化工进展 ›› 2024, Vol. 43 ›› Issue (7): 3812-3823.DOI: 10.16085/j.issn.1000-6613.2023-0936
直接甲醇燃料电池阳极催化剂的失活机制及应对策略
郭鹏1( ), 李红伟1,2(
), 李红伟1,2( ), 李贵贤1,2, 季东1,2, 王东亮1,2, 赵新红1,2(
), 李贵贤1,2, 季东1,2, 王东亮1,2, 赵新红1,2( )
)
- 1.兰州理工大学石油化工学院,甘肃 兰州 730050
2.甘肃省低碳能源化工重点实验室,甘肃 兰州 730050
-
收稿日期:2023-06-07修回日期:2023-07-11出版日期:2024-07-25发布日期:2024-08-14 -
通讯作者:李红伟,赵新红 -
作者简介:郭鹏(1997—),男,博士研究生,研究方向为低碳能源催化。E-mail:gpchem9299@163.com。 -
基金资助:甘肃省基础研究创新群体项目(22JR5RA219)
Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells
GUO Peng1( ), LI Hongwei1,2(
), LI Hongwei1,2( ), LI Guixian1,2, JI Dong1,2, WANG Dongliang1,2, ZHAO Xinhong1,2(
), LI Guixian1,2, JI Dong1,2, WANG Dongliang1,2, ZHAO Xinhong1,2( )
)
- 1.College of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
2.Key Laboratory of Low Carbon Energy and Chemical Engineering of Gansu Province, Lanzhou 730050, Gansu, China
-
Received:2023-06-07Revised:2023-07-11Online:2024-07-25Published:2024-08-14 -
Contact:LI Hongwei, ZHAO Xinhong
摘要:
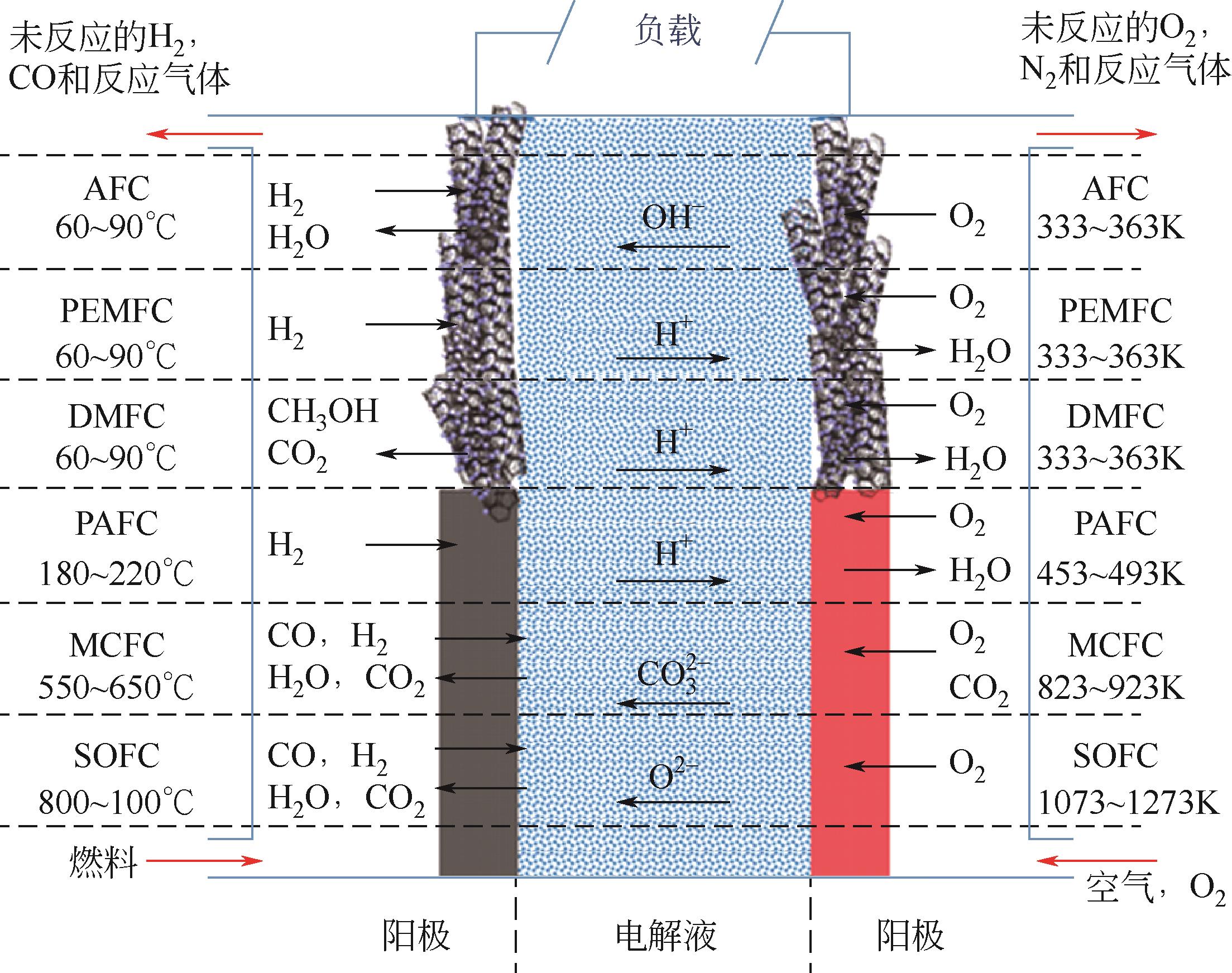
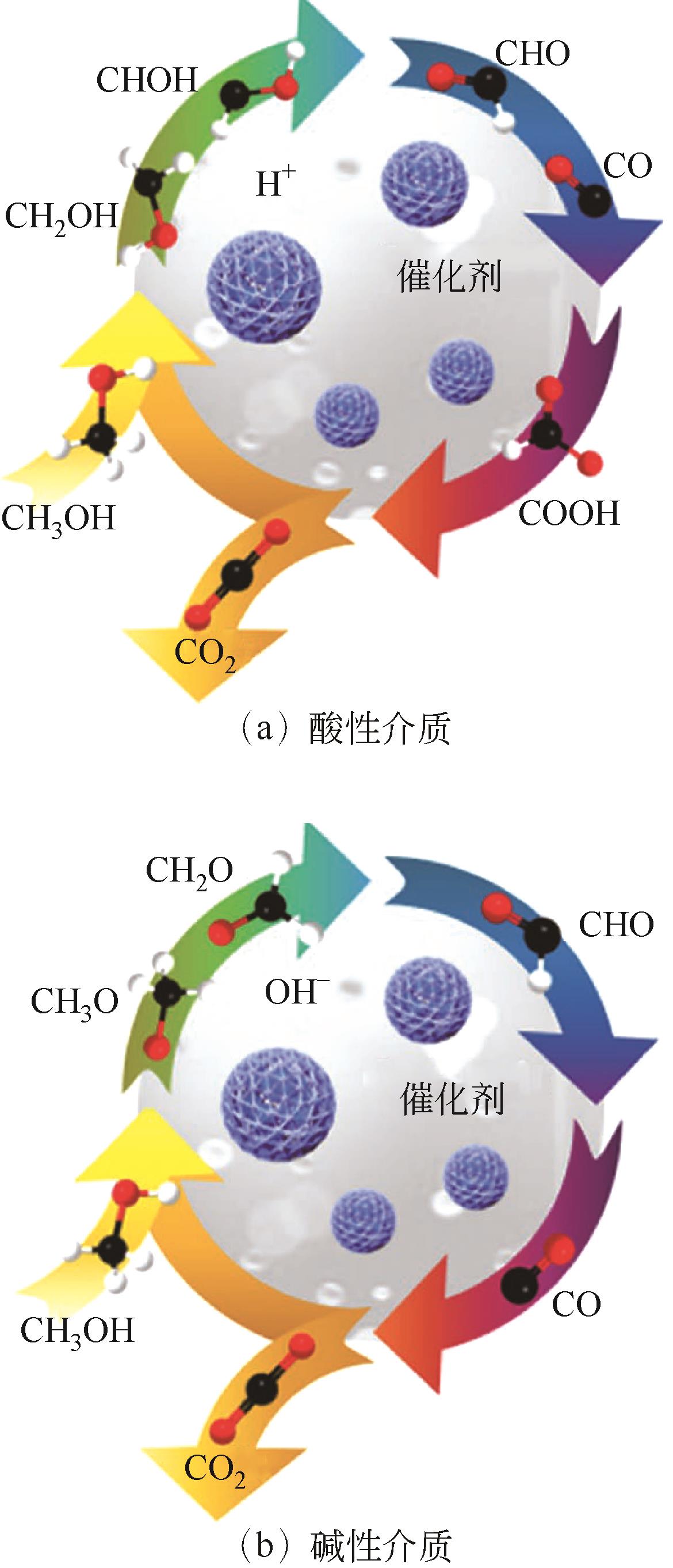
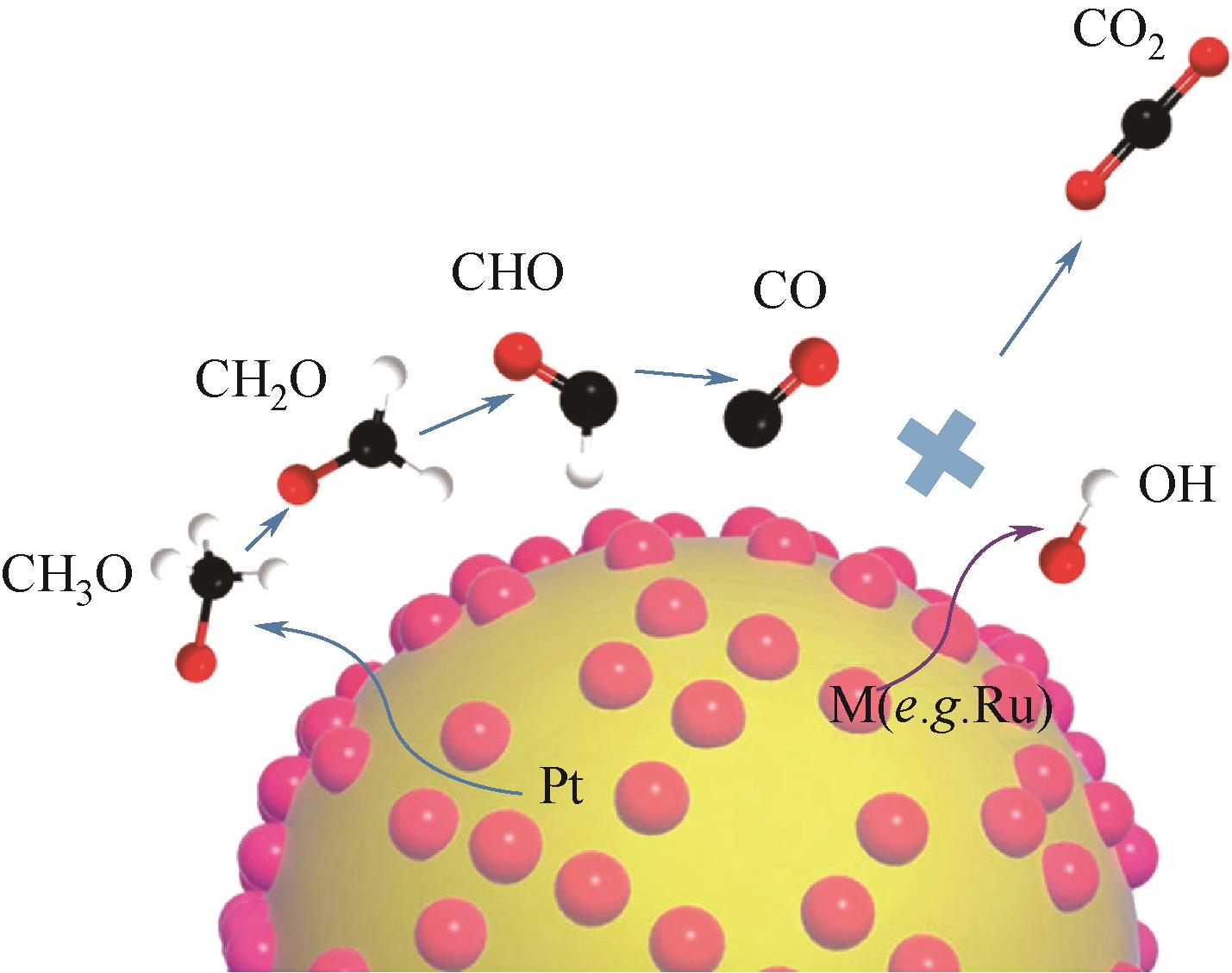
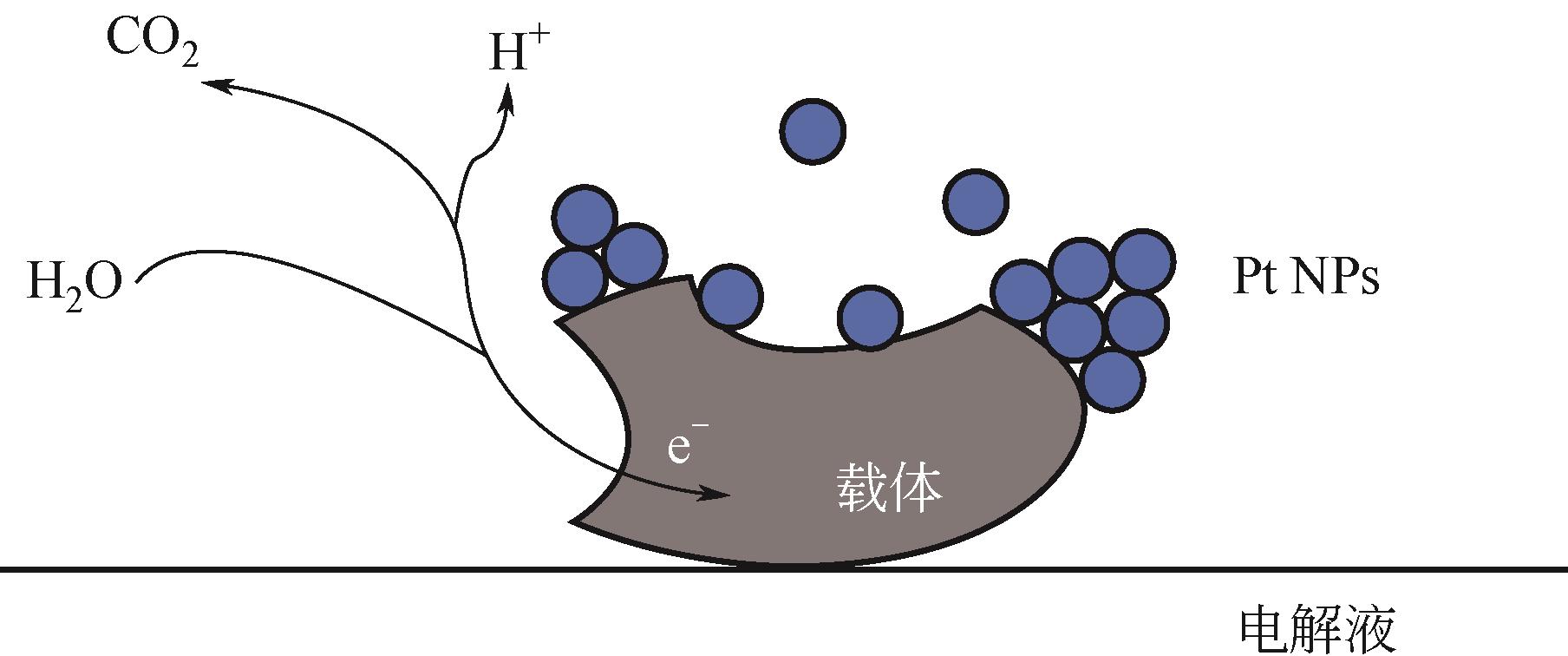
甲醇氧化电催化剂是决定直接甲醇燃料电池(direct methanol fuel cells,DMFC)性能与成本的关键。目前,铂基催化剂是最有前途的高效甲醇氧化电催化剂,但在反应过程中存在活性位的迁移、团聚与浸出、中毒以及载体的腐蚀与坍塌等原因引起的失活问题,阻碍了其进一步商业化发展。如何提高直接甲醇燃料电池阳极催化剂的稳定性是一个亟待解决的难题。本文首先总结了甲醇的电氧化原理和催化反应机理,详细综述了阳极催化剂的失活机制以及抑制改善其失活方面所取得的研究进展。最后对该领域未来的发展方向进行了展望,并指出利用限域作用限制活性金属的迁移和聚集,构建多元合金,设计增强复合型载体,将理论研究和原位表征技术相结合,是今后开发更高效、稳定阳极催化剂的重点研究方向。
中图分类号:
引用本文
郭鹏, 李红伟, 李贵贤, 季东, 王东亮, 赵新红. 直接甲醇燃料电池阳极催化剂的失活机制及应对策略[J]. 化工进展, 2024, 43(7): 3812-3823.
GUO Peng, LI Hongwei, LI Guixian, JI Dong, WANG Dongliang, ZHAO Xinhong. Mechanisms and coping strategies on deactivation of anode catalysts for direct methanol fuel cells[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3812-3823.
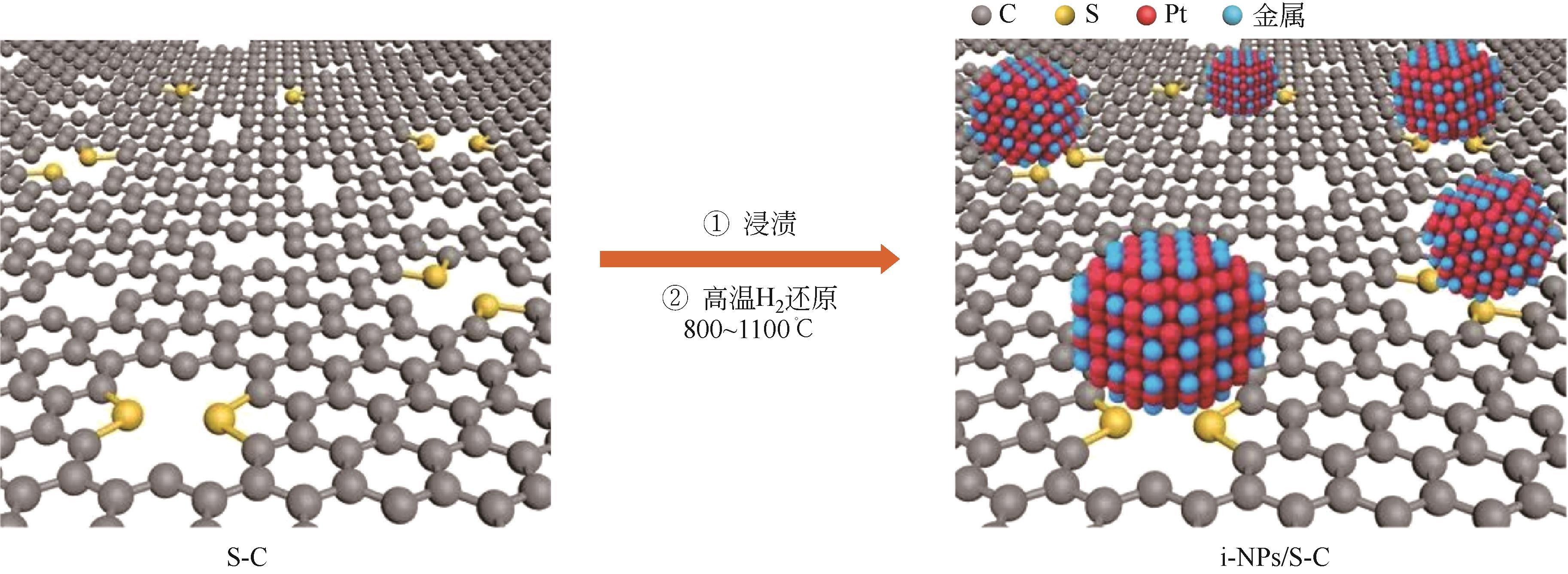
| 催化剂 | 制备方法 | 测试条件 | 稳定性 |
|---|---|---|---|
| Ce改性的Pt NPs/C[ | 乙二醇化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | -0.2V下放电1000s后保持79.2%的初始活性 |
| PtCo3@CNTs[ | g-C3N4辅助热合成法 | 0.5mol/L H2SO4 + 0.5mol/L CH3OH | 1500圈加速老化后保持87.3%的初始活性 |
| Pd-P@Pt-Ni[ | 化学还原法 | 0.5mol/L H2SO4 + 0.5mol/L CH3OH | 500圈循环后ECSA降低2% |
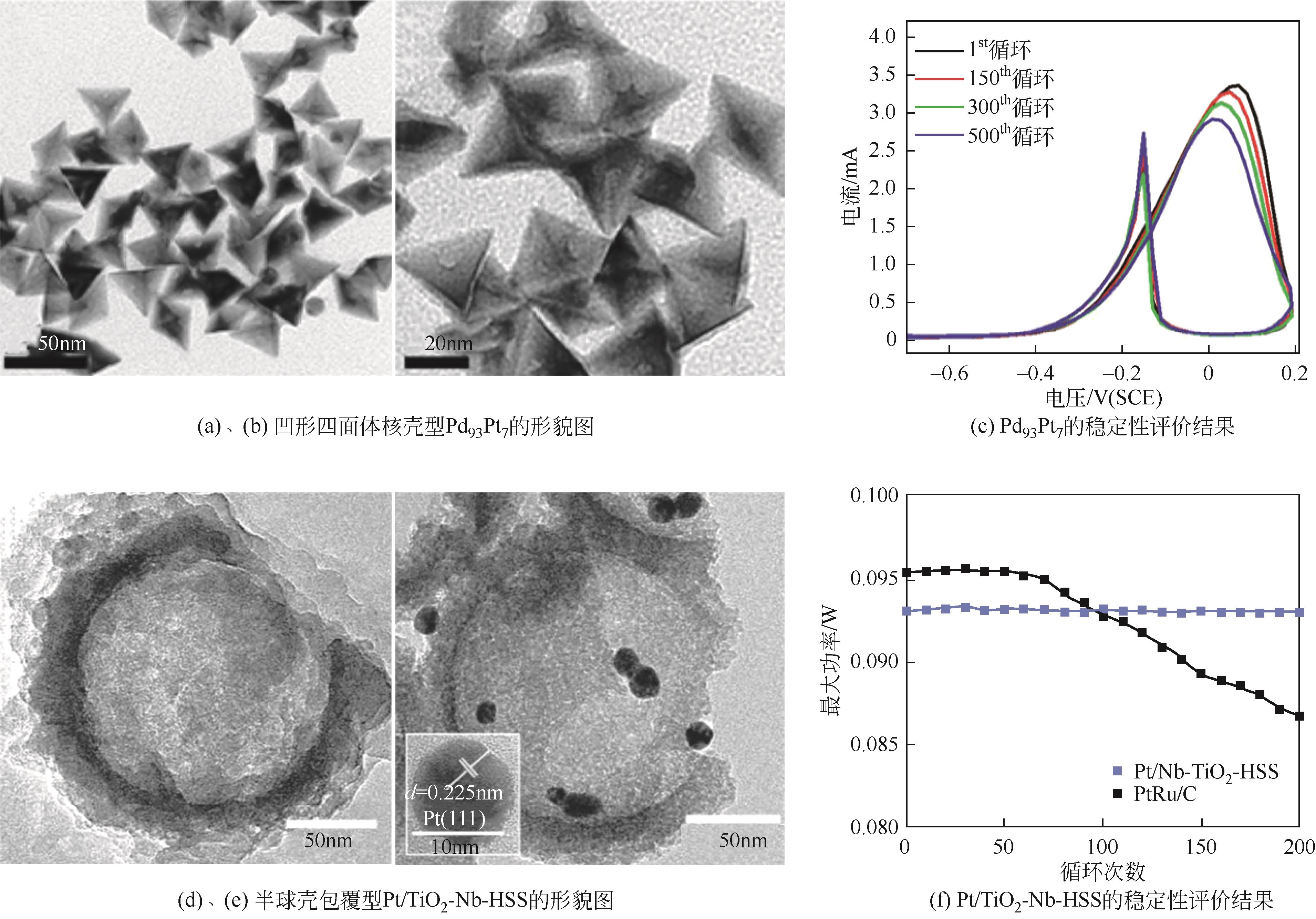
| Pd93Pt7[ | 一锅法 | 0.1mol/L NaOH + 0.5mol/LCH3OH | 500圈循环后保持86.5%的初始活性 |
| Pt/TiO2-Nb-HSS[ | 硬模板法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 80℃循环200圈活性未发生明显下降 |
| Pt-Au HNU[ | 种晶生长法 | 0.1mol/L HClO4 + 0.5mol/L CH3OH | 3000圈加速老化后ECSA降低25% |
| PtCo CNCs[ | 水热合成法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 0.6V下放电8000s保持约25%的初始活性 |
| PtSn/ATO[ | 化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 500圈加速老化后活性降低15% |
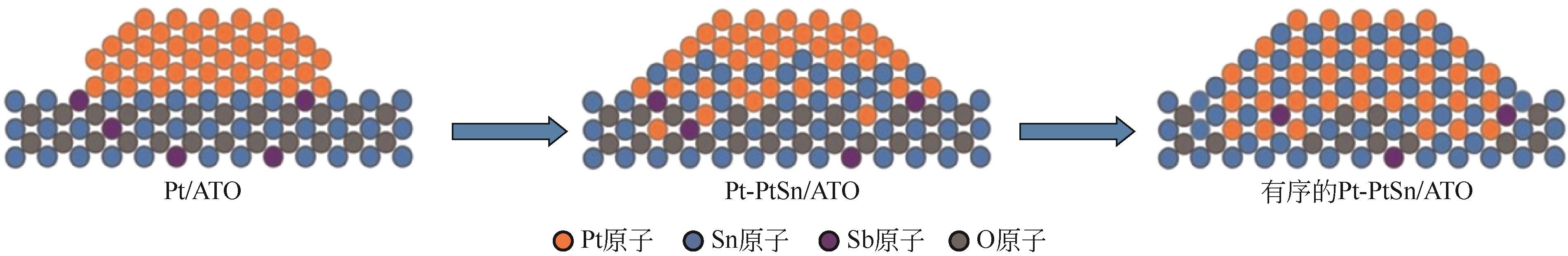
| Pt3Co/CNTs-M[ | 化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 500圈循环后质量比活性下降20.9% |
| PtRuCu[ | 电化学置换法 | 0.1mol/L HClO4 + 0.5mol/LCH3OH | 800圈循环后质量比活性下降27% |
| Pt17Pd16Ru22Te45[ | 牺牲模板法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 1000圈加速老化后质量比活性下降2.75% |
表1 代表性甲醇氧化电催化剂制备方法、测试条件及稳定性
| 催化剂 | 制备方法 | 测试条件 | 稳定性 |
|---|---|---|---|
| Ce改性的Pt NPs/C[ | 乙二醇化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | -0.2V下放电1000s后保持79.2%的初始活性 |
| PtCo3@CNTs[ | g-C3N4辅助热合成法 | 0.5mol/L H2SO4 + 0.5mol/L CH3OH | 1500圈加速老化后保持87.3%的初始活性 |
| Pd-P@Pt-Ni[ | 化学还原法 | 0.5mol/L H2SO4 + 0.5mol/L CH3OH | 500圈循环后ECSA降低2% |
| Pd93Pt7[ | 一锅法 | 0.1mol/L NaOH + 0.5mol/LCH3OH | 500圈循环后保持86.5%的初始活性 |
| Pt/TiO2-Nb-HSS[ | 硬模板法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 80℃循环200圈活性未发生明显下降 |
| Pt-Au HNU[ | 种晶生长法 | 0.1mol/L HClO4 + 0.5mol/L CH3OH | 3000圈加速老化后ECSA降低25% |
| PtCo CNCs[ | 水热合成法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 0.6V下放电8000s保持约25%的初始活性 |
| PtSn/ATO[ | 化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 500圈加速老化后活性降低15% |
| Pt3Co/CNTs-M[ | 化学还原法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 500圈循环后质量比活性下降20.9% |
| PtRuCu[ | 电化学置换法 | 0.1mol/L HClO4 + 0.5mol/LCH3OH | 800圈循环后质量比活性下降27% |
| Pt17Pd16Ru22Te45[ | 牺牲模板法 | 0.5mol/L H2SO4 + 1mol/L CH3OH | 1000圈加速老化后质量比活性下降2.75% |
| 催化剂 | 制备方法 | 测试条件 | 抗中毒性 |
|---|---|---|---|
| Pt3Rh[ | 化学还原法 | 0.5mol/L H2SO4+0.5mol/L CH3OH | If/Ib=2.61 |
| Au3Ag NFs[ | 一锅法 | 0.5mol/L KOH+2mol/L CH3OH | If/Ib=2.5 |
| Pt1Ag3 DSNCs[ | 水热法 | 0.5mol/L H2SO4+0.5mol/L CH3OH | If/Ib=1.28 |
| Pt/SiC[ | 化学还原法 | 0.5mol/L KOH+0.5mol/L CH3OH | If/Ib=1.48 |
表2 代表性催化剂抗CO中毒能力、制备方法及测试条件
| 催化剂 | 制备方法 | 测试条件 | 抗中毒性 |
|---|---|---|---|
| Pt3Rh[ | 化学还原法 | 0.5mol/L H2SO4+0.5mol/L CH3OH | If/Ib=2.61 |
| Au3Ag NFs[ | 一锅法 | 0.5mol/L KOH+2mol/L CH3OH | If/Ib=2.5 |
| Pt1Ag3 DSNCs[ | 水热法 | 0.5mol/L H2SO4+0.5mol/L CH3OH | If/Ib=1.28 |
| Pt/SiC[ | 化学还原法 | 0.5mol/L KOH+0.5mol/L CH3OH | If/Ib=1.48 |
| 1 | KAKATI Nitul, MAITI Jatindranath, LEE Seok Hee, et al. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt-Ru?[J]. Chemical Reviews, 2014, 114(24): 12397-12429. |
| 2 | STEELE B C, HEINZEL A. Materials for fuel-cell technologies[J]. Nature, 2001, 414(6861): 345-352. |
| 3 | GREELEY J, STEPHENS I E L, BONDARENKO A S, et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts[J]. Nature Chemistry, 2009, 1(7): 552-556. |
| 4 | LUO Mingchuan, ZHAO Zhonglong, ZHANG Yelong, et al. PdMo bimetallene for oxygen reduction catalysis[J]. Nature, 2019, 574(7776): 81-85. |
| 5 | WAN Xin, LIU Xiaofang, LI Yongcheng, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells[J]. Nature Catalysis, 2019, 2: 259-268. |
| 6 | ZHOU Yuanyuan, XIE Zhenyang, JIANG Jinxia, et al. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction[J]. Nature Catalysis, 2020, 3: 454-462. |
| 7 | ZHU Jiexin, XIA Lixue, YU Ruohan, et al. Ultrahigh stable methanol oxidation enabled by a high hydroxyl concentration on Pt clusters/MXene interfaces[J]. Journal of the American Chemical Society, 2022, 144(34): 15529-15538. |
| 8 | NASSR Abu Bakr Ahmed Amine, SINEV Ilya, Wolfgang GRÜUNERT, et al. PtNi supported on oxygen functionalized carbon nanotubes: In depth structural characterization and activity for methanol electrooxidation[J]. Applied Catalysis B: Environmental, 2013, 142: 849-860. |
| 9 | ZHAO Haiqiang, QI Weihong, ZHOU Xinfeng, et al. Composition-controlled synthesis of platinum and palladium nanoalloys as highly active electrocatalysts for methanol oxidation[J]. Chinese Journal of Catalysis, 2018, 39(2): 342-349. |
| 10 | HU Gaofei, SHANG Liang, SHENG Tian, et al. PtCo@NCs with short heteroatom active site distance for enhanced catalytic properties[J]. Advanced Functional Materials, 2020, 30(28): 2002281. |
| 11 | GUPTA Divyani, CHAKRABORTY Sudip, AMORIM Rodrigo G, et al. Local electrocatalytic activity of PtRu supported on nitrogen-doped carbon nanotubes towards methanol oxidation by scanning electrochemical microscopy[J]. Journal of Materials Chemistry A, 2021, 9(37): 21291-21301. |
| 12 | ZHANG Zhicheng, LUO Zhimin, CHEN Bo, et al. One-pot synthesis of highly anisotropic five-fold-twinned PtCu nanoframes used as a bifunctional electrocatalyst for oxygen reduction and methanol oxidation[J]. Advanced Materials, 2016, 28(39): 8712-8717. |
| 13 | CAI Zuocheng, KAMIKO Masao, YAMADA Ikuya, et al. PtCo3 nanoparticle-encapsulated carbon nanotubes as active catalysts for methanol fuel cell anodes[J]. ACS Applied Nano Materials, 2021, 4(2): 1445-1454. |
| 14 | LI Guoxing, LI Yuliang, LIU Huibiao, et al. Architecture of graphdiyne nanoscale films[J]. Chemical Communications, 2010, 46(19): 3256-3258. |
| 15 | HUI Lan, XUE Yurui, XING Chengyu, et al. Highly loaded independent Pt0 atoms on graphdiyne for pH-general methanol oxidation reaction[J]. Advanced Science, 2022, 9(16): e2104991. |
| 16 | ZHANG Zhiqi, LIU Jiapeng, WANG Jian, et al. Single-atom catalyst for high-performance methanol oxidation[J]. Nature Communications, 2021, 12(1): 5235. |
| 17 | MARTÍN Antonio J, MITCHELL Sharon, MONDELLI Cecilia, et al. Unifying views on catalyst deactivation[J]. Nature Catalysis, 2022, 5: 854-866. |
| 18 | MOULIJN J A, VAN DIEPEN A E, KAPTEIJN F. Catalyst deactivation: Is it predictable?[J]. Applied Catalysis A: General, 2001, 212(1/2): 3-16. |
| 19 | XIA Zhangxun, ZHANG Xiaoming, SUN Hai, et al. Recent advances in multi-scale design and construction of materials for direct methanol fuel cells[J]. Nano Energy, 2019, 65: 104048. |
| 20 | TIWARI Jitendra N, TIWARI Rajanish N, SINGH Gyan, et al. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells[J]. Nano Energy, 2013, 2(5): 553-578. |
| 21 | HAMNETT A. Mechanism and electrocatalysis in the direct methanol fuel cell[J]. Catalysis Today, 1997, 38(4): 445-457. |
| 22 | WANG Jianmei, ZHANG Bingxing, GUO Wei, et al. Toward electrocatalytic methanol oxidation reaction: Longstanding debates and emerging catalysts[J]. Advanced Materials, 2023, 35(26): e2211099. |
| 23 | LIU Hansan, SONG Chaojie, ZHANG Lei, et al. A review of anode catalysis in the direct methanol fuel cell[J]. Journal of Power Sources, 2006, 155(2): 95-110. |
| 24 | YU Eileen Hao, KREWER Ulrike, SCOTT Keith. Principles and materials aspects of direct alkaline alcohol fuel cells[J]. Energies, 2010, 3(8): 1499-1528. |
| 25 | GOODMAN Emmett D, SCHWALBE Jay A, CARGNELLO Matteo. Mechanistic understanding and the rational design of sinter-resistant heterogeneous catalysts[J]. ACS Catalysis, 2017, 7(10): 7156-7173. |
| 26 | HANSEN Thomas W, DELARIVA Andrew T, CHALLA Sivakumar R, et al. Sintering of catalytic nanoparticles: Particle migration or Ostwald ripening?[J]. Accounts of Chemical Research, 2013, 46(8): 1720-1730. |
| 27 | WANG Lingxiang, WANG Liang, MENG Xiangju, et al. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts[J]. Advanced Materials, 2019, 31(50): e1901905. |
| 28 | CHEN Ligang, LIANG Xin, LI Xiaotong, et al. Promoting electrocatalytic methanol oxidation of platinum nanoparticles by cerium modification[J]. Nano Energy, 2020, 73: 104784. |
| 29 | YANG Chenglong, WANG Lina, YIN Peng, et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells[J]. Science, 2021, 374(6566): 459-464. |
| 30 | YOO Ji Mun, SHIN Heejong, CHUNG Dong Young, et al. Carbon shell on active nanocatalyst for stable electrocatalysis[J]. Accounts of Chemical Research, 2022, 55(9): 1278-1289. |
| 31 | TONG Yueyu, YAN Xiao, LIANG Ji, et al. Metal-based electrocatalysts for methanol electro-oxidation: Progress, opportunities, and challenges[J]. Small, 2021, 17(9): e1904126. |
| 32 | LI Ziwei, LI Min, BIAN Zhoufeng, et al. Design of highly stable and selective core/yolk-shell nanocatalysts—A review[J]. Applied Catalysis B: Environmental, 2016, 188: 324-341. |
| 33 | GUO Jiangbin, ZHANG Man, XU Jing, et al. Core-shell Pd-P@Pt-Ni nanoparticles with enhanced activity and durability as anode electrocatalyst for methanol oxidation reaction[J]. RSC Advances, 2022, 12(4): 2246-2252. |
| 34 | ZHANG Ying, BU Lingzheng, JIANG Kezhu, et al. Concave Pd-Pt core-shell nanocrystals with ultrathin Pt shell feature and enhanced catalytic performance[J]. Small, 2016, 12(6): 706-712. |
| 35 | REN Bingzhi, LU Jianwei, WANG Yucheng, et al. Half-sphere shell supported Pt catalyst for electrochemical methanol oxidation[J]. Journal of the Electrochemical Society, 2020, 167(8): 084510. |
| 36 | MA Siyue, LI Huihui, HU Bicheng, et al. Synthesis of low Pt-based quaternary PtPdRuTe nanotubes with optimized incorporation of Pd for enhanced electrocatalytic activity[J]. Journal of the American Chemical Society, 2017, 139(16): 5890-5895. |
| 37 | YOU Hongjun, ZHANG Fangling, LIU Zhen, et al. Free-standing Pt-Au hollow nanourchins with enhanced activity and stability for catalytic methanol oxidation[J]. ACS Catalysis, 2014, 4(9): 2829-2835. |
| 38 | LI Zhijuan, JIANG Xian, WANG Xiaoru, et al. Concave PtCo nanocrosses for methanol oxidation reaction[J]. Applied Catalysis B: Environmental, 2020, 277: 119135. |
| 39 | CHEN Wei, LEI Zhao, ZENG Tang, et al. Structurally ordered PtSn intermetallic nanoparticles supported on ATO for efficient methanol oxidation reaction[J]. Nanoscale, 2019, 11(42): 19895-19902. |
| 40 | LI Yanru, LI Hongwei, LI Guixian, et al. Low-temperature N-anchored ordered Pt3Co intermetallic nanoparticles as electrocatalysts for methanol oxidation reaction[J]. Nanoscale, 2022, 14(38): 14199-14211. |
| 41 | XUE Shengfeng, DENG Wentao, YANG Fang, et al. Hexapod PtRuCu nanocrystalline alloy for highly efficient and stable methanol oxidation[J]. ACS Catalysis, 2018, 8(8): 7578-7584. |
| 42 | GOODMAN Emmett D, ASUNDI Arun S, HOFFMAN Adam S, et al. Monolayer support control and precise colloidal nanocrystals demonstrate metal-support interactions in heterogeneous catalysts[J]. Advanced Materials, 2021, 33(44): e2104533. |
| 43 | SHEN Shancheng, XU Shilong, ZHAO Shuai, et al. Synthesis of carbon-metal oxide composites as catalyst supports by “cooking sugar with salt”[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(2): 731-737. |
| 44 | HUANG Michael H, CHIU Chun-Ya. Achieving polyhedral nanocrystal growth with systematic shape control[J]. Journal of Materials Chemistry A, 2013, 1(28): 8081-8092. |
| 45 | NARESH N, WASIM F G S, LADEWIG B P, et al. Removal of surfactant and capping agent from Pd nanocubes (Pd-NCs) using tert-butylamine: Its effect on electrochemical characteristics[J]. Journal of Materials Chemistry A, 2013, 1(30): 8553-8559. |
| 46 | H-P DHAR, L-G CHRISTNER, A-K KUSH, et al. Performance study of a fuel cell Pt-on-C anode in presence of CO and CO2, and calculation of adsorption parameters for CO poisoning[J]. Journal of The Electrochemical Society, 1986, 133(8): 1574. |
| 47 | MANCHARAN Ramasamy, GOODENOUGH John B. Methanol oxidation in acid on ordered NiTi[J]. Journal of Materials Chemistry, 1992, 2(8): 875-887. |
| 48 | ZHANG Y, SHU G Q, SHANG Z T, et al. Electronic and cooridination effect of PtPd nanoflower alloys for the methanol electrooxidation reaction[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(24): 8958-8967. |
| 49 | LIN Shawn D, HSIAO Ting-Chou, CHANG Jen-Ray, et al. Morphology of carbon supported Pt-Ru electrocatalyst and the CO tolerance of anodes for PEM fuel cells[J]. The Journal of Physical Chemistry B, 1999, 103(1): 97-103. |
| 50 | WATANABE M. Electrocatalysis by ad-atoms: Part Ⅲ. Enhancement of the oxidation of carbon monoxide on platinum by ruthenium ad-atoms[J]. Journal of Electroanalytical Chemistry, 1975, 60(3): 275-283. |
| 51 | WATANABE Masahiro, UCHIDA Makoto, MOTOO Satoshi. Preparation of highly dispersed Pt + Ru alloy clusters and the activity for the electrooxidation of methanol[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1987, 229(1/2): 395-406. |
| 52 | NARAYANAMOORTHY Bhuvanendran, DATTA Kasibhatta Kumara Ramanatha, ESWARAMOORTHY Muthusamy, et al. Highly active and stable Pt3Rh nanoclusters as supportless electrocatalyst for methanol oxidation in direct methanol fuel cells[J]. ACS Catalysis, 2014, 4(10): 3621-3629. |
| 53 | XIONG Likun, SUN Zhongti, ZHANG Xiang, et al. Octahedral gold-silver nanoframes with rich crystalline defects for efficient methanol oxidation manifesting a CO-promoting effect[J]. Nature Communications, 2019, 10(1): 3782. |
| 54 | YAO Wenqing, JIANG Xian, LI Meng, et al. Engineering hollow porous platinum-silver double-shelled nanocages for efficient electro-oxidation of methanol[J]. Applied Catalysis B: Environmental, 2021, 282: 119595. |
| 55 | BAI Gailing, LIU Chang, GAO Zhe, et al. Atomic carbon layers supported Pt nanoparticles for minimized CO poisoning and maximized methanol oxidation[J]. Small, 2019, 15(38): e1902951. |
| 56 | GUO Lin, CHEN Siguo, LI Li, et al. A CO-tolerant PtRu catalyst supported on thiol-functionalized carbon nanotubes for the methanol oxidation reaction[J]. Journal of Power Sources, 2014, 247: 360-364. |
| 57 | QU Yongfang, ZHANG Wei, GUANG Binxiong, et al. Poly(ionic liquids) derived N, S co-doped carbon nanorod from in situ and template-free method as an efficient metal-free bifunctional electrocatalysts for direct methanol fuel cells[J]. Journal of Alloys and Compounds, 2022, 912: 165261. |
| 58 | BARAKAT Nasser A-M, MOTLAK Moaaed. Co x Ni y -decorated graphene as novel, stable and super effective non-precious electro-catalyst for methanol oxidation[J]. Applied Catalysis B: Environmental, 2014, 154: 221-231. |
| 59 | EASTWOOD B J, CHRISTENSEN P A, ARMSTRONG R D, et al. Electrochemical oxidation of a carbon black loaded polymer electrode in aqueous electrolytes[J]. Journal of Solid State Electrochemistry, 1999, 3(4): 179-186. |
| 60 | MAYRHOFER Karl J J, MEIER Josef C, ASHTON Sean J, et al. Fuel cell catalyst degradation on the nanoscale[J]. Electrochemistry Communications, 2008, 10(8): 1144-1147. |
| 61 | MAYRHOFER Karl J-J, ASHTON Sean J, MEIER Josef C, et al. Non-destructive transmission electron microscopy study of catalyst degradation under electrochemical treatment[J]. Journal of Power Sources, 2008, 185(2): 734-739. |
| 62 | SIROMA Zyun, ISHII Kenta, YASUDA Kazuaki, et al. Imaging of highly oriented pyrolytic graphite corrosion accelerated by Pt particles[J]. Electrochemistry Communications, 2005, 7(11): 1153-1156. |
| 63 | STONEHART P. Carbon substrates for phosphoric acid fuel cell cathodes[J]. Carbon, 1984, 22(4/5): 423-431. |
| 64 | CHEN Siguo, WEI Zidong, GUO Lin, et al. Enhanced dispersion and durability of Pt nanoparticles on a thiolated CNT support[J]. Chemical Communications, 2011, 47(39): 10984-10986. |
| 65 | LI L, CHEN S G, WEI Z D, et al. Experimental and DFT study of thiol-stabilized Pt/CNTs catalysts[J]. Physical Chemistry Chemical Physics: PCCP, 2012, 14(48): 16581-16587. |
| 66 | SUN Yongrong, DU Chunyu, HAN Guokang, et al. Boron, nitrogen co-doped graphene: A superior electrocatalyst support and enhancing mechanism for methanol electrooxidation[J]. Electrochimica Acta, 2016, 212: 313-321. |
| 67 | AN Meichen, DU Lei, DU Chunyu, et al. Pt nanoparticles supported by sulfur and phosphorus co-doped graphene as highly active catalyst for acidic methanol electrooxidation[J]. Electrochimica Acta, 2018, 285: 202-213. |
| 68 | HUANG Shengyang, GANESAN Prabhu, PARK Sehkyu, et al. Development of a titanium dioxide-supported platinum catalyst with ultrahigh stability for polymer electrolyte membrane fuel cell applications[J]. Journal of the American Chemical Society, 2009, 131(39): 13898-13899. |
| 69 | ABDULLAH M, KAMARUDIN S K, SHYUAN L K. TiO2 nanotube-carbon (TNT-C) as support for Pt-based catalyst for high methanol oxidation reaction in direct methanol fuel cell[J]. Nanoscale Research Letters, 2016, 11(1): 553. |
| 70 | ZHANG Dafeng, ZHANG Cuisong, CHEN Yumei, et al. Support shape effect on the catalytic performance of Pt/CeO2 nanostructures for methanol electrooxidation[J]. Electrochimica Acta, 2014, 139: 42-47. |
| 71 | DAS S, DUTTA K, KUNDU P P, et al. Nanostructured polyaniline: An efficient support matrix for platinum-ruthenium anode catalyst in direct methanol fuel cell[J]. Fuel Cells, 2018, 18(4): 369-378. |
| 72 | TINTULA K K, PITCHUMANI S, SRIDHAR P, et al. A solid-polymer-electrolyte direct methanol fuel cell (DMFC) with Pt-Ru nanoparticles supported onto poly(3,4-ethylenedioxythiophene) and polystyrene sulphonic acid polymer composite as anode[J]. Journal of Chemical Sciences, 2010, 122(3): 381-389. |
| 73 | ÇELEBI M S. Energy applications: Fuel cells[M]//Advanced Electrode Materials (Edited by TIWARI A, KURALAY F, UZUN L), Weinheim: Wiley-VCH, 2016: 397-434. |
| 74 | HUANG Kan, SASAKI Kotaro, ADZIC Radoslav R, et al. Increasing Pt oxygen reduction reaction activity and durability with a carbon-doped TiO2 nanocoating catalyst support[J]. Journal of Materials Chemistry, 2012, 22(33): 16824-16832. |
| 75 | XIA Bao yu, WANG Bao, WU Hao bin, et al. Sandwich-structured TiO2-Pt-graphene ternary hybrid electrocatalysts with high efficiency and stability[J]. Journal of Materials Chemistry, 2012, 22(32): 16499-16505. |
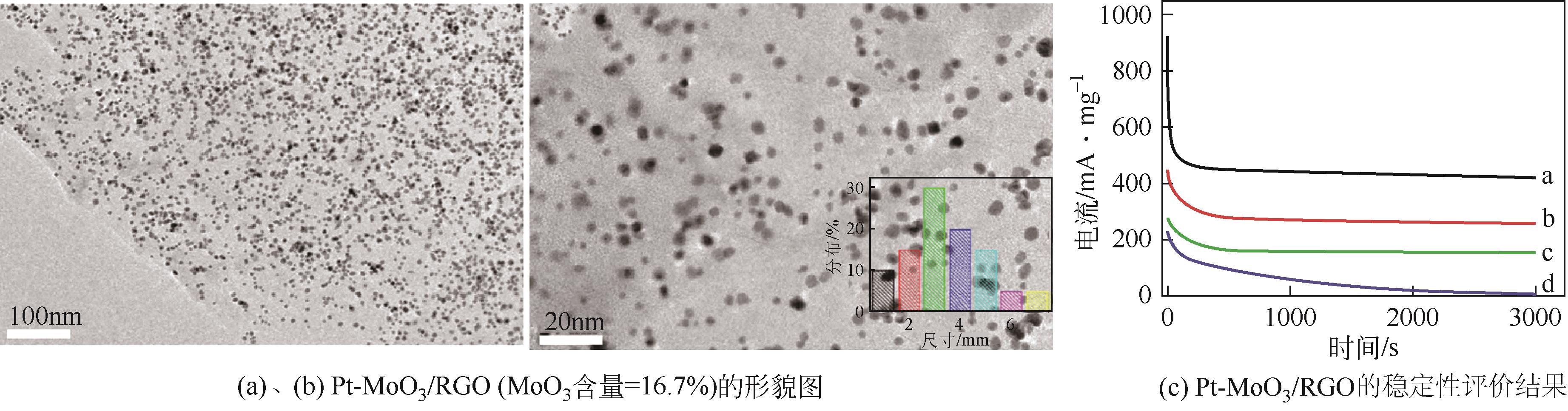
| 76 | HAO Yanfei, WANG Xudan, ZHENG Yuanyuan, et al. Uniform Pt nanoparticles incorporated into reduced graphene oxides with MoO3 as advanced anode catalysts for methanol electro-oxidation[J]. Electrochimica Acta, 2016, 198: 127-134. |
| 77 | AJENIFUJAH Olabode T, NOURALISHAHI Amideddin, CARL Sarah, et al. Platinum supported on early transition metal carbides: Efficient electrocatalysts for methanol electro-oxidation reaction in alkaline electrolyte[J]. Chemical Engineering Journal, 2021, 406: 126670. |
| [1] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [2] | 段翔, 田野, 董文威, 宋松, 李新刚. 苯酐合成的反应网络及催化反应机制研究现状与展望[J]. 化工进展, 2024, 43(5): 2587-2599. |
| [3] | 丁鑫, 张栋铭, 焦纬洲, 刘有智. 直接甲醇燃料电池阳极催化剂研究进展[J]. 化工进展, 2021, 40(9): 4918-4930. |
| [4] | 甄栋兴, 唐帅, 陈木森, 万磊, 吴雪梅, 贺高红. 硫酸化SnO2/SPPESK复合质子交换膜的制备及燃料电池性能[J]. 化工进展, 2019, 38(01): 529-537. |
| [5] | 张飞翔,齐 亮,姚克俭. 被动式DMFC气泡行为的实验研究和数值模拟[J]. 化工进展, 2013, 32(02): 283-289. |
| [6] | 邹晓燕,姚克俭. 被动式DMFC阳极通道气泡行为的实验研究 [J]. 化工进展, 2011, 30(7): 1444-. |
| [7] | 何健烽,章渊昶,朱菊香,姚克俭. 直接甲醇燃料电池阳极通道内气泡行为 [J]. 化工进展, 2010, 29(5): 831-. |
| [8] | 杨 伟1,陈胜洲2,邹汉波2,林维明1,2. 氮掺杂非贵金属氧还原催化剂研究进展 [J]. 化工进展, 2010, 29(11): 2085-. |
| [9] | 叶丁丁,李 俊,朱 恂,廖 强,黄桂兰,付 乾. 自呼吸式直接甲醇燃料电池性能及其传质特性 [J]. 化工进展, 2009, 28(6): 948-. |
| [10] | 张晓花,钟 秦. SOFC耐硫阳极催化剂La0.7Sr0.3Cr1-xFexO3-δ的制备及表征 [J]. 化工进展, 2009, 28(5): 788-. |
| [11] | 鞠剑峰,吴东辉. 直接甲醇燃料电池阳极催化剂的研究进展 [J]. 化工进展, 2009, 28(4): 646-. |
| [12] | 柯 新,姚克俭,王良华. 壁面亲水性对DMFC阳极通道内气液两相流影响的数值模拟 [J]. 化工进展, 2008, 27(2): 265-. |
| [13] | 廖 强,朱小伟,朱 恂,叶丁丁,丁玉栋. 质子交换膜燃料电池可视化研究进展 [J]. 化工进展, 2007, 26(9): 1213-. |
| [14] | 靳 豪;谢晓峰;尚玉明;冯少广;吕亚非. 直接甲醇燃料电池用有机-无机杂化质子交换膜研究进展 [J]. 化工进展, 2007, 26(4): 507-. |
| [15] | 张 颖,尹玉姬,姚康德. 直接甲醇燃料电池用阻醇质子交换膜研究进展 [J]. 化工进展, 2007, 26(4): 501-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||