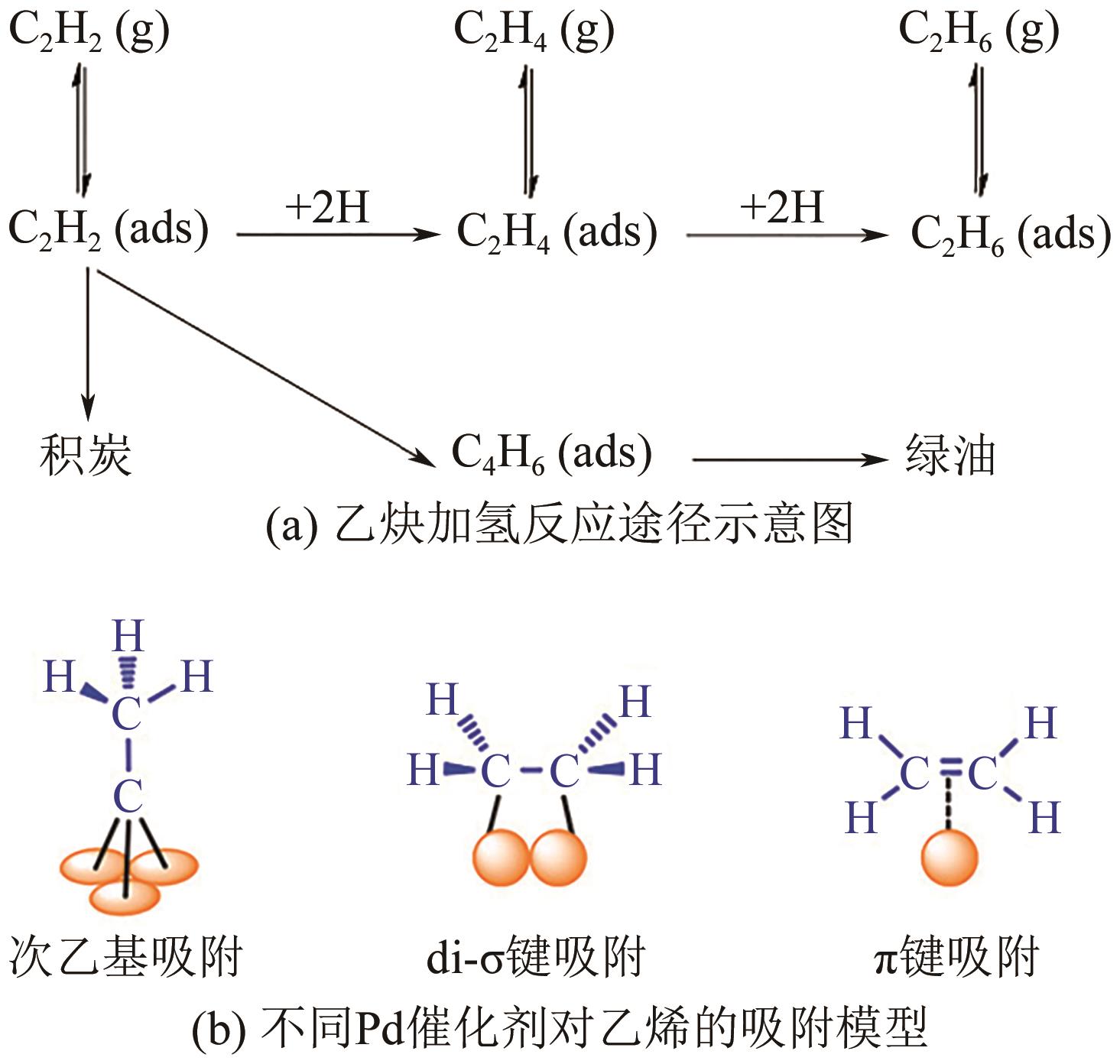
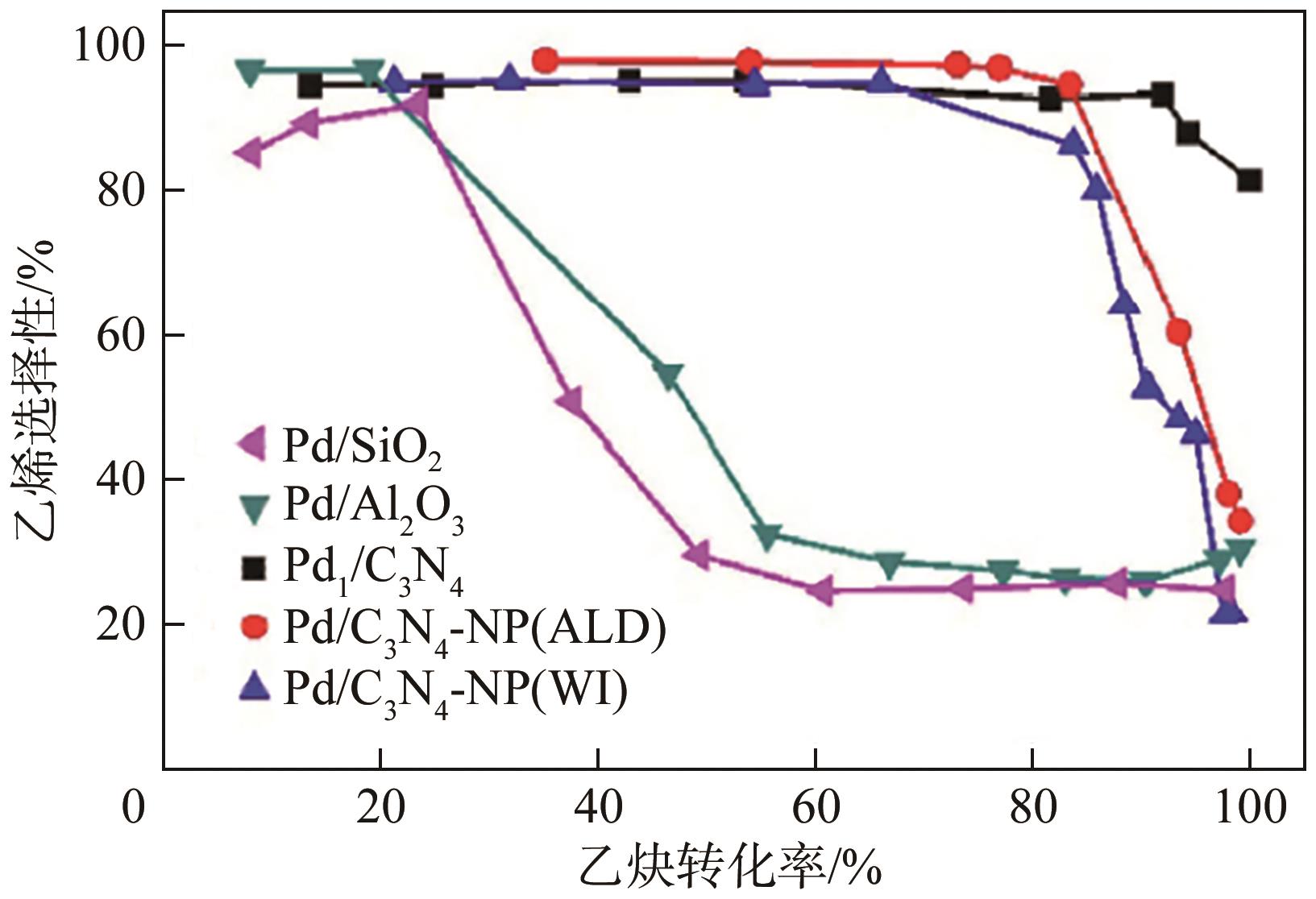
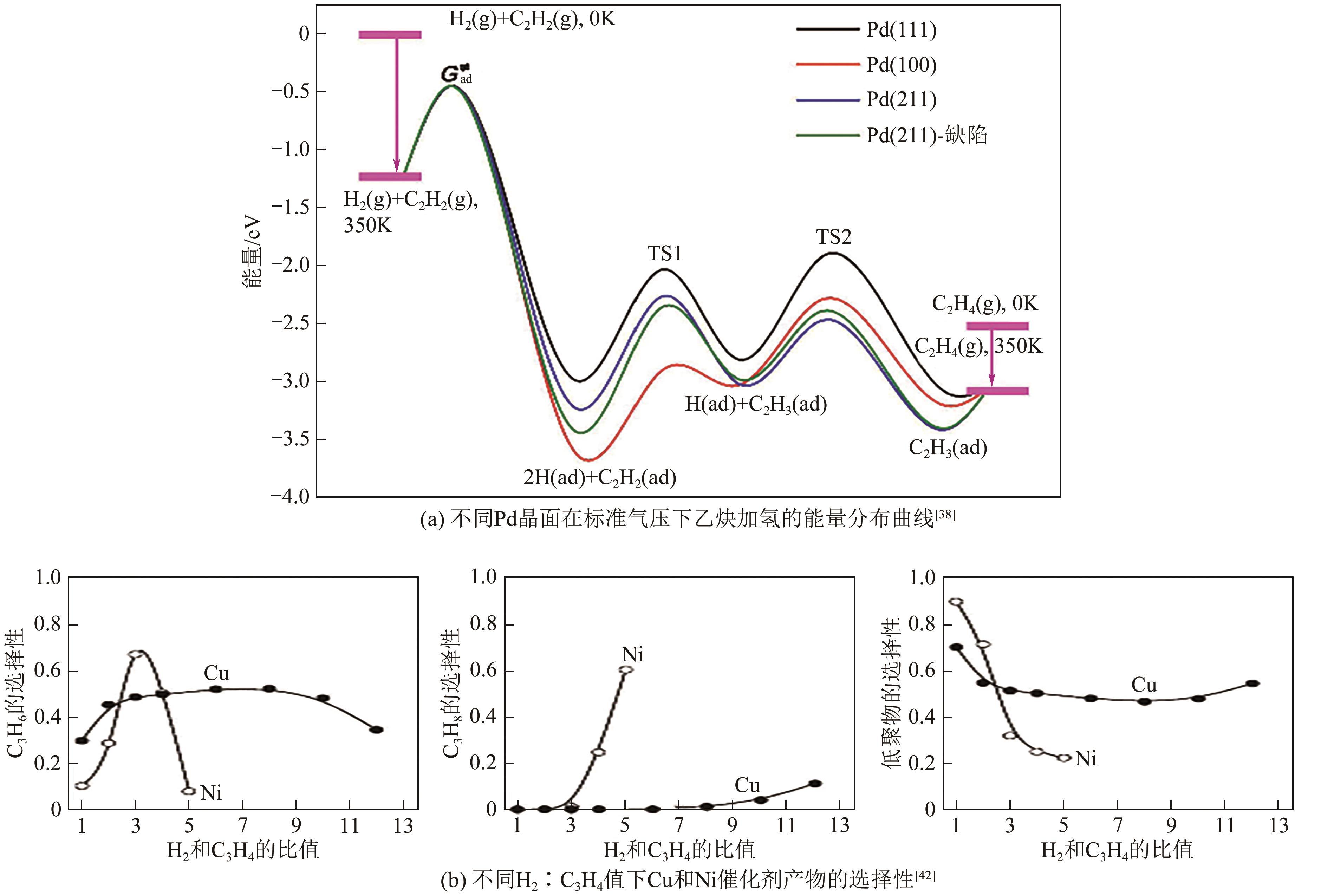
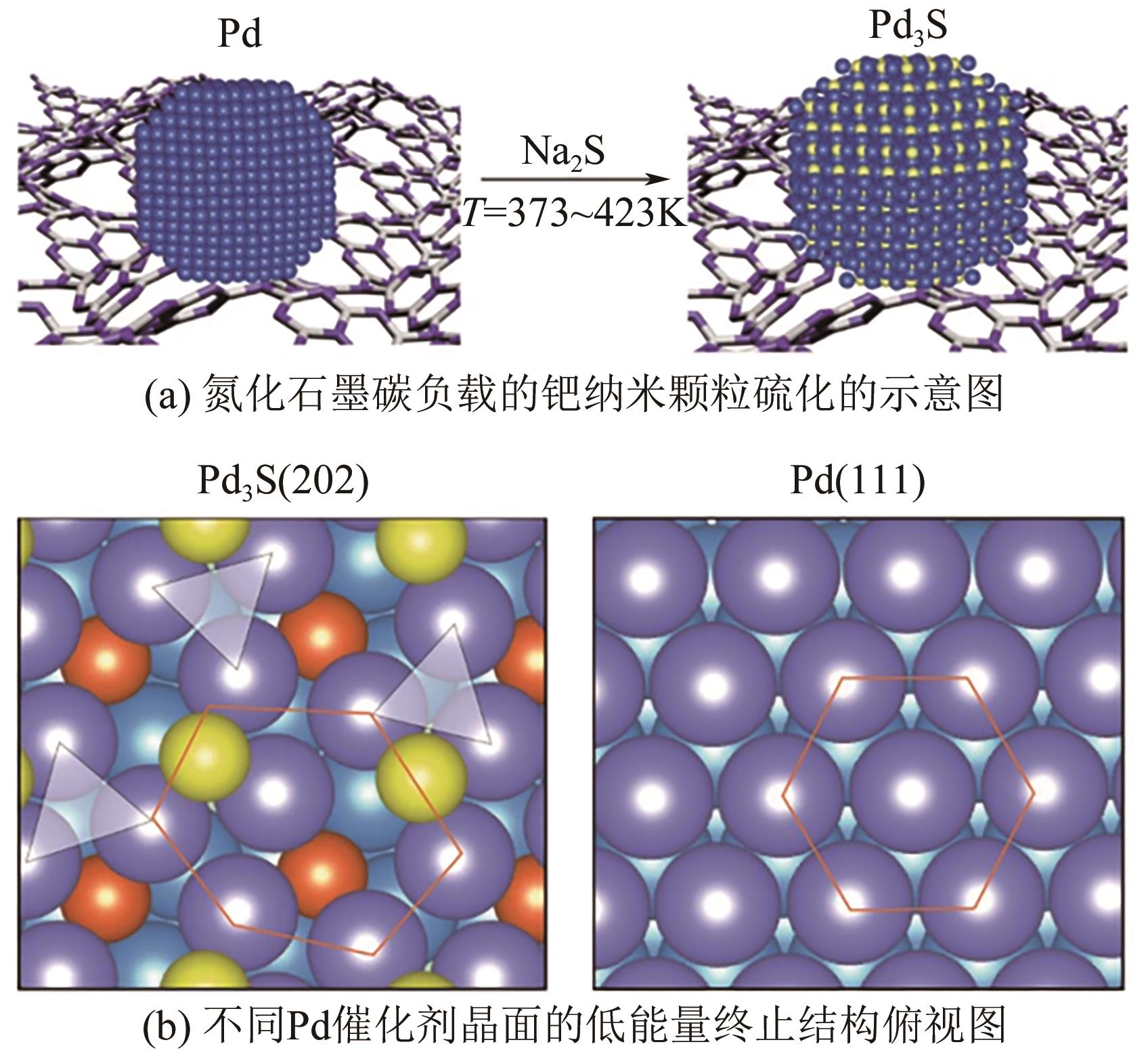
化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4366-4381.DOI: 10.16085/j.issn.1000-6613.2023-1181
• 工业催化 • 上一篇
炔烃选择性加氢制烯烃反应的研究进展
- 1.南昌大学资源与环境学院,江西 南昌 330031
2.南昌大学化学化工学院,江西 南昌 330031
-
收稿日期:2023-07-11修回日期:2023-11-12出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:王珺 -
作者简介:吴泽亮(1995—),男,博士研究生,研究方向为炔烃选择性加氢。E-mail:zeliangwu@email.ncu.edu.cn。 -
基金资助:国家自然科学基金(22322807)
Advances in selective hydrogenation of alkynes to alkenes
WU Zeliang1( ), GUAN Qihui2, CHEN Shixia2, WANG Jun2(
), GUAN Qihui2, CHEN Shixia2, WANG Jun2( )
)
- 1.School of Resources & Environment, Nanchang University, Nanchang 330031, Jiangxi, China
2.School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, Jiangxi, China
-
Received:2023-07-11Revised:2023-11-12Online:2024-08-15Published:2024-09-02 -
Contact:WANG Jun
摘要:
炔烃选择性加氢制烯烃是石油化工领域重要的反应过程。传统的热催化方法在过去的几十年中被广泛研究,而新兴光/电催化方法的研究仍处于初级阶段。得益于催化剂合成方法、结构表征技术和理论计算等的快速发展,逐步揭示了炔烃选择性加氢过程中的催化剂活性位点和反应机理。本文介绍了近年来各类催化剂在炔烃选择性加氢方面的研究进展,总结了设计高性能催化剂的相应调控策略。对比了传统热催化加氢与光/电催化选择性加氢技术路线的不同特点,重点分析了不同类型的催化加氢反应出现的问题及解决策略。最后,对该领域的研究现状进行了简要的概述,提出了该领域面临的主要挑战和未来发展趋势,并展望了未来催化剂设计和调控目标产物选择性等方向。
中图分类号:
引用本文
吴泽亮, 管琦卉, 陈世霞, 王珺. 炔烃选择性加氢制烯烃反应的研究进展[J]. 化工进展, 2024, 43(8): 4366-4381.
WU Zeliang, GUAN Qihui, CHEN Shixia, WANG Jun. Advances in selective hydrogenation of alkynes to alkenes[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4366-4381.
| 气体组成 | 催化剂 | 反应温度/℃ | 反应压力/MPa | 空速/mL·gcat-1·h-1 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| C3H4∶H2 = 2.5∶7.5 | Cu2.75Ni0.25Fe | 250 | 0.1 | 1.68 × 104 | 100 | 97 | [ |
| C2H2∶C2H4∶H2 = 0.5∶25∶1 | Pd1/g-C3N4 | 110 | 0.1 | 3 × 104 | 80 | 92 | [ |
| C3H4∶H2 = 1∶3 | Cu-Al HT | 250 | 0.1 | 1.68 × 104 | 100 | — | [ |
| C2H2∶H2 = 1∶30 | In2O3 | 350 | 0.1 | — | 100 | 85 | [ |
| C2H2∶C2H4∶H2 = 1∶9∶1.8 | Pd4S/CNFs | 250 | 0.1 | 6 × 104 | 100 | 95 | [ |
| C2H2∶C2H4∶H2 = 1∶99∶2 | Ti-Pd/PHSNs | 45 | 0.1 | 7200 | 70 | 69 | [ |
| C2H2∶C2H4∶H2 = 1∶9∶1.2 | Pd4S/CNFs | 250 | 1.8 | 2.16 × 106 | 46.6 | 96 | [ |
| C2H2∶C2H4∶H2 = 0.5∶20∶3 | Pd@C/CNT | 150 | 0.1 | 6 × 104 | 93 | 70 | [ |
| C2H2∶C2H4∶H2 = 1∶20∶20 | AuPd0.01/SiO2 | 160 | 0.1 | 6 × 104 | 86 | 56.4 | [ |
| C2H2∶C2H4∶H2 = 0.8∶83.2∶16 | Au/SiO2 | 175 | 0.1 | 9.2 × 104 | 94 | 47 | [ |
| C3H4∶H2 = 1∶30 | CeO2 | 250 | 0.1 | — | 96 | 91 | [ |
| C2H2∶C2H4∶H2 = 0.5∶50∶5 | Al13Fe4 | 200 | 0.1 | 9× 104 | 80 | 82 | [ |
| C2H2∶H2 = 1∶5 | Ni6In/SiO2 | 180 | 0.1 | 3.6× 104 | 63 | 100 | [ |
| C2H2∶H2 = 0.475∶9.25 | CrO x /(110)γ-Al2O3 | 140 | 0.1 | 2.166 × 104 | 100 | 98.5 | [ |
表1 热催化气相炔烃选择性加氢催化剂
| 气体组成 | 催化剂 | 反应温度/℃ | 反应压力/MPa | 空速/mL·gcat-1·h-1 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| C3H4∶H2 = 2.5∶7.5 | Cu2.75Ni0.25Fe | 250 | 0.1 | 1.68 × 104 | 100 | 97 | [ |
| C2H2∶C2H4∶H2 = 0.5∶25∶1 | Pd1/g-C3N4 | 110 | 0.1 | 3 × 104 | 80 | 92 | [ |
| C3H4∶H2 = 1∶3 | Cu-Al HT | 250 | 0.1 | 1.68 × 104 | 100 | — | [ |
| C2H2∶H2 = 1∶30 | In2O3 | 350 | 0.1 | — | 100 | 85 | [ |
| C2H2∶C2H4∶H2 = 1∶9∶1.8 | Pd4S/CNFs | 250 | 0.1 | 6 × 104 | 100 | 95 | [ |
| C2H2∶C2H4∶H2 = 1∶99∶2 | Ti-Pd/PHSNs | 45 | 0.1 | 7200 | 70 | 69 | [ |
| C2H2∶C2H4∶H2 = 1∶9∶1.2 | Pd4S/CNFs | 250 | 1.8 | 2.16 × 106 | 46.6 | 96 | [ |
| C2H2∶C2H4∶H2 = 0.5∶20∶3 | Pd@C/CNT | 150 | 0.1 | 6 × 104 | 93 | 70 | [ |
| C2H2∶C2H4∶H2 = 1∶20∶20 | AuPd0.01/SiO2 | 160 | 0.1 | 6 × 104 | 86 | 56.4 | [ |
| C2H2∶C2H4∶H2 = 0.8∶83.2∶16 | Au/SiO2 | 175 | 0.1 | 9.2 × 104 | 94 | 47 | [ |
| C3H4∶H2 = 1∶30 | CeO2 | 250 | 0.1 | — | 96 | 91 | [ |
| C2H2∶C2H4∶H2 = 0.5∶50∶5 | Al13Fe4 | 200 | 0.1 | 9× 104 | 80 | 82 | [ |
| C2H2∶H2 = 1∶5 | Ni6In/SiO2 | 180 | 0.1 | 3.6× 104 | 63 | 100 | [ |
| C2H2∶H2 = 0.475∶9.25 | CrO x /(110)γ-Al2O3 | 140 | 0.1 | 2.166 × 104 | 100 | 98.5 | [ |
| 底物 | 催化剂 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| 苯乙炔 | Ag/SiO2 | 100 | 10 | 30 | 100 | [ |
| 苯乙炔 | Au/CeO2 | 25 | 3 | 99 | 99 | [ |
| 苯乙炔 | Pd1/NC-PHF | 60 | 0.5 | 93.1 | 93.5 | [ |
| 苯丙炔 | Meso-PdP | 60 | 0.1 | 100 | 97.4 | [ |
| 二苯乙炔 | Pd-Pb NCs | 25 | 0.1 | 96 | 96 | [ |
| 2-甲基-3-丁炔-2-醇 | Pd3S/C3N4 | 30 | 0.1 | — | 100 | [ |
| 1-戊炔 | Pd/ZnO | 100 | 0.1 | 41 | 87 | [ |
| 1-辛炔 | Pd@Ag/HAP | 25 | 0.1 | 99 | 99 | [ |
| 苯乙炔 | H350Ni/COF | 100 | 1 | >99 | 85 | [ |
| 苯乙炔 | Ni2P@C-PPS/CNFs | 100 | 0.5 | 100 | 94.6 | [ |
| 苯乙炔 | Ni@NC-800 | 110 | 1 | 97 | 94 | [ |
表2 热催化液相炔烃选择性加氢催化剂
| 底物 | 催化剂 | 反应温度/℃ | 反应压力/MPa | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| 苯乙炔 | Ag/SiO2 | 100 | 10 | 30 | 100 | [ |
| 苯乙炔 | Au/CeO2 | 25 | 3 | 99 | 99 | [ |
| 苯乙炔 | Pd1/NC-PHF | 60 | 0.5 | 93.1 | 93.5 | [ |
| 苯丙炔 | Meso-PdP | 60 | 0.1 | 100 | 97.4 | [ |
| 二苯乙炔 | Pd-Pb NCs | 25 | 0.1 | 96 | 96 | [ |
| 2-甲基-3-丁炔-2-醇 | Pd3S/C3N4 | 30 | 0.1 | — | 100 | [ |
| 1-戊炔 | Pd/ZnO | 100 | 0.1 | 41 | 87 | [ |
| 1-辛炔 | Pd@Ag/HAP | 25 | 0.1 | 99 | 99 | [ |
| 苯乙炔 | H350Ni/COF | 100 | 1 | >99 | 85 | [ |
| 苯乙炔 | Ni2P@C-PPS/CNFs | 100 | 0.5 | 100 | 94.6 | [ |
| 苯乙炔 | Ni@NC-800 | 110 | 1 | 97 | 94 | [ |
| 气体组成 | 催化剂 | 反应温度/℃ | 转化率/% | 选择性/% | 参考文献 |
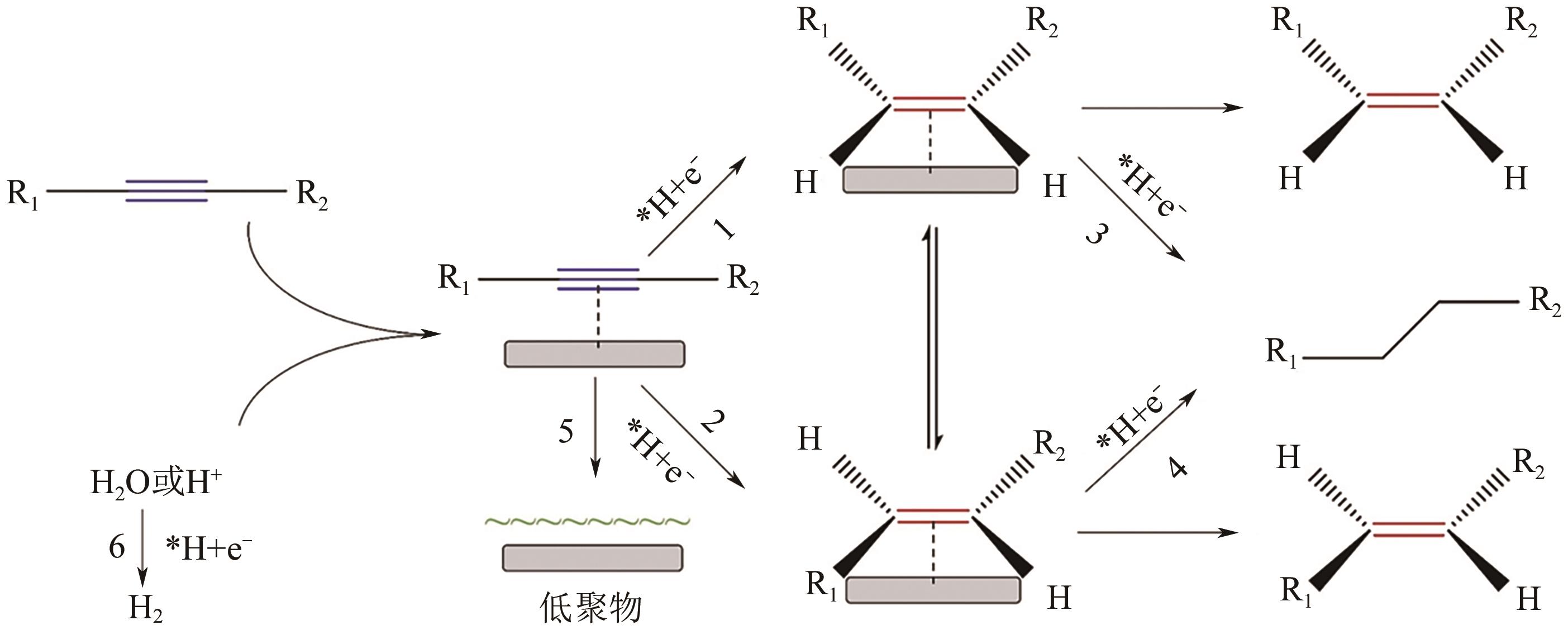
|---|---|---|---|---|---|
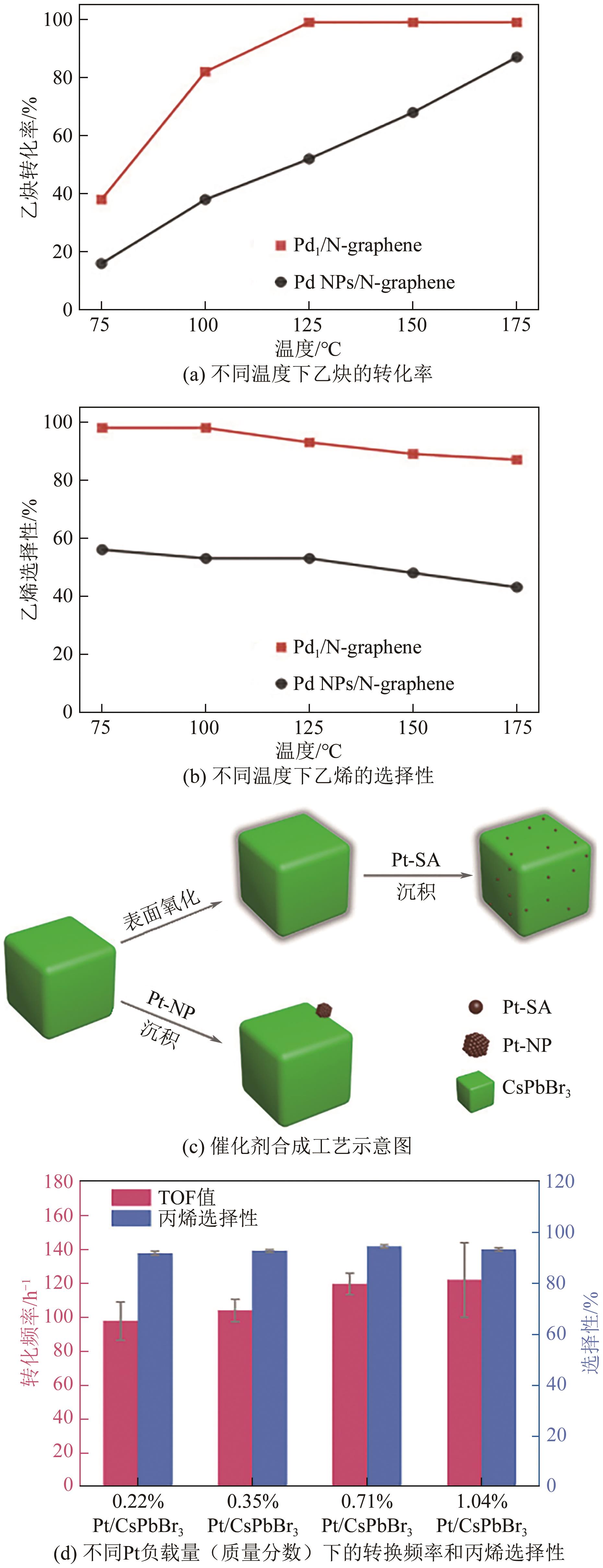
| C2H2∶C2H4:∶H2=20∶20∶1 | Pd1/N-graphene | 125 | 99 | 93.5 | [ |
| C3H4∶H2=1∶5 | Pt-SA/CsPbBr3 | 5 | — | 86.6 | [ |
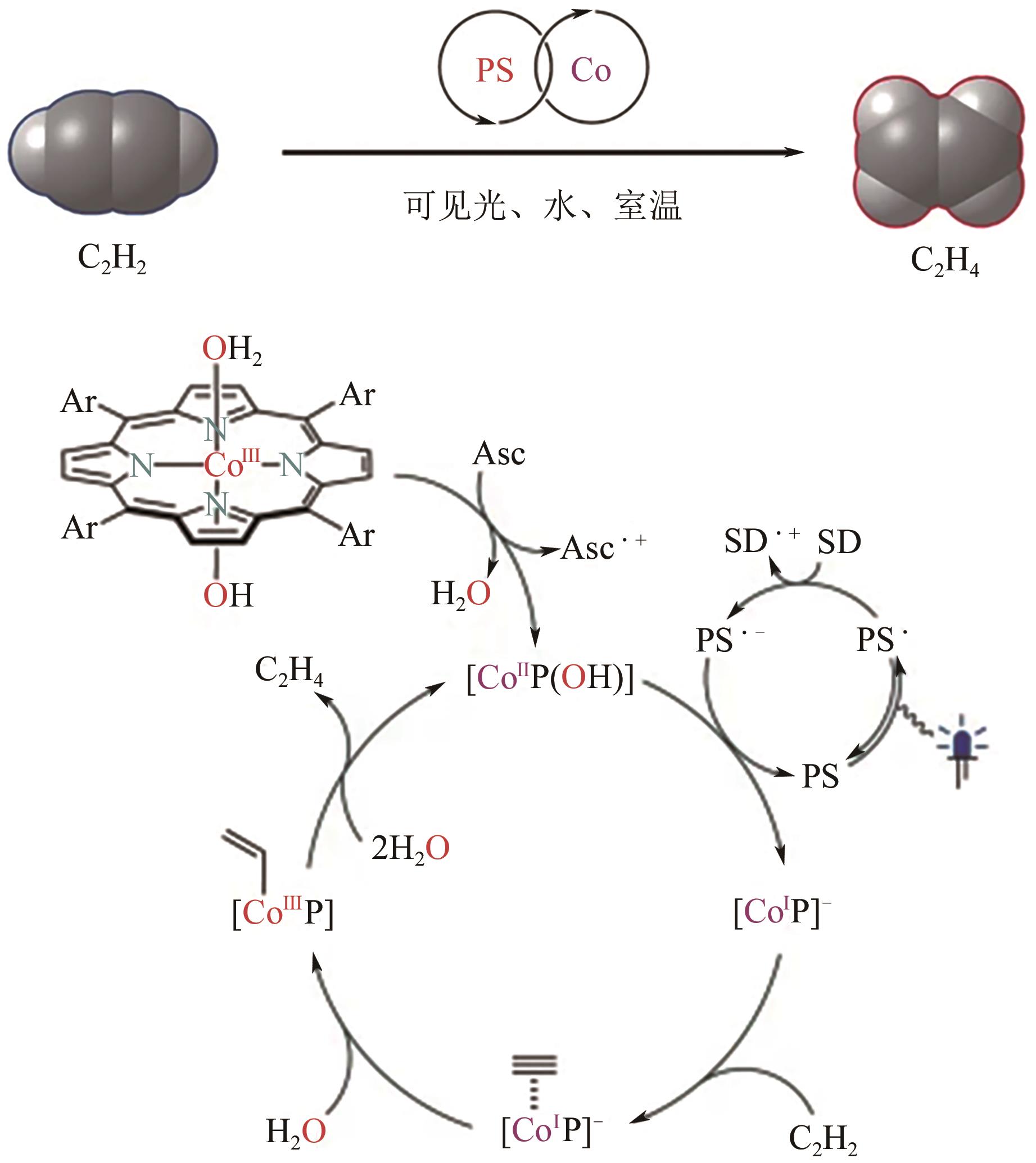
| C2H2=5%(体积分数) | [Ru(bpy)3]2+/CoTPPS | 室温 | — | 99 | [ |
| C2H2∶C2H4=1∶30 | [Ru(bpy)3]2+/CoTPPS | 室温 | 100 | 98.9 | [ |
| C3H4=5%(体积分数) | [Ru(bpy)3]2+/CoTPPS | — | 99 | [ | |
| C2H2∶C2H4∶H2=1∶20∶10 | Pd1/TiO2 SACs | 120 | 100 | >50 | [ |
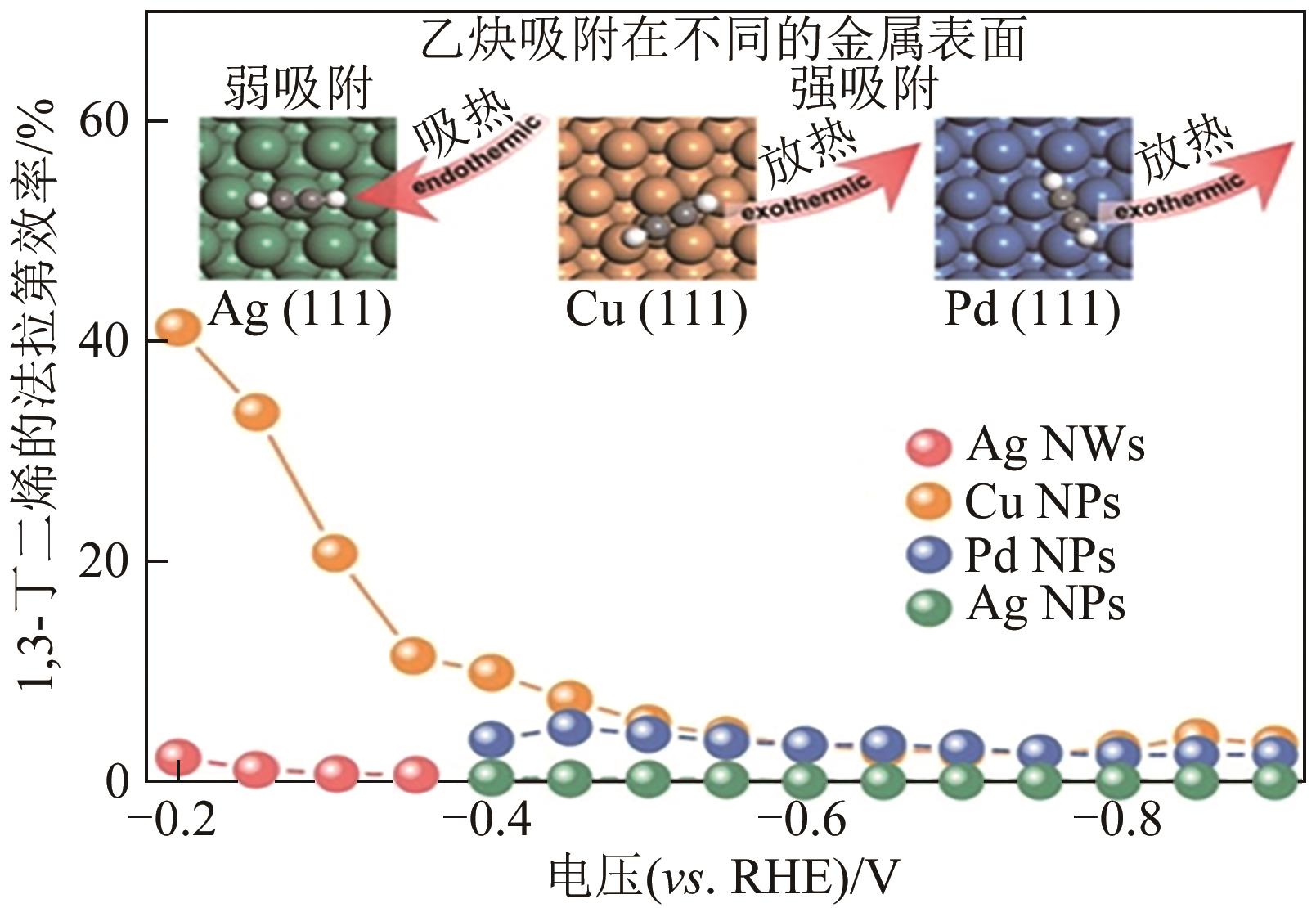
表3 光催化气相炔烃选择性加氢催化剂
| 气体组成 | 催化剂 | 反应温度/℃ | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|
| C2H2∶C2H4:∶H2=20∶20∶1 | Pd1/N-graphene | 125 | 99 | 93.5 | [ |
| C3H4∶H2=1∶5 | Pt-SA/CsPbBr3 | 5 | — | 86.6 | [ |
| C2H2=5%(体积分数) | [Ru(bpy)3]2+/CoTPPS | 室温 | — | 99 | [ |
| C2H2∶C2H4=1∶30 | [Ru(bpy)3]2+/CoTPPS | 室温 | 100 | 98.9 | [ |
| C3H4=5%(体积分数) | [Ru(bpy)3]2+/CoTPPS | — | 99 | [ | |
| C2H2∶C2H4∶H2=1∶20∶10 | Pd1/TiO2 SACs | 120 | 100 | >50 | [ |
| 底物 | 催化剂 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|
| 2-甲基-3-丁烯-2-醇 | TiO2-Pd0.5Pt0.5 NPs | 99 | 94 | [ |
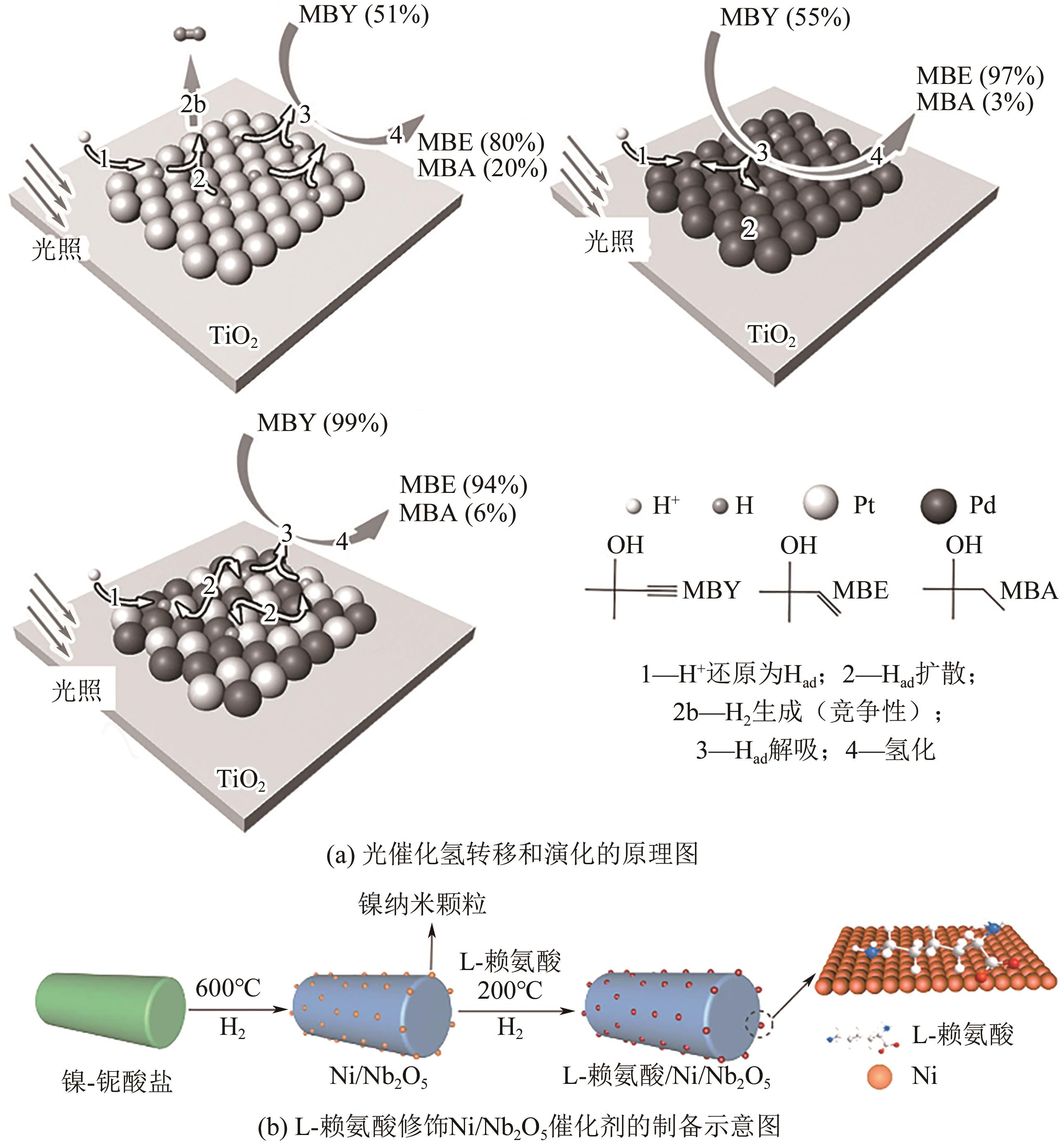
| 苯乙炔 | L-lysine/Ni/Nb2O5 | 99.9 | 95.8 | [ |
| 苯乙炔 | Pt/TiO2 | 92.4 | 91.3 | [ |
| 苯乙炔 | Ni/C3N4 | 100 | 97 | [ |
| 苯乙炔 | Au/TiO2 | 78.2 | 96.1 | [ |
表4 光催化液相炔烃选择性加氢催化剂
| 底物 | 催化剂 | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|
| 2-甲基-3-丁烯-2-醇 | TiO2-Pd0.5Pt0.5 NPs | 99 | 94 | [ |
| 苯乙炔 | L-lysine/Ni/Nb2O5 | 99.9 | 95.8 | [ |
| 苯乙炔 | Pt/TiO2 | 92.4 | 91.3 | [ |
| 苯乙炔 | Ni/C3N4 | 100 | 97 | [ |
| 苯乙炔 | Au/TiO2 | 78.2 | 96.1 | [ |
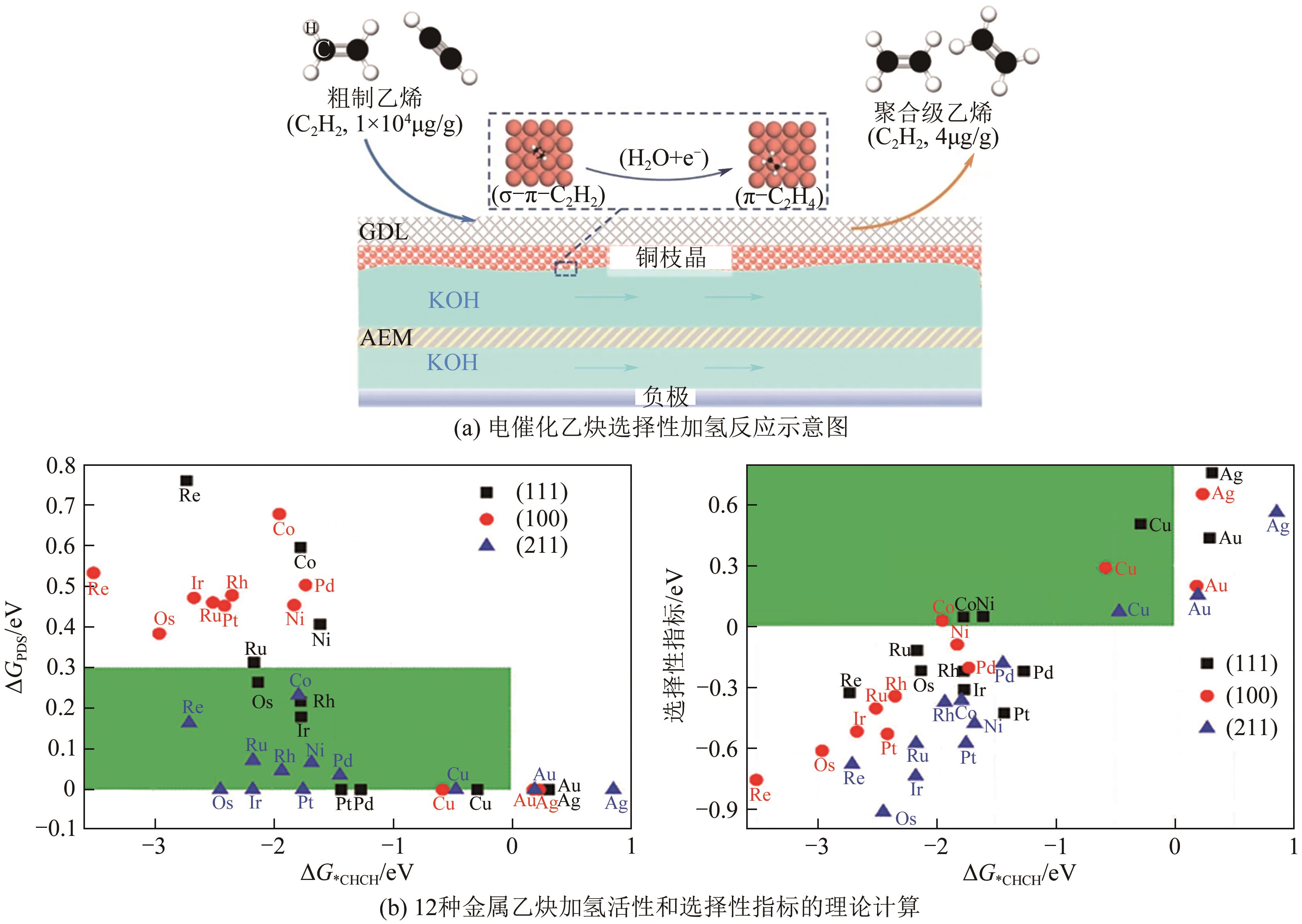
| 气体组成 | 催化剂 | 电解液 | FE/% | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| C2H2 | Cu枝晶 | 1mol/L KOH | 96 | — | — | [ |
| C2H2∶C2H4=1∶99 | Cu枝晶 | 1mol/L KOH | — | 99.96 | 97 | [ |
| C2H2∶ Ar = 5∶95 | LD-Cu | 1mol/L KOH | 74.9 | — | — | [ |
| C2H2/C2H4/Ar=0.5∶20∶79.5 | LD-Cu | 1mol/L KOH | — | 99.9 | >90 | [ |
| C2H2 | NHC-Cu | 1mol/L KOH | 98 | — | — | [ |
| C2H2∶C2H4=1∶99 | NHC-Cu | 1mol/L KOH | — | 99.7 | >99 | [ |
| C2H2 | SA-Ni-NC | 1mol/L KOH | 91.3 | — | — | [ |
| C2H2∶C2H4=1∶99 | SA-Ni-NC | 1mol/L KOH | — | 97.4 | — | [ |
| C2H2 | Ag NWs | 1mol/L KOH | 99 | — | — | [ |
| C2H2∶C2H4=1∶99 | Ag NWs | 1mol/L KOH | — | 99.8 | 99 | [ |
| C2H2 | ED-Cu | 1mol/L KOH | 97.7 | — | — | [ |
| C2H2 | Cu MPs | 1mol/L KOH | 83.2 | — | — | [ |
| C2H2 | CoPc | 1mol/L KOH | 96 | — | — | [ |
| C2H2∶C2H4=1∶99 | CoPc | 1mol/L KOH | — | 99.5 | — | [ |
表5 电催化气相炔烃选择性加氢催化剂
| 气体组成 | 催化剂 | 电解液 | FE/% | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| C2H2 | Cu枝晶 | 1mol/L KOH | 96 | — | — | [ |
| C2H2∶C2H4=1∶99 | Cu枝晶 | 1mol/L KOH | — | 99.96 | 97 | [ |
| C2H2∶ Ar = 5∶95 | LD-Cu | 1mol/L KOH | 74.9 | — | — | [ |
| C2H2/C2H4/Ar=0.5∶20∶79.5 | LD-Cu | 1mol/L KOH | — | 99.9 | >90 | [ |
| C2H2 | NHC-Cu | 1mol/L KOH | 98 | — | — | [ |
| C2H2∶C2H4=1∶99 | NHC-Cu | 1mol/L KOH | — | 99.7 | >99 | [ |
| C2H2 | SA-Ni-NC | 1mol/L KOH | 91.3 | — | — | [ |
| C2H2∶C2H4=1∶99 | SA-Ni-NC | 1mol/L KOH | — | 97.4 | — | [ |
| C2H2 | Ag NWs | 1mol/L KOH | 99 | — | — | [ |
| C2H2∶C2H4=1∶99 | Ag NWs | 1mol/L KOH | — | 99.8 | 99 | [ |
| C2H2 | ED-Cu | 1mol/L KOH | 97.7 | — | — | [ |
| C2H2 | Cu MPs | 1mol/L KOH | 83.2 | — | — | [ |
| C2H2 | CoPc | 1mol/L KOH | 96 | — | — | [ |
| C2H2∶C2H4=1∶99 | CoPc | 1mol/L KOH | — | 99.5 | — | [ |
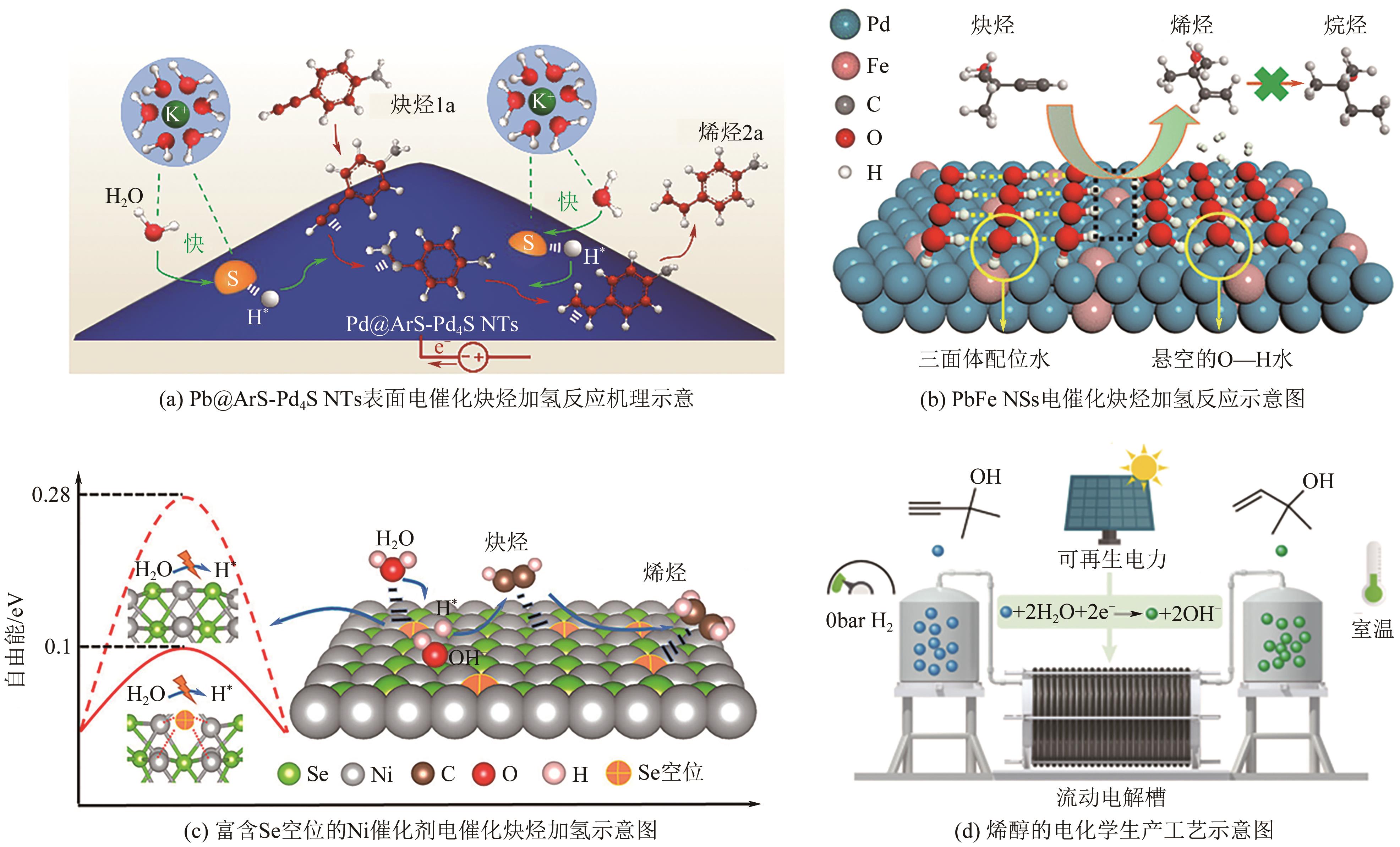
| 底物 | 催化剂 | 电解液 | FE/% | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| 二苯乙炔 | Pt1Pd99 | 1mol/L H2SO4 | 95 | — | — | [ |
| 4-乙炔基苯胺 | Pd@ArS-Pd4S PdFe NSs Ni0.85Se1-x NWs | 1mol/L KOH | 75 | 97 | 96 | [ |
| 2-甲基-3-丁烯-2-醇 | 1mol/L KOH | 49.5 | 100 | >91 | [ | |
| 4-乙炔基苯胺 | 1mol/L KOH | — | 97 | 99 | [ | |
| 2-甲基-3-丁烯-2-醇 | Cu NAs | 1mol/L KOH | 96 | — | 97 | [ |
| 2-甲基-3-丁烯-2-醇 | PdCu | 1mol/L KOH | 36 | 98.5 | 96.5 | [ |
| 4-乙炔基苯胺 | Cu-S NSs | 1mol/L KOH | — | 99 | 99 | [ |
| 4-乙炔基苯胺 | Cu-CA-CP | 1mol/L KOH | — | 97 | 99 | [ |
| 4-乙炔基苯胺 | Cu-ZnO | 1mol/L KOH | 70 | — | 99 | [ |
表6 电催化液相炔烃选择性加氢催化剂
| 底物 | 催化剂 | 电解液 | FE/% | 转化率/% | 选择性/% | 参考文献 |
|---|---|---|---|---|---|---|
| 二苯乙炔 | Pt1Pd99 | 1mol/L H2SO4 | 95 | — | — | [ |
| 4-乙炔基苯胺 | Pd@ArS-Pd4S PdFe NSs Ni0.85Se1-x NWs | 1mol/L KOH | 75 | 97 | 96 | [ |
| 2-甲基-3-丁烯-2-醇 | 1mol/L KOH | 49.5 | 100 | >91 | [ | |
| 4-乙炔基苯胺 | 1mol/L KOH | — | 97 | 99 | [ | |
| 2-甲基-3-丁烯-2-醇 | Cu NAs | 1mol/L KOH | 96 | — | 97 | [ |
| 2-甲基-3-丁烯-2-醇 | PdCu | 1mol/L KOH | 36 | 98.5 | 96.5 | [ |
| 4-乙炔基苯胺 | Cu-S NSs | 1mol/L KOH | — | 99 | 99 | [ |
| 4-乙炔基苯胺 | Cu-CA-CP | 1mol/L KOH | — | 97 | 99 | [ |
| 4-乙炔基苯胺 | Cu-ZnO | 1mol/L KOH | 70 | — | 99 | [ |
| 63 | Hao LYU, QIN Huaiyu, SUN Mingzi, et al. Mesoporosity-enabled selectivity of mesoporous palladium-based nanocrystals catalysts in semihydrogenation of alkynes[J]. Angewandte Chemie International Edition, 2022, 61(8): e202114539. |
| 64 | NIU Wenxin, GAO Yongjun, ZHANG Weiqing, et al. Pd-Pb alloy nanocrystals with tailored composition for semihydrogenation: Taking advantage of catalyst poisoning[J]. Angewandte Chemie International Edition, 2015, 54(28): 8271-8274. |
| 65 | ALBANI Davide, SHAHROKHI Masoud, CHEN Zupeng, et al. Selective ensembles in supported palladium sulfide nanoparticles for alkyne semi-hydrogenation[J]. Nature Communications, 2018, 9(1): 2634. |
| 66 | Min Wei TEW, EMERICH Herman, VAN BOKHOVEN Jeroen A. Formation and characterization of PdZn alloy: A very selective catalyst for alkyne semihydrogenation[J]. The Journal of Physical Chemistry C, 2011, 115(17): 8457-8465. |
| 67 | MITSUDOME Takato, URAYAMA Teppei, YAMAZAKI Kenji, et al. Design of core-Pd/shell-Ag nanocomposite catalyst for selective semihydrogenation of alkynes[J]. ACS Catalysis, 2016, 6(2): 666-670. |
| 68 | WANG Nan, LIU Jianguo, ZHANG Mingyue, et al. Non-noble nickel-modified covalent organic framework for partial hydrogenation of aromatic terminal alkynes[J]. ACS Applied Materials & Interfaces, 2021, 13(50): 60135-60143. |
| 69 | GUO Zhenbo, WANG Zhiqiang, ZHANG Minghui. Synthesis of metal phosphides encapsulated within defect carbon: A sulfur-tolerant and acid-resistant catalyst for selective hydrogenation[J]. Chemical Engineering Journal, 2023, 463: 142505. |
| 70 | WANG Xiaoxue, SONG Tao, FU Guangying, et al. Electronic and steric modification of Ni nanoparticle surface via N‑doped carbon layers enables highly selective semihydrogenation of alkynes[J]. ACS Catalysis, 2023, 13(17): 11634-11643. |
| 71 | LIU Huimin, SHI Lizi, ZHANG Qijian, et al. Photothermal catalysts for hydrogenation reactions[J]. Chemical Communications, 2021, 57(11): 1279-1294. |
| 72 | KATTEL Shyam, LIU Ping, CHEN Jingguang G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface[J]. Journal of the American Chemical Society, 2017, 139(29): 9739-9754. |
| 73 | Tamara BOLAÑO, CASTARLENAS Ricardo, ESTERUELAS Miguel A, et al. Sequential and selective hydrogenation of the Cα- Cβ and M-Cα double bonds of an allenylidene ligand coordinated to osmium: new reaction patterns between an allenylidene complex and alcohols[J]. Journal of the American Chemical Society, 2007, 129(28): 8850-8859. |
| 74 | LINIC Suljo, CHRISTOPHER Phillip, INGRAM David B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy[J]. Nature Materials, 2011, 10(12): 911-921. |
| 75 | CHRISTOPHER Phillip, XIN Hongliang, LINIC Suljo. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures[J]. Nature Chemistry, 2011, 3(6): 467-472. |
| 76 | LIU Wenlong, ZOU Meizhen, LIU Tao, et al. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells[J]. Advanced Materials, 2019, 31(18): e1900499. |
| 77 | ZHOU Shiqi, SHANG Lu, ZHAO Yunxuan, et al. Pd single-atom catalysts on nitrogen-doped graphene for the highly selective photothermal hydrogenation of acetylene to ethylene[J]. Advanced Materials, 2019, 31(18): 1900509. |
| 78 | HU Huicheng, GUAN Wenhao, XU Yafeng, et al. Construction of single-atom platinum catalysts enabled by CsPbBr3 nanocrystals[J]. ACS Nano, 2021, 15(8): 13129-13139. |
| 79 | ARCUDI Francesca, Luka ÐORĐEVIĆ, SCHWEITZER Neil, et al. Selective visible-light photocatalysis of acetylene to ethylene using a cobalt molecular catalyst and water as a proton source[J]. Nature Chemistry, 2022, 14(9): 1007-1012. |
| 80 | GUO Yalin, HUANG Yike, ZENG Bin, et al. Photo-thermo semi-hydrogenation of acetylene on Pd1/TiO2 single-atom catalyst[J]. Nature Communications, 2022,13(1): 2648. |
| 81 | LI Mengqiao, ZHANG Ning, LONG Ran, et al. PdPt alloy nanocatalysts supported on TiO2: Maneuvering metal-hydrogen interactions for light-driven and water-donating selective alkyne semihydrogenation[J]. Small, 2017, 13(23): 1604173. |
| 82 | WANG Jie, WANG Mengxia, LI Xincheng, et al. Bidentate ligand modification strategy on supported Ni nanoparticles for photocatalytic selective hydrogenation of alkynes[J]. Applied Catalysis B: Environmental, 2022, 313: 121449. |
| 83 | LIAN Juhong, CHAI Yuchao, QI Yu, et al. Unexpectedly selective hydrogenation of phenylacetylene to styrene on titania supported platinum photocatalyst under 385 nm monochromatic light irradiation[J]. Chinese Journal of Catalysis, 2020, 41(4): 598-603. |
| 84 | JIA Tongtong, MENG Di, JI Hongwei, et al. Visible-light-driven semihydrogenation of alkynes via proton reduction over carbon nitride supported nickel[J]. Applied Catalysis B: Environmental, 2022,304: 121004. |
| 85 | BI Qingyuan, SONG Erhong, CHEN Jiacheng, et al. Nano gold coupled black titania composites with enhanced surface plasma properties for efficient photocatalytic alkyne reduction[J]. Applied Catalysis B: Environmental, 2022, 309: 121222. |
| 86 | LIU Zhenpeng, ZHANG Lei, REN Zhipeng, et al. Advances in selective electrocatalytic hydrogenation of alkynes to alkenes[J]. Chemistry-A European Journal, 2023, 29(15): e202202979. |
| 1 | LIU Kunlong, QIN Ruixuan, ZHENG Nanfeng. Insights into the interfacial effects in heterogeneous metal nanocatalysts toward selective hydrogenation[J]. Journal of the American Chemical Society, 2021, 143(12): 4483-4499. |
| 2 | ZHAO Shu, TANG Yan, YU Xiaohu, et al. Superior reactivity of heterogeneous single-cluster catalysts for semi-hydrogenation of acetylene[J]. Science China Materials, 2023, 66(10): 3912-3921. |
| 3 | ZHANG Leilei, ZHOU Maoxiang, WANG Aiqin, et al. Selective hydrogenation over supported metal catalysts: From nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 4 | BRIDIER Blaise, Javier PÉREZ-RAMÍREZ. Cooperative effects in ternary Cu-Ni-Fe catalysts lead to enhanced alkene selectivity in alkyne hydrogenation[J]. Journal of the American Chemical Society, 2010, 132(12): 4321-4327. |
| 5 | TORRES GALVIS Hirsa M, DE JONG Krijn P. Catalysts for production of lower olefins from synthesis gas: A review[J]. ACS Catalysis, 2013, 3(9): 2130-2149. |
| 6 | BAO Zongbi, CHANG Ganggang, XING Huabin, et al. Potential of microporous metal-organic frameworks for separation of hydrocarbon mixtures[J]. Energy & Environmental Science, 2016, 9(12): 3612-3641. |
| 7 | HU Tongliang, WANG Hailong, LI Bin, et al. Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures[J]. Nature Communications, 2015, 6: 7328. |
| 8 | CUI Xili, CHEN Kaijie, XING Huabin, et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene[J]. Science, 2016, 353(6295): 141-144. |
| 9 | MCCUE Alan J, ANDERSON James A. Recent advances in selective acetylene hydrogenation using palladium containing catalysts[J]. Frontiers of Chemical Science and Engineering, 2015, 9(2):142-153. |
| 10 | BLASER Hans-Ulrich, MALAN Christophe, PUGIN Benoît, et al. Selective hydrogenation for fine chemicals: Recent trends and new developments[J]. Advanced Synthesis & Catalysis, 2003, 345(1/2): 103-151. |
| 11 | Micaela CRESPO-QUESADA, Fernando CÁRDENAS-LIZANA, DESSIMOZ Anne-Laure, et al. Modern trends in catalyst and process design for alkyne hydrogenations[J]. ACS Catalysis, 2012, 2(8): 1773-1786. |
| 12 | KLUSON Petr, CERVENY Libor. Selective hydrogenation over ruthenium catalysts[J]. Applied Catalysis A: General, 1995, 128(1): 13-31. |
| 13 | 陈志强, 车春霞, 吴登峰, 等. 乙炔选择性加氢催化剂研究进展[J]. 化工进展, 2022, 41(10): 5390-5405. |
| 87 | BU Jun, LIU Zhenpeng, MA Wenxiu, et al. Selective electrocatalytic semihydrogenation of acetylene impurities for the production of polymer-grade ethylene[J]. Nature Catalysis, 2021, 4: 557-564. |
| 88 | SHI Run, WANG Zeping, ZHAO Yunxuan, et al. Room-temperature electrochemical acetylene reduction to ethylene with high conversion and selectivity[J]. Nature Catalysis, 2021, 4: 565-574. |
| 89 | ZHANG Lei, CHEN Zhe, LIU Zhenpeng, et al. Efficient electrocatalytic acetylene semihydrogenation by electron-rich metal sites in N-heterocyclic carbene metal complexes[J]. Nature Communications, 2021, 12(1): 6574. |
| 90 | CAMPBELL Kenneth N, CAMPBELL Barbara K. The addition of hydrogen to multiple carbon-carbon bonds[J]. Chemical Reviews, 1942, 31(1): 77-175. |
| 91 | LANGER Stanley H, LANDI Henry P. The nature of electrogenerative hydrogenation[J]. Journal of the American Chemical Society, 1964, 86(21): 4694-4698. |
| 92 | Maria BEŁTOWSKA-BRZEZINSKA, Teresa ŁUCZAK, Marcin MĄCZKA, et al. Ethyne oxidation and hydrogenation on porous Pt electrode in acidic solution[J]. Journal of Electroanalytical Chemistry, 2002, 519(1/2): 101-110. |
| 93 | CHEN Zhe, CAI Cheng, WANG Tao. Identification of copper as an ideal catalyst for electrochemical alkyne semi-hydrogenation[J]. The Journal of Physical Chemistry C, 2022, 126(6): 3037-3042. |
| 94 | MA Wenxiu, CHEN Zhe, BU Jun, et al. π-Adsorption promoted electrocatalytic acetylene semihydrogenation on single-atom Ni dispersed N-doped carbon[J]. Journal of Materials Chemistry A, 2022, 10(11): 6122-6128. |
| 95 | BAI Rui, LI Jinjin, LIN Jin, et al. Weak acetylene adsorption terminated carbon-carbon coupling kinetics on silver electrocatalysts[J]. CCS Chemistry, 2023, 5(1): 200-208. |
| 96 | ZHAO Bohang, CHEN Fanpeng, WANG Mengke, et al. Economically viable electrocatalytic ethylene production with high yield and selectivity[J]. Nature Sustainability, 2023, 6: 827-837. |
| 97 | WANG Suheng, UWAKWE Kelechi, YU Liang, et al. Highly efficient ethylene production via electrocatalytic hydrogenation of acetylene under mild conditions[J]. Nature Communications, 2021, 12(1): 7072. |
| 98 | LIU Zhenpeng, CHEN Zhe, BU Jun, et al. Metal phthalocyanines as efficient electrocatalysts for acetylene semihydrogenation[J]. Chemical Engineering Journal, 2022, 431: 134129. |
| 13 | CHEN Zhiqiang, CHE Chunxia, WU Dengfeng, et al. Advances in catalysts for selective hydrogenation of acetylene[J]. Chemical Industry and Engineering Progress, 2022, 41(10): 5390-5405. |
| 14 | Gianvito VILÉ, ALBANI Davide, Neyvis ALMORA-BARRIOS, et al. Advances in the design of nanostructured catalysts for selective hydrogenation[J]. ChemCatChem, 2016, 8(1): 21-33. |
| 15 | NOYORI Ryoji, HASHIGUCHI Shohei. Asymmetric transfer hydrogenation catalyzed by chiral ruthenium complexes[J]. Accounts of Chemical Research,1997, 30(2): 97-102. |
| 16 | HAMM G, SCHMIDT T, BREITBACH J, et al. The adsorption of ethene on Pd(111) and ordered Sn/Pd(111) surface alloys[J]. Zeitschrift Für Physikalische Chemie, 2009, 223(1/2): 209-232. |
| 17 | LEAR Timothy, MARSHALL Robert, Antonio LOPEZ-SANCHEZ J, et al. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts[J]. The Journal of Chemical Physics, 2005, 123(17): 174706. |
| 18 | GU Jian, JIAN Minzhen, HUANG Li, et al. Synergizing metal-support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations[J]. Nature Nanotechnology, 2021, 16(10):1141-1149. |
| 19 | YANG Yang, ZHU Xiaojuan, WANG Lili, et al. Breaking scaling relationships in alkynol semi-hydrogenation by manipulating interstitial atoms in Pd with d-electron gain[J]. Nature Communications, 2022, 13(1): 2754. |
| 20 | LI Xiaotian, CHEN Lin, SHANG Cheng, et al. Selectivity control in alkyne semihydrogenation: Recent experimental and theoretical progress[J]. Chinese Journal of Catalysis, 2022, 43(8): 1991-2000. |
| 21 | WANG Zhe, LUO Qian, MAO Shanjun, et al. Fundamental aspects of alkyne semi-hydrogenation over heterogeneous catalysts[J]. Nano Research, 2022, 15(12): 10044-10062. |
| 22 | Gerrit WIENHÖFER, WESTERHAUS Felix A, JAGADEESH Rajenahally V, et al. Selective iron-catalyzed transfer hydrogenation of terminal alkynes[J]. Chemical Communications, 2012, 48(40): 4827-4829. |
| 23 | HORIUTI Iurô, POLANYI M. Exchange reactions of hydrogen on metallic catalysts[J]. Transactions of the Faraday Society, 1934, 30: 1164-1172. |
| 24 | 姚鹏, 史建公, 苏海霞, 等. 乙炔选择性加氢双金属催化剂研究进展[J]. 合成树脂及塑料, 2023, 40(4): 50-56. |
| YAO Peng, SHI Jiangong, SU Haixia, et al. Research progress in bimetallic catalysts for selective hydrogenation of acetylene[J]. China Synthetic Resin and Plastics, 2023, 40(4): 50-56. | |
| 25 | ZHOU Huiran, YANG Xiaofeng, LI Lin, et al. PdZn Intermetallic nanostructure with Pd-Zn-Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene[J]. ACS Catalysis, 2016, 6(2):1054-1061. |
| 26 | BENAVIDEZ Angelica D, BURTON Patrick D, NOGALES Johnny L, et al. Improved selectivity of carbon-supported palladium catalysts for the hydrogenation of acetylene in excess ethylene[J]. Applied Catalysis A: General, 2014, 482: 108-115. |
| 27 | 方洪燕, 江静静, 王定胜, 等. 乙炔半加氢催化剂设计[J]. 物理化学学报, 2023, 39(10): 31-56. |
| FANG Hongyan, JIANG Jingjing, WANG Dingsheng, et al. Catalyst design for acetylene semi-hydrogenation[J]. Acta Physico-Chimica Sinica, 2023, 39(10): 31-56. | |
| 28 | BRIDIER Blaise, Núria LÓPEZ, Javier PÉREZ-RAMÍREZ. Molecular understanding of alkyne hydrogenation for the design of selective catalysts[J]. Dalton Transactions, 2010, 39(36): 8412-8419. |
| 29 | YAN Huan, CHENG Hao, YI Hong, et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: Remarkable performance in selective hydrogenation of 1,3-butadiene[J]. Journal of the American Chemical Society, 2015, 137(33): 10484-10487. |
| 30 | HUANG Fei, DENG Yuchen, CHEN Yunlei, et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene[J]. Journal of the American Chemical Society, 2018, 140(41): 13142-13146. |
| 31 | MOLINIER M, OU J D Y, RISCH M A. Catalysts for selective hydrogenation of alkynes and alkadienes: US07153807B2[P]. 2006-12-26. |
| 32 | Gianvito VILÉ, ALBANI Davide, NACHTEGAAL Maarten, et al. A stable single-site palladium catalyst for hydrogenations[J]. Angewandte Chemie International Edition, 2015, 54(38): 11265-11269. |
| 33 | HUANG Xiaohui, XIA Yujia, CAO Yuanjie, et al. Enhancing both selectivity and coking-resistance of a single-atom Pd1/C3N4 catalyst for acetylene hydrogenation[J]. Nano Research, 2017, 10(4): 1302-1312. |
| 34 | WU Yuen, WANG Dingsheng, LI Yadong. Nanocrystals from solutions: Catalysts[J]. Chemical Society Reviews, 2014, 43(7): 2112-2124. |
| 35 | XIA Younan, XIONG Yujie, Byungkwon LIM, et al. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics?[J]. Angewandte Chemie International Edition, 2009, 48(1): 60-103. |
| 99 | NOGAMI Shuji, SHIDA Naoki, IGUCHI Shoji, et al. Mechanistic insights into the electrocatalytic hydrogenation of alkynes on Pt-Pd electrocatalysts in a proton-exchange membrane reactor[J]. ACS Catalysis, 2022, 12(9): 5430-5440. |
| 100 | GAO Ying, YANG Rong, WANG Changhong, et al. Field-induced reagent concentration and sulfur adsorption enable efficient electrocatalytic semihydrogenation of alkynes[J]. Science Advances, 2022, 8(8): eabm9477. |
| 101 | ZHU Kaili, MA Jun, CHEN Liang, et al. Unraveling the role of interfacial water structure in electrochemical semihydrogenation of alkynes[J]. ACS Catalysis, 2022, 12(9): 4840-4847. |
| 102 | LING Yangfang, WU Yongmeng, WANG Changhong, et al. Selenium vacancy promotes transfer semihydrogenation of alkynes from water electrolysis[J]. ACS Catalysis, 2021, 11(15): 9471-9478. |
| 103 | BU Jun, CHANG Siyu, LI Jinjin, et al. Highly selective electrocatalytic alkynol semi-hydrogenation for continuous production of alkenols[J]. Nature Communications, 2023, 14(1): 1533. |
| 104 | XU Xudong, MA Jun, WU Fangfang, et al. Regulating the interfacial water structure by tensile strain to boost electrochemical semi-hydrogenation of alkynes[J]. Inorganic Chemistry Frontiers, 2022, 9(14): 3444-3452. |
| 105 | WU Yongmeng, LIU Cuibo, WANG Changhong, et al. Converting copper sulfide to copper with surface sulfur for electrocatalytic alkyne semi-hydrogenation with water[J]. Nature Communications, 2021, 12: 3881. |
| 106 | 李冉. 铜基催化剂用于电催化水中炔烃转移半加氢[D]. 沈阳: 辽宁大学, 2023. |
| LI Ran. Copper-based catalyst for electrocatalytic transfer semi-hydrogenation of alkynes in water[D]. Shenyang: Liao Ning University, 2023. | |
| 36 | CHIU Chin-Yi, RUAN Lingyan, HUANG Yu. Biomolecular specificity controlled nanomaterial synthesis[J]. Chemical Society Reviews, 2013, 42(7): 2512-2527. |
| 37 | YARULIN A E, CRESPO-QUESADA R M, EGOROVA E V, et al. Structure sensitivity of selective acetylene hydrogenation over the catalysts with shape-controlled palladium nanoparticles[J]. Kinetics and Catalysis, 2012, 53(2): 253-261. |
| 38 | YANG Bo, BURCH Robbie, HARDACRE Christopher, et al. Influence of surface structures, subsurface carbon and hydrogen, and surface alloying on the activity and selectivity of acetylene hydrogenation on Pd surfaces: A density functional theory study[J]. Journal of Catalysis, 2013, 305: 264-276. |
| 39 | SEGURA Y, LÓPEZ N, PÉREZRAMÍREZ J. Origin of the superior hydrogenation selectivity of gold nanoparticles in alkyne+alkene mixtures: Triple-versus double-bond activation[J]. Journal of Catalysis, 2007, 247(2): 383-386. |
| 40 | SERNA Pedro, BORONAT Mercedes, CORMA Avelino. Tuning the behavior of Au and Pt catalysts for the chemoselective hydrogenation of nitroaromatic compounds[J]. Topics in Catalysis, 2011, 54(5): 439-446. |
| 41 | BORONAT Mercedes, CORMA Avelino. Origin of the different activity and selectivity toward hydrogenation of single metal Au and Pt on TiO2 and bimetallic Au-Pt/TiO2 catalysts[J]. Langmuir, 2010, 26(21): 16607-16614. |
| 42 | BRIDIER Blaise, Núria LÓPEZ, Javier PÉREZ-RAMÍREZ. Partial hydrogenation of propyne over copper-based catalysts and comparison with nickel-based analogues[J]. Journal of Catalysis, 2010, 269(1): 80-92. |
| 43 | 韩捷, 王天任, 孙玉珍, 等. 镍基催化剂调控的炔烃选择性加氢研究进展[J]. 精细化工, 2023, 40(10): 2161-2170, 2213. |
| HAN Jie, WANG Tianren, SUN Yuzhen, et al. Research progress in semi-hydrogenation of alkynes regulated by nickel-based catalysts[J]. Fine Chemicals, 2023, 40(10): 2161-2170, 2213. | |
| 44 | ALBANI Davide, Marçal CAPDEVILA-CORTADA, Gianvito VILÉ, et al. Semihydrogenation of acetylene on indium oxide: Proposed single-ensemble catalysis[J]. Angewandte Chemie International Edition, 2017, 56(36): 10755-10760. |
| 45 | RILEY Christopher, ZHOU Shulan, KUNWAR Deepak, et al. Design of effective catalysts for selective alkyne hydrogenation by doping of ceria with a single-atom promotor[J]. Journal of the American Chemical Society, 2018, 140(40): 12964-12973. |
| 46 | RAMOS-FERNANDEZ E V, FERREIRA A F P, SEPULVEDA-ESCRIBANO A, et al. Enhancing the catalytic performance of Pt/ZnO in the selective hydrogenation of cinnamaldehyde by Cr addition to the support[J]. Journal of Catalysis, 2008, 258(1): 52-60. |
| 47 | MCCUE Alan J, Antonio GUERRERO-RUIZ, Inmaculada RODRÍGUEZ-RAMOS, et al. Palladium sulphide-A highly selective catalyst for the gas phase hydrogenation of alkynes to alkenes[J]. Journal of Catalysis, 2016, 340: 10-16. |
| 48 | SHAO Zhengfeng, LI Chuang, CHEN Xiao, et al. A facile and controlled route to prepare an eggshell Pd catalyst for selective hydrogenation of phenylacetylene[J]. ChemCatChem, 2010, 2(12): 1555-1558. |
| 49 | MCCUE Alan J, Antonio GUERRERO-RUIZ, Carolina RAMIREZ-BARRIA, et al. Selective hydrogenation of mixed alkyne/alkene streams at elevated pressure over a palladium sulfide catalyst[J]. Journal of Catalysis, 2017, 355: 40-52. |
| 50 | ZHANG Liyun, DING Yuxiao, WU Kuang-Hsu, et al. Pd@C core-shell nanoparticles on carbon nanotubes as highly stable and selective catalysts for hydrogenation of acetylene to ethylene[J]. Nanoscale, 2017, 9(38): 14317-14321. |
| 51 | PEI Guangxian, LIU Xiaoyan, WANG Aiqin, et al. Promotional effect of Pd single atoms on Au nanoparticles supported on silica for the selective hydrogenation of acetylene in excess ethylene[J]. New Journal of Chemistry, 2014, 38(5): 2043-2051. |
| 52 | LIU Xiaoyan, MOU Chung-Yuan, LEE Szetsen, et al. Room temperature O2 plasma treatment of SiO2 supported Au catalysts for selective hydrogenation of acetylene in the presence of large excess of ethylene[J]. Journal of Catalysis, 2012, 285(1): 152-159. |
| 53 | Gianvito VILÉ, BRIDIER Blaise, WICHERT Jonas, et al. Ceria in hydrogenation catalysis: High selectivity in the conversion of alkynes to olefins[J]. Angewandte Chemie International Edition, 2012, 51(34): 8620-8623. |
| 54 | ARMBRÜSTER M, KOVNIR K, FRIEDRICH M, et al. Al13Fe4 as a low-cost alternative for palladium in heterogeneous hydrogenation[J]. Nature Materials, 2012, 11(8): 690-693. |
| 55 | CHEN Yanjun, CHEN Jixiang. Selective hydrogenation of acetylene on SiO2 supported Ni-In bimetallic catalysts: Promotional effect of In[J]. Applied Surface Science, 2016, 387: 16-27. |
| 56 | WANG Yibo, YANG Jie, GU Rongtian, et al. Crystal-facet effect of γ-Al2O3 on supporting CrO x for catalytic semihydrogenation of acetylene[J]. ACS Catalysis, 2018, 8(7): 6419-6425. |
| 57 | CHEN Xiao, SHI Chuang, LIANG Changhai. Highly selective catalysts for the hydrogenation of alkynols: A review[J]. Chinese Journal of Catalysis, 2021, 42(12): 2105-2121. |
| 58 | LINDLAR H. Ein neuer katalysator für selektive hydrierungen[J]. Helvetica Chimica Acta, 1952, 35(2): 446-450. |
| 59 | BEIER Matthias J, ANDANSON Jean-Michel, BAIKER Alfons. Tuning the chemoselective hydrogenation of nitrostyrenes catalyzed by ionic liquid-supported platinum nanoparticles[J]. ACS Catalysis, 2012, 2(12): 2587-2595. |
| 60 | Gianvito VILÉ, Javier PÉREZ-RAMÍREZ. Beyond the use of modifiers in selective alkyne hydrogenation: Silver and gold nanocatalysts in flow mode for sustainable alkene production[J]. Nanoscale, 2014, 6(22): 13476-13482. |
| 61 | MITSUDOME Takato, YAMAMOTO Masaaki, MAENO Zen, et al. One-step synthesis of core-gold/shell-ceria nanomaterial and its catalysis for highly selective semihydrogenation of alkynes[J]. Journal of the American Chemical Society, 2015, 137(42): 13452-13455. |
| 62 | LI Shuai, YUE Guichu, LI Huaike, et al. Pd single atom stabilized on multiscale porous hollow carbon fibers for phenylacetylene semi-hydrogenation reaction[J]. Chemical Engineering Journal, 2023, 454: 140031. |
| [1] | 张世蕊, 范朕连, 宋慧平, 张丽娜, 高宏宇, 程淑艳, 程芳琴. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| [2] | 朱连燕, 周幸福. 锰掺杂DSA电极及其对印染废水处理的过程优化[J]. 化工进展, 2024, 43(6): 3459-3467. |
| [3] | 陈富强, 仲兆平, 戚仁志. 铜基催化剂电还原二氧化碳为甲酸研究进展[J]. 化工进展, 2024, 43(6): 3051-3060. |
| [4] | 蔚德磊, 韩康顺, 陈瑶, 刘祥春, 崔平. 单原子Ni、N共掺杂碳材料基催化剂电还原CO2制CO研究进展[J]. 化工进展, 2024, 43(6): 3174-3186. |
| [5] | 解仲凯, 施伟东. 电荷极化光催化剂光转化二氧化碳制多碳化学品的研究进展[J]. 化工进展, 2024, 43(5): 2714-2722. |
| [6] | 姚乃瑜, 曹景沛, 庞新博, 赵小燕, 蔡士杰, 徐敏, 赵静平, 冯晓博, 伊凤娇. 低阶煤热解挥发分热催化重整研究进展[J]. 化工进展, 2024, 43(5): 2279-2293. |
| [7] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [8] | 徐诗琪, 朱颖, 陈宁华, 陆彩妹, 江露莹, 王俊辉, 覃岳隆, 张寒冰. 环境因素对水体中四环素光催化降解行为的影响[J]. 化工进展, 2024, 43(1): 551-559. |
| [9] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [10] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [11] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [12] | 郭立行, 庞蔚莹, 马克遥, 杨镓涵, 孙泽辉, 张盼, 付东, 赵昆. 层序空间多孔结构TiO2实现高效光催化CO2还原[J]. 化工进展, 2023, 42(7): 3643-3651. |
| [13] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [14] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [15] | 杨状, 李闰华, 强增寿, 王雅君, 姚文清. 废弃制冷剂R134a的光催化降解[J]. 化工进展, 2023, 42(4): 2109-2114. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||