化工进展 ›› 2024, Vol. 43 ›› Issue (1): 551-559.DOI: 10.16085/j.issn.1000-6613.2023-0217
• 资源与环境化工 • 上一篇
环境因素对水体中四环素光催化降解行为的影响
徐诗琪1( ), 朱颖1, 陈宁华2, 陆彩妹1, 江露莹1, 王俊辉1, 覃岳隆2(
), 朱颖1, 陈宁华2, 陆彩妹1, 江露莹1, 王俊辉1, 覃岳隆2( ), 张寒冰1
), 张寒冰1
- 1.广西大学资源环境与材料学院,广西 南宁 530004
2.广西环境科学保护研究院,广西 南宁 530022
-
收稿日期:2023-02-17修回日期:2023-05-04出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:覃岳隆 -
作者简介:徐诗琪(1999—),女,硕士研究生,研究方向为水文学及水资源。E-mail:myy163052@163.com。 -
基金资助:国家自然科学基金地区项目(52263029);广西自然科学基金(2020GXNSFAA297036);广西自然科学基金(2021GXNSFAA220035);广西石化资源加工及过程强化技术重点实验室(2022Z005)
Effect of environmental factors on the photocatalytic degradation behavior of tetracycline in water
XU Shiqi1( ), ZHU Ying1, CHEN Ninghua2, LU Caimei1, JIANG Luying1, WANG Junhui1, QIN Yuelong2(
), ZHU Ying1, CHEN Ninghua2, LU Caimei1, JIANG Luying1, WANG Junhui1, QIN Yuelong2( ), ZHANG Hanbing1
), ZHANG Hanbing1
- 1.School of Resources, Environment and Materials, Guangxi University, Nanning 530004, Guangxi, China
2.Scientific Research Academy of Guangxi Environmental Protection, Nanning 530022, Guangxi, China
-
Received:2023-02-17Revised:2023-05-04Online:2024-01-20Published:2024-02-05 -
Contact:QIN Yuelong
摘要:
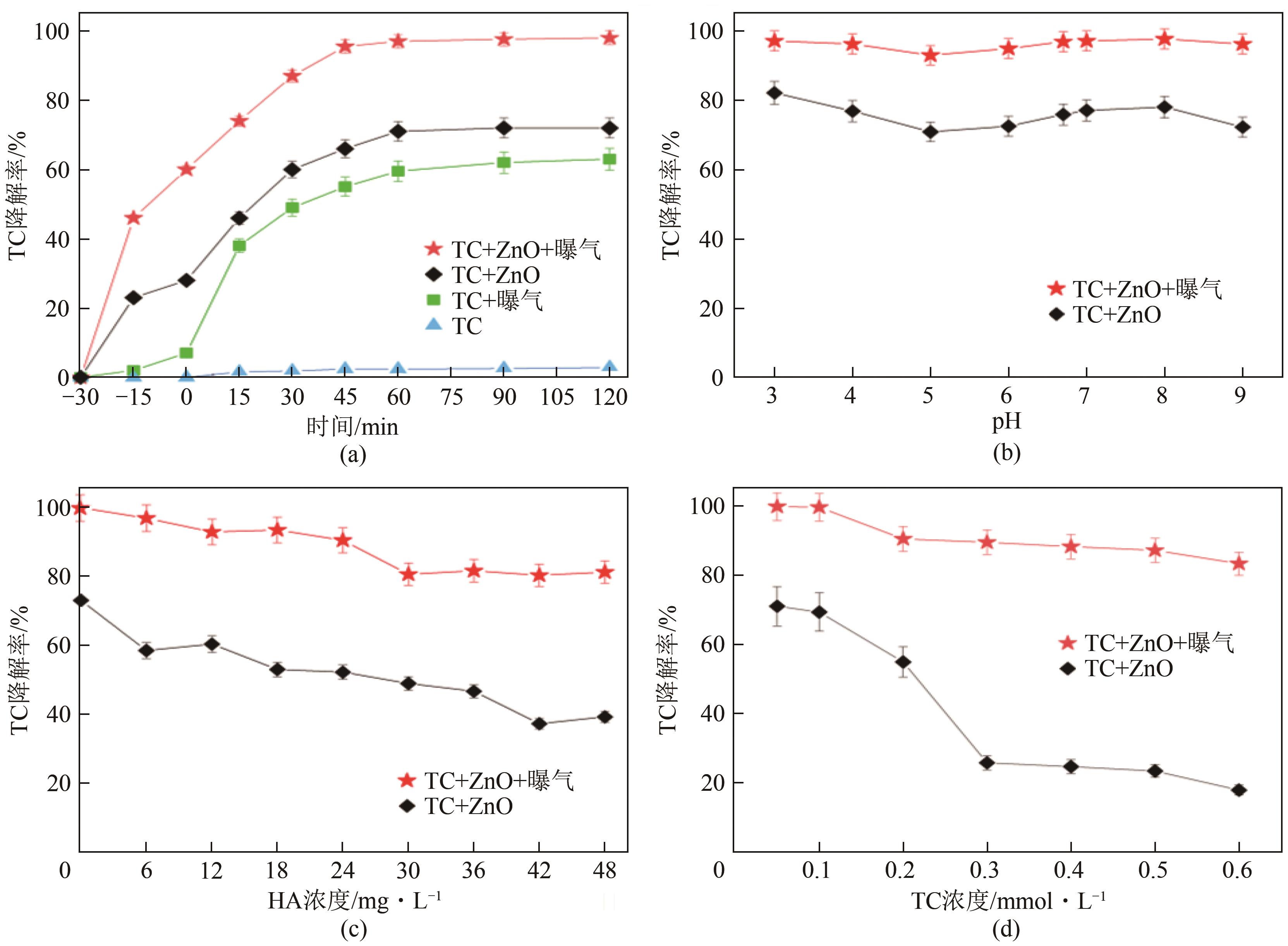
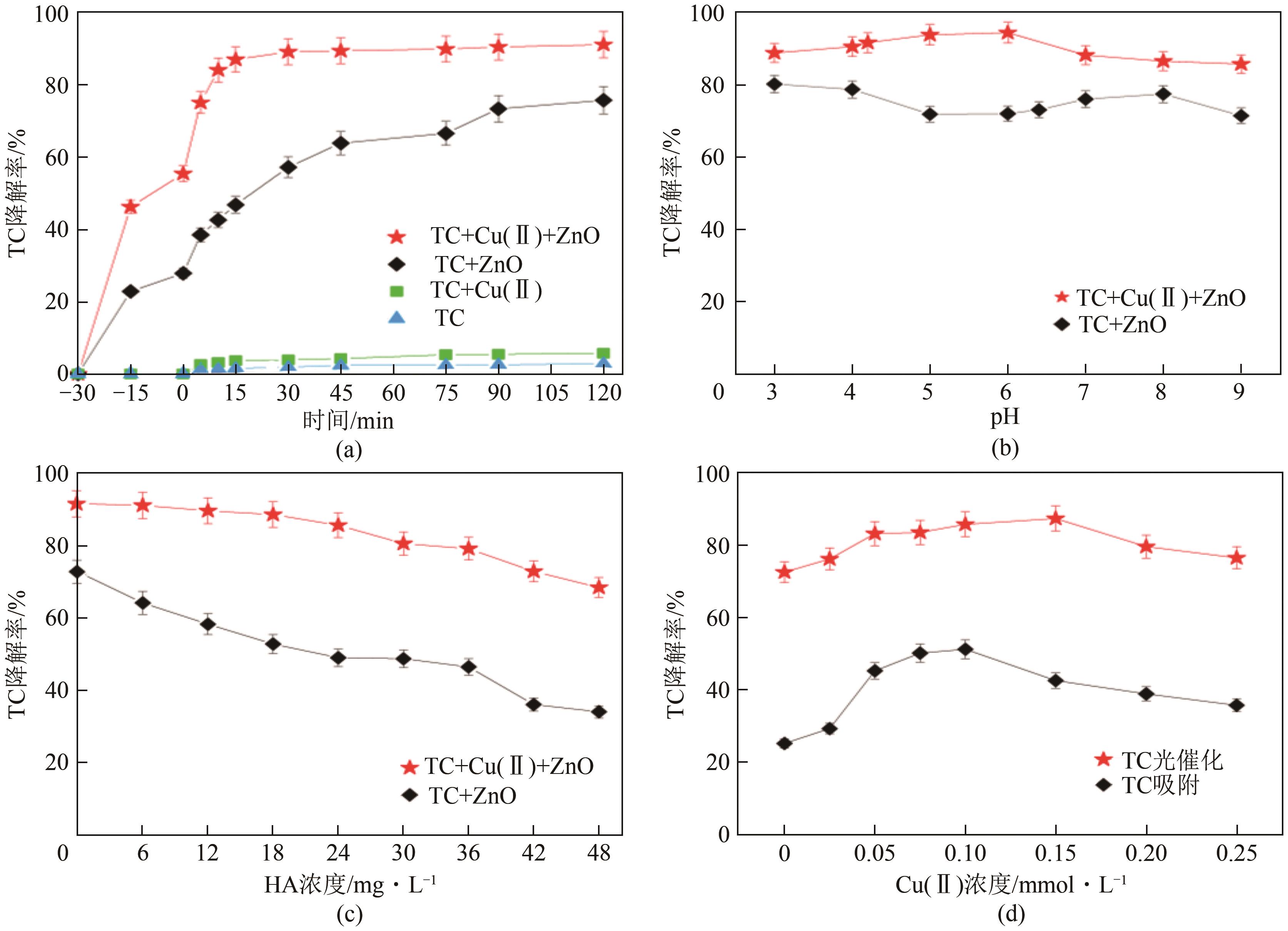
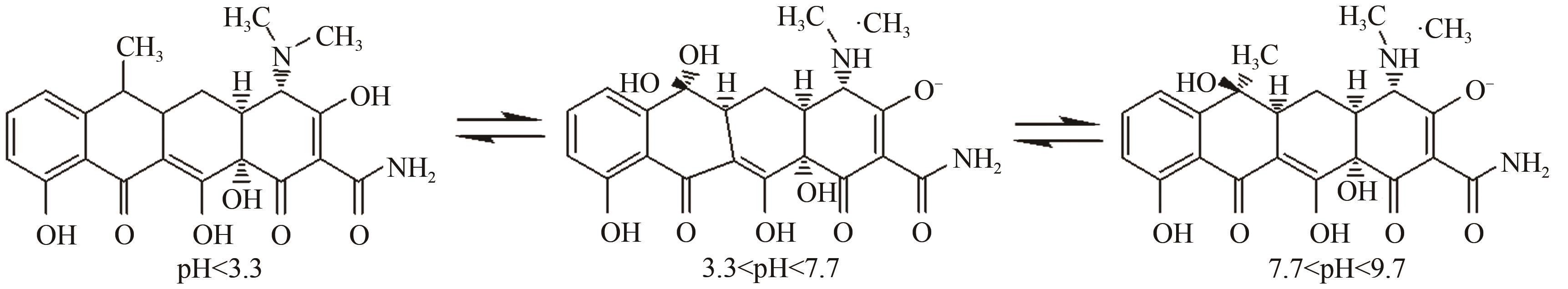
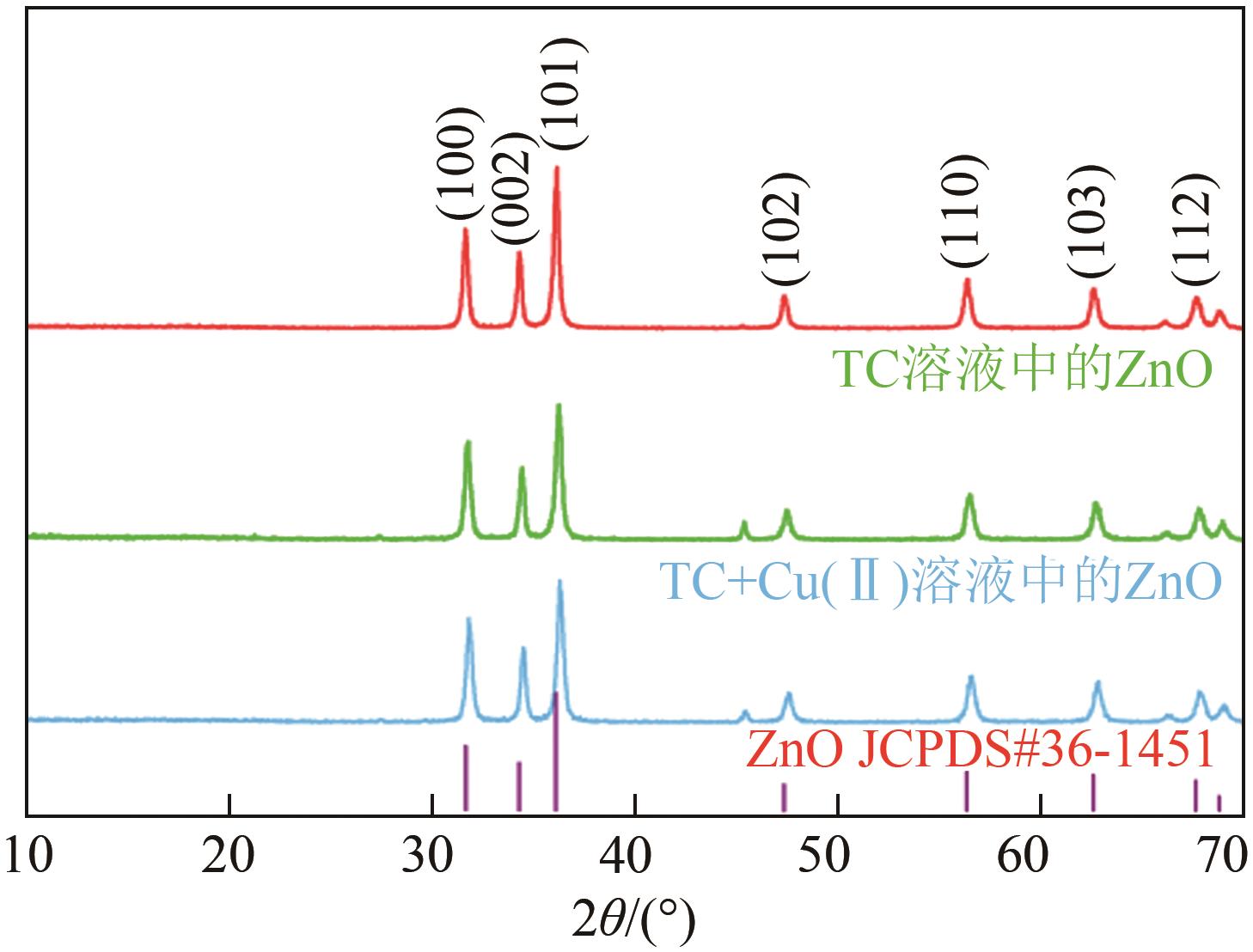
为探索实际水体中四环素(tetracycline,TC)的降解规律,以ZnO作为光催化剂研究四环素在复杂的自然环境条件(曝气、重金属、光照)下反应时间、pH、腐殖酸(humic acid,HA)浓度及四环素浓度对光催化降解过程的影响。结果表明,3种环境条件均促进了四环素的降解:曝气情况下大量的溶解氧会和催化剂协同促进超氧自由基和羟基自由基的生成,使TC达到99%的光催化降解率;重金属Cu(Ⅱ)的加入使溶液中形成TC-Cu(Ⅱ)-ZnO络合物,显著提高了ZnO对TC的降解效率,在30min时达到89%的降解率;自然光拥有全光谱,相比可见光展现出更强的TC降解作用,TC降解率达到86%,比可见光下降解率提高了14%。三因素协同作用可以有效降低TC的降解时间,在75min时达到降解平衡,降解率为99%。通过动力学分析比较了不同环境状态下的光催化活性,结果为:曝气>重金属>光照。
中图分类号:
引用本文
徐诗琪, 朱颖, 陈宁华, 陆彩妹, 江露莹, 王俊辉, 覃岳隆, 张寒冰. 环境因素对水体中四环素光催化降解行为的影响[J]. 化工进展, 2024, 43(1): 551-559.
XU Shiqi, ZHU Ying, CHEN Ninghua, LU Caimei, JIANG Luying, WANG Junhui, QIN Yuelong, ZHANG Hanbing. Effect of environmental factors on the photocatalytic degradation behavior of tetracycline in water[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 551-559.
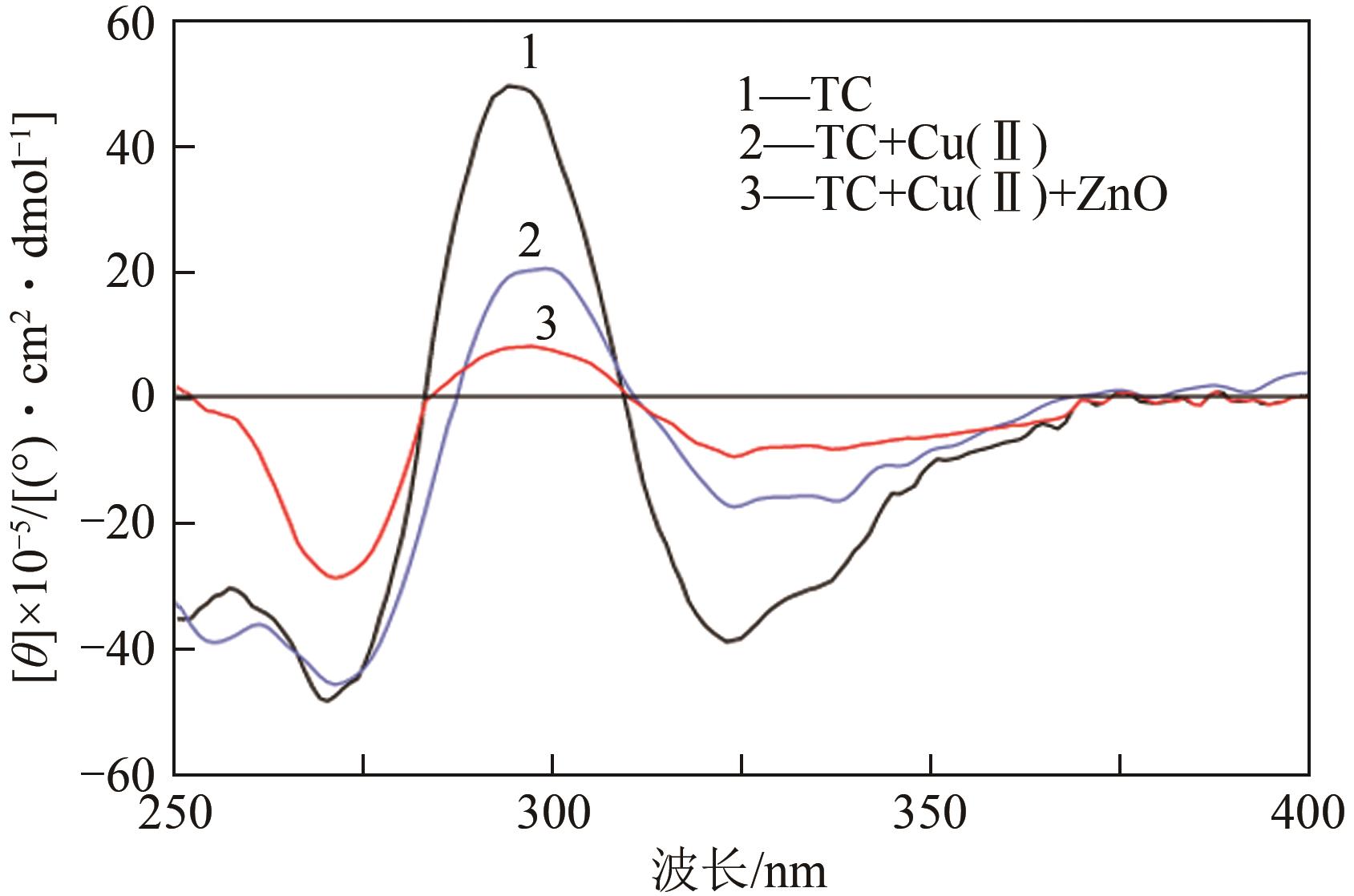
| 样品 | 最大带宽 | 最小带宽 | ||
|---|---|---|---|---|
| λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | |
| TC | 295 | 49.8 | 325 | 38.8 |
| TC‒Cu(Ⅱ) | 300 | 20.6 | 338 | 16.3 |
| TC‒Cu(Ⅱ)‒ZnO | 307 | 2.4 | 338 | 7.1 |
表2 Cu(Ⅱ)和ZnO对TC摩尔椭圆率的影响
| 样品 | 最大带宽 | 最小带宽 | ||
|---|---|---|---|---|
| λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | |
| TC | 295 | 49.8 | 325 | 38.8 |
| TC‒Cu(Ⅱ) | 300 | 20.6 | 338 | 16.3 |
| TC‒Cu(Ⅱ)‒ZnO | 307 | 2.4 | 338 | 7.1 |
| 编号 | 曝气 | Cu(Ⅱ)浓度 | 自然光 | 降解平衡 时间/min | 降解平衡时 降解率/% |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 120 | 99 |
| 2 | 1 | 2 | 1 | 90 | 91 |
| 3 | 1 | 1 | 2 | 120 | 86 |
| 4 | 2 | 2 | 1 | 90 | 99 |
| 5 | 2 | 1 | 2 | 90 | 99 |
| 6 | 1 | 2 | 2 | 90 | 94 |
| 7 | 2 | 2 | 2 | 75 | 99 |
表3 正交实验结果
| 编号 | 曝气 | Cu(Ⅱ)浓度 | 自然光 | 降解平衡 时间/min | 降解平衡时 降解率/% |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 120 | 99 |
| 2 | 1 | 2 | 1 | 90 | 91 |
| 3 | 1 | 1 | 2 | 120 | 86 |
| 4 | 2 | 2 | 1 | 90 | 99 |
| 5 | 2 | 1 | 2 | 90 | 99 |
| 6 | 1 | 2 | 2 | 90 | 94 |
| 7 | 2 | 2 | 2 | 75 | 99 |
| 反应条件 | 拟合方程 | 回归系数R2 | 动力学常数k/min-1 |
|---|---|---|---|
| TC | y=0.110x-0.101 | 0.979 | 0.110 |
| TC+Cu(Ⅱ) | y=0.489x+0.430 | 0.951 | 0.489 |
| TC+曝气 | y=0.769x-1.057 | 0.960 | 0.769 |
| TC+自然光 | y=0.286x-0.130 | 0.944 | 0.286 |
表4 不同条件下TC的光催化降解动力学拟合结果
| 反应条件 | 拟合方程 | 回归系数R2 | 动力学常数k/min-1 |
|---|---|---|---|
| TC | y=0.110x-0.101 | 0.979 | 0.110 |
| TC+Cu(Ⅱ) | y=0.489x+0.430 | 0.951 | 0.489 |
| TC+曝气 | y=0.769x-1.057 | 0.960 | 0.769 |
| TC+自然光 | y=0.286x-0.130 | 0.944 | 0.286 |
| 1 | 汤睿, 张寒冰, 陆彩妹, 等. 有机磁性膨润土对环丙沙星和四环素的吸附性能[J]. 化工进展, 2021, 40(11): 6235-6245. |
| TANG Rui, ZHANG Hanbing, LU Caimei, et al. Adsorption of ciprofloxacin and tetracycline by organically modified magnetic bentonite[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6235-6245. | |
| 2 | JIANG Liushan, XIE Yu, HE Fan, et al. Facile synthesis of GO as middle carrier modified flower-like BiOBr and C3N4 nanosheets for simultaneous treatment of chromium(Ⅵ) and tetracycline[J]. Chinese Chemical Letters, 2021, 32(7): 2187-2191. |
| 3 | ZHANG Yanan, ZHAO Yangguo, YANG Dexiang, et al. Insight into the removal of tetracycline-resistant bacteria and resistance genes from mariculture wastewater by ultraviolet/persulfate advanced oxidation process[J]. Journal of Hazardous Materials Advances, 2022, 7: 100129. |
| 4 | 郭新兴, 刘建国, 王鹏, 等. 电化学沉积法制备ZIF-8及其对四环素的吸附[J]. 环境化学, 2020, 39(3): 581-592. |
| GUO Xinxing, LIU Jianguo, WANG Peng, et al. Electrochemical synthesis of ZIF-8 for adsorption of tetracycline[J]. Environmental Chemistry, 2020, 39(3): 581-592. | |
| 5 | 张畅滕, 申乾宏, 郑素华, 等. 三维阶层结构BiOBr/Bi2WO6多孔微球制备及其对盐酸四环素的可见光催化降解性能[J]. 材料科学与工程学报, 2022, 40(4): 567-574. |
| ZHANG Changteng, SHEN Qianhong, ZHENG Suhua, et al. Preparation of three-dimensional hierarchical BiOBr/Bi2WO6 porous microsphere and its visible-light photocatalytic degradation for tetracycline hydrochloride[J]. Journal of Materials Science and Engineering, 2022, 40(4): 567-574. | |
| 6 | 石宇, 杨晓婷, 兰贵红, 等. MnO x 掺杂纳米石墨阴极的制备及其对盐酸四环素的降解[J]. 精细化工, 2022, 39(4): 798-805. |
| SHI Yu, YANG Xiaoting, LAN Guihong, et al. Preparation of nano graphite cathode doped with MnO x and its degradation for tetracycline hydrochloride[J]. Fine Chemicals, 2022, 39(4): 798-805. | |
| 7 | 李佳泽, 吴宝利, 刘富荣, 等. BAF工艺深度处理四环素类制药废水研究[J]. 中国给水排水, 2022, 38(5): 24-31. |
| LI Jiaze, WU Baoli, LIU Furong, et al. Treatment of tetracyclines antibiotics pharmaceutical wastewater by BAF process[J]. China Water & Wastewater, 2022, 38(5): 24-31. | |
| 8 | 陈杰, 李明明, 刘治刚, 等. Fe3O4@TiO2核壳微球吸附-光催化联合去除四环素性能[J]. 化学研究与应用, 2022, 34(8): 1803-1812. |
| CHEN Jie, LI Mingming, LIU Zhigang, et al. Removal of tetracycline by Fe3O4@TiO2 core-shell microsphere combined with adsorption-photocatalysis[J]. Chemical Research and Application, 2022, 34(8): 1803-1812. | |
| 9 | 宋继梅, 朱婉蓉, 杨捷, 等. Ag/AgBr/BiOBr的制备及其快速光催化降解四环素[J]. 安徽大学学报(自然科学版), 2022, 46(5): 71-82. |
| SONG Jimei, ZHU Wanrong, YANG Jie, et al. Preparation of Ag/AgBr/BiOBr and their prominent photocatalytic activity for the degradation of tetracycline under visible light[J]. Journal of Anhui University (Natural Science Edition), 2022, 46(5): 71-82. | |
| 10 | 仇思, 李小明, 罗琨, 等. g-C3N4/Ag3PO4/CNT对亚甲基蓝和四环素光催化的降解[J]. 环境化学, 2022, 41(7): 2414-2424. |
| CHOU Si, LI Xiaoming, LUO Kun, et al. Study on the photocatalytic degradation performance of g-C3N4/Ag3PO4/CNT on methylene blue and tetracycline[J]. Environmental Chemistry, 2022, 41(7): 2414-2424. | |
| 11 | 邓郁蓉, 欧阳卓智, 杨琛, 等. 四环素在水体中的自然光解作用机制[J]. 环境科学学报, 2022, 42(9): 40-50. |
| DENG Yurong, OUYANG Zhuozhi, YANG Chen, et al. Photolysis of tetracycline in aqueous environment[J]. Acta Scientiae Circumstantiae, 2022, 42(9): 40-50. | |
| 12 | ZOUHEIR M, TANJI K, NAVIO J A, et al. Effective photocatalytic conversion of formic acid using iron, copper and sulphate doped TiO2 [J]. Journal of Central South University, 2022, 29(11): 3592-3607. |
| 13 | HU Xueli, LU Peng, FU Min, et al. Activating the photocatalytic activity of insulator Barium silicate: A liquid-phase alkalized tetracycline photosensitizer and its self-destruction[J]. Chemical Engineering Journal, 2023, 454: 140281. |
| 14 | 刘怡璇, 林跃朝, 马伟芳. 可见光催化降解水中卤代有机污染物的研究进展[J]. 化工进展, 2022, 41(S1): 571-579. |
| LIU Yixuan, LIN Yuechao, MA Weifang. Research progress on degradation of halogenated organic contaminants in water by visible light photocatalysis[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 571-579. | |
| 15 | 乔波, 欧阳紫菱, 郭丽, 等. 曝气量对PCMR处理腐殖酸废水中膜污染控制研究[J]. 水处理技术, 2022, 48(5): 81-84. |
| QIAO Bo, OUYANG Ziling, GUO Li, et al. Study on the control of membrane fouling in humic acid wastewater treated by PCMR with aeration volume[J]. Technology of Water Treatment, 2022, 48(5): 81-84. | |
| 16 | ZHU Ying, LIU Kun, MUHAMMAD Y, et al. Effects of divalent copper on tetracycline degradation and the proposed transformation pathway[J]. Environmental Science and Pollution Research, 2020, 27(5): 5155-5167. |
| 17 | GOPAL G, NATARAJAN C, MUKHERJEE A. Synergistic removal of tetracycline and copper(Ⅱ) by in-situ B-Fe/Ni nanocomposite—A novel and an environmentally sustainable green nanomaterial[J]. Environmental Technology & Innovation, 2022, 25: 102187. |
| 18 | AI Yuejie, ZHAO Chaofeng, SUN Lu, et al. Coagulation mechanisms of humic acid in metal ions solution under different pH conditions: A molecular dynamics simulation[J]. Science of the Total Environment, 2020, 702: 135072. |
| 19 | MUÑOZ-FLORES P, POON P S, ANIA C O, et al. Performance of a C-containing Cu-based photocatalyst for the degradation of tartrazine: Comparison of performance in a slurry and CPC photoreactor under artificial and natural solar light[J]. Journal of Colloid and Interface Science, 2022, 623: 646-659. |
| 20 | FILIPE O M S, SANTOS E B H, OTERO Marta, et al. Photodegradation of metoprolol in the presence of aquatic fulvic acids. Kinetic studies, degradation pathways and role of singlet oxygen, OH radicals and fulvic acids triplet states[J]. Journal of Hazardous Materials, 2020, 385: 121523. |
| 21 | HUANG Hua, NIU Zhirui, SHI Ruru, et al. Thermal oxidation activation of hydrochar for tetracycline adsorption: The role of oxygen concentration and temperature[J]. Bioresource Technology, 2020, 306: 123096. |
| 22 | YANG Bo, WANG Chengjin, CHENG Xin, et al. Interactions between the antibiotic tetracycline and humic acid: Examination of the binding sites, and effects of complexation on the oxidation of tetracycline[J]. Water Research, 2021, 202: 117379. |
| 23 | 潘柳疏, 焦春霖, 熊建华, 等. 蔗渣纤维基碳球负载TiO2复合材料的制备表征及对四环素的去除研究[J]. 水处理技术, 2022, 48(5): 85-89. |
| PAN Liushu, JIAO Chunlin, XIONG Jianhua, et al. Preparation, characterization and tetracycline removal of bagasse cellulose carbon spheres supported nano-TiO2 composites[J]. Technology of Water Treatment, 2022, 48(5): 85-89. | |
| 24 | CAI Ying, SHEN Shihao, FAN Jinhong. Enhanced degradation of tetracycline by Cu(Ⅱ) complexation in the FeS/sulfite system[J]. Journal of Hazardous Materials, 2022, 421: 126673. |
| 25 | 饶涵, 马永梅, 李思悦. NaYF4: Yb, Tm@TiO2复合催化剂光催化降解盐酸四环素[J]. 功能材料, 2022, 53(3): 3011-3019. |
| RAO Han, MA Yongmei, LI Siyue. Photocatalytic degradation of tetracycline hydrochloride by NaYF4: Yb, Tm@TiO2 composite catalyst[J]. Journal of Functional Materials, 2022, 53(3): 3011-3019. | |
| 26 | 段毅, 邹烨, 周书葵, 等. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158. |
| DUAN Yi, ZOU Ye, ZHOU Shukui, et al. Progress in the degradation of organic pollutants by H2O2/PMS/PDS activated by transition metal single-atom catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4147-4158. | |
| 27 | GONG Yinan, WANG Ying, TANG Miaomiao, et al. A two-step process coupling photocatalysis with adsorption to treat tetracycline-Copper(Ⅱ) hybrid wastewaters[J]. Journal of Water Process Engineering, 2022, 47: 102710. |
| 28 | 黄翔峰, 熊永娇, 彭开铭, 等. 金属离子络合对抗生素去除特性的影响研究进展[J]. 环境化学, 2016, 35(1): 133-140. |
| HUANG Xiangfeng, XIONG Yongjiao, PENG Kaiming, et al. The progress of antibiotics removal performance under the complexion effect of metal ions[J]. Environmental Chemistry, 2016, 35(1): 133-140. | |
| 29 | JIANG Chenxiao, CHEN Hanlin, ZHANG Yilue, et al. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter[J]. Chemical Engineering Journal, 2018, 348: 952-959. |
| 30 | FATIMA B, SIDDIQUI S I, RAJOR H K, et al. Photocatalytic removal of organic dye using green synthesized zinc oxide coupled cadmium tungstate nanocomposite under natural solar light irradiation[J]. Environmental Research, 2023, 216: 114534. |
| 31 | CIONTI C, PARGOLETTI E, FALLETTA E, et al. Combining pH-triggered adsorption and photocatalysis for the remediation of complex water matrices[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108468. |
| 32 | 陈佳昆, 汤健, 夏恒, 等. 城市固废炉排炉焚烧过程二𫫇英排放浓度数值仿真[J]. 化工进展, 2023, 42(2): 1061-1072. |
| CHEN Jiakun, TANG Jian, XIA Heng, et al. Numerical simulation of dioxin emission concentration in grate furnace incineration processes for municipal solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1061-1072. | |
| 33 | VILLARREAL R C, LUQUE-MORALES M, CHINCHILLAS-CHINCHILLAS M J, et al. Langmuir-Hinshelwood-Hougen-Watson model for the study of photodegradation properties of zinc oxide semiconductor nanoparticles synthetized by Peumus boldus [J]. Results in Physics, 2022, 36: 105421. |
| 34 | 蒋柱武, 史安童, 沈俊宏. Cu-ZnO/g-C3N4复合材料可见光催化降解环丙沙星效率及机理研究[J]. 材料导报, 2022, 36(20): 84-90. |
| JIANG Zhuwu, SHI Antong, SHEN Junhong. Study on efficiency and mechanism of visible-light photocatalytic degradation of ciprofloxacin by using Cu-ZnO/g-C3N4 composite[J]. Materials Reports, 2022, 36(20): 84-90. |
| [1] | 裴文艺, 陈子阳, 赵萌, 姜红, 陈日志. 预润湿对Janus陶瓷膜制备及布气性能的影响[J]. 化工进展, 2024, 43(1): 353-363. |
| [2] | 王莹, 韩云平, 李琳, 李衍博, 李慧丽, 颜昌仁, 李彩侠. 城市污水厂病毒气溶胶逸散特征研究现状与未来展望[J]. 化工进展, 2023, 42(S1): 439-446. |
| [3] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [4] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [5] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [6] | 郭立行, 庞蔚莹, 马克遥, 杨镓涵, 孙泽辉, 张盼, 付东, 赵昆. 层序空间多孔结构TiO2实现高效光催化CO2还原[J]. 化工进展, 2023, 42(7): 3643-3651. |
| [7] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [8] | 刘佳, 梁德青, 李君慧, 林德才, 吴思婷, 卢富勤. 油水体系水合物浆液流动保障研究进展[J]. 化工进展, 2023, 42(4): 1739-1759. |
| [9] | 杨状, 李闰华, 强增寿, 王雅君, 姚文清. 废弃制冷剂R134a的光催化降解[J]. 化工进展, 2023, 42(4): 2109-2114. |
| [10] | 胥生元, 郝玮, 王杰, 高文生, 谢克锋. 半导体光催化剂BiOCl异质结的构建及应用[J]. 化工进展, 2023, 42(3): 1493-1507. |
| [11] | 陈邦富, 欧阳平, 李宇涵, 段有雨, 董帆. ZnSn(OH)6 基纳米材料在环境光催化中的应用[J]. 化工进展, 2023, 42(2): 756-764. |
| [12] | 姚稳, 张雨晨, 滕文馨, 黎江玲. 表面活性剂对制备Ca掺杂β-In2S3微观结构的影响及其光催化降解甲基橙性能[J]. 化工进展, 2023, 42(2): 774-782. |
| [13] | 程荣, 邓子祺, 夏锦程, 李江, 石磊, 郑祥. 光催化系统灭活微生物气溶胶的研究进展[J]. 化工进展, 2023, 42(2): 957-968. |
| [14] | 吴新波, 党鸿钟, 马娇, 严渊, 曾天续, 李维维, 张国珍, 陈永志. A2/O-BAF工艺短程硝化模式下反硝化除磷效能[J]. 化工进展, 2023, 42(2): 1089-1097. |
| [15] | 多佳, 姚国栋, 王英霁, 曾旭, 金滨滨. 改性Au-TiO2光降解废水中诺氟沙星的影响[J]. 化工进展, 2023, 42(2): 624-630. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||