化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4757-4765.DOI: 10.16085/j.issn.1000-6613.2023-1190
• 资源与环境化工 • 上一篇
生物炭光催化氧化-吸附协同降解亚硝酸盐
毛华恺1( ), 余洋1, 张悦1, 夏广坤1, 吴赟韬1, 楼乐瑶1, 牛文娟1,2,3, 刘念1,2,3(
), 余洋1, 张悦1, 夏广坤1, 吴赟韬1, 楼乐瑶1, 牛文娟1,2,3, 刘念1,2,3( )
)
- 1.华中农业大学工学院,湖北 武汉 430070
2.农业农村部水产养殖设施工程重点实验室,湖北 武汉 430070
3.农业农村部长江中下游农业装备重点实验室,湖北 武汉 430070
-
收稿日期:2023-07-13修回日期:2023-09-07出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:刘念 -
作者简介:毛华恺,(2002—),男,本科生,研究方向为种养废水光催化降解。E-mail:mhk@webmail.hzau.edu.cn。 -
基金资助:国家自然科学基金(32201685);湖北省创新创业训练项目(S202310504186)
Synergistic biochar photocatalytic oxidation-adsorption for nitrite degradation
MAO Huakai1( ), YU Yang1, ZHANG Yue1, XIA Guangkun1, WU Yuntao1, LOU Leyao1, NIU Wenjuan1,2,3, LIU Nian1,2,3(
), YU Yang1, ZHANG Yue1, XIA Guangkun1, WU Yuntao1, LOU Leyao1, NIU Wenjuan1,2,3, LIU Nian1,2,3( )
)
- 1.College of Engineering, Huazhong Agricultural University, Wuhan 430070, Hubei, China
2.Key Laboratory of Aquaculture Facilities Engineering, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China
3.Agricultural Equipment Laboratory of the Middle and Lower Reaches of the Yangtze River, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China
-
Received:2023-07-13Revised:2023-09-07Online:2024-08-15Published:2024-09-02 -
Contact:LIU Nian
摘要:
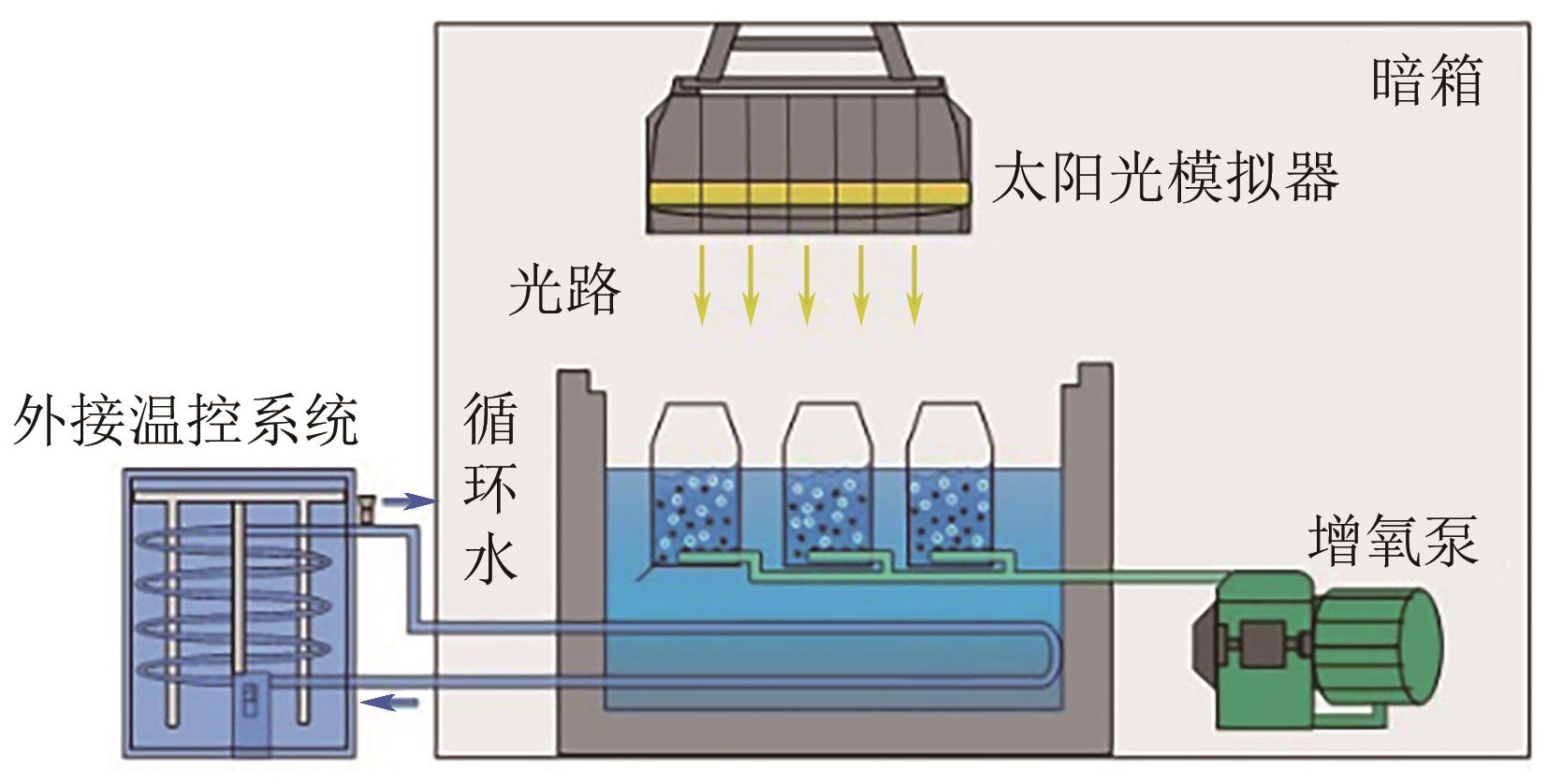
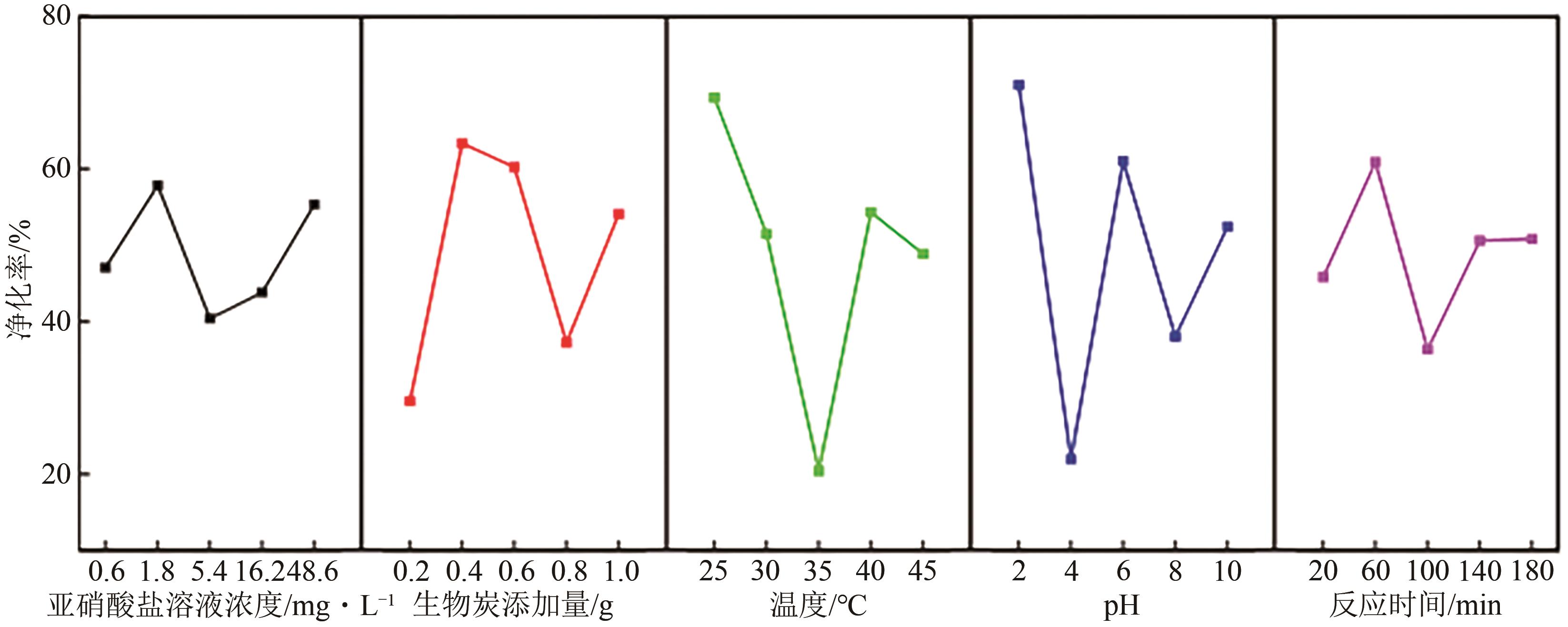
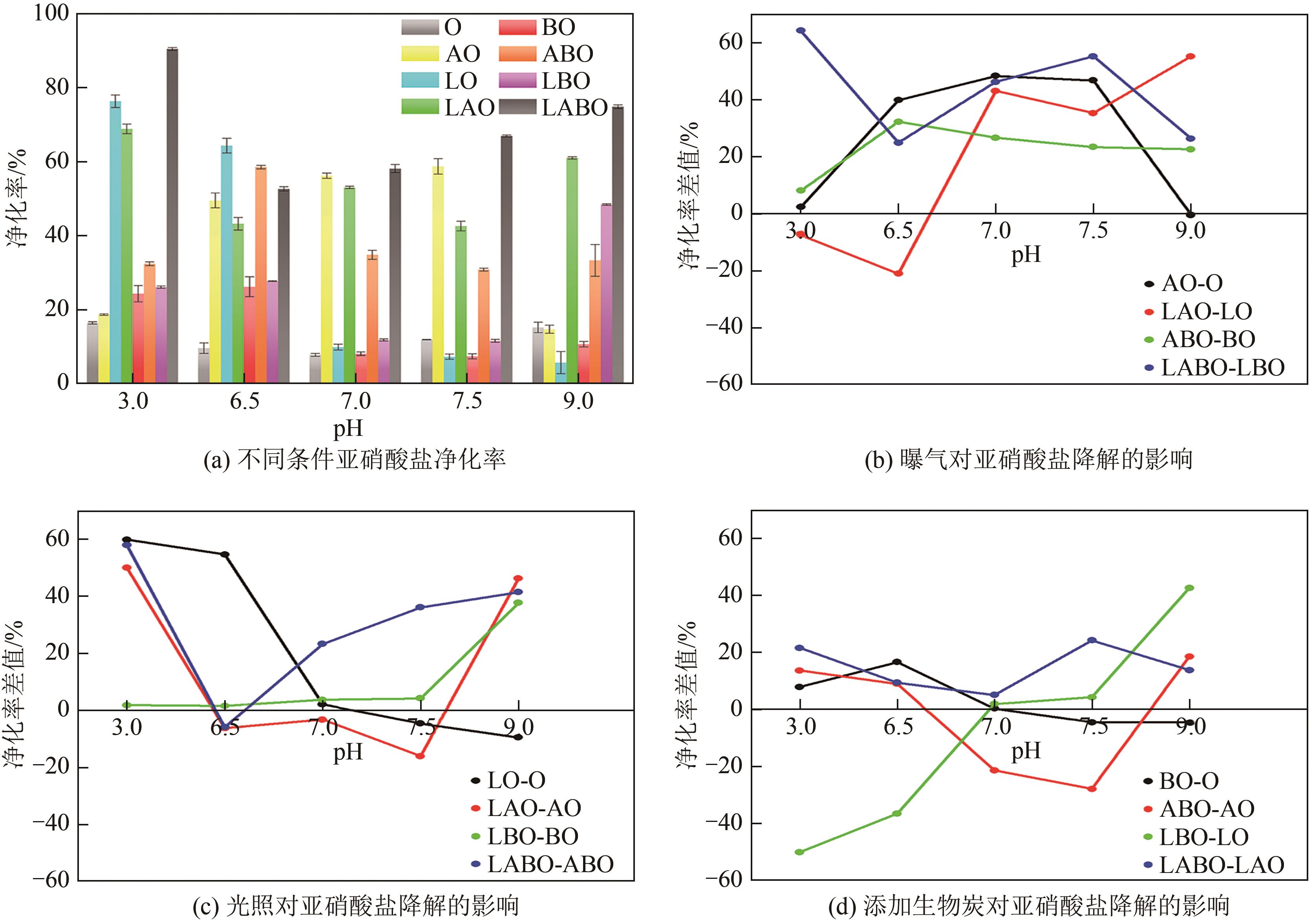
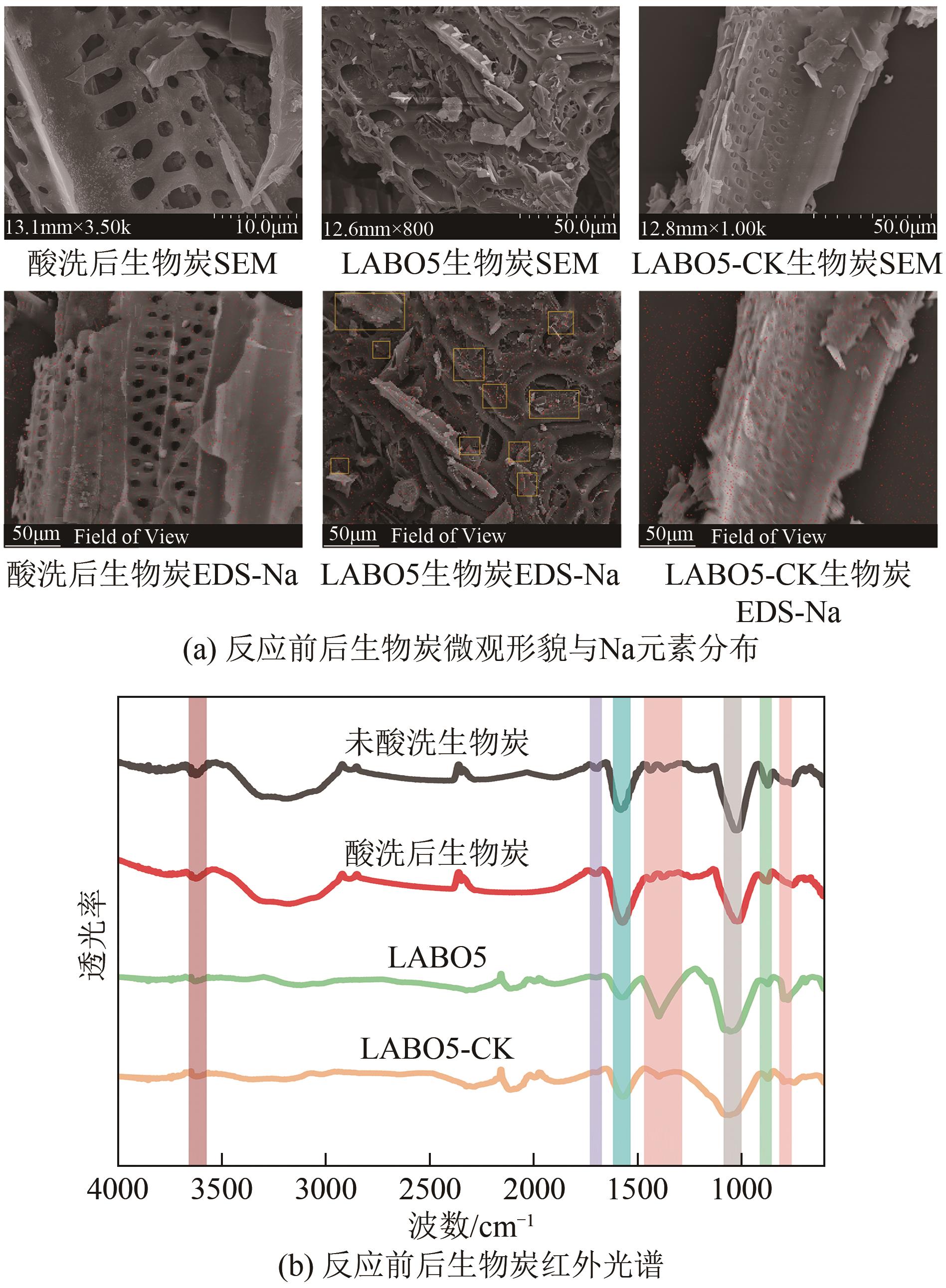
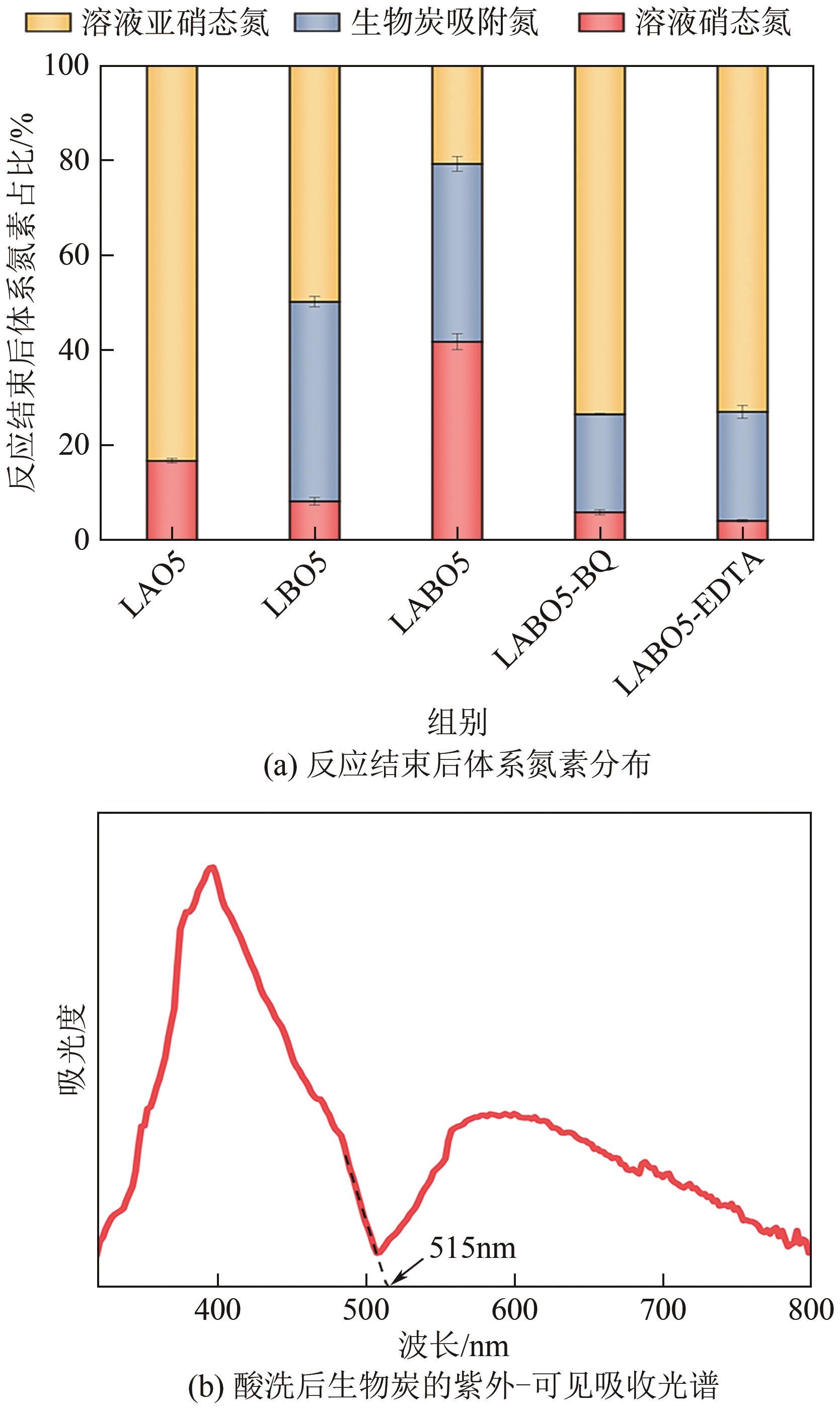
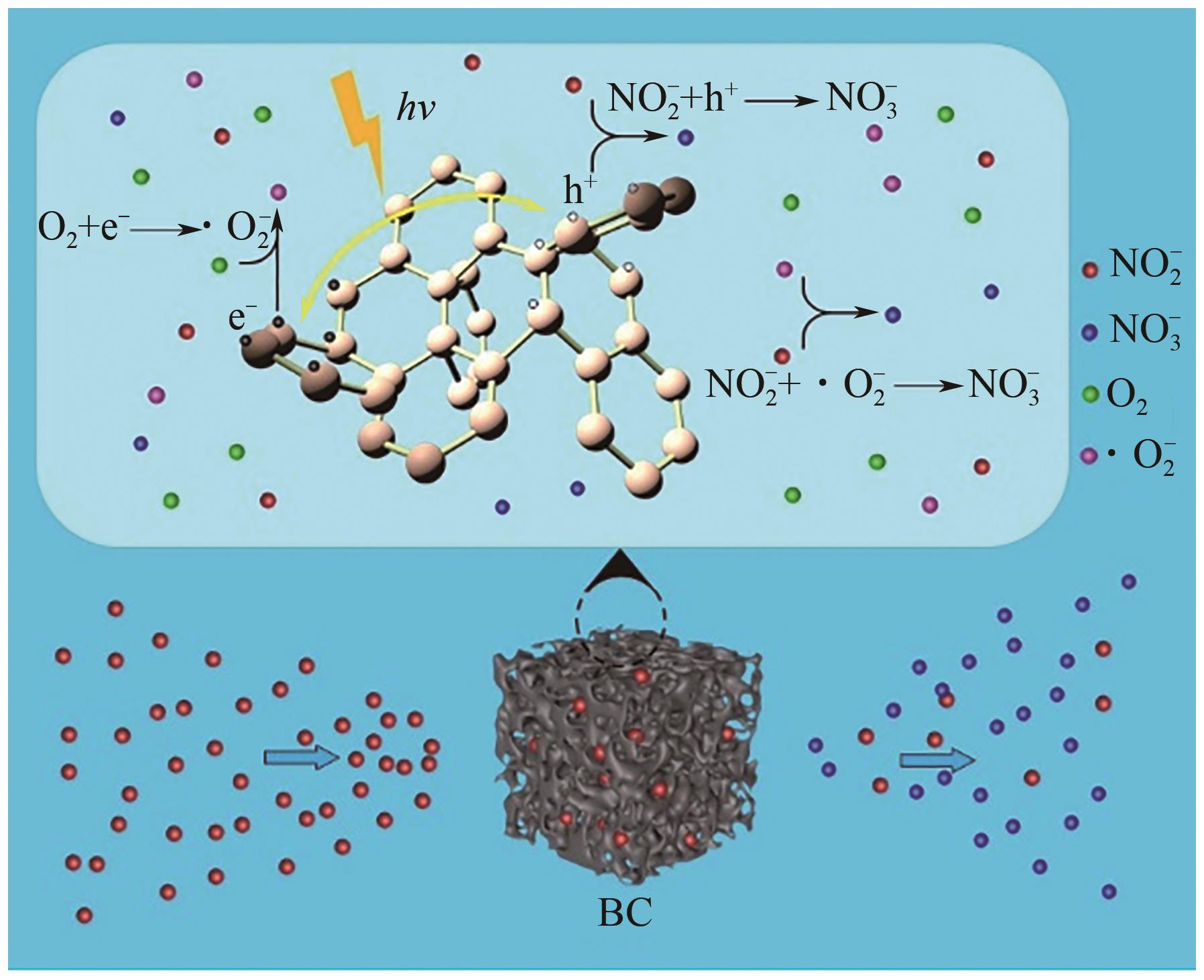
通过正交实验研究生物炭-光-氧共同作用下不同环境亚硝酸盐净化规律,结合多重对照实验,解析光照、曝气、添加生物炭对亚硝酸盐降解的影响差异,揭示亚硝态氮迁移路径与降解机制。结果表明,亚硝酸盐净化效果主要受环境酸碱度和温度影响,其中酸碱度为主控因素。当亚硝酸盐浓度1.8mg/L、生物炭添加量0.4g、温度25℃、反应时间60min、pH=2时,净化率最高可达98.25%。多数情况下,曝气能够显著提升亚硝酸盐净化率,较之未曝气处理最高提升9.7倍。光照则更适合用于强酸、强碱环境中亚硝酸盐的去除,较之无光照条件净化率最高提升5.7倍。而单纯添加生物炭对亚硝酸盐降解助力效果有限,需配合光照和曝气共同作用。三种条件共存,产生生物炭光催化氧化-吸附协同作用,促使亚硝态氮经由氧化、光催化、吸附等路径降解、迁移,获得良好净化效果。
中图分类号:
引用本文
毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765.
MAO Huakai, YU Yang, ZHANG Yue, XIA Guangkun, WU Yuntao, LOU Leyao, NIU Wenjuan, LIU Nian. Synergistic biochar photocatalytic oxidation-adsorption for nitrite degradation[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4757-4765.
| 元素组成 | 质量分数/% |
|---|---|
| C | 42.72 |
| H | 2.58 |
| O | 47.79 |
| N | 1.20 |
| S | 0.55 |
| K | 1.43 |
| Ca | 0.57 |
| Mg | 0.78 |
表1 玉米秸秆生物炭元素组成
| 元素组成 | 质量分数/% |
|---|---|
| C | 42.72 |
| H | 2.58 |
| O | 47.79 |
| N | 1.20 |
| S | 0.55 |
| K | 1.43 |
| Ca | 0.57 |
| Mg | 0.78 |
| 水平 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min |
|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 |
| 2 | 1.8 | 0.4 | 30 | 4 | 60 |
| 3 | 5.4 | 0.6 | 35 | 6 | 100 |
| 4 | 16.2 | 0.8 | 40 | 8 | 140 |
| 5 | 48.6 | 1.0 | 45 | 10 | 180 |
表2 正交试验因素水平设计表
| 水平 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min |
|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 |
| 2 | 1.8 | 0.4 | 30 | 4 | 60 |
| 3 | 5.4 | 0.6 | 35 | 6 | 100 |
| 4 | 16.2 | 0.8 | 40 | 8 | 140 |
| 5 | 48.6 | 1.0 | 45 | 10 | 180 |
| 试验组 | 编号 | L | A | B | pH | 酸碱度 |
|---|---|---|---|---|---|---|
| LABO | LABO1 | √ | √ | √ | 3 | 强酸 |
| LABO | LABO2 | √ | √ | √ | 6.5 | 弱酸 |
| LABO | LABO3 | √ | √ | √ | 7 | 中性 |
| LABO | LABO4 | √ | √ | √ | 7.5 | 弱碱 |
| LABO | LABO5 | √ | √ | √ | 9 | 强碱 |
| ABO | ABO1 | × | √ | √ | 3 | 强酸 |
| ABO | ABO2 | × | √ | √ | 6.5 | 弱酸 |
| ABO | ABO3 | × | √ | √ | 7 | 中性 |
| ABO | ABO4 | × | √ | √ | 7.5 | 弱碱 |
| ABO | ABO5 | × | √ | √ | 9 | 强碱 |
| LBO | LBO1 | √ | × | √ | 3 | 强酸 |
| LBO | LBO2 | √ | × | √ | 6.5 | 弱酸 |
| LBO | LBO3 | √ | × | √ | 7 | 中性 |
| LBO | LBO4 | √ | × | √ | 7.5 | 弱碱 |
| LBO | LBO5 | √ | × | √ | 9 | 强碱 |
| LAO | LAO1 | √ | √ | × | 3 | 强酸 |
| LAO | LAO2 | √ | √ | × | 6.5 | 弱酸 |
| LAO | LAO3 | √ | √ | × | 7 | 中性 |
| LAO | LAO4 | √ | √ | × | 7.5 | 弱碱 |
| LAO | LAO5 | √ | √ | × | 9 | 强碱 |
| BO | BO1 | × | × | √ | 3 | 强酸 |
| BO | BO2 | × | × | √ | 6.5 | 弱酸 |
| BO | BO3 | × | × | √ | 7 | 中性 |
| BO | BO4 | × | × | √ | 7.5 | 弱碱 |
| BO | BO5 | × | × | √ | 9 | 强碱 |
| AO | AO1 | × | √ | × | 3 | 强酸 |
| AO | AO2 | × | √ | × | 6.5 | 弱酸 |
| AO | AO3 | × | √ | × | 7 | 中性 |
| AO | AO4 | × | √ | × | 7.5 | 弱碱 |
| AO | AO5 | × | √ | × | 9 | 强碱 |
| LO | LO1 | √ | × | × | 3 | 强酸 |
| LO | LO2 | √ | × | × | 6.5 | 弱酸 |
| LO | LO3 | √ | × | × | 7 | 中性 |
| LO | LO4 | √ | × | × | 7.5 | 弱碱 |
| LO | LO5 | √ | × | × | 9 | 强碱 |
| O | O1 | × | × | × | 3 | 强酸 |
| O | O2 | × | × | × | 6.5 | 弱酸 |
| O | O3 | × | × | × | 7 | 中性 |
| O | O4 | × | × | × | 7.5 | 弱碱 |
| O | O5 | × | × | × | 9 | 强碱 |
表3 单因素平行实验因素水平设计表
| 试验组 | 编号 | L | A | B | pH | 酸碱度 |
|---|---|---|---|---|---|---|
| LABO | LABO1 | √ | √ | √ | 3 | 强酸 |
| LABO | LABO2 | √ | √ | √ | 6.5 | 弱酸 |
| LABO | LABO3 | √ | √ | √ | 7 | 中性 |
| LABO | LABO4 | √ | √ | √ | 7.5 | 弱碱 |
| LABO | LABO5 | √ | √ | √ | 9 | 强碱 |
| ABO | ABO1 | × | √ | √ | 3 | 强酸 |
| ABO | ABO2 | × | √ | √ | 6.5 | 弱酸 |
| ABO | ABO3 | × | √ | √ | 7 | 中性 |
| ABO | ABO4 | × | √ | √ | 7.5 | 弱碱 |
| ABO | ABO5 | × | √ | √ | 9 | 强碱 |
| LBO | LBO1 | √ | × | √ | 3 | 强酸 |
| LBO | LBO2 | √ | × | √ | 6.5 | 弱酸 |
| LBO | LBO3 | √ | × | √ | 7 | 中性 |
| LBO | LBO4 | √ | × | √ | 7.5 | 弱碱 |
| LBO | LBO5 | √ | × | √ | 9 | 强碱 |
| LAO | LAO1 | √ | √ | × | 3 | 强酸 |
| LAO | LAO2 | √ | √ | × | 6.5 | 弱酸 |
| LAO | LAO3 | √ | √ | × | 7 | 中性 |
| LAO | LAO4 | √ | √ | × | 7.5 | 弱碱 |
| LAO | LAO5 | √ | √ | × | 9 | 强碱 |
| BO | BO1 | × | × | √ | 3 | 强酸 |
| BO | BO2 | × | × | √ | 6.5 | 弱酸 |
| BO | BO3 | × | × | √ | 7 | 中性 |
| BO | BO4 | × | × | √ | 7.5 | 弱碱 |
| BO | BO5 | × | × | √ | 9 | 强碱 |
| AO | AO1 | × | √ | × | 3 | 强酸 |
| AO | AO2 | × | √ | × | 6.5 | 弱酸 |
| AO | AO3 | × | √ | × | 7 | 中性 |
| AO | AO4 | × | √ | × | 7.5 | 弱碱 |
| AO | AO5 | × | √ | × | 9 | 强碱 |
| LO | LO1 | √ | × | × | 3 | 强酸 |
| LO | LO2 | √ | × | × | 6.5 | 弱酸 |
| LO | LO3 | √ | × | × | 7 | 中性 |
| LO | LO4 | √ | × | × | 7.5 | 弱碱 |
| LO | LO5 | √ | × | × | 9 | 强碱 |
| O | O1 | × | × | × | 3 | 强酸 |
| O | O2 | × | × | × | 6.5 | 弱酸 |
| O | O3 | × | × | × | 7 | 中性 |
| O | O4 | × | × | × | 7.5 | 弱碱 |
| O | O5 | × | × | × | 9 | 强碱 |
| 误差源 | SS | df | MS | F | 显著性 |
|---|---|---|---|---|---|
| α | 2275.34 | 4 | 568.84 | 1.51 | - |
| β | 8851.34 | 4 | 2212.84 | 5.87 | - |
| γ | 12785.26 | 4 | 3196.31 | 8.48 | ** |
| δ | 14891.02 | 4 | 3722.75 | 9.88 | ** |
| ε | 3113.10 | 4 | 778.28 | 2.06 | - |
| 空列 | 10879.21 | 4 | 2719.80 | 7.22 | - |
| 误差 | 10922.71 | 25 | 376.65 | — | — |
| 总和 | 52825.84 | 49 | — | — | — |
表4 各因素对净化率的方差分析
| 误差源 | SS | df | MS | F | 显著性 |
|---|---|---|---|---|---|
| α | 2275.34 | 4 | 568.84 | 1.51 | - |
| β | 8851.34 | 4 | 2212.84 | 5.87 | - |
| γ | 12785.26 | 4 | 3196.31 | 8.48 | ** |
| δ | 14891.02 | 4 | 3722.75 | 9.88 | ** |
| ε | 3113.10 | 4 | 778.28 | 2.06 | - |
| 空列 | 10879.21 | 4 | 2719.80 | 7.22 | - |
| 误差 | 10922.71 | 25 | 376.65 | — | — |
| 总和 | 52825.84 | 49 | — | — | — |
| 实验 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min | ζ(空列) | η/% | 标准偏差 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 | 1 | 42.50 | 0.000 |
| 2 | 0.6 | 0.4 | 30 | 4 | 60 | 2 | 54.00 | 0.000 |
| 3 | 0.6 | 0.6 | 35 | 6 | 100 | 3 | 29.85 | 0.017 |
| 4 | 0.6 | 0.8 | 40 | 8 | 140 | 4 | 30.14 | 0.014 |
| 5 | 0.6 | 1.0 | 45 | 10 | 180 | 5 | 78.87 | 0.004 |
| 6 | 1.8 | 0.2 | 30 | 6 | 140 | 5 | 76.11 | 0.010 |
| 7 | 1.8 | 0.4 | 35 | 8 | 180 | 1 | 10.16 | 0.006 |
| 8 | 1.8 | 0.6 | 40 | 10 | 20 | 2 | 79.88 | 0.007 |
| 9 | 1.8 | 0.8 | 45 | 2 | 60 | 3 | 80.55 | 0.005 |
| 10 | 1.8 | 1.0 | 25 | 1 | 100 | 4 | 42.50 | 0.000 |
| 11 | 5.4 | 0.2 | 35 | 10 | 60 | 4 | 6.62 | 0.019 |
| 12 | 5.4 | 0.4 | 40 | 2 | 100 | 5 | 91.06 | 0.003 |
| 13 | 5.4 | 0.6 | 45 | 4 | 140 | 1 | 1.77 | 0.011 |
| 14 | 5.4 | 0.8 | 25 | 6 | 180 | 2 | 68.06 | 0.000 |
| 15 | 5.4 | 1.0 | 30 | 8 | 20 | 3 | 34.51 | 0.016 |
| 16 | 16.2 | 0.2 | 40 | 4 | 180 | 3 | 5.23 | 0.005 |
| 17 | 16.2 | 0.4 | 45 | 6 | 20 | 4 | 65.78 | 0.011 |
| 18 | 16.2 | 0.6 | 25 | 8 | 60 | 5 | 97.93 | 0.001 |
| 19 | 16.2 | 0.8 | 30 | 10 | 100 | 1 | 0.97 | 0.009 |
| 20 | 16.2 | 1.0 | 35 | 2 | 140 | 2 | 49.14 | 0.001 |
| 21 | 48.6 | 0.2 | 45 | 8 | 100 | 2 | 17.38 | 0.004 |
| 22 | 48.6 | 0.4 | 25 | 10 | 140 | 3 | 95.78 | 0.000 |
| 23 | 48.6 | 0.6 | 30 | 2 | 180 | 4 | 91.77 | 0.002 |
| 24 | 48.6 | 0.8 | 35 | 4 | 20 | 5 | 6.42 | 0.023 |
| 25 | 48.6 | 1.0 | 40 | 6 | 60 | 1 | 65.34 | 0.000 |
表5 正交实验结果
| 实验 | α/mg·L-1 | β/g | γ/℃ | δ | ε/min | ζ(空列) | η/% | 标准偏差 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.2 | 25 | 2 | 20 | 1 | 42.50 | 0.000 |
| 2 | 0.6 | 0.4 | 30 | 4 | 60 | 2 | 54.00 | 0.000 |
| 3 | 0.6 | 0.6 | 35 | 6 | 100 | 3 | 29.85 | 0.017 |
| 4 | 0.6 | 0.8 | 40 | 8 | 140 | 4 | 30.14 | 0.014 |
| 5 | 0.6 | 1.0 | 45 | 10 | 180 | 5 | 78.87 | 0.004 |
| 6 | 1.8 | 0.2 | 30 | 6 | 140 | 5 | 76.11 | 0.010 |
| 7 | 1.8 | 0.4 | 35 | 8 | 180 | 1 | 10.16 | 0.006 |
| 8 | 1.8 | 0.6 | 40 | 10 | 20 | 2 | 79.88 | 0.007 |
| 9 | 1.8 | 0.8 | 45 | 2 | 60 | 3 | 80.55 | 0.005 |
| 10 | 1.8 | 1.0 | 25 | 1 | 100 | 4 | 42.50 | 0.000 |
| 11 | 5.4 | 0.2 | 35 | 10 | 60 | 4 | 6.62 | 0.019 |
| 12 | 5.4 | 0.4 | 40 | 2 | 100 | 5 | 91.06 | 0.003 |
| 13 | 5.4 | 0.6 | 45 | 4 | 140 | 1 | 1.77 | 0.011 |
| 14 | 5.4 | 0.8 | 25 | 6 | 180 | 2 | 68.06 | 0.000 |
| 15 | 5.4 | 1.0 | 30 | 8 | 20 | 3 | 34.51 | 0.016 |
| 16 | 16.2 | 0.2 | 40 | 4 | 180 | 3 | 5.23 | 0.005 |
| 17 | 16.2 | 0.4 | 45 | 6 | 20 | 4 | 65.78 | 0.011 |
| 18 | 16.2 | 0.6 | 25 | 8 | 60 | 5 | 97.93 | 0.001 |
| 19 | 16.2 | 0.8 | 30 | 10 | 100 | 1 | 0.97 | 0.009 |
| 20 | 16.2 | 1.0 | 35 | 2 | 140 | 2 | 49.14 | 0.001 |
| 21 | 48.6 | 0.2 | 45 | 8 | 100 | 2 | 17.38 | 0.004 |
| 22 | 48.6 | 0.4 | 25 | 10 | 140 | 3 | 95.78 | 0.000 |
| 23 | 48.6 | 0.6 | 30 | 2 | 180 | 4 | 91.77 | 0.002 |
| 24 | 48.6 | 0.8 | 35 | 4 | 20 | 5 | 6.42 | 0.023 |
| 25 | 48.6 | 1.0 | 40 | 6 | 60 | 1 | 65.34 | 0.000 |
| 参数 | α | β | γ | δ | ε | ζ |
|---|---|---|---|---|---|---|
| K1 | 235.36 | 147.83 | 346.77 | 355.01 | 235.36 | 120.74 |
| K2 | 289.18 | 316.77 | 257.36 | 109.92 | 289.18 | 268.46 |
| K3 | 202.01 | 301.20 | 102.19 | 305.13 | 202.01 | 245.92 |
| K4 | 219.06 | 186.13 | 271.64 | 190.12 | 219.06 | 236.80 |
| K5 | 276.68 | 270.37 | 244.34 | 262.11 | 276.68 | 350.39 |
| R | 87.17 | 168.93 | 244.58 | 245.09 | 122.68 | 229.65 |
表6 极差分析表
| 参数 | α | β | γ | δ | ε | ζ |
|---|---|---|---|---|---|---|
| K1 | 235.36 | 147.83 | 346.77 | 355.01 | 235.36 | 120.74 |
| K2 | 289.18 | 316.77 | 257.36 | 109.92 | 289.18 | 268.46 |
| K3 | 202.01 | 301.20 | 102.19 | 305.13 | 202.01 | 245.92 |
| K4 | 219.06 | 186.13 | 271.64 | 190.12 | 219.06 | 236.80 |
| K5 | 276.68 | 270.37 | 244.34 | 262.11 | 276.68 | 350.39 |
| R | 87.17 | 168.93 | 244.58 | 245.09 | 122.68 | 229.65 |
| 1 | BASAK Bikram, BHUNIA Biswanath, DUTTA Subhasish, et al. Enhanced biodegradation of 4-chlorophenol by Candida tropicalis PHB5 via optimization of physicochemical parameters using Taguchi orthogonal array approach[J]. International Biodeterioration & Biodegradation, 2013, 78: 17-23. |
| 2 | AMORIM Camila C, LEÃO Mônica M D, MOREIRA Regina F P M, et al. Performance of blast furnace waste for azo dye degradation through photo-Fenton-like processes[J]. Chemical Engineering Journal, 2013, 224(1): 59-66. |
| 3 | 于兵川, 吴洪特, 张万忠. 光催化纳米材料在环境保护中的应用[J]. 石油化工, 2005, 34(5): 491-495. |
| YU Bingchuan, WU Hongte, ZHANG Wanzhong. Application of nano-photocatalysts in environmental protection[J]. Petrochemical Technology, 2005, 34(5): 491-495. | |
| 4 | WANG Hao, WANG Jing, XIANG Xin, et al. Preparation of PVDF/CdS/Bi2WO6/ZnO hybrid membrane with enhanced visible-light photocatalytic activity for degrading nitrite in water[J]. Environmental Research, 2020, 191: 110036. |
| 5 | BOUKHEMIKHEM Z, REKHILA G, BRAHIMI R, et al. Photocatalytic NO2-oxidation on the hetero-junction Ag(5%)/NiFe2O4 prepared by sol gel route[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2020, 394: 112454. |
| 6 | FU Dongying, HAN Gaoyi, CHANG Yunzhen, et al. The synthesis and properties of ZnO-graphene nano hybrid for photodegradation of organic pollutant in water[J]. Materials Chemistry and Physics, 2012, 132(2/3): 673-681. |
| 7 | 魏宏斌, 徐迪民. 固定相TiO2膜的制备及其光催化活性[J]. 中国给水排水, 2002, 18(7): 57-59. |
| WEI Hongbin, XU Dimin. Preparation and photocatalytic activity of fixed phase TiO2 film[J]. China Water & Wastewater, 2002,18(7): 57-59. | |
| 8 | ZHANG Lei, JIANG Daochuan, IRFAN Rana Muhammad, et al. Highly efficient and selective photocatalytic dehydrogenation of benzyl alcohol for simultaneous hydrogen and benzaldehyde production over Ni-decorated Zn0.5Cd0.5S solid solution[J]. Journal of Energy Chemistry, 2019, 30(3): 71-77. |
| 9 | LI Xin, LIN Jing, ZHANG Di, et al. Material flow analysis of titanium dioxide and sustainable policy suggestion in China[J]. Resources Policy, 2020, 67: 101685. |
| 10 | SAJAN Chimmikuttanda Ponnappa, WAGEH Swelm, AL-GHAMDI Ahmed A, et al. TiO2 nanosheets with exposed {001} facets for photocatalytic applications[J]. Nano Research, 2016, 9(1): 3-27. |
| 11 | LU Haiqiang, ZHAO Jianghong, LI Li, et al. Selective oxidation of sacrificial ethanol over TiO2-based photocatalysts during water splitting[J]. Energy & Environmental Science, 2011, 4(9): 3384-3388. |
| 12 | YASUDA Masahide, MATSUMOTO Tomoko, YAMASHITA Toshiaki. Sacrificial hydrogen production over TiO2-based photocatalysts: Polyols, carboxylic acids, and saccharides[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 1627-1635. |
| 13 | TESTA Juan J, GRELA María A, LITTER Marta I. Heterogeneous photocatalytic reduction of chromium(Ⅵ) over TiO2 particles in the presence of oxalate: Involvement of Cr(Ⅴ) species[J]. Environmental Science & Technology, 2004, 38(5): 1589-1594. |
| 14 | 陈杰, 肖玉婷, 汪楠, 等. 简易合成具有增强电荷转移的Z型CeO2/C3N4异质结用于光催化CO2还原[J]. Science China Materials, 2023, 66(8): 3165-3175. |
| CHEN Jie, XIAO Yuting, WANG Nan, et al. Facile synthesis of a Z-scheme CeO2/C3N4 heterojunction with enhanced charge transfer for CO2 photoreduction[J]. Science China Materials, 2023, 66(8): 3165-3175. | |
| 15 | LUO Jinhua, WU Yaohui, JIANG Mengzhu, et al. Novel ZnFe2O4/BC/ZnO photocatalyst for high-efficiency degradation of tetracycline under visible light irradiation[J]. Chemosphere, 2023, 311(1): 137041. |
| 16 | XIA Qi, HUANG Binbin, YUAN Xingzhong, et al. Modified stannous sulfide nanoparticles with metal-organic framework: Toward efficient and enhanced photocatalytic reduction of chromium (Ⅵ) under visible light[J]. Journal of Colloid and Interface Science, 2018, 530: 481-492. |
| 17 | DJELLABI Ridha, YANG Bo, WANG Yan, et al. Carbonaceous biomass-titania composites with TiOC bonding bridge for efficient photocatalytic reduction of Cr(Ⅵ) under narrow visible light[J]. Chemical Engineering Journal, 2019, 366: 172-180. |
| 18 | Dinh Ngoc Giao NGO, CHUANG Xiangying, HUANG Chin-Pao, et al. Compositional characterization of nine agricultural waste biochars: The relations between alkaline metals and cation exchange capacity with ammonium adsorption capability[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 110003. |
| 19 | HUFF Matthew D, LEE James W. Biochar-surface oxygenation with hydrogen peroxide[J]. Journal of Environmental Management, 2016, 165: 17-21. |
| 20 | LIU Yurong, PASKEVICIUS Mark, Veronica SOFIANOS M, et al. A SAXS study of the pore structure evolution in biochar during gasification in H2O, CO2 and H2O/CO2 [J]. Fuel, 2021, 292: 120384. |
| 21 | 周石洋, 陈玲. 食品中亚硝酸盐含量测定的研究[J]. 食品安全质量检测学报, 2011, 2(6): 285-289. |
| ZHOU Shiyang, CHEN Ling. Study on the determination of nitrite in food[J]. Journal of Food Safety & Quality, 2011, 2(6): 285-289. | |
| 22 | 中华人民共和国自然资源部. 地下水质分析方法 硝酸盐的测定紫外分光光度法: [S]. 北京: 中国标准出版社, 2021. |
| Ministry of Natural Resources of the People’s Republic of China. Analysis methods for groundwater quality—Determination of nitrate ultraviolet spectrophotometric method: [S]. Beijing: Standards Press of China, 2021. | |
| 23 | 栗秀萍, 于洋, 何旺, 等. 超重力强化AMP-PZ复合溶液脱碳技术及表观动力学[J]. 化工进展, 2022, 41(S1): 22-28. |
| LI Xiuping, YU Yang, HE Wang, et al. High-gravity intensified decarburization process and apparent kinetics of AMP-PZ composite solution[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 22-28. | |
| 24 | 中本一雄, 黄德如. 无机和配位化合物的红外和拉曼光谱[M]. 北京: 化学工业出版社,1986. |
| KAZUO Nakamoto, HUANG Deru. Inrared and Raman spectra of inorganic and coordination compounds[M]. Beijing: Chemical Industry Press, 1986. | |
| 25 | 黄安香, 杨定云, 杨守禄, 等. 改性生物炭对土壤重金属污染修复研究进展[J]. 化工进展, 2020, 39(12): 5266-5274. |
| HUANG Anxiang, YANG Dingyun, YANG Shoulu, et al. Advance in remediation of heavy metal pollution in soil by modified biochar[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5266-5274. | |
| 26 | 宋江生, 管益东, 师杨杰, 等. 杨木基生物炭吸附去除水溶液中磺胺吡啶[J]. 净水技术, 2023, 42(7): 90-97. |
| SONG Jiangsheng, GUAN Yidong, SHI Yangjie, et al. Poplar based biochars for adsorption and removal of sulfapyridine in aqueous solution[J]. Water Purification Technology, 2023, 42(7): 90-97. | |
| 27 | 钟来元, 廖荣骏, 刘付宇杰, 等. KOH改性花生壳生物炭对盐酸四环素的吸附性能及其机理[J]. 农业环境科学学报, 2023, 42(9): 2038-2048. |
| ZHONG Laiyuan, LIAO Rongjun, LIUFU Yujie, et al. Adsorption of tetracycline hydrochloride by KOH modified peanut shell biochar and its mechanism[J]. Journal of Agro-Environment Science, 2023, 42(9): 2038-2048. | |
| 28 | 杨婷婷, 黄艳艳, 柳维扬, 等. 三种改性小麦秸秆生物炭表征及其对Cu2+的吸附性能[J]. 农业工程学报, 2023, 39(8): 222-230. |
| YANG Tingting, HUANG Yanyan, LIU Weiyang, et al. Characterization of three kinds of modified wheat straw derived biochars and their sorption capacity for Cu2+ [J]. Transactions of the Chinese Society of Agricultural Engineering, 2023, 39(8): 222-230. | |
| 29 | 王金玉, 王赛男, 卢佳宏, 等. 盐酸改性豆渣不溶性膳食纤维对亚硝酸盐的吸附特性及机理[J]. 大豆科学, 2022, 41(4): 463-471. |
| WANG Jinyu, WANG Sainan, LU Jiahong, et al. Adsorption characteristics and mechanism of hydrochloric acid modified okara insoluble dietary fiber to nitrite[J]. Soybean Science, 2022, 41(4): 463-471. | |
| 30 | 庄远红, 刘静娜, 费鹏, 等. 模拟胃环境下柚皮果胶对亚硝酸根的吸附动力学[J]. 西南大学学报(自然科学版), 2018, 40(12): 65-72. |
| ZHUANG Yuanhong, LIU Jingna, FEI Peng, et al. Adsorption kinetics of pectin from pomelo peelon nitrite in a simulated gastric environment[J]. Journal of Southwest University(Natural Science Edition), 2018, 40(12): 65-72. | |
| 31 | 吴丹萍, 陈全, 李东梅, 等. 生物炭含氧官能团的生成溯源及其在污染物吸附-降解过程中的作用[J]. 环境化学, 2021, 40(10): 3190-3198. |
| WU Danping, CHEN Quan, LI Dongmei, et al. Traceability of oxygen-containing functional groups in biochars and their roles in the adsorption-degradation of contaminants[J]. Environmental Chemistry, 2021, 40(10): 3190-3198. | |
| 32 | 向欣. CdS/Bi2S3/Bi2MoO6光催化膜耦合芽孢杆菌协同降解亚硝酸盐[D]. 南宁: 广西大学, 2021. |
| XIANG Xin. Synergistic Degradation of nitrite by CdS/Bi2S3/Bi2MoO6 photocatalytic membrane coupled bacillus[D]. Nanning: Guangxi University, 2021. | |
| 33 | GOMAA Hosam M, SAUDI H A, YAHIA I S, et al. Influence of graphene nanopowder impurities on the structural and optical properties of sodium borate copper-based glass: Towards UV/NIR shielding materials[J]. Solid State Sciences, 2022, 129: 106911. |
| 34 | 李法云, 李佳宇, 吝美霞, 等. 大豆秸秆生物炭负载石墨相氮化碳对土壤石油烃的光催化降解[J]. 应用基础与工程科学学报, 2022, 30(3): 519-529. |
| LI Fayun, LI Jiayu, LIN Meixia, et al. Photocatalytic degradation of soil petroleum hydrocarbons by biochar supported graphite phase carbon nitride[J]. Journal of Basic Science and Engineering, 2022, 30(3): 519-529. | |
| 35 | 曹雪娟, 单柏林, 邓梅, 等. Fe掺杂g-C3N4光催化剂的制备及光催化性能研究[J]. 重庆交通大学学报(自然科学版), 2019, 38(11): 52-57. |
| CAO Xuejuan, SHAN Bailin, DENG Mei, et al. Preparation and photocatalytic properties of Fe-doped g-C3N4 photocatalyst[J]. Journal of Chongqing Jiaotong University(Natural Science), 2019, 38(11): 52-57. | |
| 36 | 高晓明, 付峰, 吕磊, 等. 光催化剂Cu-BiVO4的制备及其光催化降解含酚废水[J]. 化工进展, 2012, 31(5): 1039-1042, 1087. |
| GAO Xiaoming, FU Feng, Lei LYU, et al. Preparation of Cu-BiVO4 photocatalyst and its application in the treatment of phenol-containing wastewater[J]. Chemical Industry and Engineering Progress, 2012, 31(5): 1039-1042, 1087. | |
| 37 | 王宏娟, 郑楠, 董晓丽. 碘掺杂氯氧化铋光催化剂的制备及性能[J]. 大连工业大学学报, 2020, 39(6): 419-423. |
| WANG Hongjuan, ZHENG Nan, DONG Xiaoli. Preparation and properties of I-doped bismuth chloride oxide photocatalyst[J]. Journal of Dalian Polytechnic University, 2020, 39(6): 419-423. | |
| 38 | 李红亮, 张涛, 付贤军, 等. ZnO/g-C3N4复合光催化材料降解四环素研究[J]. 能源与环保, 2023, 45(8): 163-169. |
| LI Hongliang, ZHANG Tao, FU Xianjun, et al. Study on degradation of tetracycline by ZnO/g-C3N4 composite photocatalytic materials[J]. China Energy and Environmental Protection, 2023, 45(8): 163-169. | |
| 39 | 赵文武, 周海静, 黄雁, 等. 稀土掺杂硼酸盐Bi2ZnB2O7∶xDy3+光催化剂的合成及其催化机理[J]. 化工进展, 2022, 41(11): 5843-5849. |
| ZHAO Wenwu, ZHOU Haijing, HUANG Yan, et al. Photocatalytic mechanism and synthesis of rare earth doped borate Bi2ZnB2O7∶xDy3+ photocatalyst[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5843-5849. |
| [1] | 吴泽亮, 管琦卉, 陈世霞, 王珺. 炔烃选择性加氢制烯烃反应的研究进展[J]. 化工进展, 2024, 43(8): 4366-4381. |
| [2] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [3] | 付涛, 李立, 高莉宁, 朱富维, 曹炜烨, 陈华鑫. 水泥基硼掺杂石墨相氮化碳降解NO[J]. 化工进展, 2024, 43(8): 4403-4410. |
| [4] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [5] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
| [6] | 宋占龙, 汤涛, 潘蔚, 赵希强, 孙静, 毛岩鹏, 王文龙. 微纳米气泡强化臭氧氧化降解含酚废水[J]. 化工进展, 2024, 43(8): 4614-4623. |
| [7] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [8] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [9] | 武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693. |
| [10] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [11] | 郭长滨, 李蒙蒙, 冯梦晗, 原田, 张克强, 罗艳丽, 王风. 铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能[J]. 化工进展, 2024, 43(8): 4748-4756. |
| [12] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [13] | 李文哲, 申淼, 王建强. 熔盐法制备新型二维层状金属碳/氮化物(MXene)的研究进展[J]. 化工进展, 2024, 43(7): 3660-3671. |
| [14] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| [15] | 罗丛佳, 豆义波, 卫敏. 水滑石光催化剂结构调控用于二氧化碳还原的研究进展[J]. 化工进展, 2024, 43(7): 3891-3909. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||