化工进展 ›› 2024, Vol. 43 ›› Issue (3): 1637-1647.DOI: 10.16085/j.issn.1000-6613.2023-1600
• 资源与环境化工 • 上一篇
γ-Al2O3/CuO-ACF电吸附除盐的影响因素及反应动力学
柴多生1( ), 高峰2, 吴友兵3, 孙昕1(
), 高峰2, 吴友兵3, 孙昕1( ), 郝然1, 杨宇2, 焦翔飞1
), 郝然1, 杨宇2, 焦翔飞1
- 1.西安建筑科技大学环境与市政工程学院,陕西 西安 710055
2.内蒙古自治区水利科学研究院,内蒙古 呼和浩特 010011
3.马鞍山市城乡规划设计院有限责任公司,安徽 马鞍山 243011
-
收稿日期:2023-09-11修回日期:2023-11-20出版日期:2024-03-10发布日期:2024-04-11 -
通讯作者:孙昕 -
作者简介:柴多生(1995—),男,硕士研究生,主要研究方向为微咸水淡化。E-mail:cds1712@126.com。 -
基金资助:内蒙古自治区水利科技项目(NSK202002);陕西省自然科学基金重点项目(2023-JC-ZD-28);陕西省重点研发计划(2023GXLH-056)
Reaction dynamics and influencing factors of capacitive deionization desalination using γ-Al2O3 / CuO-ACF
CHAI Duosheng1( ), GAO Feng2, WU Youbing3, SUN Xin1(
), GAO Feng2, WU Youbing3, SUN Xin1( ), HAO Ran1, YANG Yu2, JIAO Xiangfei1
), HAO Ran1, YANG Yu2, JIAO Xiangfei1
- 1.School of Environmental and Municipal Engineering, Xi’an University of Architecture and Technology, Xi’an 710055,Shaanxi, China
2.Inner Mongolia Hydraulic Research Institute, Hohhot 010011, Inner Mongolia, China
3.Urban & Rural Planning & Design Institute of Ma’anshan, Ma’anshan 243011, Anhui, China
-
Received:2023-09-11Revised:2023-11-20Online:2024-03-10Published:2024-04-11 -
Contact:SUN Xin
摘要:
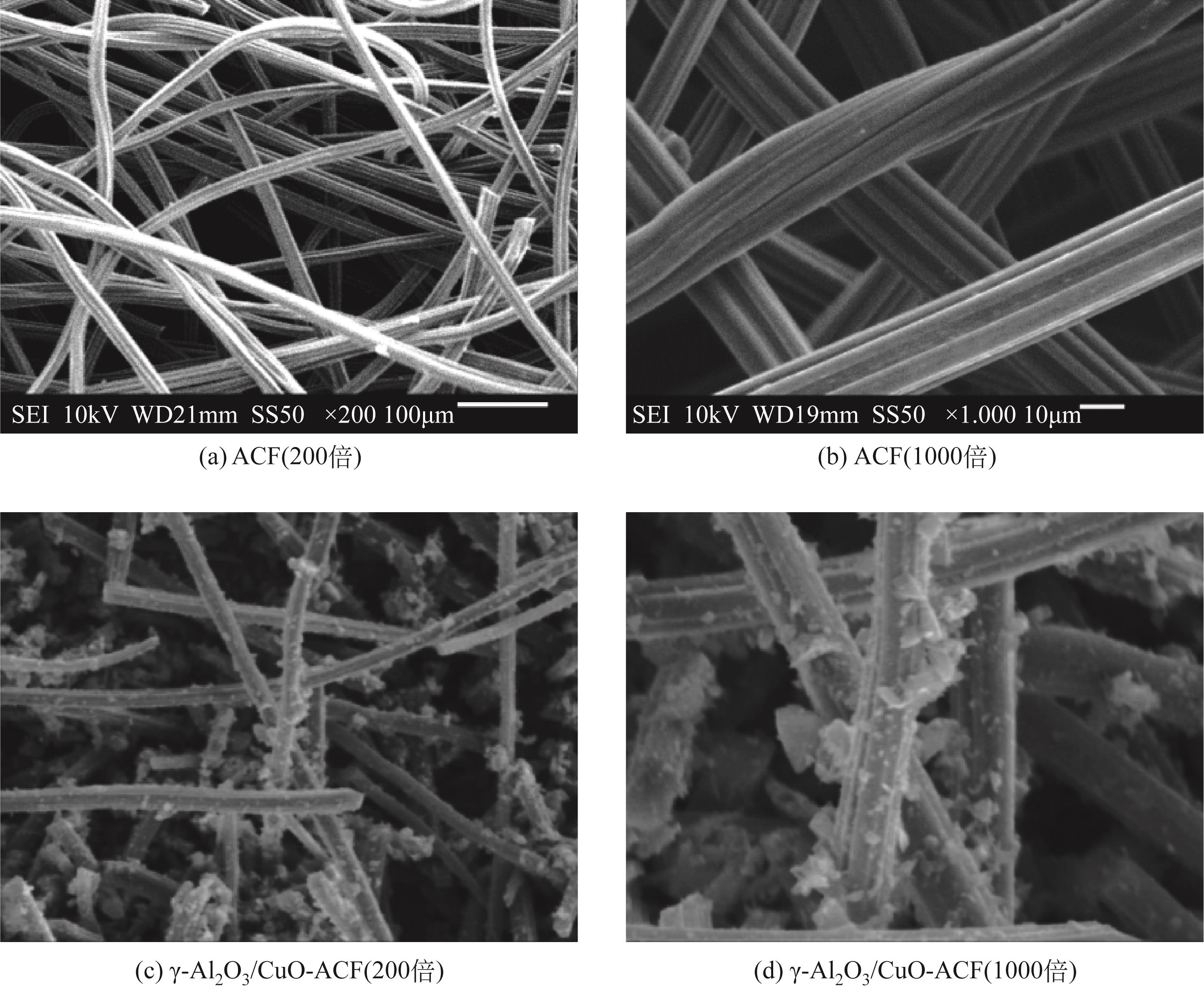
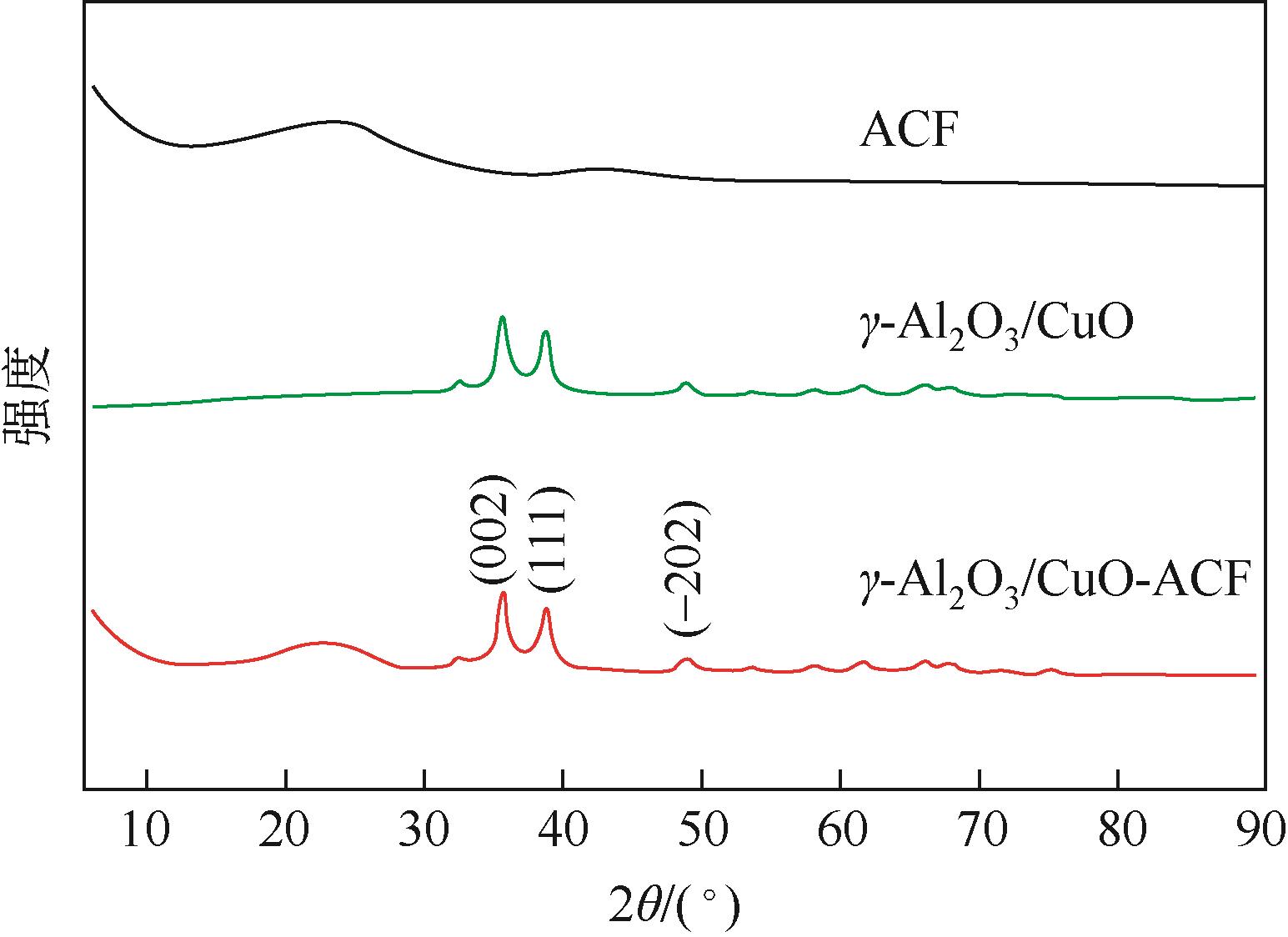
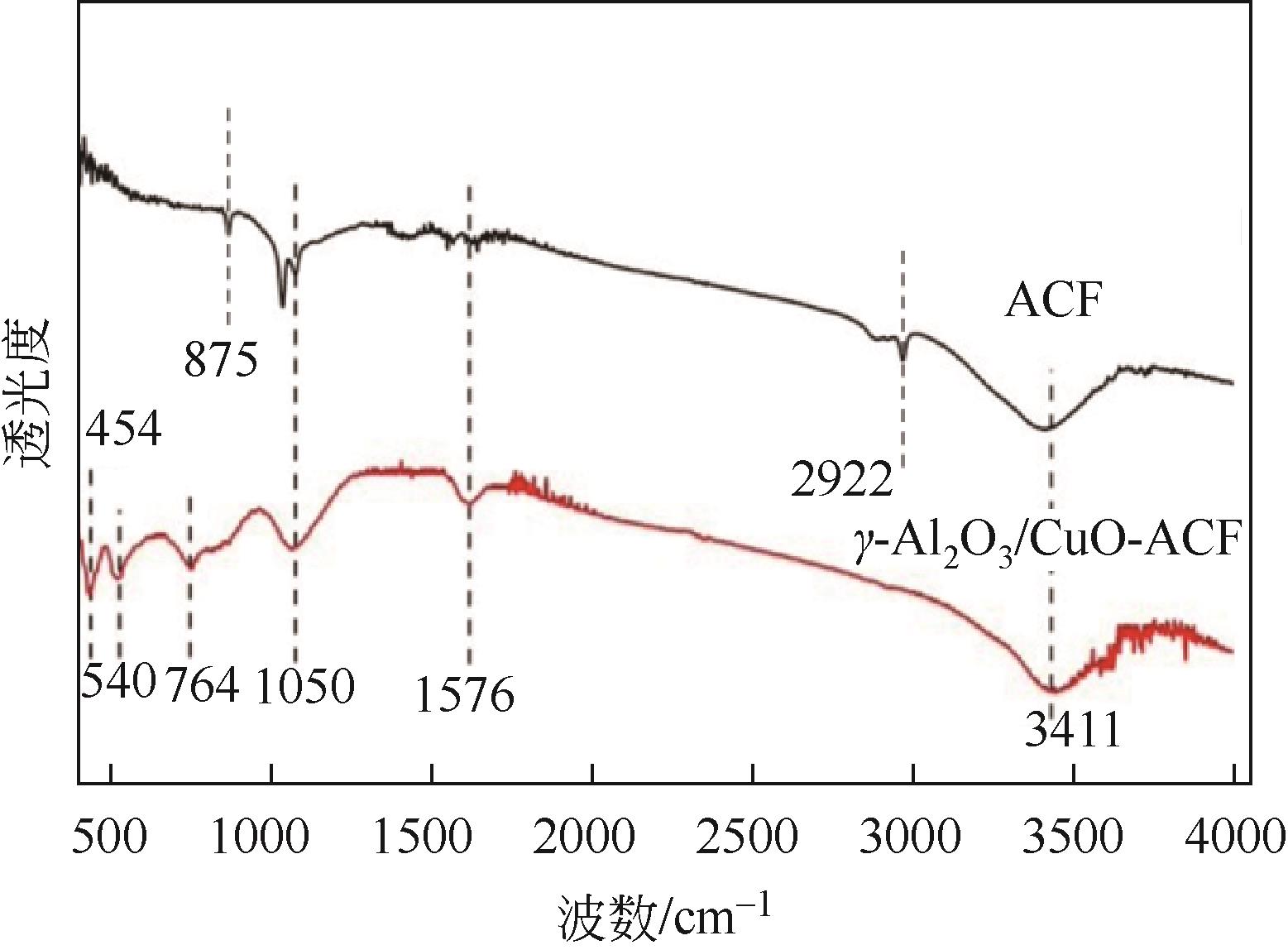
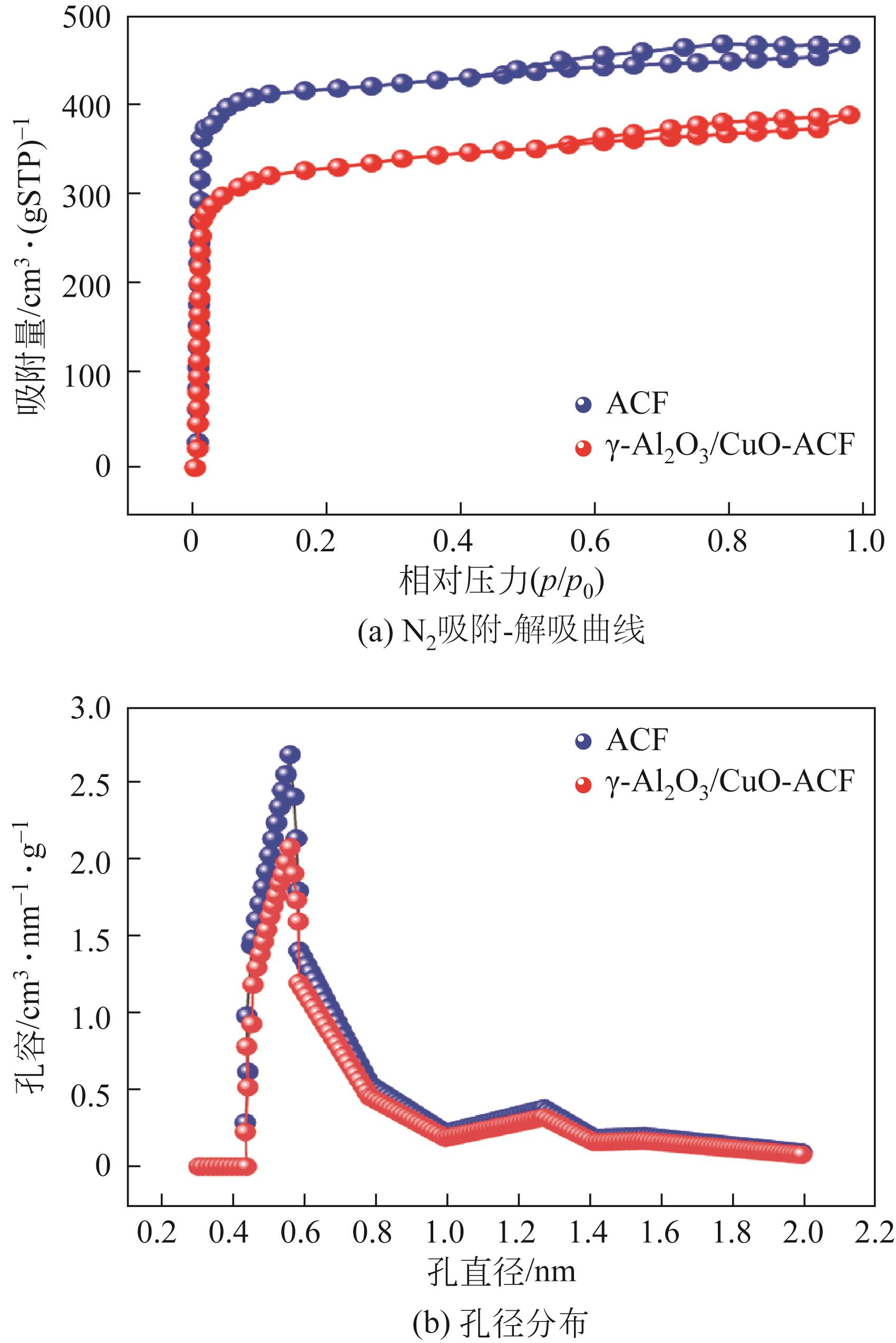
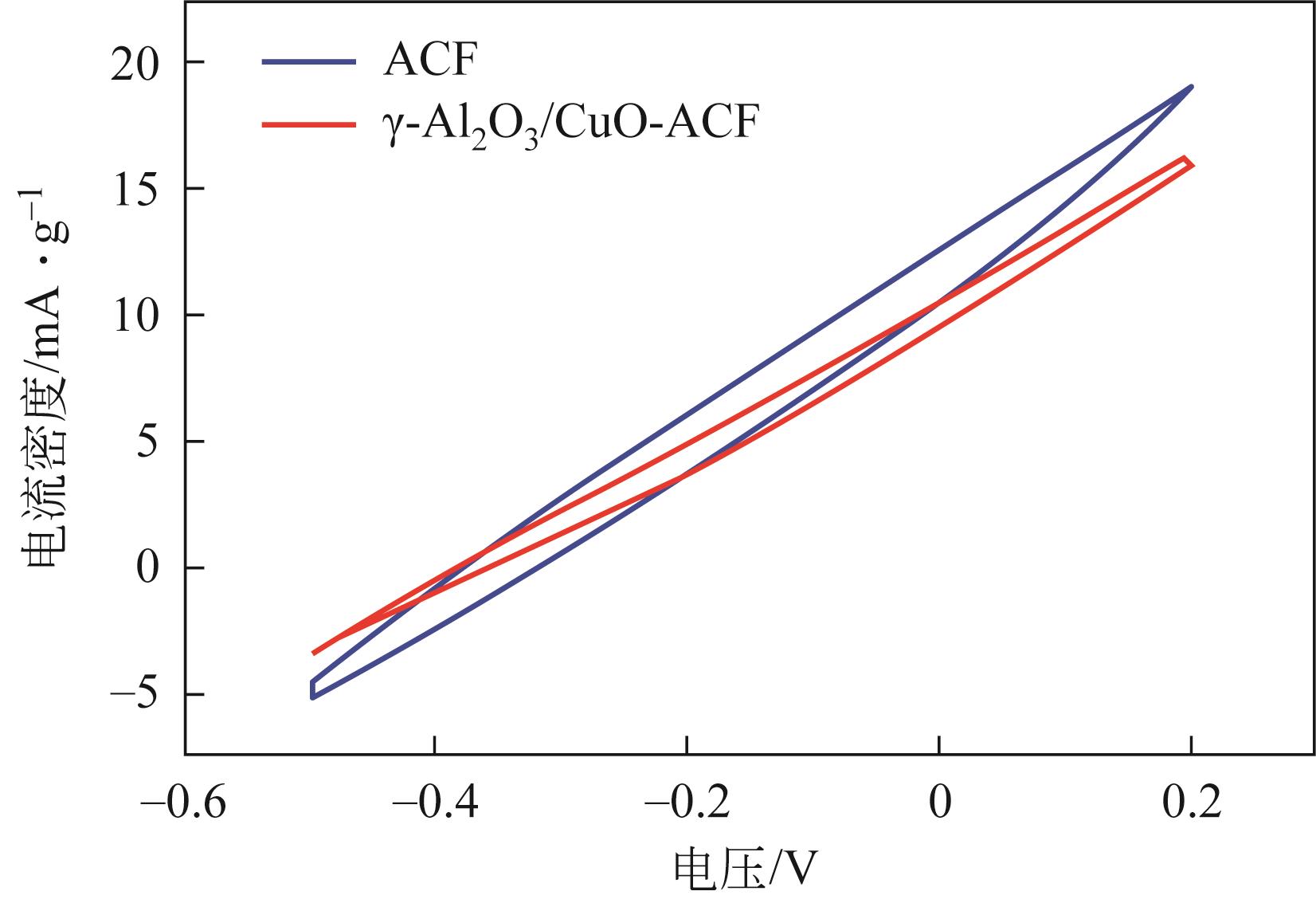
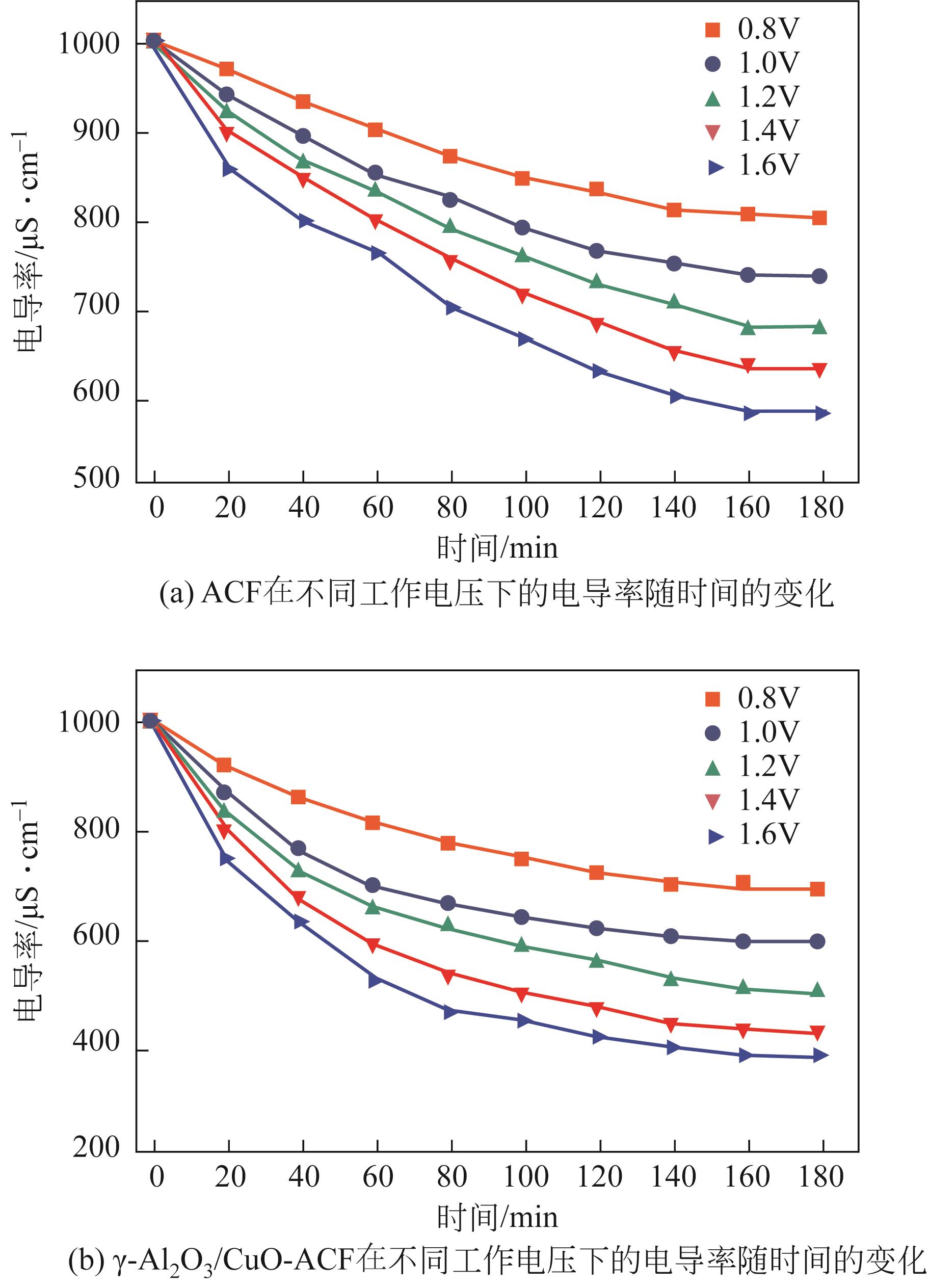
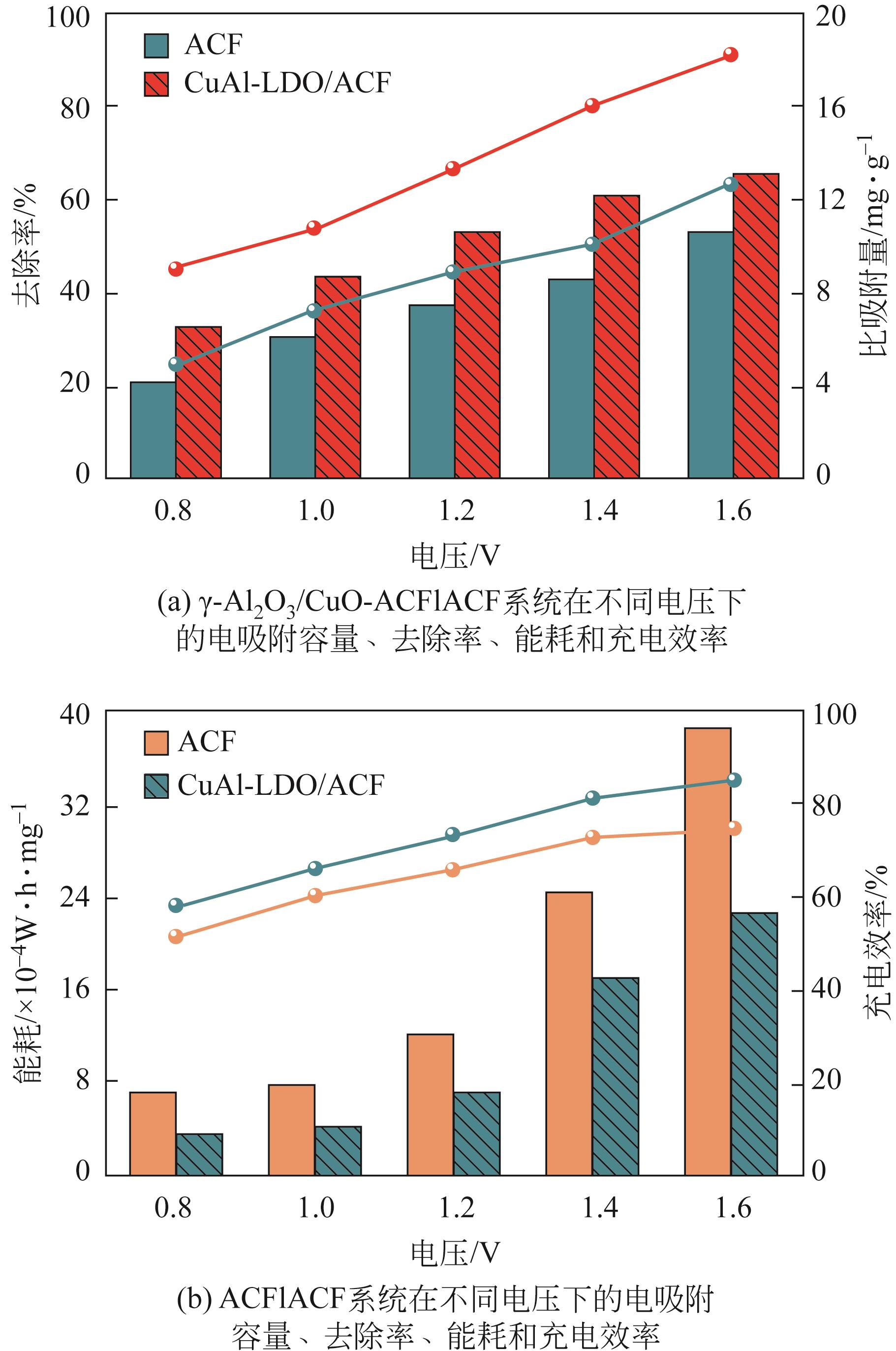
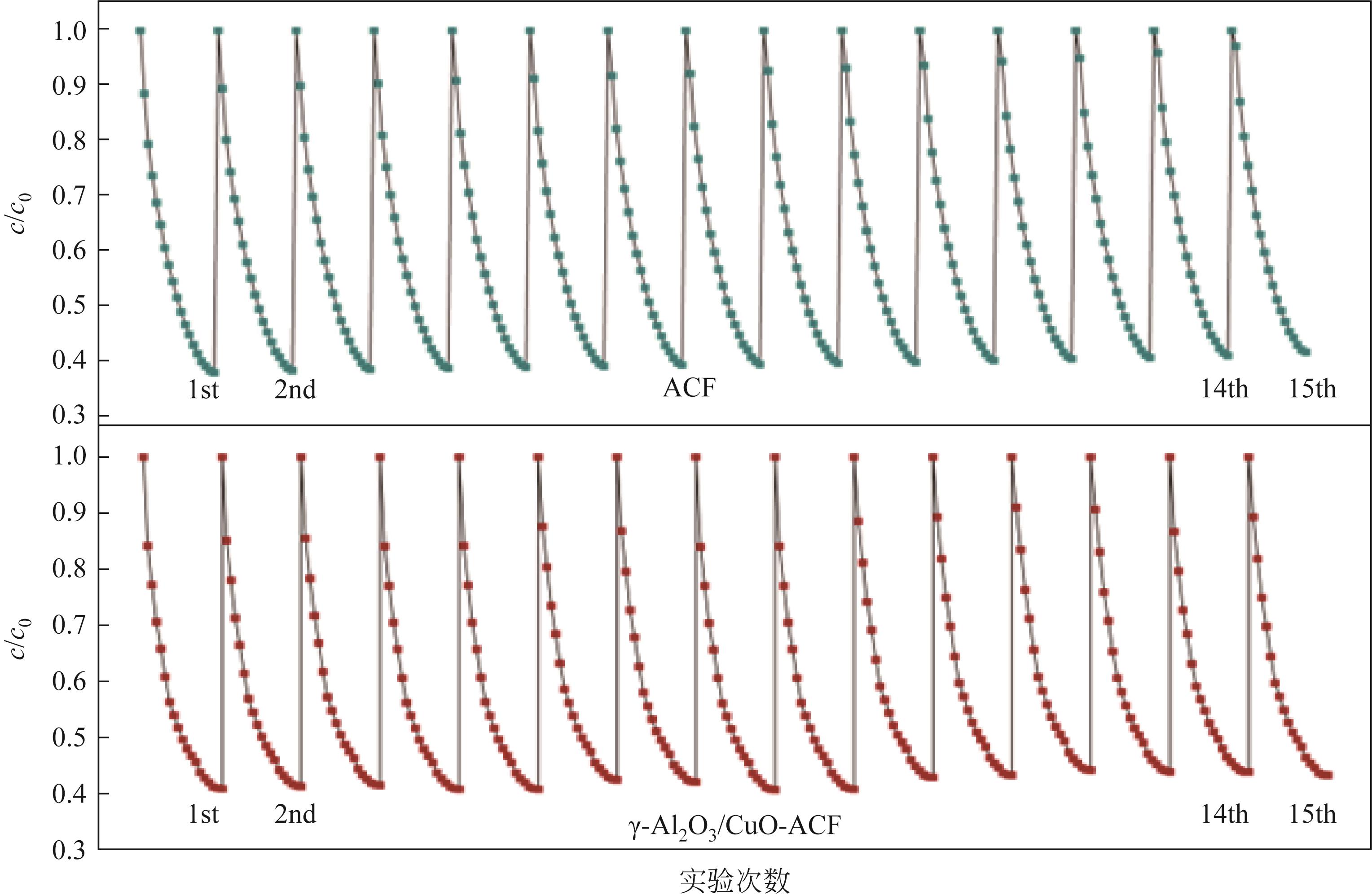
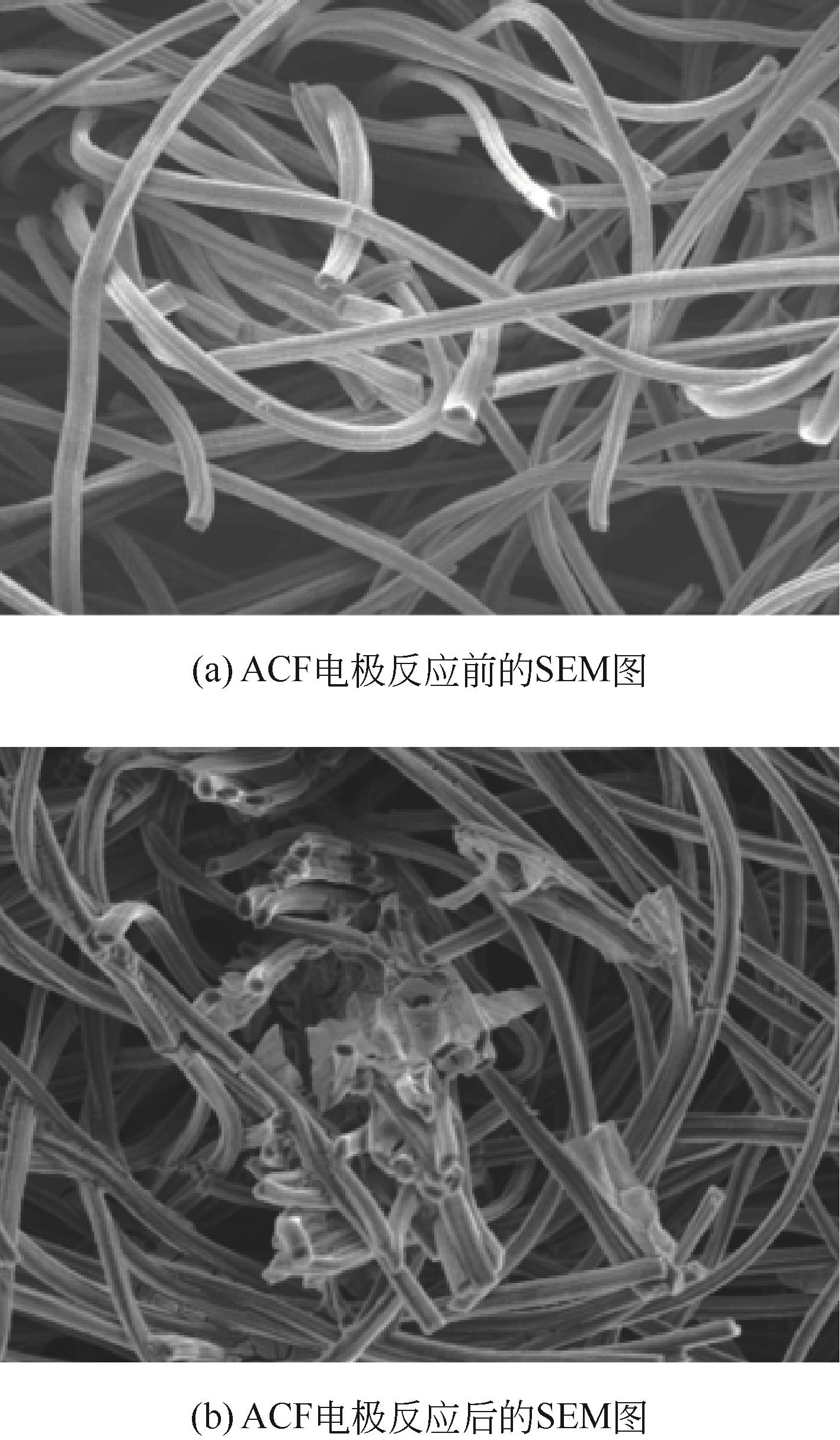
开发脱盐率高、寿命长的电极材料是电容去离子(CDI)水处理技术的研究热点之一。通过一锅水热法将层状CuAl双金属氧化物与活性碳纤维复合,成功制备了CDI电极(γ-Al2O3/CuO-ACF)。采用SEM、XRD、FTIR和CV测试对样品的形貌、结构和电极性能进行了表征。当初始NaCl浓度为500mg/L时,随着电压从0.8V逐渐增加到1.6V,两种电极的比吸附量、脱盐效率、电流效率和电耗均有所增加,γ-Al2O3/CuO-ACF的四项参数依次比ACF提高23.4%~55.3%、44.8%~82.0%、65.5%~90.0%和降低15.0%~21.4%。当腐殖酸浓度为5~10mg/L时,ACF脱盐效率下降明显,而γ-Al2O3/CuO-ACF脱盐效率仅在腐殖酸浓度10mg/L时略有下降。在15次循环后,NaCl溶液体系的脱盐效率保留率为96%;但由于腐殖酸的存在,该值下降为92%。两种电极的电吸附除盐过程遵循Langmuir等温吸附方程,表示盐离子在电极表面为单分子层物理吸附。与传统ACF电极相比,γ-Al2O3/CuO-ACF电极具有优异的可回收性、稳定性和增强的电化学特性。
中图分类号:
引用本文
柴多生, 高峰, 吴友兵, 孙昕, 郝然, 杨宇, 焦翔飞. γ-Al2O3/CuO-ACF电吸附除盐的影响因素及反应动力学[J]. 化工进展, 2024, 43(3): 1637-1647.
CHAI Duosheng, GAO Feng, WU Youbing, SUN Xin, HAO Ran, YANG Yu, JIAO Xiangfei. Reaction dynamics and influencing factors of capacitive deionization desalination using γ-Al2O3 / CuO-ACF[J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1637-1647.
| 电极材料 | 外加电压 /V | 初始浓度 /mg·L-1 | SAC /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| ACF-HNO3 | 1.2 | 500 | 12.80 | [ |
| NPC@ACF | 1.2 | 500 | 14.63 | [ |
| Al2O3-ACF | 2.0 | 500 | 12.25 | [ |
| ACF | 1.4 | 500 | 10.16 | 本工作 |
| γ-Al2O3/CuO-ACF | 1.4 | 500 | 16.97 | 本工作 |
表1 不同电极材料除盐能力的比较
| 电极材料 | 外加电压 /V | 初始浓度 /mg·L-1 | SAC /mg·g-1 | 参考 文献 |
|---|---|---|---|---|
| ACF-HNO3 | 1.2 | 500 | 12.80 | [ |
| NPC@ACF | 1.2 | 500 | 14.63 | [ |
| Al2O3-ACF | 2.0 | 500 | 12.25 | [ |
| ACF | 1.4 | 500 | 10.16 | 本工作 |
| γ-Al2O3/CuO-ACF | 1.4 | 500 | 16.97 | 本工作 |
外加电压 /V | ACF | γ-Al2O3/CuO-ACF | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Qm /mg·g-1 | KL | RL | R2 | Qm /mg·g-1 | KL | RL | |
| 0.8 | 0.9944 | 13.26 | 0.0048 | 0.8065 | 0.9988 | 11.12 | 0.0089 | 0.1841 |
| 1.0 | 0.9935 | 13.81 | 0.0053 | 0.7891 | 0.9960 | 14.06 | 0.0102 | 0.1633 |
| 1.2 | 0.9931 | 13.94 | 0.0067 | 0.7491 | 0.9966 | 15.20 | 0.0148 | 0.1189 |
| 1.4 | 0.9925 | 14.48 | 0.0080 | 0.7135 | 0.9912 | 15.73 | 0.0292 | 0.0640 |
| 1.6 | 0.9923 | 16.25 | 0.0086 | 0.6993 | 0.9946 | 19.05 | 0.0410 | 0.0465 |
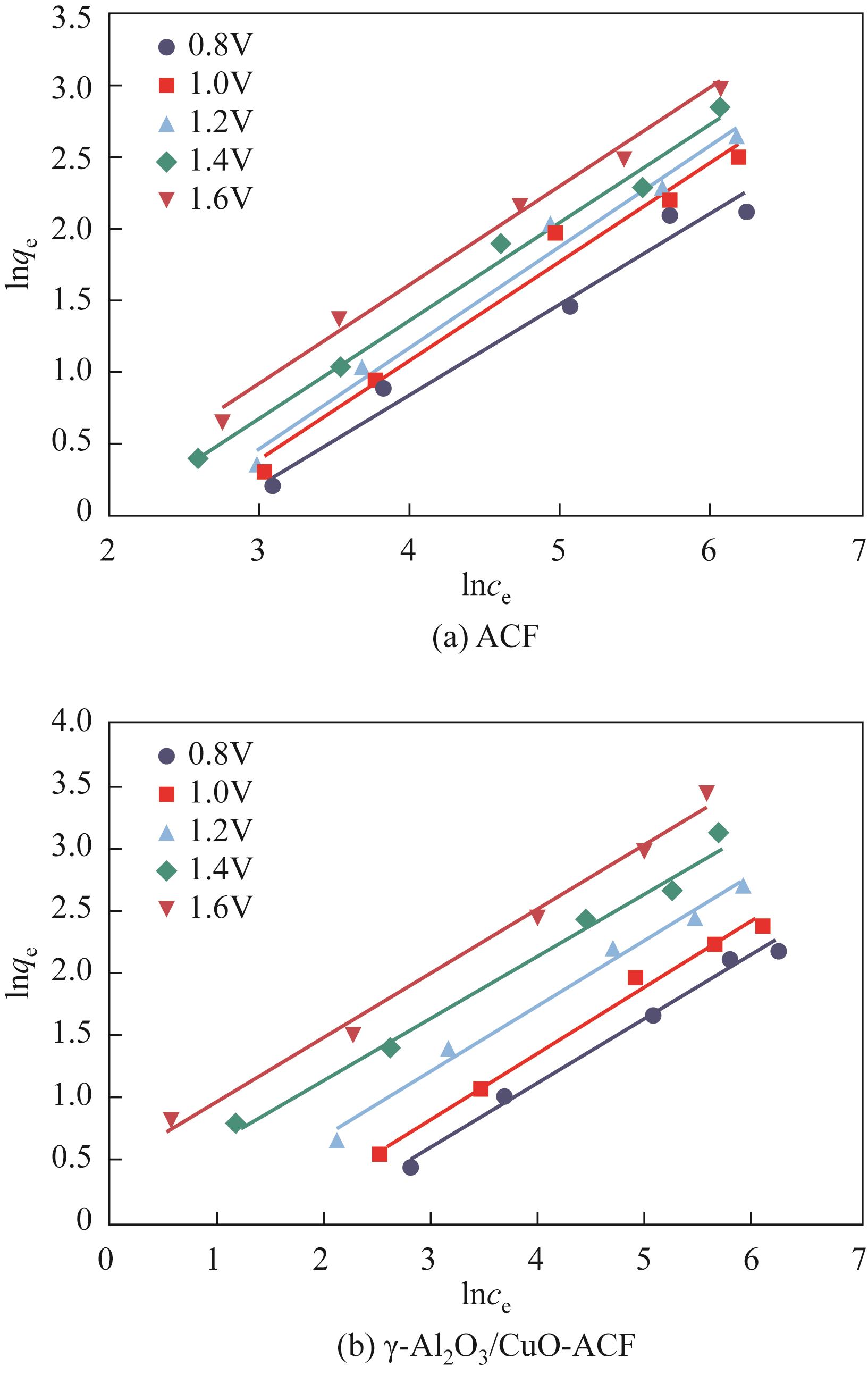
表2 不同电压下ACF和γ-Al2O3/CuO-ACF电极的Langmuir等温线参数比较
外加电压 /V | ACF | γ-Al2O3/CuO-ACF | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Qm /mg·g-1 | KL | RL | R2 | Qm /mg·g-1 | KL | RL | |
| 0.8 | 0.9944 | 13.26 | 0.0048 | 0.8065 | 0.9988 | 11.12 | 0.0089 | 0.1841 |
| 1.0 | 0.9935 | 13.81 | 0.0053 | 0.7891 | 0.9960 | 14.06 | 0.0102 | 0.1633 |
| 1.2 | 0.9931 | 13.94 | 0.0067 | 0.7491 | 0.9966 | 15.20 | 0.0148 | 0.1189 |
| 1.4 | 0.9925 | 14.48 | 0.0080 | 0.7135 | 0.9912 | 15.73 | 0.0292 | 0.0640 |
| 1.6 | 0.9923 | 16.25 | 0.0086 | 0.6993 | 0.9946 | 19.05 | 0.0410 | 0.0465 |
外加电压 /V | ACF | γ-Al2O3/CuO-ACF | ||||
|---|---|---|---|---|---|---|
| R2 | KF | n | R2 | KF | n | |
| 0.8 | 0.9689 | 0.1485 | 1.4858 | 0.9888 | 0.3918 | 1.9290 |
| 1.0 | 0.9706 | 0.1891 | 1.4570 | 0.9832 | 0.5065 | 1.9488 |
| 1.2 | 0.9765 | 0.1945 | 1.4270 | 0.9855 | 0.7124 | 1.9222 |
| 1.4 | 0.9894 | 0.2504 | 1.4531 | 0.9845 | 1.1903 | 2.0099 |
| 1.6 | 0.9915 | 0.2718 | 1.3894 | 0.9854 | 1.4228 | 1.9410 |
表3 不同电压下ACF和γ-Al2O3/CuO-ACF电极的Freundlich等温线参数比较
外加电压 /V | ACF | γ-Al2O3/CuO-ACF | ||||
|---|---|---|---|---|---|---|
| R2 | KF | n | R2 | KF | n | |
| 0.8 | 0.9689 | 0.1485 | 1.4858 | 0.9888 | 0.3918 | 1.9290 |
| 1.0 | 0.9706 | 0.1891 | 1.4570 | 0.9832 | 0.5065 | 1.9488 |
| 1.2 | 0.9765 | 0.1945 | 1.4270 | 0.9855 | 0.7124 | 1.9222 |
| 1.4 | 0.9894 | 0.2504 | 1.4531 | 0.9845 | 1.1903 | 2.0099 |
| 1.6 | 0.9915 | 0.2718 | 1.3894 | 0.9854 | 1.4228 | 1.9410 |
| 1 | ZHANG Douqing, LI Mingjun, JI Xiang, et al. Revealing potential of energy-saving behind emission reduction: A DEA-based empirical study[J]. Management of Environmental Quality: an International Journal, 2019, 30(4): 714-730. |
| 2 | TONG Yongjuan, ZHANG Qi, CAI Jiuju, et al. Water consumption and wastewater discharge in China’s steel industry[J]. Ironmaking & Steelmaking, 2018, 45(10): 868-877. |
| 3 | GENDEL Youri, ROMMERSKIRCHEN Alexandra Klara Elisabeth, DAVID Oana, et al. Batch mode and continuous desalination of water using flowing carbon deionization (FCDI) technology[J]. Electrochemistry Communications, 2014, 46: 152-156. |
| 4 | YANG Zhiyu, JIN Linjian, LU Guoqian, et al. Sponge-templated preparation of high surface area graphene with ultrahigh capacitive deionization performance[J]. Advanced Functional Materials, 2014, 24(25): 3917-3925. |
| 5 | BLAIR John W, MURPHY George W. Electrochemical demineralization of water with porous electrodes of large surface area[M]//Advances in Chemistry. Washington D C: American Chemical Society, 1960: 206-223. |
| 6 | HAN Linchen, KARTHIKEYAN K G, ANDERSON Marc A, et al. Exploring the impact of pore size distribution on the performance of carbon electrodes for capacitive deionization[J]. Journal of Colloid and Interface Science, 2014, 430: 93-99. |
| 7 | WANG Hui, SHI Liyi, YAN Tingting, et al. Design of graphene-coated hollow mesoporous carbon spheres as high performance electrodes for capacitive deionization[J]. Journal of Materials Chemistry A, 2014, 2(13): 4739-4750. |
| 8 | ZORNITTA Rafael L, GARCÍA-MATEOS Francisco J, LADO Julio J, et al. High-performance activated carbon from polyaniline for capacitive deionization[J]. Carbon, 2017, 123: 318-333. |
| 9 | ZHOU Jianen, YANG Qingyun, XIE Qiongyi, et al. Recent progress in Co-based metal-organic framework derivatives for advanced batteries[J]. Journal of Materials Science & Technology, 2022, 96: 262-284. |
| 10 | RASINES G, LAVELA P, MACÍAS C, et al. Mesoporous carbon black-aerogel composites with optimized properties for the electro-assisted removal of sodium chloride from brackish water[J]. Journal of Electroanalytical Chemistry, 2015, 741: 42-50. |
| 11 | LUO Guoming, WANG Yuzhi, GAO Lixin, et al. Graphene bonded carbon nanofiber aerogels with high capacitive deionization capability[J]. Electrochimica Acta, 2018, 260: 656-663. |
| 12 | ZHAO Xiaoyu, WEI Hongxin, ZHAO Huachao, et al. Electrode materials for capacitive deionization: A review[J]. Journal of Electroanalytical Chemistry, 2020, 873: 114416. |
| 13 | CHU Dawei, SONG Xiumei, TAN Lichao, et al. Polyvinyl pyrrolidone-induced assembly of NiCo-LDHs nanosheets: A facile method for fabricating three-dimensional flower-like microspheres with excellent supercapacitor performance[J]. Inorganic Chemistry Communications, 2019, 110: 107587. |
| 14 | ZHU Fangfang, LIU Weijing, LIU Yu, et al. Construction of porous interface on CNTs@NiCo-LDH core-shell nanotube arrays for supercapacitor applications[J]. Chemical Engineering Journal, 2020, 383: 123150. |
| 15 | 郭宁, 郭婷, 陈方方. 电容去离子脱盐影响因素的研究进展[J]. 四川化工, 2023, 26(3): 11-15. |
| GUO Ning, GUO Ting, CHEN Fangfang. Research progress on influencing factors of capacitive deionization desalination[J]. Sichuan Chemical Industry, 2023, 26(3): 11-15. | |
| 16 | XU Lina, LI Jiao, SUN Haibin, et al. In situ growth of Cu2O/CuO nanosheets on Cu coating carbon cloths as a binder-free electrode for asymmetric supercapacitors[J]. Frontiers in Chemistry, 2019, 7: 420. |
| 17 | 李谦, 刘志英, 许海辉, 等. 负载Al2O3的活性碳纤维电吸附除盐研究[J]. 工业水处理, 2017, 37(9): 48-52. |
| LI Qian, LIU Zhiying, XU Haihui, et al. Research on Al2O3-loaded activated carbon fiber for the electro-adsorptive desalination[J]. Industrial Water Treatment, 2017, 37(9): 48-52. | |
| 18 | FAN Guoli, LI Feng, EVANS David G, et al. Catalytic applications of layered double hydroxides: Recent advances and perspectives[J]. Chemical Society Reviews, 2014, 43(20): 7040-7066. |
| 19 | LI Changming, WEI Min, EVANS David G, et al. Layered double hydroxide-based nanomaterials as highly efficient catalysts and adsorbents[J]. Small, 2014, 10(22): 4469-4486. |
| 20 | REN Qidi, WANG Gang, WU Tingting, et al. Calcined MgAl-layered double hydroxide/graphene hybrids for capacitive deionization[J]. Industrial & Engineering Chemistry Research, 2018, 57(18): 6417-6425. |
| 21 | LI Kaijian, GUO Dengfeng, LIN Furong, et al. Electrosorption of copper ions by poly(m-phenylenediamine)/reduced graphene oxide synthesized via a one-step in situ redox strategy[J]. Electrochimica Acta, 2015, 166: 47-53. |
| 22 | LEI Xiaodong, WANG Bo, LIU Junfeng, et al. Three-dimensional NiAl-mixed metal oxide film: Preparation and capacitive deionization performances[J]. RSC Advances, 2014, 4(78): 41642-41648. |
| 23 | WU Qinghao, LIANG Dawei, AVRAHAM Eran, et al. Enhanced capacitive deionization of an integrated membrane electrode by thin layer spray-coating of ion exchange polymers on activated carbon electrode[J]. Desalination, 2020, 491: 114460. |
| 24 | ELANG BARRUNA A G, MUHAMAD NAUFAL R, NUGRAHA Mohammad Ridho, et al. Material characteristics and electrochemical performance of lithium-ion capacitor with activated carbon cathode derived from sugarcane bagasse[J]. IOP Conference Series: Earth and Environmental Science, 2021, 673(1): 012018. |
| 25 | Abdul HAI, ALQASSEM Bayan, BHARATH G, et al. Cobalt and nickel ferrites based capacitive deionization electrode materials for water desalination applications[J]. Electrochimica Acta, 2020, 363: 137083. |
| 26 | JABBAR Saja Mohsen. Synthesis of CuO nano structure via sol-gel and precipitation chemical methods[J]. Al-Khwarizmi Engineering Journal, 2017, 12(4): 126-131. |
| 27 | KANWAL Aisha, SAJJAD Shamaila, LEGHARI Sajjad Ahmed Khan, et al. Cascade electron transfer in ternary CuO/α-Fe2O3/γ-Al2O3 nanocomposite as an effective visible photocatalyst[J]. Journal of Physics and Chemistry of Solids, 2021, 151: 109899. |
| 28 | ZHENG Run, LIN Qixuan, MENG Ling, et al. Flexible phosphorus-doped activated carbon fiber paper in situ loading of CuO for degradation of phenol[J]. Separation and Purification Technology, 2022, 298: 121619. |
| 29 | CHEN Baoliang, CHEN Zaiming, Shaofang LYU. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate[J]. Bioresource Technology, 2011, 102(2): 716-723. |
| 30 | DALMASCHIO Cleocir José, MASTELARO Valmor R, NASCENTE Pedro, et al. Oxide surface modification: Synthesis and characterization of zirconia-coated alumina[J]. Journal of Colloid and Interface Science, 2010, 343(1): 256-262. |
| 31 | KOTOMIN E A, STASHANS A, KANTOROVICH L N, et al. Calculations of the geometry and optical properties of FMg centers and dimer (F2-type) centers in corundum crystals[J]. Physical Review B, 1995, 51(14): 8770-8778. |
| 32 | XI Wen, LI Haibo. Vertically-aligned growth of CuAl-layered double oxides on reduced graphene oxide for hybrid capacitive deionization with superior performance[J]. Environmental Science Nano, 2020, 7: 764-772. |
| 33 | Vinod Vellora Thekkae Padil, Černík Miroslav. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application[J]. International Journal of Nanomedicine, 2013, 8: 889-898. |
| 34 | SHARMA Ajit, LEE Byeong-Kyu. Integrated ternary nanocomposite of TiO2/NiO/reduced graphene oxide as a visible light photocatalyst for efficient degradation of o-chlorophenol[J]. Journal of Environmental Management, 2016, 181: 563-573. |
| 35 | LIU Shiqi, ZHANG Zichen, HUANG Fei, et al. Carbonized polyaniline activated peroxymonosulfate (PMS) for phenol degradation: Role of PMS adsorption and singlet oxygen generation[J]. Applied Catalysis B: Environmental, 2021, 286: 119921. |
| 36 | HUPP Joseph T, POEPPELMEIER Kenneth R. Better living through nanopore chemistry[J]. Science, 2005, 309(5743): 2008-2009. |
| 37 | MAO Ping, QI Bingbing, LIU Ying, et al. AgII doped MIL-101 and its adsorption of iodine with high speed in solution[J]. Journal of Solid State Chemistry, 2016, 237: 274-283. |
| 38 | WU Tingting, WANG Gang, DONG Qiang, et al. Asymmetric capacitive deionization utilizing nitric acid treated activated carbon fiber as the cathode[J]. Electrochimica Acta, 2015, 176: 426-433. |
| 39 | Lijuan MEN, CHEN Chunyu, LIU An, et al. N-doped porous carbon-based capacitive deionization electrode materials loaded with activated carbon fiber for water desalination applications[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107943. |
| 40 | TANG Chuyang Y, KWON Young-Nam, LECKIE James O. Fouling of reverse osmosis and nanofiltration membranes by humic acid—Effects of solution composition and hydrodynamic conditions[J]. Journal of Membrane Science, 2007, 290(1/2): 86-94. |
| 41 | GABELICH Christopher J, TRAN Tri D, I H Mel SUFFET. Electrosorption of inorganic salts from aqueous solution using carbon aerogels[J]. Environmental Science & Technology, 2002, 36(13): 3010-3019. |
| 42 | WANG Changming, SONG Haiou, ZHANG Quanxing, et al. Parameter optimization based on capacitive deionization for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration[J]. Desalination, 2015, 365: 407-415. |
| 43 | WIMALASIRI Yasodinee, MOSSAD Mohamed, ZOU Linda. Thermodynamics and kinetics of adsorption of ammonium ions by graphene laminate electrodes in capacitive deionization[J]. Desalination, 2015, 357: 178-188. |
| [1] | 张祚群, 高扬, 白超杰, 薛立新. 二次界面聚合同步反扩散原位生长ZIF-8纳米粒子制备聚酰胺混合基质反渗透(RO)膜[J]. 化工进展, 2023, 42(S1): 364-373. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 宗悦, 张瑞君, 高珊珊, 田家宇. “特殊稳定型”压力驱动薄膜复合(TFC)脱盐膜的研究进展[J]. 化工进展, 2023, 42(4): 2058-2067. |
| [4] | 陈仪, 郭耀励, 叶海星, 李宇璇, 牛青山. 二维纳米材料在渗透汽化脱盐膜中的应用[J]. 化工进展, 2023, 42(3): 1437-1447. |
| [5] | 张赫, 李小可, 熊颖, 文劲. 基于水凝胶界面光蒸发的压裂返排液脱盐降污处理[J]. 化工进展, 2023, 42(2): 1073-1079. |
| [6] | 孙孟威, 刘壮, 谢锐, 巨晓洁, 汪伟, 褚良银. 镧离子插层MoS2膜的制备及印染高盐水处理[J]. 化工进展, 2023, 42(1): 346-353. |
| [7] | 张辛亥, 赵思琛, 朱辉, 张首石, 王凯. 多种碳材料与碳酸钠复合后脱硫性能对比[J]. 化工进展, 2022, 41(S1): 424-435. |
| [8] | 张辛亥, 赵思琛, 朱辉, 王凯, 张首石. 活性碳纤维负载型脱硫剂在矿井气体环境条件下的应用[J]. 化工进展, 2022, 41(S1): 415-423. |
| [9] | 祁元, 徐欣蓉, 阮玮, 吴昊, 吴科, 周亚明, 杨宏旻. 改性活性碳纤维对苯胺吸附特性分析[J]. 化工进展, 2022, 41(S1): 622-630. |
| [10] | 张华, 刘光全, 张晓飞, 罗臻. 电脱盐废水稳定性分析及破乳技术[J]. 化工进展, 2022, 41(9): 5047-5054. |
| [11] | 袁权, 李海红, 刘浩杰. HNO3改性活性炭对不同价态离子的电吸附规律[J]. 化工进展, 2022, 41(9): 4986-4994. |
| [12] | 洪波. 取消药剂消耗的电脱盐污水物理法预处理[J]. 化工进展, 2022, 41(5): 2788-2796. |
| [13] | 石一慈, 潘艳秋, 王成宇, 范嘉禾, 俞路. 焦耳效应强化气隙式膜蒸馏脱盐过程的实验研究[J]. 化工进展, 2022, 41(5): 2285-2291. |
| [14] | 周永泉, 张艾, 刘亚男, 王铮. 等离子体射流耦合活性碳纤维去除水中糖皮质激素[J]. 化工进展, 2022, 41(4): 2209-2215. |
| [15] | 张群, 陈重军, 谢嘉玮, 邹馨怡. 高盐废水微生物脱盐池处理研究进展[J]. 化工进展, 2022, 41(2): 974-980. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||