化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4587-4600.DOI: 10.16085/j.issn.1000-6613.2023-1013
• 资源与环境化工 • 上一篇
基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物
张茜1( ), 李皓芯1, 张天阳2,3, 李子富1, 孙文俊4, 敖秀玮1(
), 李皓芯1, 张天阳2,3, 李子富1, 孙文俊4, 敖秀玮1( )
)
- 1.北京科技大学能源与环境工程学院,北京 100083
2.同济大学环境科学与工程学院,上海 200092
3.水利部 长三角城镇供水节水及水环境治理重点实验室,上海 200092
4.清华大学环境学院,北京 100084
-
收稿日期:2023-06-20修回日期:2023-09-25出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:敖秀玮 -
作者简介:张茜(1999—),女,硕士研究生,研究方向为紫外线高级氧化技术去除水中新污染物。E-mail:zx877592915@163.com。 -
基金资助:国家自然科学基金(52270020);中央高校基本科研业务费项目(06500211)
Degradation of per- and polyfluoroalkyl substances in water by UV-based advanced oxidation or advanced reduction processes
ZHANG Xi1( ), LI Haoxin1, ZHANG Tianyang2,3, LI Zifu1, SUN Wenjun4, AO Xiuwei1(
), LI Haoxin1, ZHANG Tianyang2,3, LI Zifu1, SUN Wenjun4, AO Xiuwei1( )
)
- 1.School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China
2.College of Environmental Science & Engineering, Tongji University, Shanghai 200092, China
3.Key Laboratory of Urban Water Supply, Water Saving and Water Environment Governance in the Yangtze River Delta of Ministry of Water Resources, Shanghai 200092, China
4.School of Environment, Tsinghua University, Beijing 100084, China
-
Received:2023-06-20Revised:2023-09-25Online:2024-08-15Published:2024-09-02 -
Contact:AO Xiuwei
摘要:
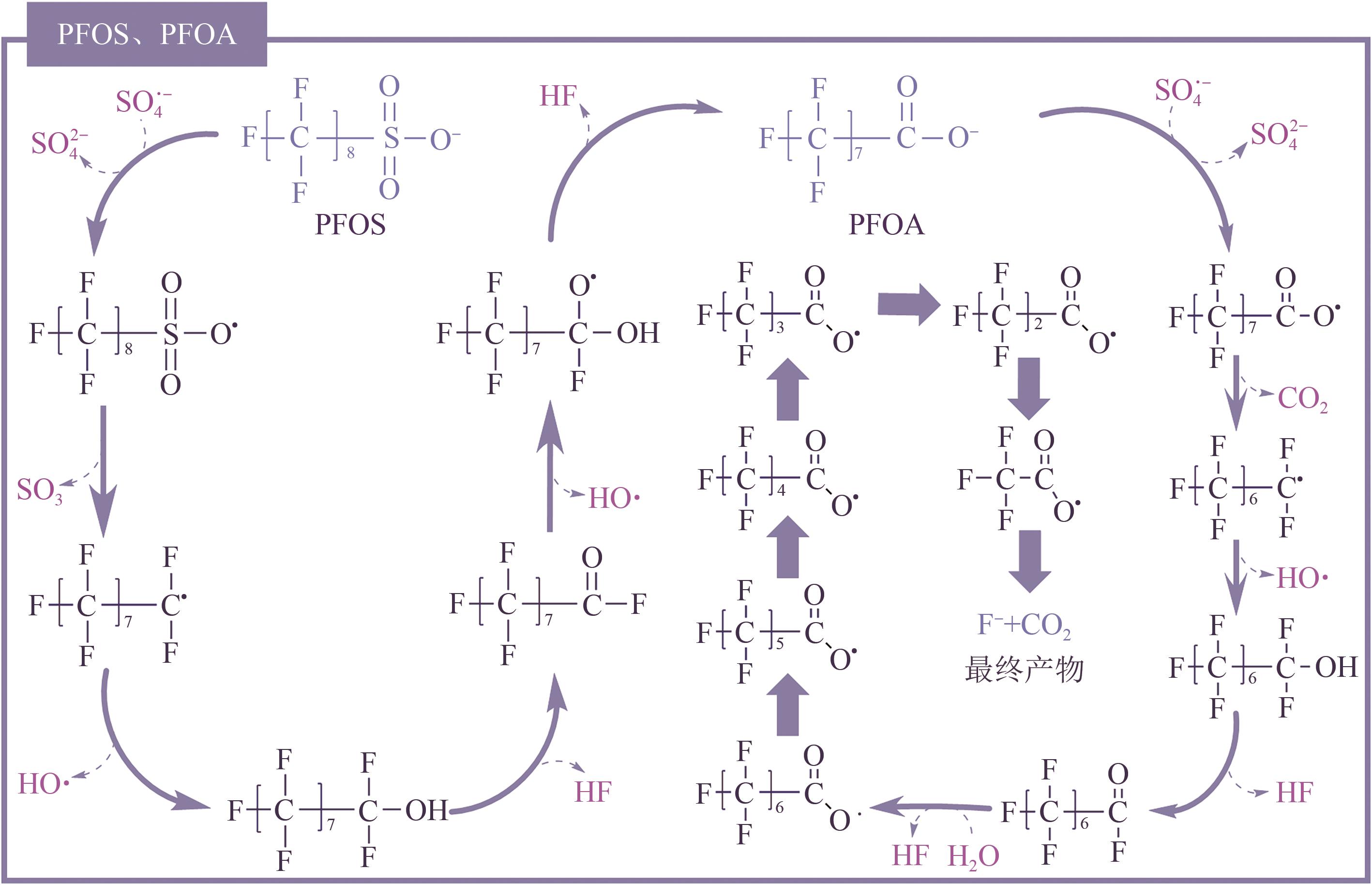
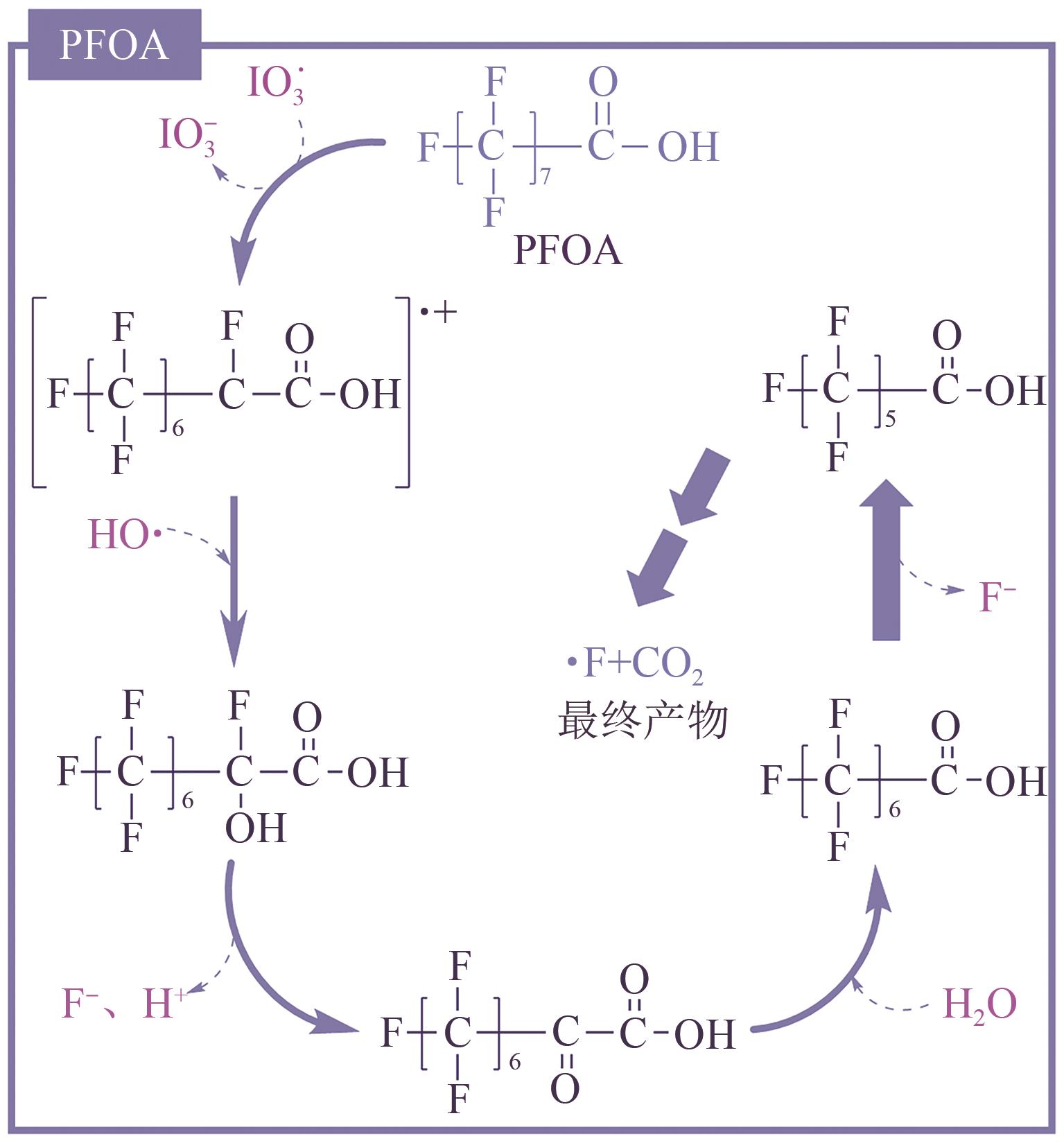
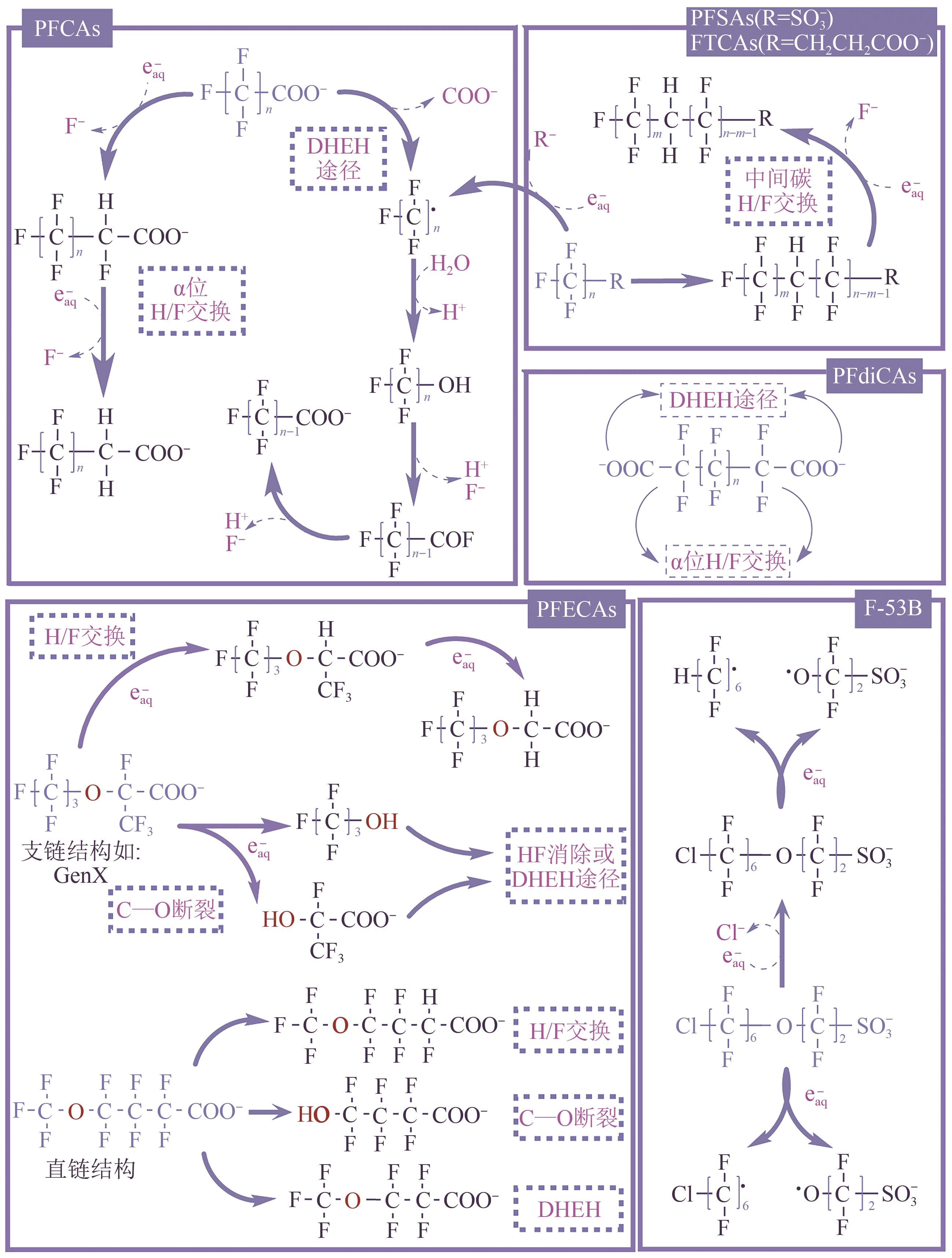
全氟或多氟烷基化合物(PFASs)是一类具有难降解性、生物蓄积性和潜在毒性的持久性有机污染物,广泛存在于各类环境介质中。水环境是PFASs最重要的归趋之一,然而水处理常规技术难以去除PFASs,近年来,基于紫外线的高级氧化或高级还原技术在PFASs的降解中表现出巨大的发展潜力和良好的应用前景。本文针对目前对于直接紫外光降解、紫外高级氧化技术和紫外高级还原技术降解PFASs的相关研究,重点从降解机理(包括活性物种产生机理和反应机理)、降解效率(包括降解率和脱氟率)和影响因素(包括光波长、pH、溶解氧、无机离子和腐殖酸等)三个维度对比了不同反应体系下PFASs的去除效能,并总结了基于紫外线的高级氧化或高级还原技术应用于去除实际水体中PFASs时需克服的难点,以期为该类技术在PFASs降解方面的发展提供参考。
中图分类号:
引用本文
张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600.
ZHANG Xi, LI Haoxin, ZHANG Tianyang, LI Zifu, SUN Wenjun, AO Xiuwei. Degradation of per- and polyfluoroalkyl substances in water by UV-based advanced oxidation or advanced reduction processes[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4587-4600.
| 化合物 | 光波长/nm | 灯功率/W | 反应时间/h | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|
| PFOA | 254 | 200 | 72 | 89.5 | 33 | [ |
| PFOA | 185 | 20 | 3 | 87 | 21 | [ |
| PFOA | 254 | 23 | 2 | 9 | — | [ |
| 185 | 87 | 25 | ||||
| PFOA | 185 | 15 | 2 | 61.7 | 17.1 | [ |
| PFOS | 185 | 23 | 12 | 8 | — | [ |
| PFOS(水溶液) | 254 | 32 | 24 | 8 | — | [ |
| PFOS(碱性2-丙醇溶液) | 76 | — | ||||
| 6∶2 FTUCA | 254 | — | 2 | <7 | — | [ |
表1 紫外线直接光解PFASs的研究总结
| 化合物 | 光波长/nm | 灯功率/W | 反应时间/h | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|
| PFOA | 254 | 200 | 72 | 89.5 | 33 | [ |
| PFOA | 185 | 20 | 3 | 87 | 21 | [ |
| PFOA | 254 | 23 | 2 | 9 | — | [ |
| 185 | 87 | 25 | ||||
| PFOA | 185 | 15 | 2 | 61.7 | 17.1 | [ |
| PFOS | 185 | 23 | 12 | 8 | — | [ |
| PFOS(水溶液) | 254 | 32 | 24 | 8 | — | [ |
| PFOS(碱性2-丙醇溶液) | 76 | — | ||||
| 6∶2 FTUCA | 254 | — | 2 | <7 | — | [ |
| 性质 | PFOS | PFOA |
|---|---|---|
| CAS号 | 176-23-1 | 335-67-1 |
| 物理状态(室温、标准大气压) | 白色粉末 | 白色粉末/蜡状白色固体 |
| 分子式 | CF3(CF2)7SO3H | CF3(CF2)6COOH |
| 分子量 | 500.130 | 414.068 |
| 蒸气压/Pa | 3.31×10-4(20℃) | 4.21(25℃) |
| 水中溶解度/g·L-1 | 0.57 | 3.3 |
| pKa | 3.27 | 2.5 |
| 半衰期(水中,25℃)/年 | >41 | >92 |
表2 PFOS和PFOA主要理化性质
| 性质 | PFOS | PFOA |
|---|---|---|
| CAS号 | 176-23-1 | 335-67-1 |
| 物理状态(室温、标准大气压) | 白色粉末 | 白色粉末/蜡状白色固体 |
| 分子式 | CF3(CF2)7SO3H | CF3(CF2)6COOH |
| 分子量 | 500.130 | 414.068 |
| 蒸气压/Pa | 3.31×10-4(20℃) | 4.21(25℃) |
| 水中溶解度/g·L-1 | 0.57 | 3.3 |
| pKa | 3.27 | 2.5 |
| 半衰期(水中,25℃)/年 | >41 | >92 |
| 化合物 | 光波长/nm | 灯功率/W | 反应时间/h | UV-AOPs | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| PFOA | 254 | 24 | 24 | UV/H2O2 | 19.7 | 6.8 | [ |
| PFOA | 254 | 400 | 12 | UV/H2O2(HCO3-共存) | 100 | 82.3 | [ |
| PFOA | 254 | 28 | 4 | UV/O3 | 27.1 | 10.6 | [ |
| PFASs | 185/254 | 300 | 20min | UV/O3 | 95 | — | [ |
| PFOA | 220~460 | 200 | 2 | UV/Na2S2O8 | 99.1 | — | [ |
| 12 | — | 73.8 | |||||
| OBS | 254 | — | 5 | UV/Na2S2O8 | 100 | 27.6 | [ |
| PFBA | 254~400 | 1000 | 5 | UV/Na2S2O8 | 88.0 | 39.6 | [ |
| PFOA | 254 | 23 | 2 | UV/NaIO4 | 70 | 17 | [ |
| 185 | 60 | 13 | |||||
| PFOA | 254 | 15 | 2 | UV/NaIO4(通O2) | 47 | 12.5 | [ |
| UV/NaIO4(通N2) | 69 | 17 | |||||
| PFOA | 254 | 24 | 30min | UV/NaClO | 6 | — | [ |
表3 均相UV-AOPs去除PFASs的研究总结
| 化合物 | 光波长/nm | 灯功率/W | 反应时间/h | UV-AOPs | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| PFOA | 254 | 24 | 24 | UV/H2O2 | 19.7 | 6.8 | [ |
| PFOA | 254 | 400 | 12 | UV/H2O2(HCO3-共存) | 100 | 82.3 | [ |
| PFOA | 254 | 28 | 4 | UV/O3 | 27.1 | 10.6 | [ |
| PFASs | 185/254 | 300 | 20min | UV/O3 | 95 | — | [ |
| PFOA | 220~460 | 200 | 2 | UV/Na2S2O8 | 99.1 | — | [ |
| 12 | — | 73.8 | |||||
| OBS | 254 | — | 5 | UV/Na2S2O8 | 100 | 27.6 | [ |
| PFBA | 254~400 | 1000 | 5 | UV/Na2S2O8 | 88.0 | 39.6 | [ |
| PFOA | 254 | 23 | 2 | UV/NaIO4 | 70 | 17 | [ |
| 185 | 60 | 13 | |||||
| PFOA | 254 | 15 | 2 | UV/NaIO4(通O2) | 47 | 12.5 | [ |
| UV/NaIO4(通N2) | 69 | 17 | |||||
| PFOA | 254 | 24 | 30min | UV/NaClO | 6 | — | [ |
| 化合物 | 光波长/nm | 灯功率/W | 反应时间 | UV-AOPs | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| GenX | 230~300 | 15 | 48h | UV/TiO2 | 37 | 2.8 | [ |
| PFOA | 310~400 | 500 | 6h | UV/TiO2 | 30 | — | [ |
| PFOA | 254 | 400 | 12h | UV/Cu-TiO2 | 91 | 19 | [ |
| PFASs | 185/254 | 300 | 20min | UV/O3 | 95 | — | [ |
| PFOA | 365 | 125 | 7h | UV/TiO2 | 31.3 | 3.3 | [ |
| UV/Ag-TiO2 | 57.7 | 8.7 | |||||
| UV/Pd-TiO2 | 94.2 | 25.9 | |||||
| UV/Pt-TiO2 | 100 | 34.8 | |||||
| PFOA | 200~600 | 150 | 8h | UV/TiO2-rGO | 86 | 30 | [ |
| PFOA | 365 | 24 | 30min | UV/BN/TiO2 | 100 | — | [ |
| 60min | — | >50 | |||||
| PFOA | 254 | 32 | 4h | UV/In2O3-300℃ | 100 | 35 | [ |
| UV/In2O3-400℃ | 100 | 38 | |||||
| PFOA | 185 | 14 | 65min | UV/纳米针状β-Ga2O3 | 100 | — | [ |
| PFOA | 254 | 50 | 90min | UV/纳米棒状β-Ga2O3 | 98.8 | 56.2 | [ |
| PFOA | 254 | 32 | 90min | UV/Ga2O3/PMS | 100 | — | [ |
| 185 | 60min | UV/Ga2O3/PMS | 100 | — | |||
| PFOA | 254 | 200 | 60min | UV/In-Ga2O3 | 100 | — | [ |
| 5∶3 FTCA | 365 | — | 3d | UV/ZnO | 96 | 14.9 | [ |
| PFOA | 254 | — | 4h | UV/ZnO-rGO/Na2S2O8/O3 | 99.2 | 60 | [ |
| PFOA | 185/254 | 8 | 4h | UV/H3PW12O40/BMS | — | 52.6 | [ |
| PFOA | 365 | 500 | 40min | UV/{0 1 0}-BiOCl | 100 | — | [ |
| 4h | — | 41 | |||||
| PFOA | 254 | 500 | 6h | UV/BiOCl/Zn-Al水滑石(BHZA) | 94 | — | [ |
| GenX | 254 | — | 4h | UV/Bi/TnTs@AC | 70.0 | 42.7 | [ |
| PFOA | 254 | 10 | 12h | UV/BiOCl | 100 | 59.3 | [ |
表4 非均相UV-AOPs去除PFASs的研究总结
| 化合物 | 光波长/nm | 灯功率/W | 反应时间 | UV-AOPs | 降解率/% | 脱氟率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| GenX | 230~300 | 15 | 48h | UV/TiO2 | 37 | 2.8 | [ |
| PFOA | 310~400 | 500 | 6h | UV/TiO2 | 30 | — | [ |
| PFOA | 254 | 400 | 12h | UV/Cu-TiO2 | 91 | 19 | [ |
| PFASs | 185/254 | 300 | 20min | UV/O3 | 95 | — | [ |
| PFOA | 365 | 125 | 7h | UV/TiO2 | 31.3 | 3.3 | [ |
| UV/Ag-TiO2 | 57.7 | 8.7 | |||||
| UV/Pd-TiO2 | 94.2 | 25.9 | |||||
| UV/Pt-TiO2 | 100 | 34.8 | |||||
| PFOA | 200~600 | 150 | 8h | UV/TiO2-rGO | 86 | 30 | [ |
| PFOA | 365 | 24 | 30min | UV/BN/TiO2 | 100 | — | [ |
| 60min | — | >50 | |||||
| PFOA | 254 | 32 | 4h | UV/In2O3-300℃ | 100 | 35 | [ |
| UV/In2O3-400℃ | 100 | 38 | |||||
| PFOA | 185 | 14 | 65min | UV/纳米针状β-Ga2O3 | 100 | — | [ |
| PFOA | 254 | 50 | 90min | UV/纳米棒状β-Ga2O3 | 98.8 | 56.2 | [ |
| PFOA | 254 | 32 | 90min | UV/Ga2O3/PMS | 100 | — | [ |
| 185 | 60min | UV/Ga2O3/PMS | 100 | — | |||
| PFOA | 254 | 200 | 60min | UV/In-Ga2O3 | 100 | — | [ |
| 5∶3 FTCA | 365 | — | 3d | UV/ZnO | 96 | 14.9 | [ |
| PFOA | 254 | — | 4h | UV/ZnO-rGO/Na2S2O8/O3 | 99.2 | 60 | [ |
| PFOA | 185/254 | 8 | 4h | UV/H3PW12O40/BMS | — | 52.6 | [ |
| PFOA | 365 | 500 | 40min | UV/{0 1 0}-BiOCl | 100 | — | [ |
| 4h | — | 41 | |||||
| PFOA | 254 | 500 | 6h | UV/BiOCl/Zn-Al水滑石(BHZA) | 94 | — | [ |
| GenX | 254 | — | 4h | UV/Bi/TnTs@AC | 70.0 | 42.7 | [ |
| PFOA | 254 | 10 | 12h | UV/BiOCl | 100 | 59.3 | [ |
| 化合物 | 光波长 /nm | 灯功率 /W | 反应时间/h | UV-AOPs | 降解率 /% | 脱氟率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|
| GenX | 254 | — | 2 | UV/SO | 100 | — | [ |
| 6 | — | 90 | |||||
| PFOA | 254 | 10 | 1 | UV/SO | 100 | — | [ |
| 24 | — | 88.5 | |||||
| PFOA | 254 | 250 | 10min | UV/SO | 100 | 45.7 | [ |
| PFOS | 185 | 10 | 4 | UV/SO | 97.3 | 68.5 | [ |
| 254 | 85.8 | 64.6 | |||||
| PFOS | 185 | — | 3 | UV/SO | — | 53.5 | [ |
| 254 | — | 40.2 | |||||
| F-53B | 254 | — | 1min | UV/SO | 99 | — | [ |
| 8 | — | 90 | |||||
| PFOA | 254 | 15 | 6 | UV/I- | 93.9 | 76.8 | [ |
| PFOS | 254 | 14 | 1.5 | UV/I-(HA共存) | 86.0 | 55.6 | [ |
| PFOA | 254 | 14 | 1.5 | UV/I- | 67.5 | 23.5 | [ |
| PFOS | 254 | 36 | 8 | UV/IAA | 95 | — | [ |
| PFOA | 254 | 36 | 2 | UV/DIHA | 95 | 51 | [ |
| PFOS | 254 | 14 | 0.5 | UV/NTA | 45.1 | 18.6 | [ |
| 10 | 85.4 | 46.8 |
表5 UV-ARPs去除PFASs的研究总结
| 化合物 | 光波长 /nm | 灯功率 /W | 反应时间/h | UV-AOPs | 降解率 /% | 脱氟率 /% | 参考文献 |
|---|---|---|---|---|---|---|---|
| GenX | 254 | — | 2 | UV/SO | 100 | — | [ |
| 6 | — | 90 | |||||
| PFOA | 254 | 10 | 1 | UV/SO | 100 | — | [ |
| 24 | — | 88.5 | |||||
| PFOA | 254 | 250 | 10min | UV/SO | 100 | 45.7 | [ |
| PFOS | 185 | 10 | 4 | UV/SO | 97.3 | 68.5 | [ |
| 254 | 85.8 | 64.6 | |||||
| PFOS | 185 | — | 3 | UV/SO | — | 53.5 | [ |
| 254 | — | 40.2 | |||||
| F-53B | 254 | — | 1min | UV/SO | 99 | — | [ |
| 8 | — | 90 | |||||
| PFOA | 254 | 15 | 6 | UV/I- | 93.9 | 76.8 | [ |
| PFOS | 254 | 14 | 1.5 | UV/I-(HA共存) | 86.0 | 55.6 | [ |
| PFOA | 254 | 14 | 1.5 | UV/I- | 67.5 | 23.5 | [ |
| PFOS | 254 | 36 | 8 | UV/IAA | 95 | — | [ |
| PFOA | 254 | 36 | 2 | UV/DIHA | 95 | 51 | [ |
| PFOS | 254 | 14 | 0.5 | UV/NTA | 45.1 | 18.6 | [ |
| 10 | 85.4 | 46.8 |
| 1 | BUCK R C, FRANKLIN J, BERGER U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins[J]. Integrated Environmental Assessment and Management, 2011, 7(4): 513-541. |
| 2 | LEE Young-Min, LEE Ji-Young, KIM Moon-Kyung, et al. Concentration and distribution of per- and polyfluoroalkyl substances (PFAS) in the Asan Lake area of South Korea[J]. Journal of Hazardous Materials, 2020, 381: 120909. |
| 3 | TANG Aiping, ZHANG Xinghui, LI Rongfu, et al. Spatiotemporal distribution, partitioning behavior and flux of per- and polyfluoroalkyl substances in surface water and sediment from Poyang Lake, China[J]. Chemosphere, 2022, 295: 133855. |
| 4 | WONG F, SHOEIB M, KATSOYIANNIS A, et al. Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP)[J]. Atmospheric Environment, 2018, 172: 65-73. |
| 5 | BRUSSEAU M L, ANDERSON R H, GUO Bo. PFAS concentrations in soils: Background levels versus contaminated sites[J]. Science of the Total Environment, 2020, 740: 140017. |
| 6 | OJO A F, PENG Cheng, NG J C. Combined effects and toxicological interactions of perfluoroalkyl and polyfluoroalkyl substances mixtures in human liver cells (HepG2)[J]. Environmental Pollution, 2020, 263: 114182. |
| 7 | LIANG Luyun, PAN Yongling, Lihua BIN, et al. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS[J]. Chemosphere, 2022, 291: 132892. |
| 8 | DOMINGO J L, NADAL M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature[J]. Environmental Research, 2019, 177: 108648. |
| 9 | EVICH M G, DAVIS M J B, MCCORD J P, et al. Per- and polyfluoroalkyl substances in the environment[J]. Science, 2022, 375(6580): eabg9065. |
| 10 | RAHMAN M F, PELDSZUS S, ANDERSON W B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review[J]. Water Research, 2014, 50: 318-340. |
| 11 | APPLEMAN T D, HIGGINS C P, QUIÑONES O, et al. Treatment of poly- and perfluoroalkyl substances in U. S. full-scale water treatment systems[J]. Water Research, 2014, 51: 246-255. |
| 12 | DIXIT F, BARBEAU B, MOSTAFAVI S G, et al. Efficient removal of GenX (HFPO-DA) and other perfluorinated ether acids from drinking and recycled waters using anion exchange resins[J]. Journal of Hazardous Materials, 2020, 384: 121261. |
| 13 | LEWIS A J, JOYCE T, HADAYA M, et al. Rapid degradation of PFAS in aqueous solutions by reverse vortex flow gliding arc plasma[J]. Environmental Science: Water Research & Technology, 2020, 6(4): 1044-1057. |
| 14 | SungJu IM, LEE Hyeonho, Hojung RHO. The fouling layers characteristics of osmotically driven membranes affect transport behaviors of reverse salt permeation and per-fluorinated compounds[J]. Desalination, 2022, 540: 116001. |
| 15 | LENKA S P, KAH M, PADHYE L P. A review of the occurrence, transformation, and removal of poly- and perfluoroalkyl substances (PFAS) in wastewater treatment plants[J]. Water Research, 2021, 199: 117187. |
| 16 | BARISCI S, SURI R. Electrooxidation of short- and long-chain perfluoroalkyl substances (PFASs) under different process conditions[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105323. |
| 17 | LIU Xiaoqing, WEI Wei, XU Juan, et al. Photochemical decomposition of perfluorochemicals in contaminated water[J]. Water Research, 2020, 186: 116311. |
| 18 | UMAR M. Reductive and oxidative UV degradation of PFAS — Status, needs and future perspectives[J]. Water, 2021, 13(22): 3185. |
| 19 | BECK S E, WRIGHT H B, HARGY T M, et al. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems[J]. Water Research, 2015, 70: 27-37. |
| 20 | BANAYAN ESFAHANI E, MOHSENI M. Fluence-based photo-reductive decomposition of PFAS using vacuum UV (VUV) irradiation: Effects of key parameters and decomposition mechanism[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 107050. |
| 21 | ANUMOL T, DAGNINO S, VANDERVORT D R, et al. Transformation of Polyfluorinated compounds in natural waters by advanced oxidation processes[J]. Chemosphere, 2016, 144: 1780-1787. |
| 22 | YAMAMOTO T, NOMA Y, SAKAI S, et al. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol[J]. Environmental Science & Technology, 2007, 41(16): 5660-5665. |
| 23 | GIRI R R, OZAKI H, OKADA T, et al. Factors influencing UV photodecomposition of perfluorooctanoic acid in water[J]. Chemical Engineering Journal, 2012, 180: 197-203. |
| 24 | CHEN J, ZHANG P Y. Photodegradation of perfluorooctanoic acid in water under irradiation of 254nm and 185nm light by use of persulfate[J]. Water Science and Technology, 2006, 54(11/12): 317-325. |
| 25 | CHEN Jing, ZHANG Pengyi, LIU Jian. Photodegradation of perfluorooctanoic acid by 185nm vacuum ultraviolet light[J]. Journal of Environmental Sciences, 2007, 19(4): 387-390. |
| 26 | CAO M H, WANG B B, YU H S, et al. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 1143-1146. |
| 27 | HORI H, HAYAKAWA E, EINAGA H, et al. Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches[J]. Environmental Science & Technology, 2004, 38(22): 6118-6124. |
| 28 | JIN Ling, ZHANG Pengyi. Photochemical decomposition of perfluorooctane sulfonate (PFOS) in an anoxic alkaline solution by 185nm vacuum ultraviolet[J]. Chemical Engineering Journal, 2015, 280: 241-247. |
| 29 | WANG Xuelin, CHEN Zhongyun, WANG Yonglei, et al. A review on degradation of perfluorinated compounds based on ultraviolet advanced oxidation[J]. Environmental Pollution, 2021, 291: 118014. |
| 30 | LIANG Xiaoyan, CHENG Jianhua, YANG Cao, et al. Factors influencing aqueous perfluorooctanoic acid (PFOA) photodecomposition by VUV irradiation in the presence of ferric ions[J]. Chemical Engineering Journal, 2016, 298: 291-299. |
| 31 | CUI Junkui, GAO Panpan, DENG Yang. Destruction of per- and polyfluoroalkyl substances (PFAS) with advanced reduction processes (ARPs): A critical review[J]. Environmental Science & Technology, 2020, 54(7): 3752-3766. |
| 32 | CHOWDHURY N, PRABAKAR S, CHOI Hyeok. Dependency of the photocatalytic and photochemical decomposition of per- and polyfluoroalkyl substances (PFAS) on their chain lengths, functional groups, and structural properties[J]. Water Science and Technology, 2021, 84(12): 3738-3754. |
| 33 | JAVED H, Cong LYU, SUN Ruonan, et al. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation[J]. Chemosphere, 2020, 247: 125883. |
| 34 | PHAN THI Lan-Anh, Huu-Tuan DO, LEE Yuchi, et al. Photochemical decomposition of perfluorooctanoic acids in aqueous carbonate solution with UV irradiation[J]. Chemical Engineering Journal, 2013, 221: 258-263. |
| 35 | HUANG Jiye, WANG Xi, PAN Zhaoqi, et al. Efficient degradation of perfluorooctanoic acid (PFOA) by photocatalytic ozonation[J]. Chemical Engineering Journal, 2016, 296: 329-334. |
| 36 | DAI Xiaodong, XIE Zongli, DORIAN Brian, et al. Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air[J]. Environmental Science: Water Research & Technology, 2019, 5(11): 1897-1907. |
| 37 | HORI H, YAMAMOTO A, HAYAKAWA E, et al. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant[J]. Environmental Science & Technology, 2005, 39(7): 2383-2388. |
| 38 | LIU Liquan, DENG Shanshan, BAO Yixiang, et al. Degradation of OBS (sodium p-perfluorous nonenoxybenzenesulfonate) as a novel per- and polyfluoroalkyl substance by UV/persulfate and UV/sulfite: Fluorinated intermediates and treatability in fluoroprotein foam[J]. Environmental Science & Technology, 2022, 56(10): 6201-6211. |
| 39 | WANG Mingran, WANG Qianyu, CAI Yanping, et al. Efficient degradation and defluorination of perfluorobutyric acid under UV irradiation in the presence of persulfate[J]. Journal of Cleaner Production, 2021, 327: 129472. |
| 40 | 曹梦华, 王贝贝, 朱湖地, 等. 高碘酸盐光化学降解水中PFOA研究[J]. 环境科学, 2011, 32(1): 130-134. |
| CAO Menghua, WANG Beibei, ZHU Hudi, et al. Photo-chemical decomposition of perfluorooctanoic acids in aqueous periodate[J]. Environmental Science, 2011, 32(1): 130-134. | |
| 41 | METZ J, ZUO Pengxiao, WANG Bo, et al. Perfluorooctanoic acid degradation by UV/chlorine[J]. Environmental Science & Technology Letters, 2022, 9(8): 673-679. |
| 42 | SANSOTERA M, PERSICO F, PIROLA C, et al. Decomposition of perfluorooctanoic acid photocatalyzed by titanium dioxide: Chemical modification of the catalyst surface induced by fluoride ions[J]. Applied Catalysis B: Environmental, 2014, 148/149: 29-35. |
| 43 | CHEN Mengjia, Shang-Lien LO, LEE Yuchi, et al. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide[J]. Journal of Hazardous Materials, 2015, 288: 168-175. |
| 44 | LI Mingjie, YU Zebin, LIU Qing, et al. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2 [J]. Chemical Engineering Journal, 2016, 286: 232-238. |
| 45 | RIVERO M J, RIBAO P, GOMEZ-RUIZ B, et al. Comparative performance of TiO2-rGO photocatalyst in the degradation of dichloroacetic and perfluorooctanoic acids[J]. Separation and Purification Technology, 2020, 240: 116637. |
| 46 | DUAN L J, WANG B, HECK K N, et al. Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid[J]. Chemical Engineering Journal, 2022, 448: 137735. |
| 47 | LIU Xiaoqing, CHEN Zhijie, TIAN Ke, et al. Fe3+ promoted the photocatalytic defluorination of perfluorooctanoic acid (PFOA) over In2O3 [J]. ACS ES&T Water, 2021, 1(11): 2431-2439. |
| 48 | SHAO Tian, ZHANG Pengyi, LI Zhenmin, et al. Photocatalytic decomposition of perfluorooctanoic acid in pure water and wastewater by needle-like nanostructured gallium oxide[J]. Chinese Journal of Catalysis, 2013, 34(8): 1551-1559. |
| 49 | ZHANG Weilan, ZHANG Dongqing, LIANG Yanna. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: A review[J]. Environmental Pollution, 2019, 247: 266-276. |
| 50 | XU Bentuo, ZHOU J L, ALTAEE A, et al. Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate[J]. Chemosphere, 2020, 239: 124722. |
| 51 | Huu-Tuan DO, PHAN THI Lan-Anh, NGUYEN Ngoc Han DAO, et al. Tailoring photocatalysts and elucidating mechanisms of photocatalytic degradation of perfluorocarboxylic acids (PFCAs) in water: A comparative overview[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(10): 2569-2578. |
| 52 | ABADA B, ALIVIO T E G, SHAO Yiru, et al. Photodegradation of fluorotelomer carboxylic 5∶3 acid and perfluorooctanoic acid using zinc oxide[J]. Environmental Pollution, 2018, 243: 637-644. |
| 53 | WU Dan, LI Xukai, ZHANG Jingxian, et al. Efficient PFOA degradation by persulfate-assisted photocatalytic ozonation[J]. Separation and Purification Technology, 2018, 207: 255-261. |
| 54 | YOU Xiazhou, YU Linlong, XIAO Fangfang, et al. Synthesis of phosphotungstic acid-supported bimodal mesoporous silica-based catalyst for defluorination of aqueous perfluorooctanoic acid under vacuum UV irradiation[J]. Chemical Engineering Journal, 2018, 335: 812-821. |
| 55 | WU Yaoyao, HU Yunxuan, HAN Muqiao, et al. Mechanism insights into the facet-dependent photocatalytic degradation of perfluorooctanoic acid on BiOCl nanosheets[J]. Chemical Engineering Journal, 2021, 425: 130672. |
| 56 | YANG Yiqiong, ZHENG Zenghui, YANG Minhui, et al. In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology[J]. Journal of Hazardous Materials, 2020, 399: 123070. |
| 57 | SONG Zhou, DONG Xuelin, WANG Nan, et al. Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism[J]. Chemical Engineering Journal, 2017, 317: 925-934. |
| 58 | SCHRÖDER H F, MEESTERS R J W. Stability of fluorinated surfactants in advanced oxidation processes—A follow up of degradation products using flow injection-mass spectrometry, liquid chromatography-mass spectrometry and liquid chromatography-multiple stage mass spectrometry[J]. Journal of Chromatography A, 2005, 1082(1): 110-119. |
| 59 | AKTER J, LEE Jai-Yeop, Hyun-Ju HA, et al. Degradation of organics and change concentration in per-fluorinated compounds (PFCs) during ozonation and UV/H2O2 advanced treatment of tertiary-treated sewage[J]. Sustainability, 2022, 14(9): 5597. |
| 60 | YANG Lie, HE Liuyang, XUE Jianming, et al. Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: Review on influences, mechanisms and prospective[J]. Journal of Hazardous Materials, 2020, 393: 122405. |
| 61 | LUTZE H V, BREKENFELD J, NAUMOV S, et al. Degradation of perfluorinated compounds by sulfate radicals—New mechanistic aspects and economical considerations[J]. Water Research, 2018, 129: 509-519. |
| 62 | 詹宇航, 秦雅鑫, 陈博磊, 等. 全氟辛酸和全氟辛基磺酸的光降解技术及机理研究进展[J]. 环境化学, 2022, 41(1): 46-56. |
| ZHAN Yuhang, QIN Yaxin, CHEN Bolei, et al. Photodegradation technology and mechanism of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid(PFOS): A critical review[J]. Environmental Chemistry, 2022, 41(1): 46-56. | |
| 63 | ZHANG Xi, YU Xiaobin, YU Xingyue, et al. Efficiency and mechanism of 2,4-dichlorophenol degradation by the UV/IO4 - process[J]. Science of the Total Environment, 2021, 782: 146781. |
| 64 | 朱晓伟, 肖广锋, 周婷, 等. 高碘酸盐的活化及降解水体有机污染物研究进展[J]. 水处理技术, 2021, 47(3): 7-11. |
| ZHU Xiaowei, XIAO Guangfeng, ZHOU Ting, et al. Research progress of periodate activation and degradation of organic pollutants in water[J]. Technology of Water Treatment, 2021, 47(3): 7-11. | |
| 65 | DJABALLAH M L, MEROUANI S, BENDJAMA H, et al. Development of a free radical-based kinetics model for the oxidative degradation of chlorazol black in aqueous solution using periodate photoactivated process[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2021, 408: 113102. |
| 66 | LI Simiao, AO Xiuwei, LI Chen, et al. Insight into PPCP degradation by UV/NH2Cl and comparison with UV/NaClO: Kinetics, reaction mechanism, and DBP formation[J]. Water Research, 2020, 182: 115967. |
| 67 | MCINTYRE H, MINDA V, HAWLEY E, et al. Coupled photocatalytic alkaline media as a destructive technology for per- and polyfluoroalkyl substances in aqueous film-forming foam impacted stormwater[J]. Chemosphere, 2022, 291: 132790. |
| 68 | ALALM M GAR, BOFFITO D C. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review[J]. Chemical Engineering Journal, 2022, 450: 138352. |
| 69 | LIU Fuqiang, GUAN Xiaohong, XIAO Feng. Photodegradation of per- and polyfluoroalkyl substances in water: A review of fundamentals and applications[J]. Journal of Hazardous Materials, 2022, 439: 129580. |
| 70 | 刘晴, 喻泽斌, 张睿涵, 等. 钯掺TiO2光催化降解全氟辛酸[J]. 环境科学, 2015, 36(6): 2138-2146. |
| LIU Qing, YU Zebin, ZHANG Ruihan, et al. Photocatalytic degradation of perfluorooctanoic acid by Pd-TiO2 photocatalyst[J]. Environmental Science, 2015, 36(6): 2138-2146. | |
| 71 | 袁雅静. 全氟或多氟烷基物质水处理技术研究进展[J]. 化工进展, 2021, 40(S1): 397-403. |
| YUAN Yajing. Progress in water treatment technology for perfluorinated or polyfluorinated alkyl substances[J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 397-403. | |
| 72 | XU Bentuo, AHMED M B, ZHOU J L, et al. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors[J]. Chemosphere, 2017, 189: 717-729. |
| 73 | 许骐, 周琴, 王乐阳, 等. 纳米材料对水体中PFCs的去除行为及机制[J]. 地球科学, 2018, 43(5): 1725-1736. |
| XU Qi, ZHOU Qin, WANG Leyang, et al. Removal behavior and mechanism of perfluorinated compounds from water by nano-materials[J]. Earth Science, 2018, 43(5): 1725-1736. | |
| 74 | LI Xiaoyun, ZHANG Pengyi, JIN Ling, et al. Efficient photocatalytic decomposition of perfluorooctanoic acid by indium oxide and its mechanism[J]. Environmental Science & Technology, 2012, 46(10): 5528-5534. |
| 75 | LI Zhenmin, ZHANG Pengyi, LI Jinge, et al. Synthesis of In2O3-graphene composites and their photocatalytic performance towards perfluorooctanoic acid decomposition[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2013, 271: 111-116. |
| 76 | WANG Qi, CHEN Yajie, LIU Xiu, et al. Sulfur doped In2O3-CeO2 hollow hexagonal prisms with carbon coating for efficient photocatalytic CO2 reduction[J]. Chemical Engineering Journal, 2021, 421: 129968. |
| 77 | FU Caixia, XU Xiuru, ZHENG Chunmiao, et al. Photocatalysis of aqueous PFOA by common catalysts of In2O3, Ga2O3, TiO2, CeO2 and CdS: Influence factors and mechanistic insights[J]. Environmental Geochemistry and Health, 2022, 44(9): 2943-2953. |
| 78 | JAMWAL N S, KIANI A. Gallium oxide nanostructures: A review of synthesis, properties and applications[J]. Nanomaterials, 2022, 12(12): 2061. |
| 79 | ZHAO Baoxiu, LI Xiang, YANG Long, et al. β-Ga2O3 nanorod synthesis with a one-step microwave irradiation hydrothermal method and its efficient photocatalytic degradation for perfluorooctanoic acid[J]. Photochemistry and Photobiology, 2015, 91(1): 42-47. |
| 80 | ZHAO Baoxiu, Mou LYU, ZHOU Li. Photocatalytic degradation of perfluorooctanoic acid with β-Ga2O3 in anoxic aqueous solution[J]. Journal of Environmental Sciences, 2012, 24(4): 774-780. |
| 81 | SHAO Tian, ZHANG Pengyi, JIN Ling, et al. Photocatalytic decomposition of perfluorooctanoic acid in pure water and sewage water by nanostructured gallium oxide[J]. Applied Catalysis B: Environmental, 2013, 142/143: 654-661. |
| 82 | TAN Xianjun, CHEN Guanhan, XING Dingyu, et al. Indium-modified Ga2O3 hierarchical nanosheets as efficient photocatalysts for the degradation of perfluorooctanoic acid[J]. Environmental Science: Nano, 2020, 7(8): 2229-2239. |
| 83 | WU Dan, LI Xukai, TANG Yiming, et al. Mechanism insight of PFOA degradation by ZnO assisted-photocatalytic ozonation: Efficiency and intermediates[J]. Chemosphere, 2017, 180: 247-252. |
| 84 | 游霞, 肖芳芳, 程建华, 等. VUV/HPW/BMMs体系对全氟辛酸(PFOA)的脱氟研究[J]. 环境科学学报, 2017, 37(11): 4064-4070. |
| YOU Xia, XIAO Fangfang, CHENG Jianhua, et al. Defluorination of aqueous perfluorooctanoic acid by combination of vacuum ultraviolet and bimodal mesoporous materials supported phosphotungstic acid catalyst[J]. Acta Scientiae Circumstantiae, 2017, 37(11): 4064-4070. | |
| 85 | ZHU Yangmo, JI Haodong, HE Ke, et al. Photocatalytic degradation of GenX in water using a new adsorptive photocatalyst[J]. Water Research, 2022, 220: 118650. |
| 86 | 段钰汀, 于水利, 肖倩. 高级还原技术去除有机消毒副产物的研究进展[J]. 中国给水排水, 2022, 38(6): 38-43. |
| DUAN Yuting, YU Shuili, XIAO Qian. Research progress on advanced reduction processes for degradation of organic disinfection by-products[J]. China Water & Wastewater, 2022, 38(6): 38-43. | |
| 87 | WANG Shana, YANG Qi, CHEN Fei, et al. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review[J]. Chemical Engineering Journal, 2017, 328: 927-942. |
| 88 | BAO Yixiang, DENG Shanshan, JIANG Xinshu, et al. Degradation of PFOA substitute: GenX (HFPO-DA ammonium salt): Oxidation with UV/persulfate or reduction with UV/sulfite?[J]. Environmental Science & Technology, 2018, 52(20): 11728-11734. |
| 89 | SONG Zhou, TANG Heqing, WANG Nan, et al. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system[J]. Journal of Hazardous Materials, 2013, 262: 332-338. |
| 90 | GU Yurong, LIU Tongzhou, ZHANG Qian, et al. Efficient decomposition of perfluorooctanoic acid by a high photon flux UV/sulfite process: Kinetics and associated toxicity[J]. Chemical Engineering Journal, 2017, 326: 1125-1133. |
| 91 | GU Yurong, LIU Tongzhou, WANG Hongjie, et al. Hydrated electron based decomposition of perfluorooctane sulfonate (PFOS) in the VUV/sulfite system[J]. Science of the Total Environment, 2017, 607/608: 541-548. |
| 92 | 宋洲, 周茜, 方晓青. 真空紫外光解SO 3 2 - 高效还原降解全氟辛烷磺酸[J]. 环境污染与防治, 2018, 40(6): 645-651. |
| SONG Zhou, ZHOU Qian, FANG Xiaoqing. Efficient reductive degradation of perfluorooctane sulfonate in a sulfite-mediated vacuum ultraviolet photolysis system[J]. Environmental Pollution & Control, 2018, 40(6): 645-651. | |
| 93 | BAO Yixiang, HUANG Jun, CAGNETTA G, et al. Removal of F-53B as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction[J]. Water Research, 2019, 163: 114907. |
| 94 | QU Yan, ZHANG Chaojie, LI Fei, et al. Photo-reductive defluorination of perfluorooctanoic acid in water[J]. Water Research, 2010, 44(9): 2939-2947. |
| 95 | SUN Zhuyu, ZHANG Chaojie, CHEN Pei, et al. Impact of humic acid on the photoreductive degradation of perfluorooctane sulfonate (PFOS) by UV/Iodide process[J]. Water Research, 2017, 127: 50-58. |
| 96 | GUO Chenxi, ZHANG Chaojie, SUN Zhuyu, et al. Synergistic impact of humic acid on the photo-reductive decomposition of perfluorooctanoic acid[J]. Chemical Engineering Journal, 2019, 360: 1101-1110. |
| 97 | KUGLER A, DONG Hailiang, LI Chen, et al. Reductive defluorination of perfluorooctanesulfonic acid (PFOS) by hydrated electrons generated upon UV irradiation of 3-Indole-acetic-acid in 12-Aminolauric-Modified montmorillonite[J]. Water Research, 2021, 200: 117221. |
| 98 | JIN Xin, WANG Zhe, HONG Ran, et al. Supramolecular assemblies of a newly developed indole derivative for selective adsorption and photo-destruction of perfluoroalkyl substances[J]. Water Research, 2022, 225: 119147. |
| 99 | SUN Zhuyu, ZHANG Chaojie, XING Lu, et al. UV/nitrilotriacetic acid process as a novel strategy for efficient photoreductive degradation of perfluorooctanesulfonate[J]. Environmental Science & Technology, 2018, 52(5): 2953-2962. |
| 100 | BANAYAN ESFAHANI E, ASADI ZEIDABADI F, ZHANG Shengyang, et al. Photo-chemical/catalytic oxidative/reductive decomposition of per- and poly-fluoroalkyl substances (PFAS), decomposition mechanisms and effects of key factors: A review[J]. Environmental Science: Water Research & Technology, 2022, 8(4): 698-728. |
| 101 | BENTEL M J, LIU Zekun, YU Yaochun, et al. Enhanced degradation of perfluorocarboxylic acids (PFCAs) by UV/sulfite treatment: Reaction mechanisms and system efficiencies at pH 12[J]. Environmental Science & Technology Letters, 2020, 7(5): 351-357. |
| 102 | ABUSALLOUT I, WANG Junli, HANIGAN D. Emerging investigator series: Rapid defluorination of 22 per- and polyfluoroalkyl substances in water using sulfite irradiated by medium-pressure UV[J]. Environmental Science: Water Research & Technology, 2021, 7(9): 1552-1562. |
| 103 | REN Zhongfei, BERGMANN U, LEIVISKÄ T. Reductive degradation of perfluorooctanoic acid in complex water matrices by using the UV/sulfite process[J]. Water Research, 2021, 205: 117676. |
| 104 | LIU C J, MCKAY G, JIANG D, et al. Pilot-scale field demonstration of a hybrid nanofiltration and UV-sulfite treatment train for groundwater contaminated by per- and polyfluoroalkyl substances (PFASs) [J]. Water Research, 2021, 205: 117677. |
| 105 | CAO Huimin, ZHANG Weilan, WANG Cuiping, et al. Photodegradation of F-53B in aqueous solutions through an UV/iodide system[J]. Chemosphere, 2022, 292: 133436. |
| 106 | PARK Hyunwoong, VECITIS C D, CHENG Jie, et al. Reductive degradation of perfluoroalkyl compounds with aquated electrons generated from iodide photolysis at 254nm[J]. Photochemical & Photobiological Sciences, 2011, 10(12): 1945-1953. |
| 107 | HUANG Li, DONG Wenbo, HOU Huiqi. Investigation of the reactivity of hydrated electron toward perfluorinated carboxylates by laser flash photolysis[J]. Chemical Physics Letters, 2007, 436(1/2/3): 124-128. |
| 108 | TIAN Haoting, GAO Juan, LI Hui, et al. Complete defluorination of perfluorinated compounds by hydrated electrons generated from 3-indole-acetic-acid in organomodified montmorillonite[J]. Scientific Reports, 2016, 6: 32949. |
| 109 | CHEN Zhanghao, LI Chen, GAO Juan, et al. Efficient reductive destruction of perfluoroalkyl substances under self-assembled micelle confinement[J]. Environmental Science & Technology, 2020, 54(8): 5178-5185. |
| 110 | SUN Zhuyu, ZHANG Chaojie, JIANG Jinchi, et al. UV/FeⅡNTA as a novel photoreductive system for the degradation of perfluorooctane sulfonate (PFOS) via a photoinduced intramolecular electron transfer mechanism[J]. Chemical Engineering Journal, 2022, 427: 130923. |
| 111 | ATEIA M, MAROLI A, THARAYIL N, et al. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review[J]. Chemosphere, 2019, 220: 866-882. |
| [1] | 靳立军, 刘铮铮, 李扬, 杨赫, 胡浩权. 耦合富氢小分子催化活化的煤热解提高焦油产率策略与实践[J]. 化工进展, 2024, 43(7): 3613-3619. |
| [2] | 罗丛佳, 豆义波, 卫敏. 水滑石光催化剂结构调控用于二氧化碳还原的研究进展[J]. 化工进展, 2024, 43(7): 3891-3909. |
| [3] | 何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088. |
| [4] | 刘梦凡, 王华伟, 王亚楠, 张艳茹, 蒋旭彤, 孙英杰. Bio-FeMnCeO x 活化PMS降解四环素效能与机制[J]. 化工进展, 2024, 43(6): 3492-3502. |
| [5] | 肖自胜, 李金玲, 陈伊睿, 兰支利, 尹笃林. g-C3N4锚定Cu(Ⅰ)高选择性催化CCl4合成2,4,4,4-四氯丁腈[J]. 化工进展, 2024, 43(6): 3293-3300. |
| [6] | 黄淄博, 周文静, 魏进家. 基于ReaxFF MD模拟的低阶煤热解产物演化规律及反应机理[J]. 化工进展, 2024, 43(5): 2409-2419. |
| [7] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [8] | 孙月, 王斯佳, 吴明侠, 宋先雨, 徐首红. pH/温度响应型聚合物PMAA-b-PDMAEMA的合成、性能调控及应用[J]. 化工进展, 2024, 43(1): 480-489. |
| [9] | 于笑笑, 巢艳红, 刘海燕, 朱文帅, 刘植昌. D-A共轭聚合强化光电性能及光催化CO2转化[J]. 化工进展, 2024, 43(1): 292-301. |
| [10] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [11] | 吕程远, 张函, 杨明旺, 杜健军, 樊江莉. 生物成像用二氧杂环丁烷余辉发光体系的研究进展[J]. 化工进展, 2023, 42(8): 4108-4122. |
| [12] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [13] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [14] | 范思涵, 于国熙, 来超超, 何欢, 黄斌, 潘学军. 非生物改性对厌氧微生物产物光化学活性影响[J]. 化工进展, 2023, 42(4): 2180-2189. |
| [15] | 闫博引, 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成. UV/H2O2和UV/NaClO工艺降解吉非罗齐的比较[J]. 化工进展, 2023, 42(11): 6102-6112. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||