]. Journal of the American Chemical Society, 1922, 44(12): 2665-2685.
|
| 20 |
SCHRADER M E. Young-dupre revisited[J]. Langmuir, 1995, 11(9): 3585-3589.
|
| 21 |
HUANG S-D, VALSARAJ K T, Wilson D J. Removal of refractory organics by aeration ( Ⅴ ): Solvent sublation of naphthalene and phenanthrene[J]. Separation Science and Technology, 1983, 18(10): 941-968.
|
 ), 王君妍1,2, 何林1,2,3(
), 王君妍1,2, 何林1,2,3( ), 隋红1,2, 李鑫钢1,2,3
), 隋红1,2, 李鑫钢1,2,3
 ), WANG Junyan1,2, HE Lin1,2,3(
), WANG Junyan1,2, HE Lin1,2,3( ), SUI Hong1,2, LI Xingang1,2,3
), SUI Hong1,2, LI Xingang1,2,3