化工进展 ›› 2024, Vol. 43 ›› Issue (9): 4941-4950.DOI: 10.16085/j.issn.1000-6613.2023-1289
• 工业催化 • 上一篇
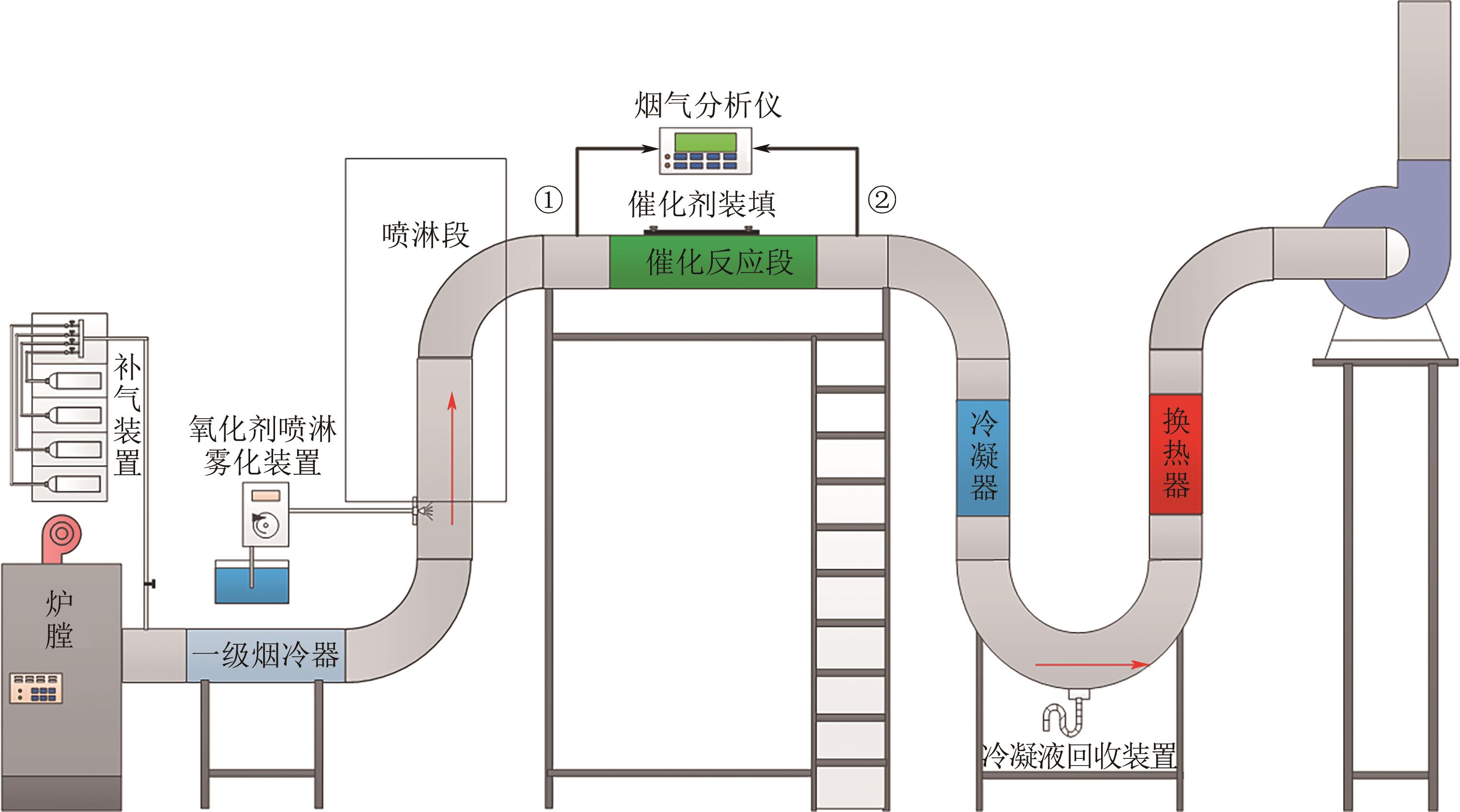
H2O2低温催化氧化法脱硫脱硝中试实验特性
修浩然( ), 王云刚(
), 王云刚( ), 白彦渊, 刘涛, 张兴邦, 张益嘉
), 白彦渊, 刘涛, 张兴邦, 张益嘉
- 西安交通大学热流科学与工程教育部重点实验室,陕西 西安 710049
-
收稿日期:2023-07-26修回日期:2023-12-25出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:王云刚 -
作者简介:修浩然(1998—),男,硕士研究生,研究方向为清洁能源高效利用及处理。E-mail:1010345641@qq.com。 -
基金资助:国家重点研发计划(2021YFC3001803);国家自然科学基金面上项目(52276085);王宽诚教育基金
Pilot test of H2O2 low temperature catalytic oxidation for desulfurization and denitrification
XIU Haoran( ), WANG Yungang(
), WANG Yungang( ), BAI Yanyuan, LIU Tao, ZHANG Xingbang, ZHANG Yijia
), BAI Yanyuan, LIU Tao, ZHANG Xingbang, ZHANG Yijia
- Key Laboratory of Thermo-Fluid Science and Engineering (Ministry of Education), Xi'an Jiaotong University, Xi'an 710049, Shaanxi, China
-
Received:2023-07-26Revised:2023-12-25Online:2024-09-15Published:2024-09-30 -
Contact:WANG Yungang
摘要:
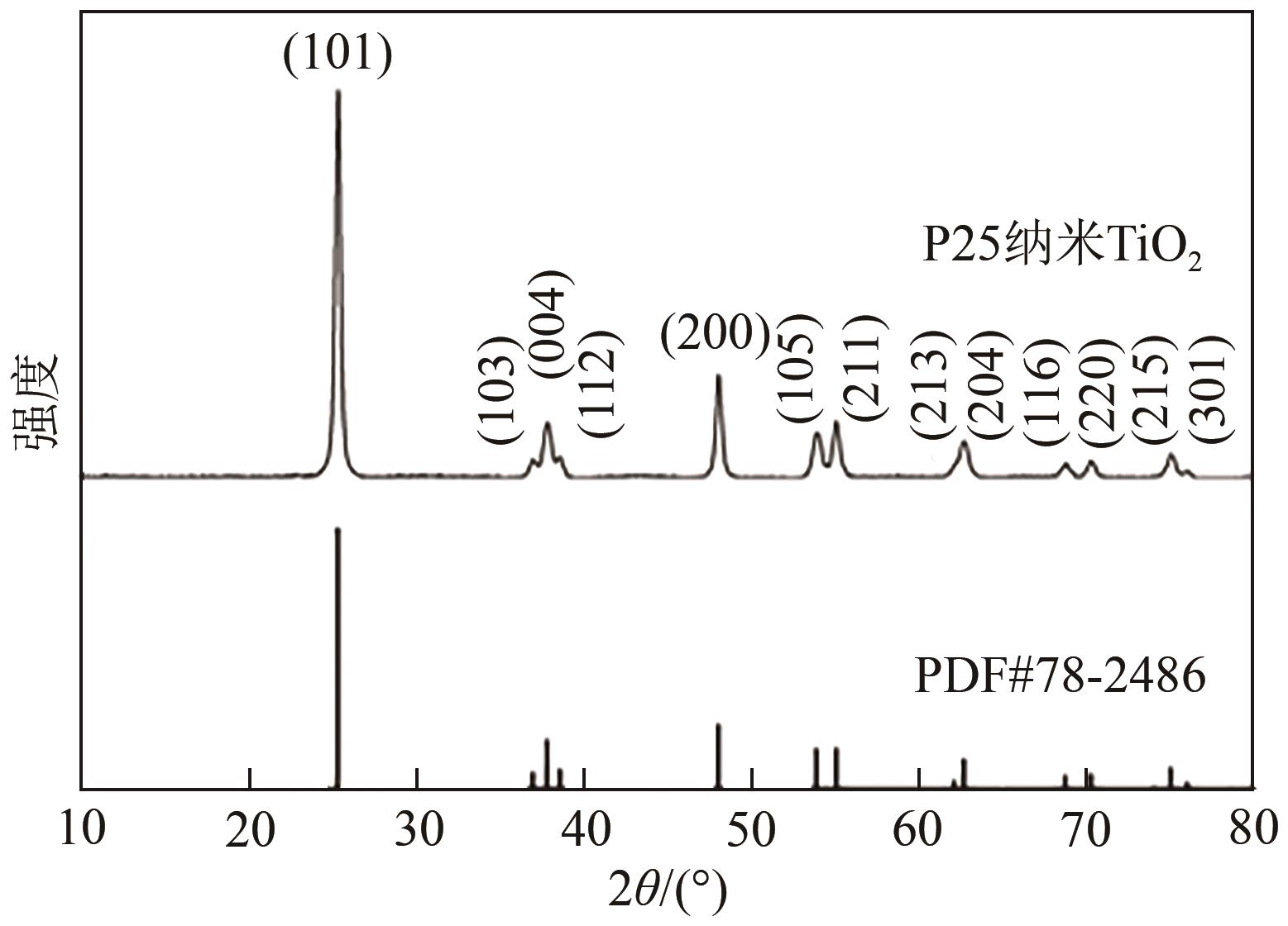
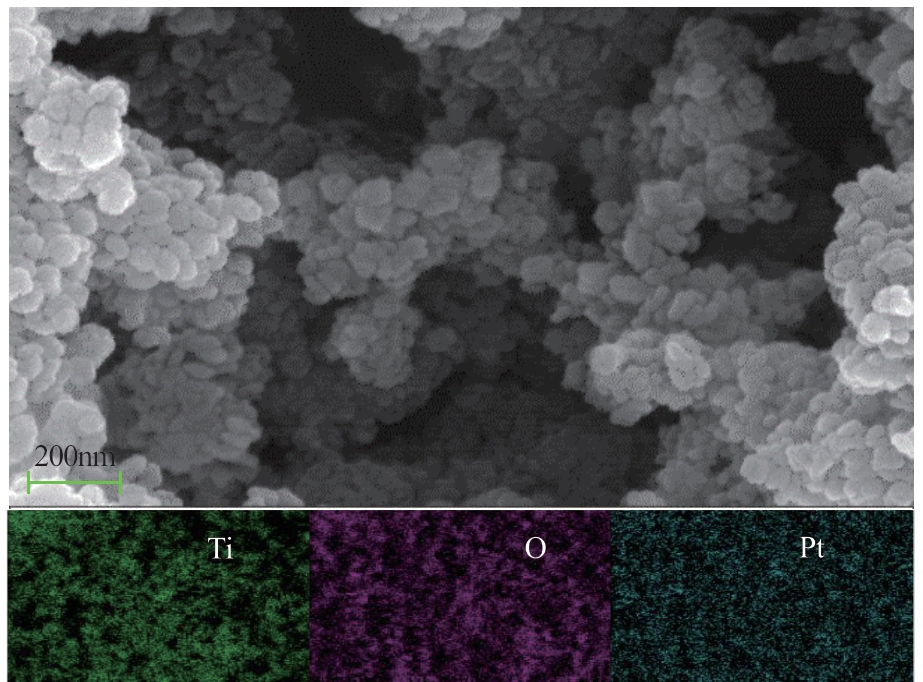
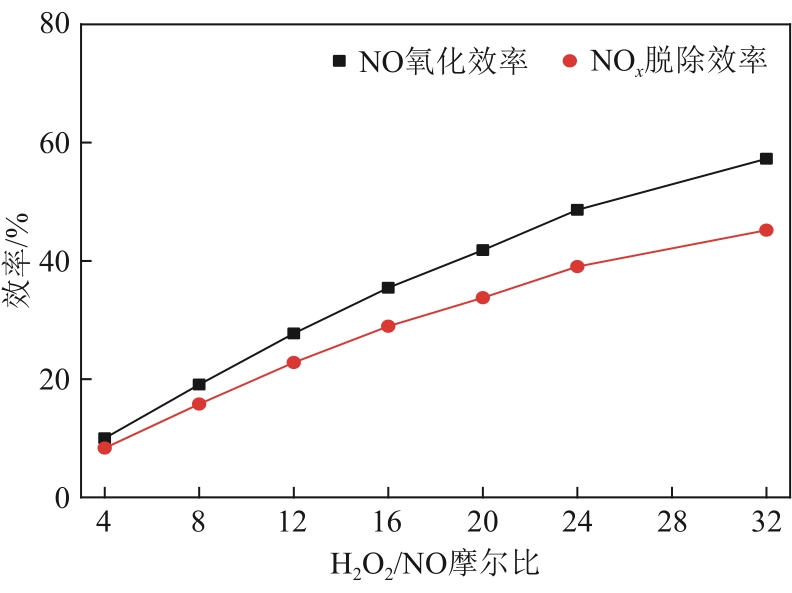
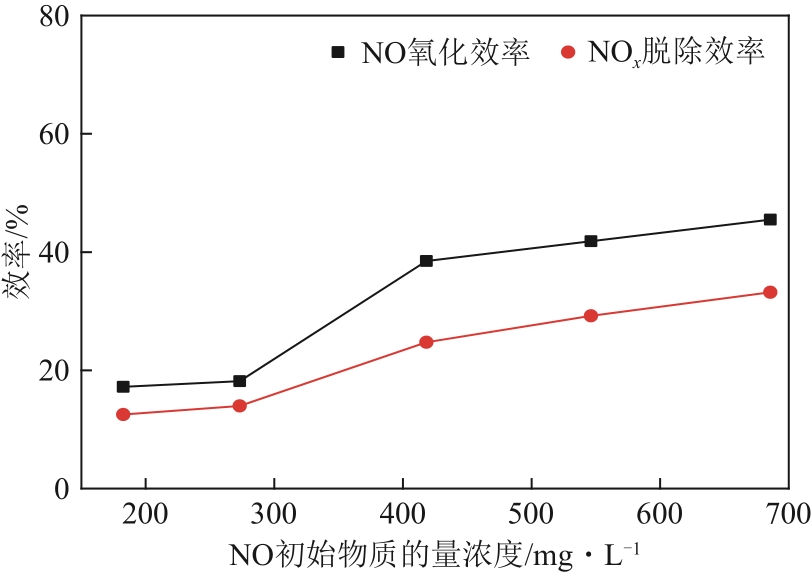
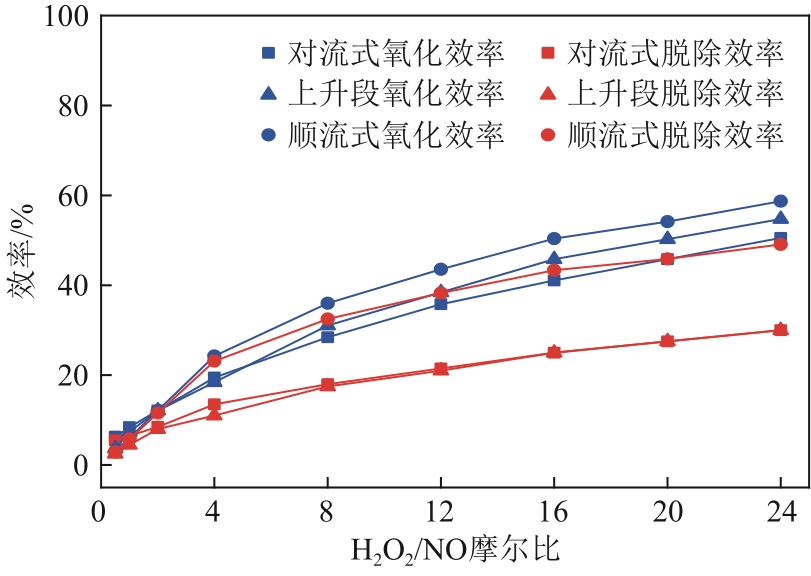
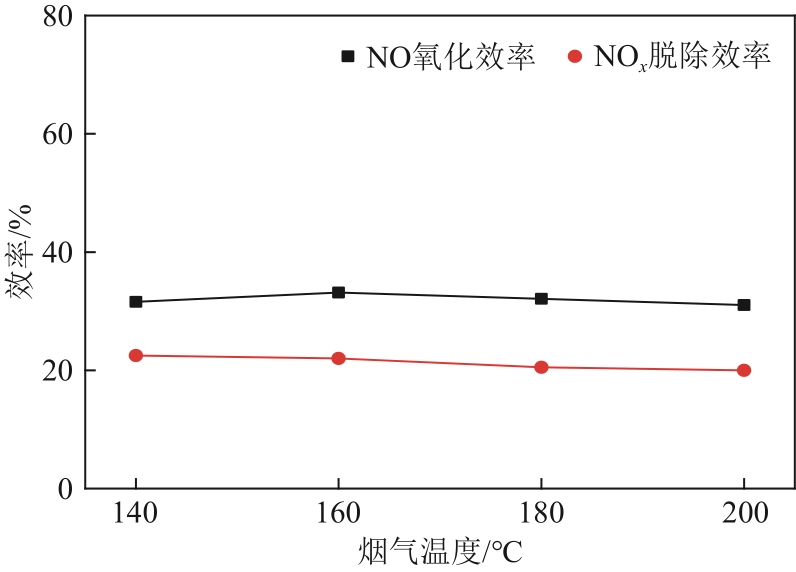
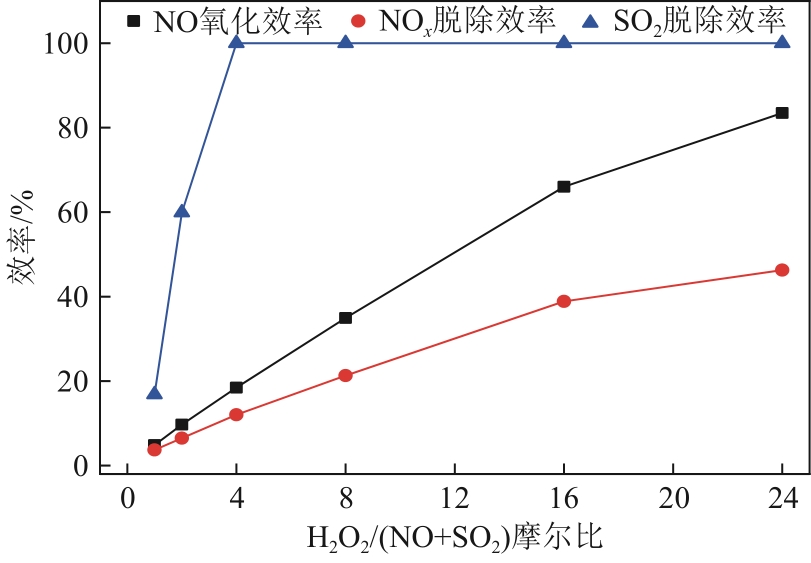
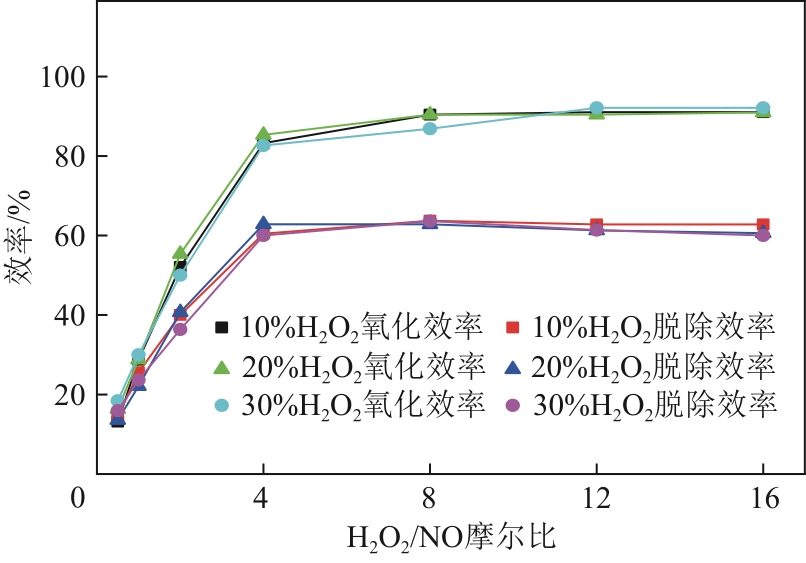
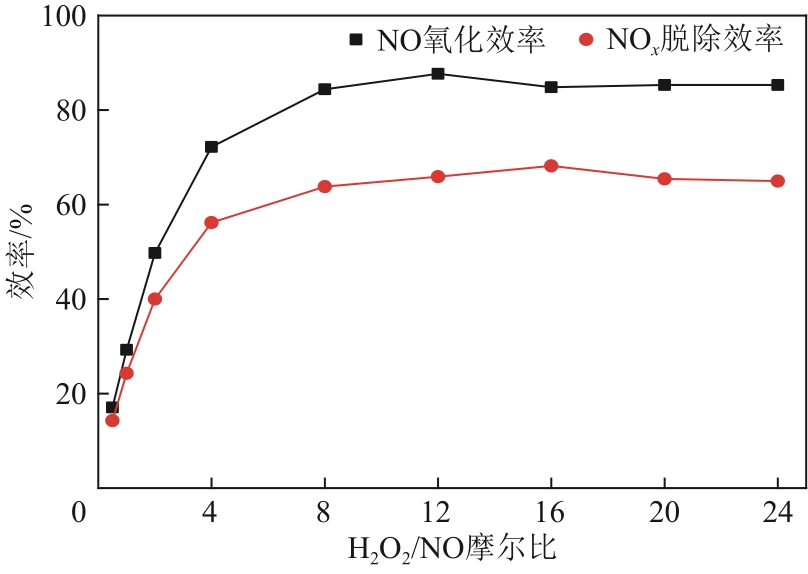
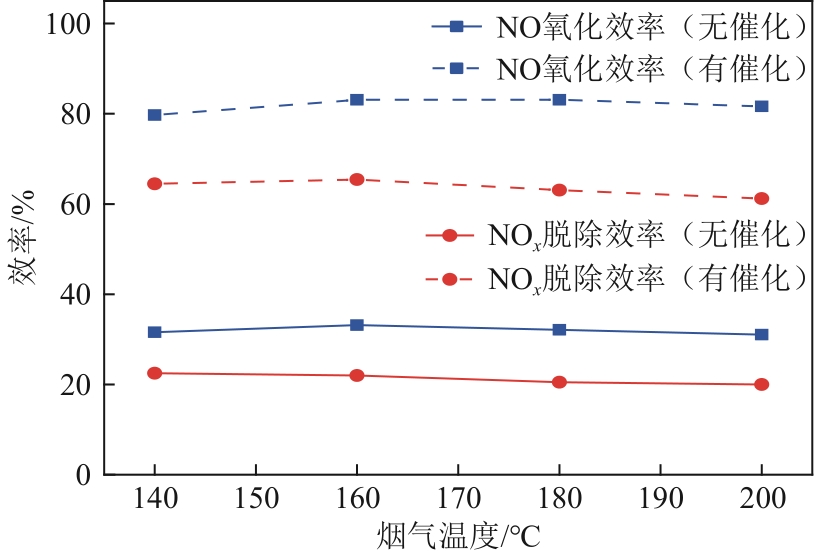
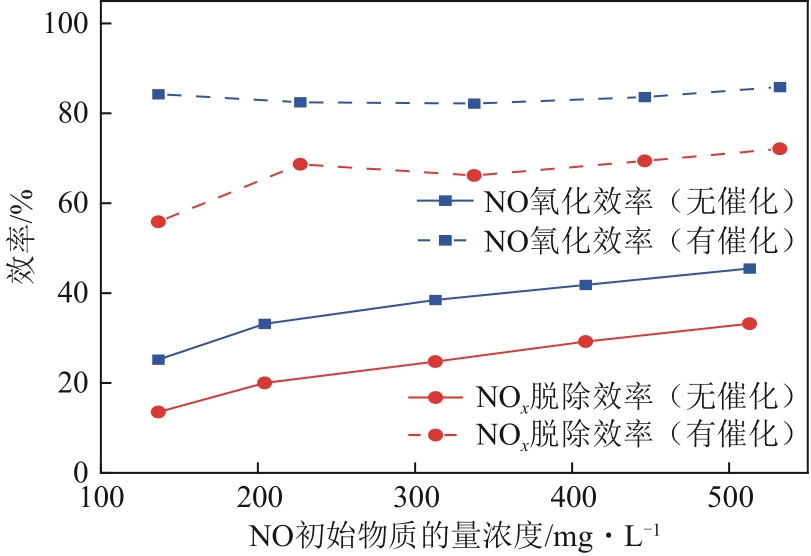
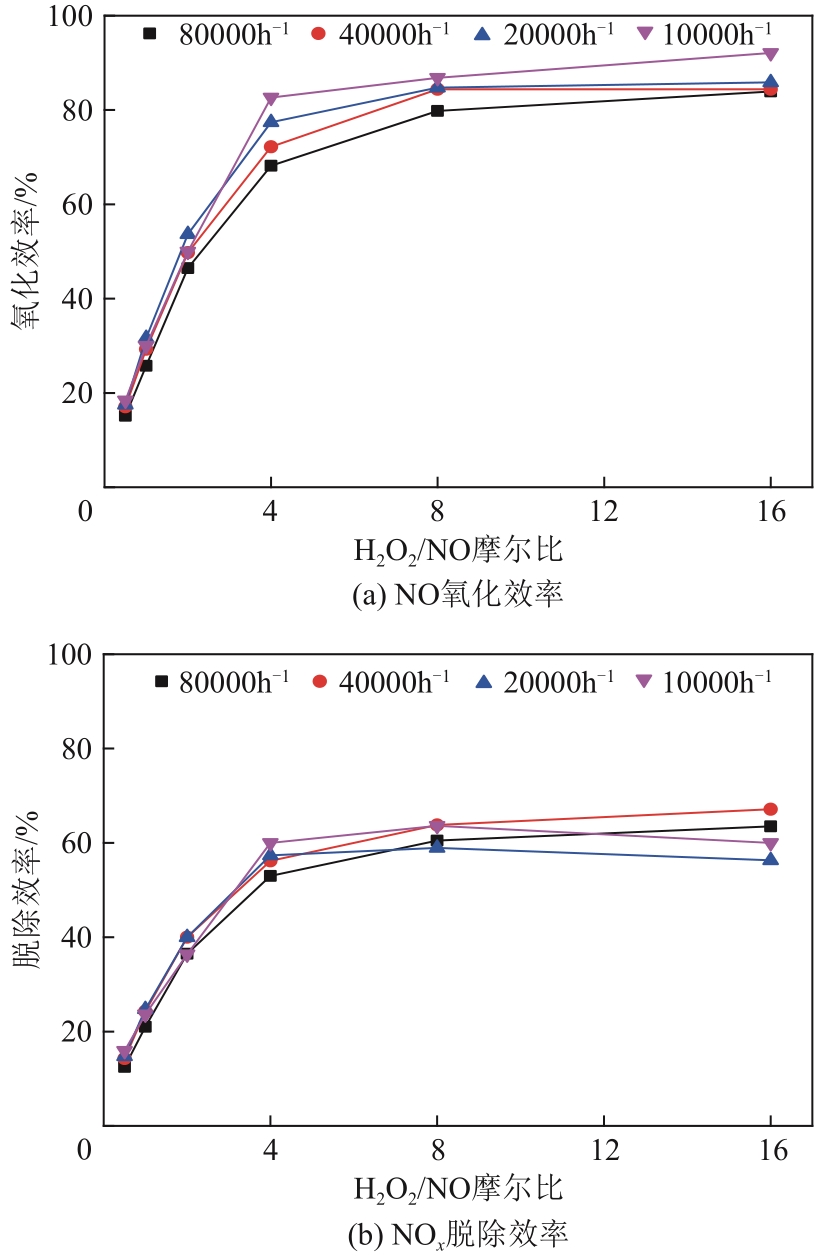
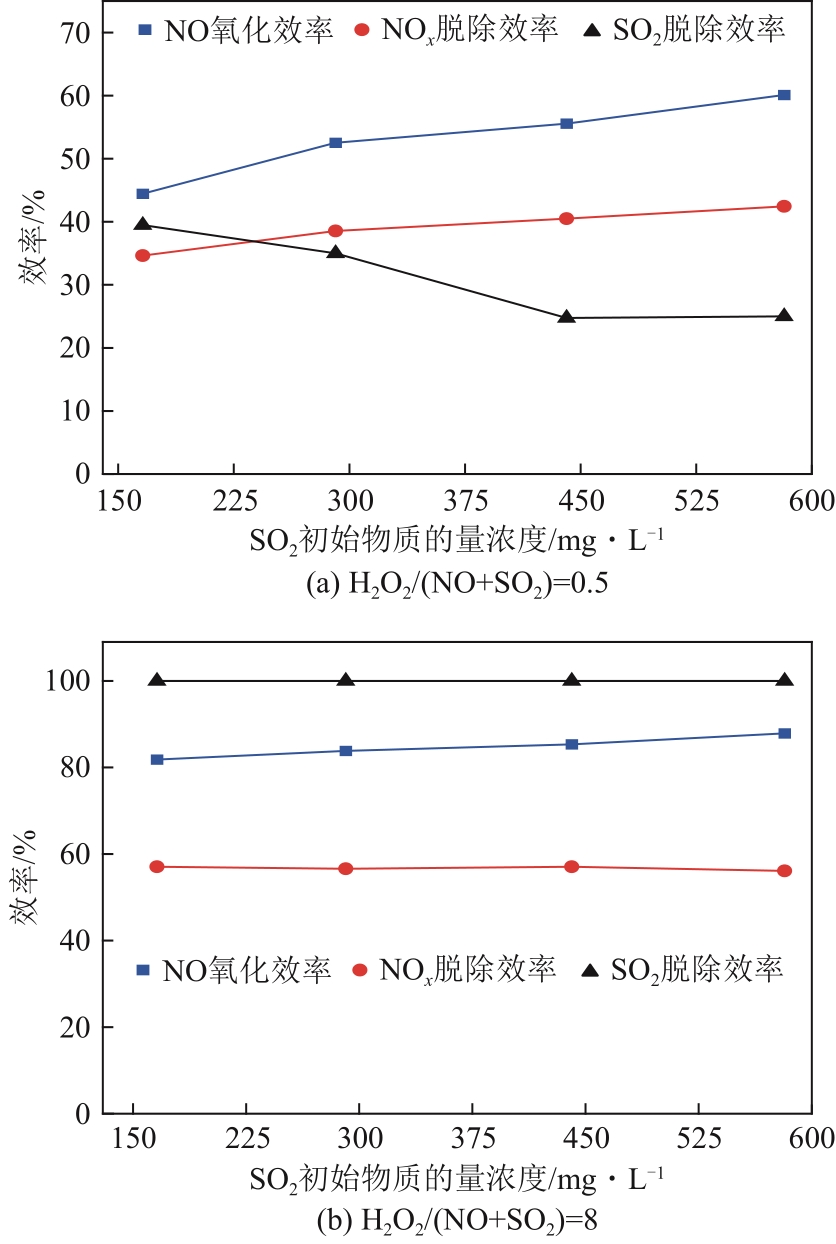
H2O2低温催化氧化法脱硫脱硝技术在燃煤电站深度调峰过程中可以应用于低温烟气。本文搭建中试热态实验系统,选取工业蜂窝状TiO2催化剂,通过X射线衍射(XRD)、X射线荧光光谱(XRF)和扫描电子显微镜(SEM)等对其进行表征,进行脱硫脱硝一体化实验研究,探究各因素对H2O2低温催化氧化脱硝效率的影响规律。结果表明:微观下纳米TiO2颗粒分布均匀、分散性好、纯度高。在单独脱硝实验中,温度对NO氧化和脱除效率影响较小;不采用催化剂时,NO的氧化脱除主要依靠H2O2自身的氧化性以及少量H2O2分解生成的‧HO2,效果较差。采用催化剂后,随着H2O2/NO摩尔比增加,NO氧化及脱除效率与无催化剂时相比大幅提升。在脱硫脱硝实验中,无催化剂条件下SO2对NO的氧化和脱除效率有明显促进作用;有催化剂条件下,当H2O2/(NO+SO2)摩尔比为0.5时,NO与SO2对‧OH的利用存在竞争效应,随着SO2浓度的增加,NO氧化及脱除效率逐渐提升,SO2脱除效率逐渐降低。采用蜂窝TiO2催化剂,当H2O2/NO摩尔比为8时,NO氧化效率达90.4%,NO x 脱除效率达63.7%,SO2脱除效率可达到100%。
中图分类号:
引用本文
修浩然, 王云刚, 白彦渊, 刘涛, 张兴邦, 张益嘉. H2O2低温催化氧化法脱硫脱硝中试实验特性[J]. 化工进展, 2024, 43(9): 4941-4950.
XIU Haoran, WANG Yungang, BAI Yanyuan, LIU Tao, ZHANG Xingbang, ZHANG Yijia. Pilot test of H2O2 low temperature catalytic oxidation for desulfurization and denitrification[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 4941-4950.
| 主要成分 | 质量分数/% |
|---|---|
| CaO | 0.032 |
| SiO2 | 0.039 |
| P2O5 | 0.044 |
| TiO2 | 98.90 |
表1 纳米TiO2的XRF分析
| 主要成分 | 质量分数/% |
|---|---|
| CaO | 0.032 |
| SiO2 | 0.039 |
| P2O5 | 0.044 |
| TiO2 | 98.90 |
| 1 | KRISHNA K, MAKKEE M. Coke formation over zeolites and CeO2-z eolites and its influence on selective catalytic reduction of NO x [J]. Applied Catalysis B: Environmental, 2005, 59(1/2): 35-44. |
| 2 | WU Zhongbiao, JIANG Boqiong, LIU Yue. Effect of transition metals addition on the catalyst of manganese/titania for low-temperature selective catalytic reduction of nitric oxide with ammonia[J]. Applied Catalysis B: Environmental, 2008, 79(4): 347-355. |
| 3 | 顾甜, 高凤雨, 唐晓龙, 等. 炭基材料负载型低温NH3-SCR脱硝催化剂的研究进展[J]. 化工进展, 2019, 38(5): 2329-2338. |
| GU Tian, GAO Fengyu, TANG Xiaolong, et al. Research progress on carbon-based material supported catalysts for the selective catalytic reduction of NO x by NH3 at low temperature[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2329-2338. | |
| 4 | WANG Han, YUAN Bo, HAO Runlong, et al. A critical review on the method of simultaneous removal of multi-air-pollutant in flue gas[J]. Chemical Engineering Journal, 2019, 378: 122155. |
| 5 | 樊庆锌, 王明轩, 关心, 等. 某燃煤电厂300MW机组SCR烟气脱硝装置结构优化[J]. 化工进展, 2014, 33(10): 2806-2814. |
| FAN Qingxin, WANG Mingxuan, GUAN Xin, et al. Optimal design of a SCR-DeNO x system for a 300MW coal-fired power plant[J]. Chemical Industry and Engineering Progress, 2014, 33(10): 2806-2814. | |
| 6 | LIU Yangxian, ADEWUYI Yusuf G. A review on removal of elemental mercury from flue gas using advanced oxidation process: Chemistry and process[J]. Chemical Engineering Research and Design, 2016, 112: 199-250. |
| 7 | 杨加强, 梅毅, 王驰, 等. 湿法烟气脱硝技术现状及发展[J]. 化工进展, 2017, 36(2): 695-704. |
| YANG Jiaqiang, MEI Yi, WANG Chi, et al. Current status and trends on wet flue gas denitration technology[J]. Chemical Industry and Engineering Progress, 2017, 36(2): 695-704. | |
| 8 | CHEN Luke, HSU Chung-Hao, YANG Chenlu. Oxidation and absorption of nitric oxide in a packed tower with sodium hypochlorite aqueous solutions[J]. Environmental Progress, 2005, 24(3): 279-288. |
| 9 | CHU H, LI S Y, CHIEN T W. The absorption kinetics of no from flue gas in a stirred tank reactor with KMnO4/NaOH solutions[J]. Journal of Environmental Science and Health, Part A, 1998, 33(5): 801-827. |
| 10 | JAKUBIAK Maciej P, KORDYLEWSKI. Pilot-scale studies on NO x removal from flue gas via NO ozonation and absorption into NaOH solution[J]. Chemical and Process Engineering, 2012, 33(3): 345-358. |
| 11 | 丁杰. H2O2/O3催化分解及其氧化烟气脱硝研究[D]. 南京: 南京理工大学, 2017. |
| DING Jie. Reserches on catalytic decomposition of H2O2/O3 and oxidation of flue gas denitration[D]. Nanjing: Nanjing University of Science and Technology, 2017. | |
| 12 | 张杰, 邓云波. NaClO氧化法脱硝及与NaOH同时脱硫脱硝技术研究[J]. 节能与环保, 2020(11): 72-73. |
| ZHANG Jie, DENG Yunbo. Study on NaClO xidative denitration and simultaneous desulfurization and denitration with NaOH[J]. Energy Conservation & Environmental Protection, 2020(11): 72-73. | |
| 13 | DE PAIVA J L, KACHAN G C. Modeling and simulation of a packed column for NO x absorption with hydrogen peroxide[J]. Industrial & Engineering Chemistry Research, 1998, 37(2): 609-614. |
| 14 | 苑鹏, 卢凤菊, 梅雪, 等. 高级氧化法在烟气脱硫脱硝脱汞中的应用研究进展[J]. 化工进展, 2016, 35(10): 3313-3322. |
| YUAN Peng, LU Fengju, MEI Xue, et al. Recent progress on application of advanced oxidation processes (AOPs) to remove SO2, NO x and Hg0 from flue gas[J]. Chemical Industry and Engineering Progress, 2016, 35(10): 3313-3322. | |
| 15 | 朱贤. 过氧化氢水溶液强化吸收SO2/NO x 及其反应动力学的实验研究[D]. 上海: 上海交通大学, 2010. |
| ZHU Xian. Experimental and kinetic study on the enhanced absorption of SO2/NO x into aqueous solution containing hydrogen peroxide[D]. Shanghai: Shanghai Jiao Tong University, 2010. | |
| 16 | BAVEJA K K, RAO D Subba, SARKAR M K. Kinetics of absorption of nitric oxide in hydrogen peroxide solutions[J]. Journal of Chemical Engineering of Japan, 1979, 12(4): 322-325. |
| 17 | HAYWOOD J M, COOPER C D. The economic feasibility of using hydrogen peroxide for the enhanced oxidation and removal of nitrogen oxides from coal-fired power plant flue gases[J]. Journal of the Air & Waste Management Association, 1998, 48(3): 238-246. |
| 18 | CHEN Lei, XU Zhiwen, HE Chi, et al. Gas-phase total oxidation of nitric oxide using hydrogen peroxide vapor over Pt/TiO2 [J]. Applied Surface Science, 2018, 457: 821-830. |
| 19 | 柏源, 李忠华, 薛建明, 等. 燃煤烟气H2O2脱硝性能影响因素的实验研究[J]. 化工进展, 2012, 31(1): 208-212. |
| BAI Yuan, LI Zhonghua, XUE Jianming, et al. Experimental study on affecting factors for the performance of denitrification using H2O2 solution in coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2012, 31(1): 208-212. | |
| 20 | 马双忱, 马京香, 赵毅, 等. 采用UV/H2O2体系进行烟气脱硫脱硝的实验研究[J]. 中国电机工程学报, 2009, 29(5): 27-31. |
| MA Shuangchen, MA Jingxiang, ZHAO Yi, et al. Experimental study on desulfurization and denitrification using UV/H2O2 system[J]. Proceedings of the CSEE, 2009, 29(5): 27-31. | |
| 21 | LIU Yangxian, ZHANG Jun, SHENG Changdong, et al. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process[J]. Chemical Engineering Journal, 2010, 162(3): 1006-1011. |
| 22 | MA Jinzhu, HE Hong, LIU Fudong. Effect of Fe on the photocatalytic removal of NO x over visible light responsive Fe/TiO2 catalysts[J]. Applied Catalysis B: Environmental, 2015(179) 21-28. |
| 23 | 王琦. 基于TiO2改性催化剂催化H2O2氧化低温脱硝的实验研究[D]. 北京: 华北电力大学, 2018. |
| WANG Qi. Experimental study on low temperature H2O2-based oxidized denitration catalyzed by TiO2 modified catalyst[D]. Beijing: North China Electric Power University, 2018. | |
| 24 | 茹晋波. 基于H2O2催化预氧化NO的湿法烟气同时脱硫脱硝特性研究[D]. 南京: 东南大学, 2017. |
| RU Jinbo. Simultaneous desulfurization and denitrification from flue gas by catalytic preoxidation of NO with H2O2 [D]. Nanjing: Southeast University, 2017. | |
| 25 | JIA Shuaihui, PU Ge, XIONG Weicheng, et al. Investigation on simultaneous removal of SO2 and NO over a Cu-Fe/TiO2 catalyst using vaporized H2O2: An analysis on SO2 effect[J]. Industrial & Engineering Chemistry Research, 2021, 60(37): 13474-13484. |
| 26 | MENG Fanyu, ZHANG Shule, ZHANG Mingjia, et al. The mechanism of Ce-MCM-41 catalyzed peroxone reaction into ·OH and ·O 2 - radicals for enhanced NO oxidation[J]. Molecular Catalysis, 2022, 518: 112110. |
| 27 | LIU Xuan, WANG Changan, ZHU Tao, et al. Simultaneous removal of NO x and SO2 from coal-fired flue gas based on the catalytic decomposition of H2O2 over Fe2(MoO4)3 [J]. Chemical Engineering Journal, 2019, 371: 486-499. |
| 28 | 涂汉超. 含氮生物质气化气燃烧的NO x 排放特性研究[D]. 杭州: 浙江大学, 2018. |
| TU Hanchao. Study on NO x emission characteristics of biomass gasification gas combustion with nitrogenous compounds[D]. Hangzhou: Zhejiang University, 2018. | |
| 29 | 吕博, 涂淑平, 孙文哲, 等. H2O2低温脱硫脱硝研究及应用进展[J]. 应用化工, 2020, 49(10): 2654-2656, 2661. |
| Bo LYU, TU Shuping, SUN Wenzhe, et al. Research and application progress of H2O2 low temperature desulfurization and denitrification[J]. Applied Chemical Industry, 2020, 49(10): 2654-2656, 2661. | |
| 30 | QI Yongfeng, GE Panle, WANG Meiting, et al. Experimental investigation and numerical simulation of simultaneous desulfurization and denitrification by H2O2 solution assisted with microwave and additive[J]. Chemical Engineering Journal, 2020, 391: 123559. |
| 31 | YANG Bingchuan, MA Suxia, CUI Rongji, et al. Novel low-cost simultaneous removal of NO and SO2 with ·OH from decomposition of H2O2 catalyzed by alkali-magnetic modified fly ash[J]. Industrial & Engineering Chemistry Research, 2019, 58(13): 5339-5347. |
| 32 | MATTHEWS Ralph W. Hydroxylation reactions induced by near-ultraviolet photolysis of aqueous titanium dioxide suspensions[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1984, 80(2): 457. |
| [1] | 高玉李, 王红秋, 黄格省, 鲜楠莹, 师晓玉. 全固态锂电池的产业化和技术研究进展[J]. 化工进展, 2024, 43(9): 4767-4778. |
| [2] | 刘振涛, 梅金林, 王春雅, 段爱军, 巩雁军, 徐春明, 王喜龙. 一步法加氢制生物航煤催化剂研究进展[J]. 化工进展, 2024, 43(9): 4909-4924. |
| [3] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| [4] | 胡婷霞, 赵立欣, 姚宗路, 霍丽丽, 贾吉秀, 谢腾. 双金属催化剂在生物质焦油催化蒸汽重整领域的研究进展[J]. 化工进展, 2024, 43(8): 4354-4365. |
| [5] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [6] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [7] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
| [8] | 宋占龙, 汤涛, 潘蔚, 赵希强, 孙静, 毛岩鹏, 王文龙. 微纳米气泡强化臭氧氧化降解含酚废水[J]. 化工进展, 2024, 43(8): 4614-4623. |
| [9] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [10] | 毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765. |
| [11] | 李文哲, 申淼, 王建强. 熔盐法制备新型二维层状金属碳/氮化物(MXene)的研究进展[J]. 化工进展, 2024, 43(7): 3660-3671. |
| [12] | 张子杭, 王树荣. 生物质热解转化与产物低碳利用研究进展[J]. 化工进展, 2024, 43(7): 3692-3708. |
| [13] | 龚德成, 沈倩, 朱贤青, 黄云, 夏奡, 张敬苗, 朱恂, 廖强. 微藻超临界水气化制取富氢合成气的研究进展[J]. 化工进展, 2024, 43(7): 3709-3728. |
| [14] | 郭鹏, 李红伟, 李贵贤, 季东, 王东亮, 赵新红. 直接甲醇燃料电池阳极催化剂的失活机制及应对策略[J]. 化工进展, 2024, 43(7): 3812-3823. |
| [15] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||