化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2409-2419.DOI: 10.16085/j.issn.1000-6613.2023-2004
• 化石能源的清洁高效转化利用 • 上一篇
基于ReaxFF MD模拟的低阶煤热解产物演化规律及反应机理
- 1.西安交通大学化学工程与技术学院,陕西 西安 710049
2.西安交通大学动力工程多相流国家重点实验室,陕西 西安 710049
-
收稿日期:2023-11-27修回日期:2024-01-07出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:周文静 -
作者简介:黄淄博(1995—),男,博士研究生,研究方向为煤与生物质热解。E-mail:zbhuang1128@163.com。 -
基金资助:中国华能集团能源安全技术专项(HNKJ20-H87-03)
Product evolution and reaction mechanism of low-rank coal pyrolysis based on ReaxFF MD simulation
HUANG Zibo1( ), ZHOU Wenjing1(
), ZHOU Wenjing1( ), WEI Jinjia1,2
), WEI Jinjia1,2
- 1.School of Chemical Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
2.State Key Laboratory of Multiphase Flow in Power Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2023-11-27Revised:2024-01-07Online:2024-05-15Published:2024-06-15 -
Contact:ZHOU Wenjing
摘要:
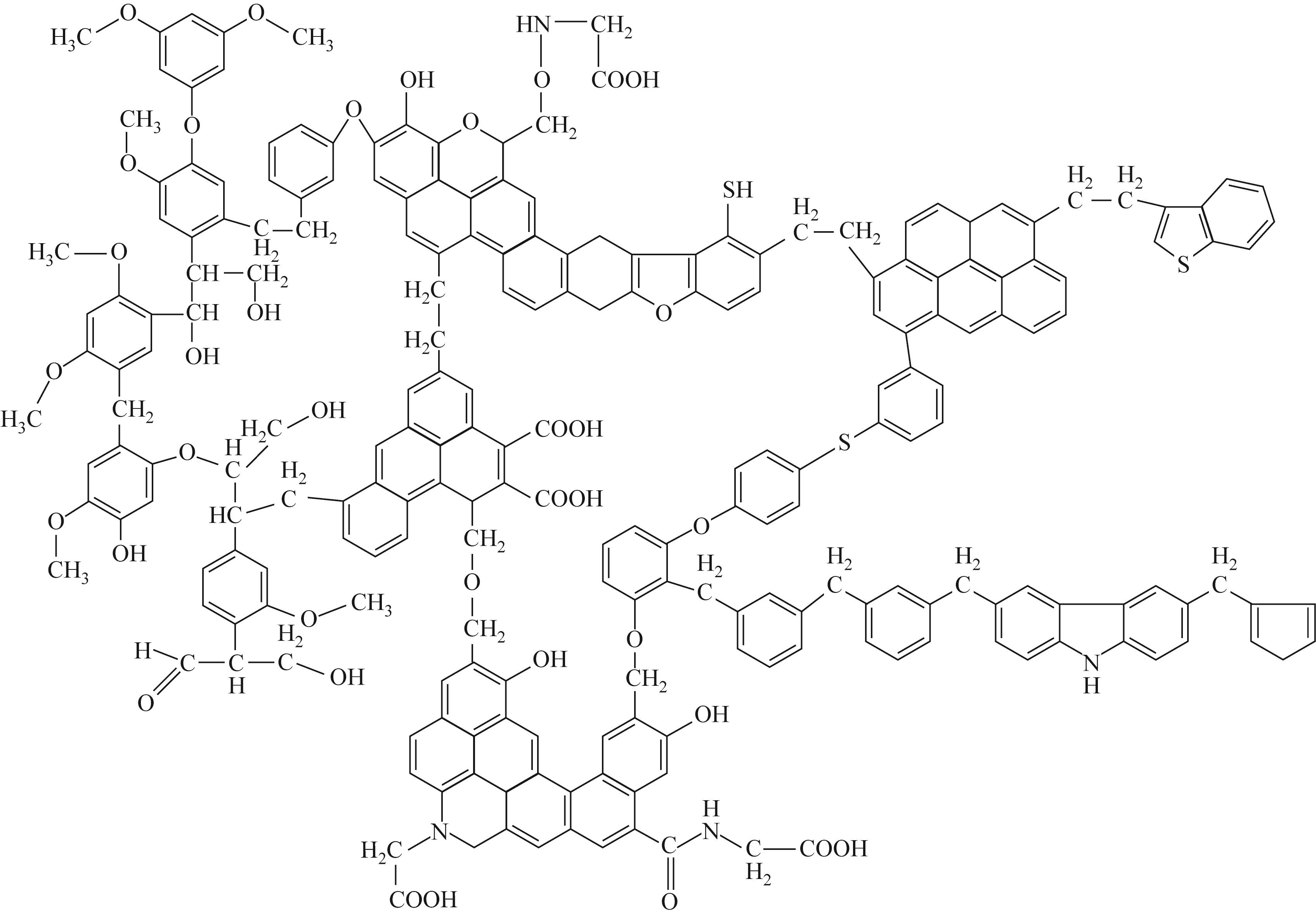
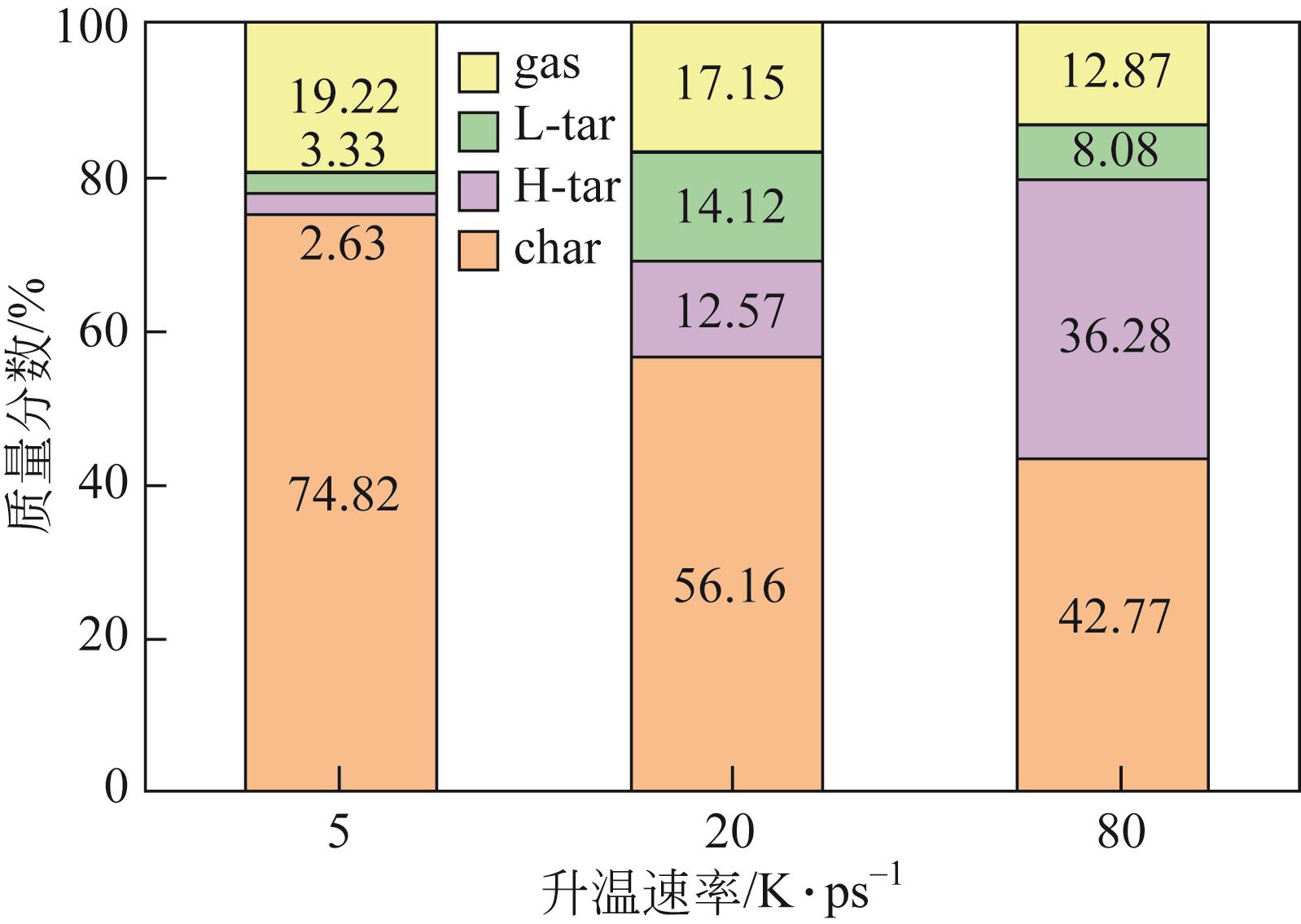
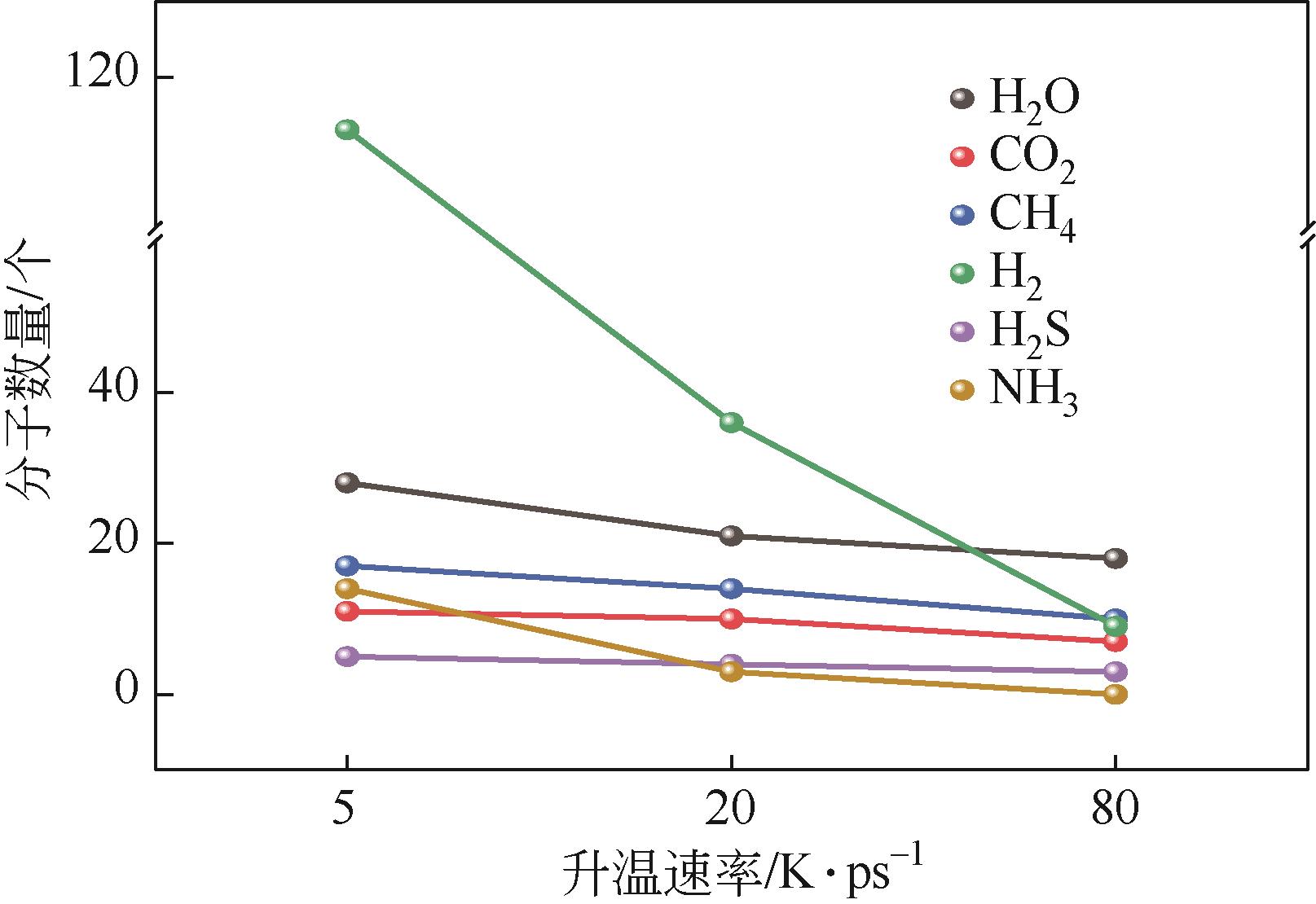
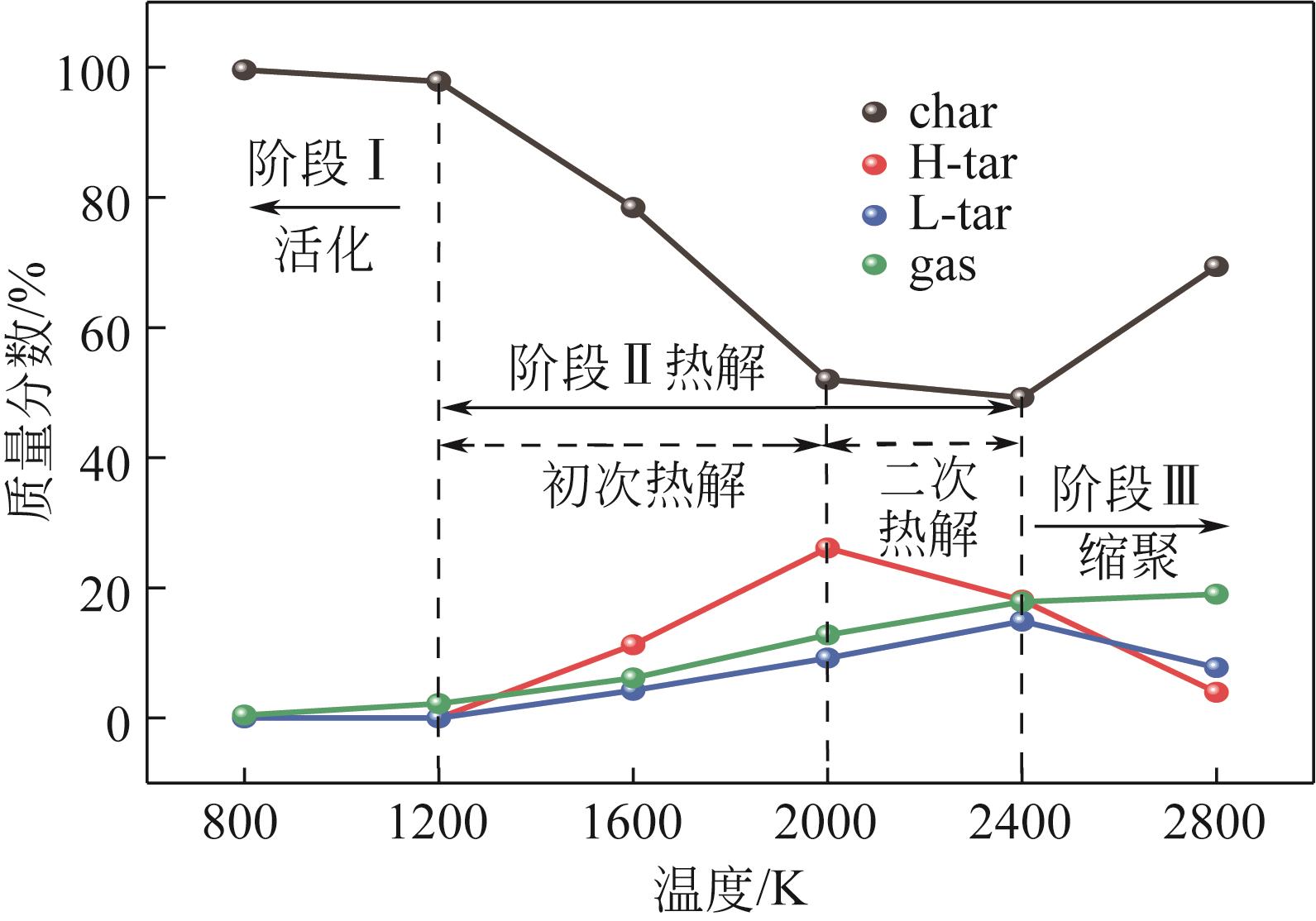
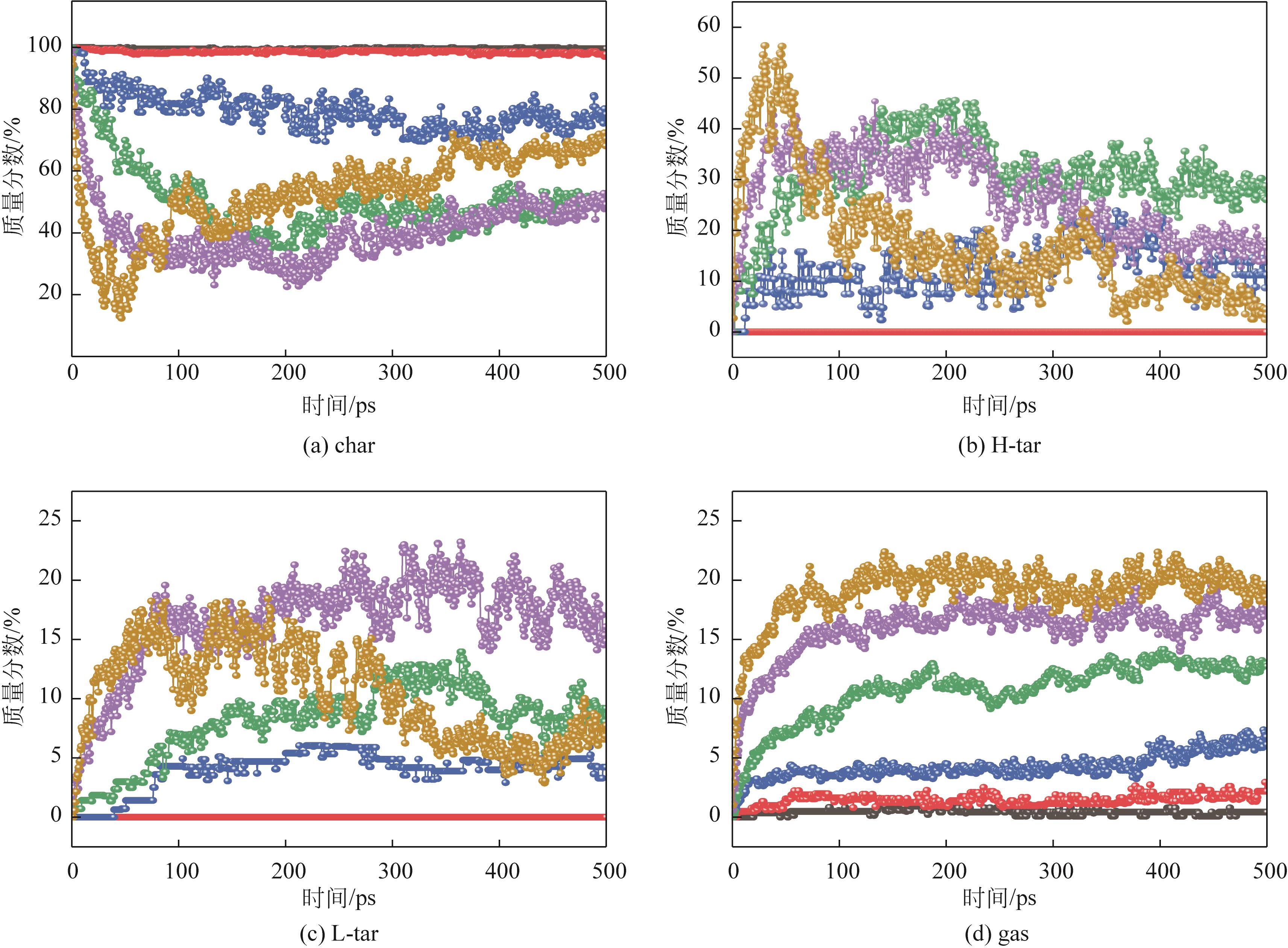
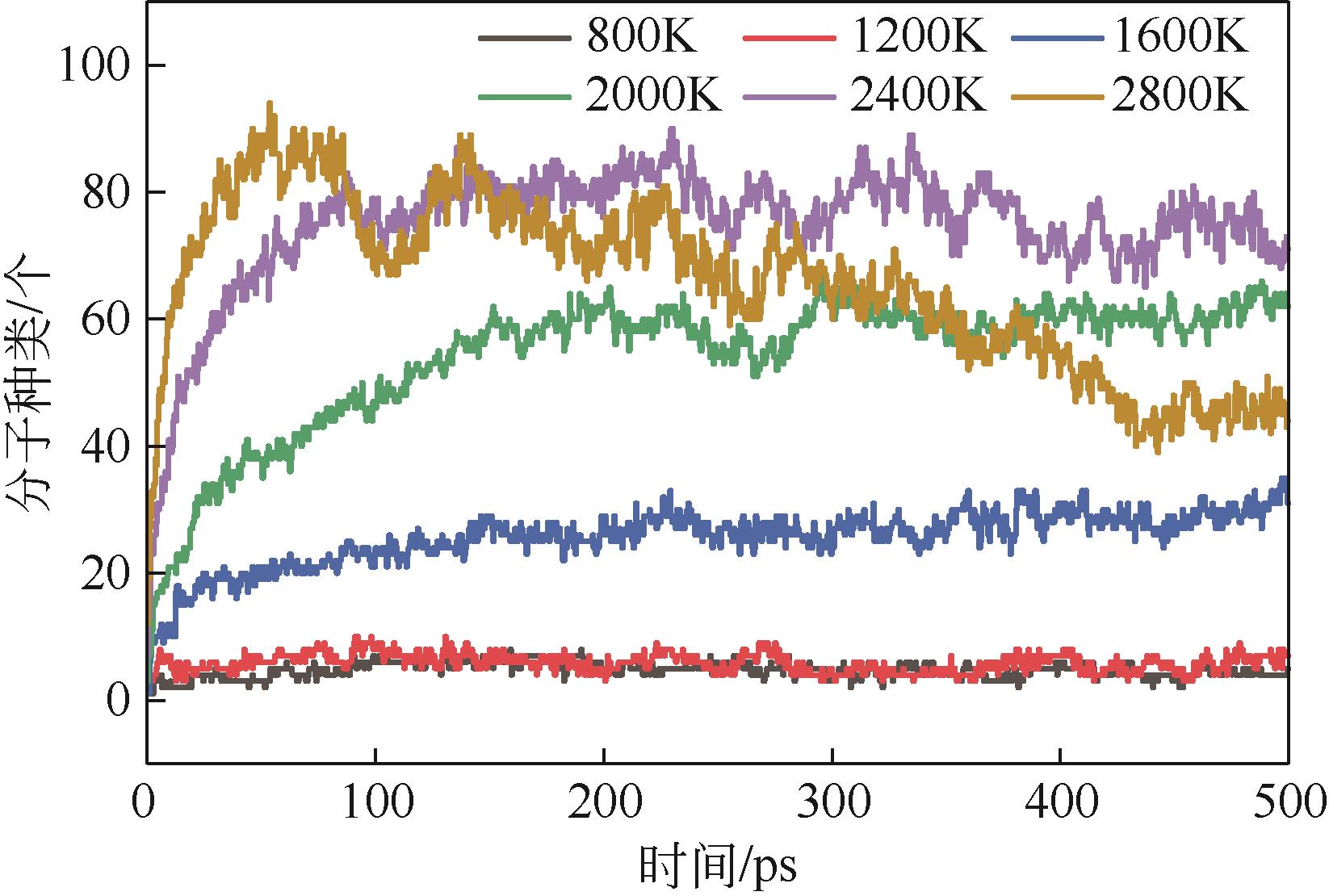
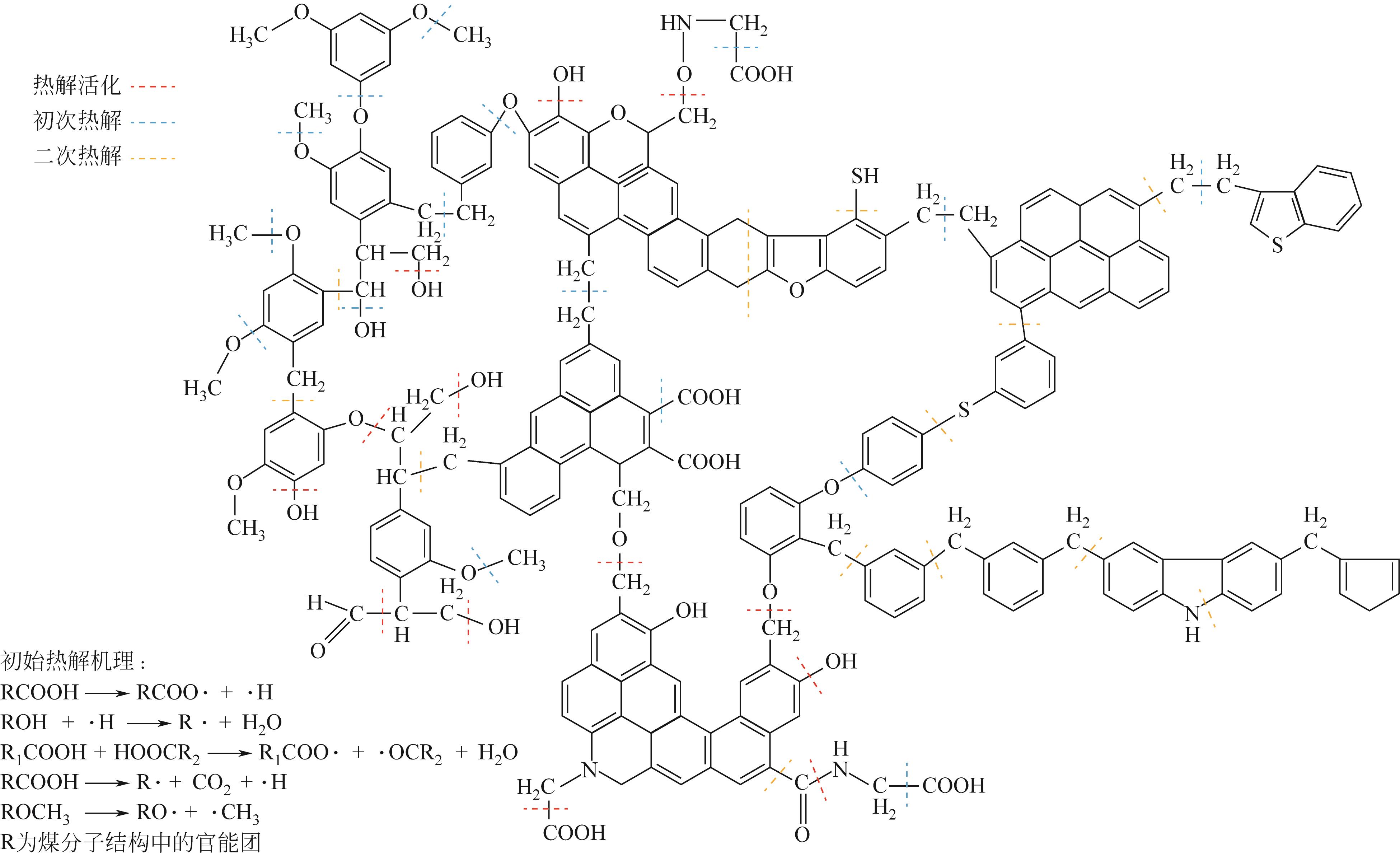
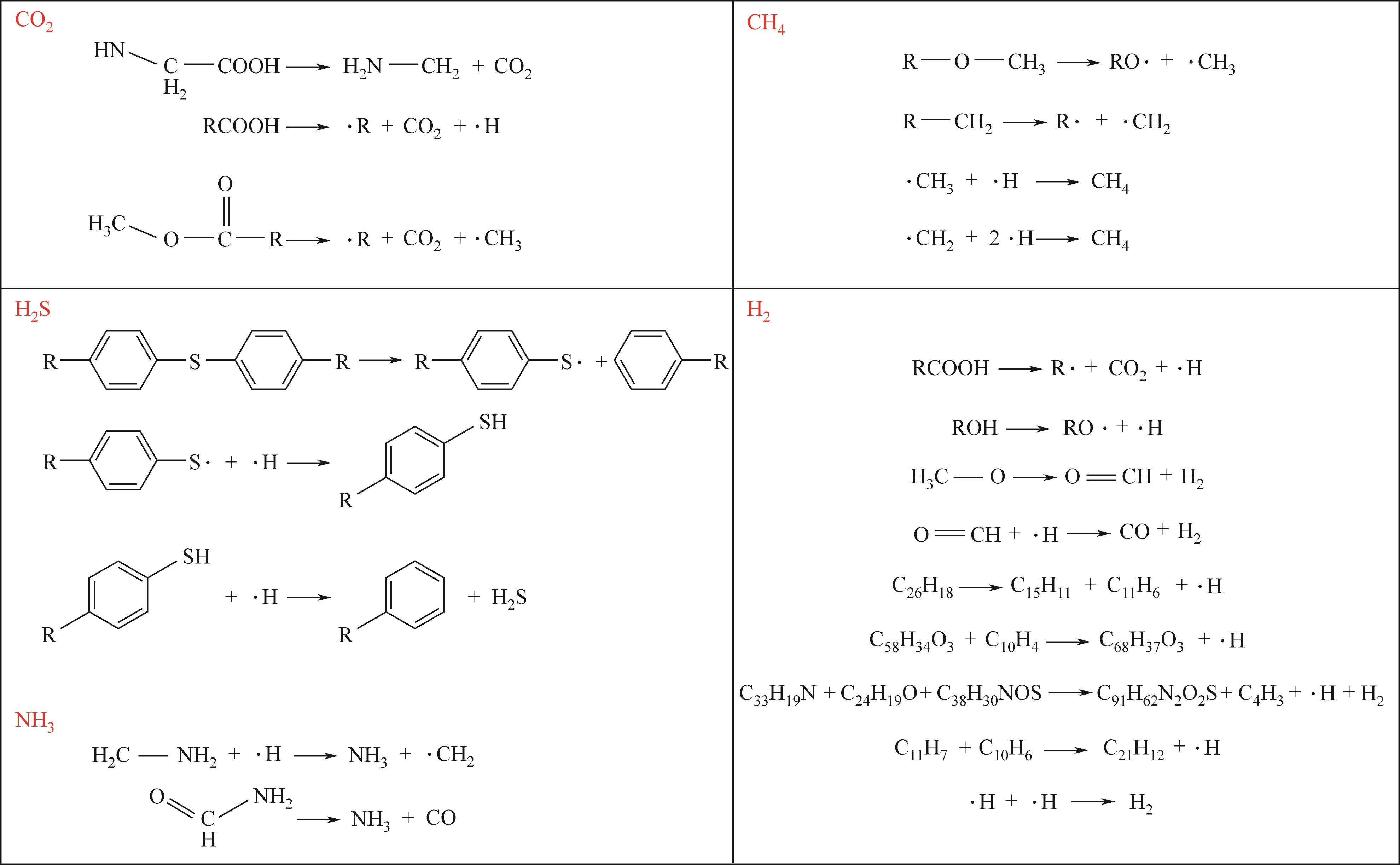
热解是实现煤炭资源清洁高效利用的重要途径,深入认识煤热解过程中挥发分自由基的变化规律对调控热解产物至关重要,但实验方法难以捕捉其细节。本文选用经典的褐煤分子模型,结合反应分子动力学(ReaxFF MD)模拟探究了低阶煤热解过程中挥发分自由基的演化规律及反应机理。ReaxFF MD模拟结果表明,挥发分产物的收率随升温速率的增大而增加,较高的升温速率抑制了气体产物的生成、提高了焦油产物的收率,但焦油的重质化严重。含氧官能团的裂解是煤热解的触发机制,热解过程主要分为活化(800~1200K)、热解(1200~2400K)和缩聚(2400~2800K)三个阶段。在高温缩聚阶段,焦油片段之间更容易交联,进而发生缩聚反应形成焦炭,并伴随着气体生成,导致焦油收率降低,气体和焦炭产率增加。因此,改善焦油收率和品质的关键是促进焦油片段的裂解,抑制其缩聚。分析了气相产物的形成机理,CO2主要来自羧基和酯基的裂解;甲氧基侧链和桥键裂解形成·CH3和·CH2自由基并捕获·H,最终形成CH4分子;焦油的二次热解和缩聚释放大量·H和H2,·H之间进一步反应生成H2;而煤中的硫醚结构与含氮支链裂解后,进而被·H自由基稳定为H2S和NH3。这些从分子层面获得的机理认识,可为实验或工业调控热解产物提供重要的参考依据。
中图分类号:
引用本文
黄淄博, 周文静, 魏进家. 基于ReaxFF MD模拟的低阶煤热解产物演化规律及反应机理[J]. 化工进展, 2024, 43(5): 2409-2419.
HUANG Zibo, ZHOU Wenjing, WEI Jinjia. Product evolution and reaction mechanism of low-rank coal pyrolysis based on ReaxFF MD simulation[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2409-2419.
| 状态 | Etotal/kcal·mol–1 | EA/kcal·mol–1 | EB/kcal·mol–1 | ET/kcal·mol–1 | EW/kcal·mol–1 | EH/kcal·mol–1 |
|---|---|---|---|---|---|---|
| 初始结构 | 9696.05 | 142.40 | 3719.14 | 279.65 | 5540.87 | –0.30 |
| 几何优化 | 858.17 | 163.41 | 97.53 | 140.86 | 453.71 | –0.84 |
| 退火优化 | 720.90 | 120.75 | 91.97 | 120.75 | 373.64 | –4.68 |
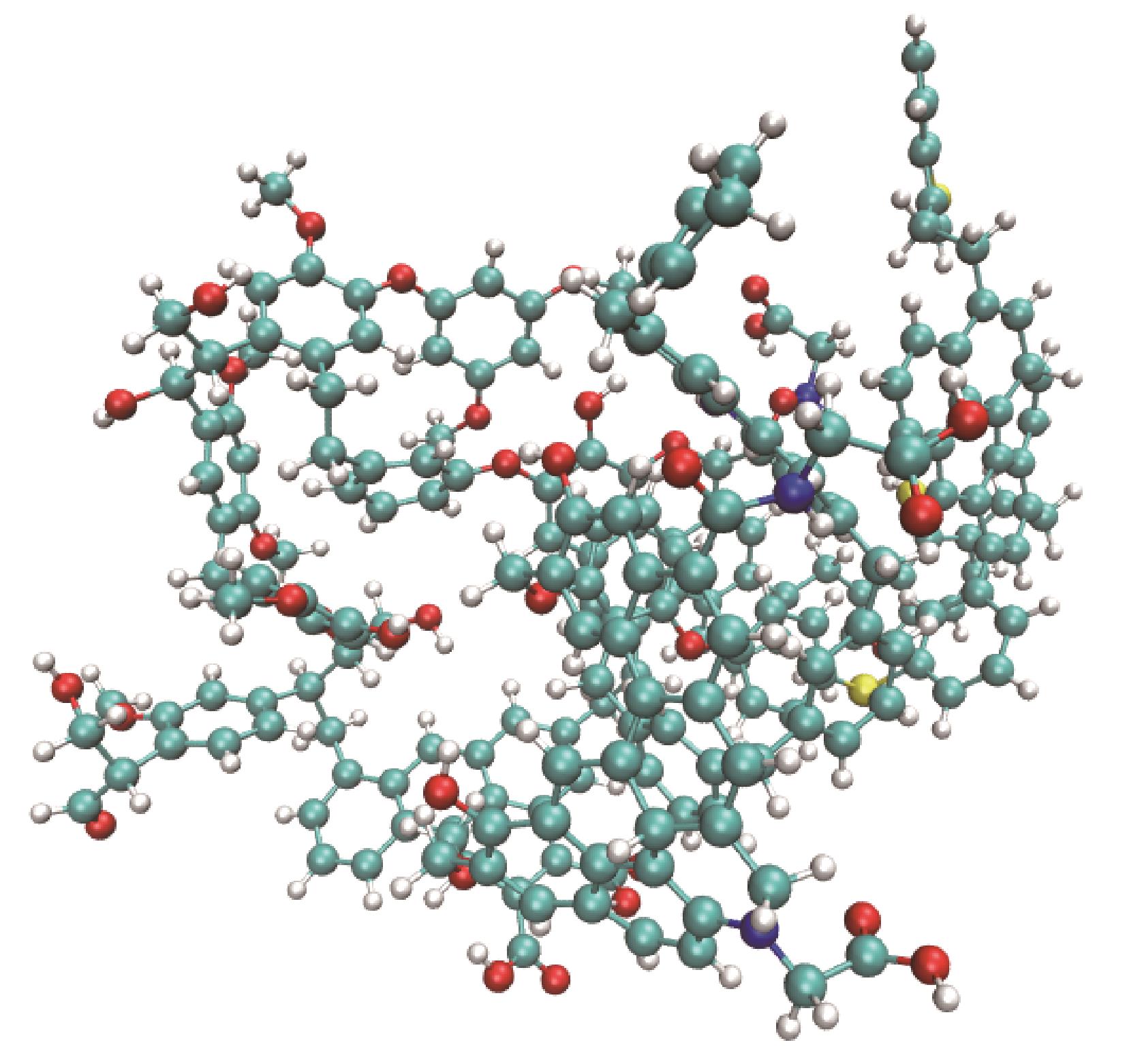
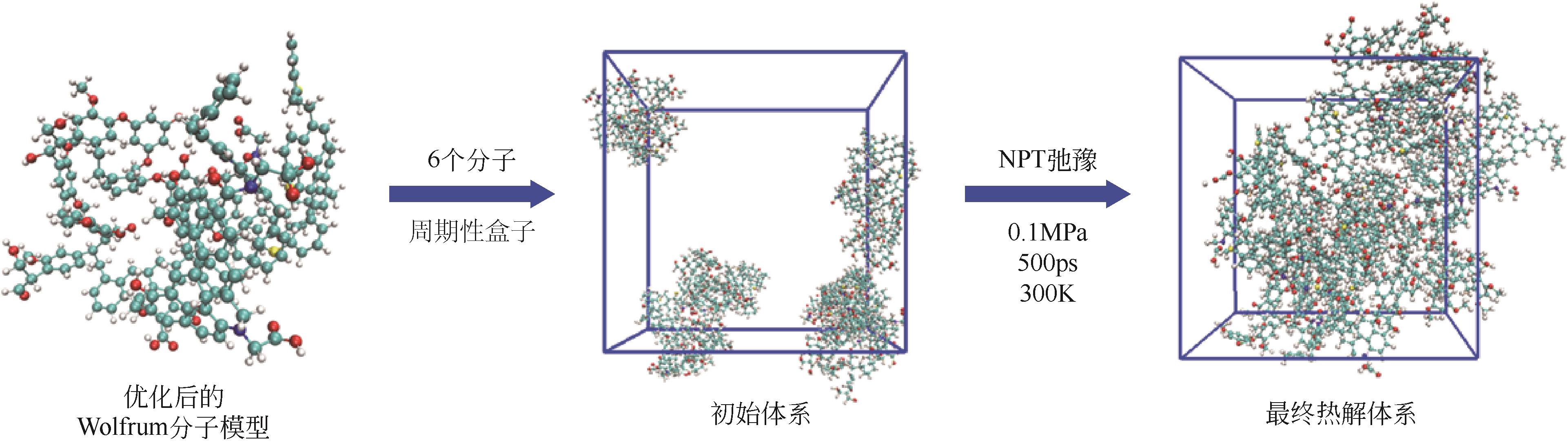
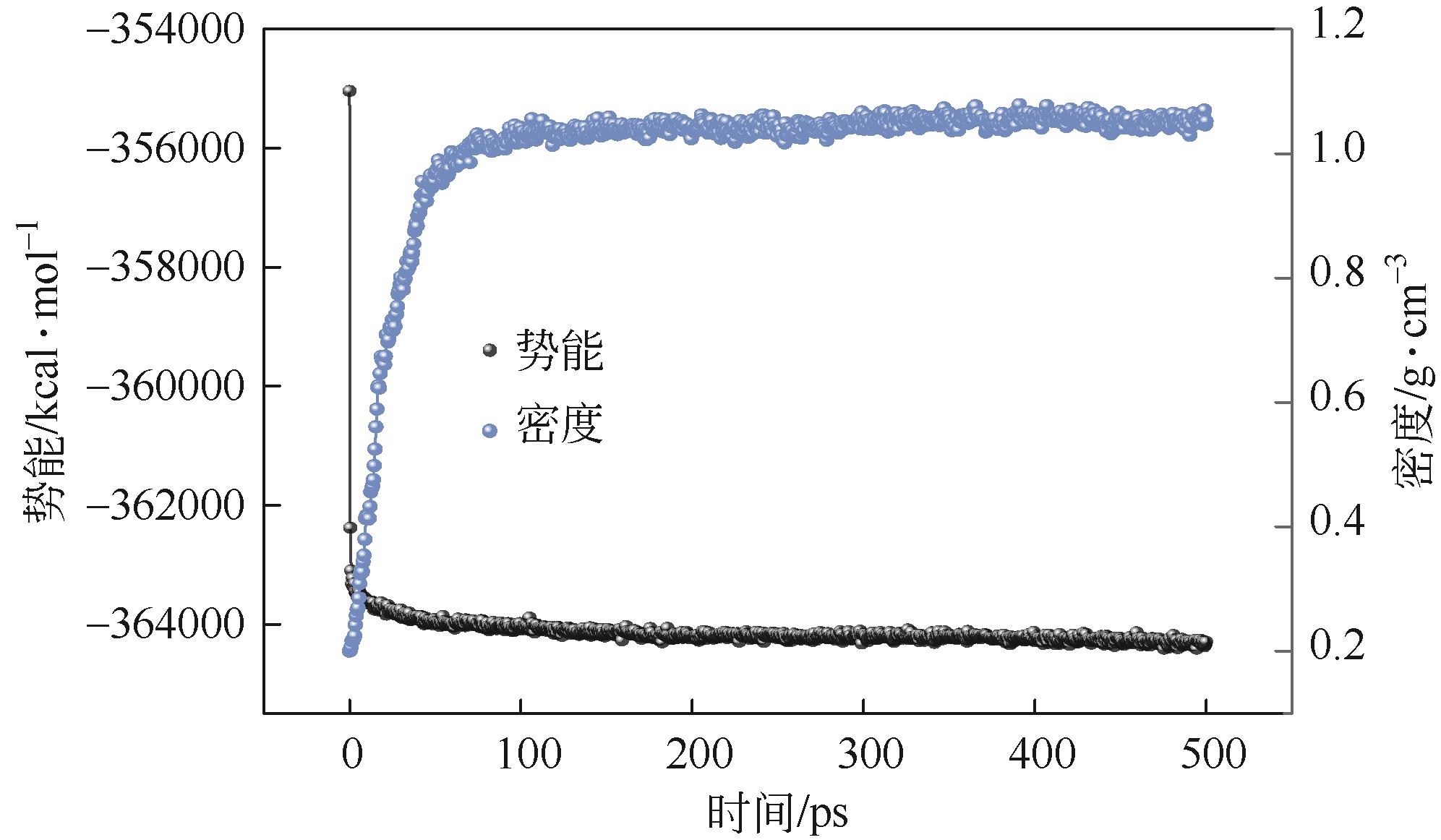
表1 Wolfrum模型优化前后的能量
| 状态 | Etotal/kcal·mol–1 | EA/kcal·mol–1 | EB/kcal·mol–1 | ET/kcal·mol–1 | EW/kcal·mol–1 | EH/kcal·mol–1 |
|---|---|---|---|---|---|---|
| 初始结构 | 9696.05 | 142.40 | 3719.14 | 279.65 | 5540.87 | –0.30 |
| 几何优化 | 858.17 | 163.41 | 97.53 | 140.86 | 453.71 | –0.84 |
| 退火优化 | 720.90 | 120.75 | 91.97 | 120.75 | 373.64 | –4.68 |
| 物质 | 800K | 1200K | 1600K | 2000K | 2400K | 2800K |
|---|---|---|---|---|---|---|
| char | 2 C225H182O36N4S3 | 1 C225H182O36N4S3 | 1 C217H161O28N3S3 | 1 C135H78O14S2 | 1 C388H211O25N5S2 | 1 C631H307O48N4S4 |
| 2 C225H181O36N4S3 | 1 C223H173O33N4S3 | 1 C187H139O23N3S3 | 1 C113H75O14NS | 1 C131H78O11N2 | 1 C229H103O18S | |
| 2 C225H181O35N4S3 | 1 C222H168O28N4S3 | 1 C186H146O24N3S3 | 1 C72H50O7NS | 1 C60H38O4N | 1 C189H85O13N | |
| 1 C222H177O33N3S3 | 1 C138H96O22S3 | 1 C69H46O4S | 1 C59H37O4 | 1 C48H27O5N | ||
| 1 C221H178O35N2S3 | 1 C137H99O8NS3 | 1 C65H42O8N | 1 C49H27O5S | |||
| 1 C163H142O22N2S3 | 1 C115H76O15N2 | 1 C64H42O10S2 | 1 C45H28O2S | |||
| 1 C66H44O15N2 | 1 C66H52O16N2 | 1 C62H38O6N2S | ||||
| 1 C52H33O2S3 | 1 C60H40O6S2 | |||||
| 1 C59H42O13 | ||||||
| 1 C52H30O4 | ||||||
| H-tar | 1 C39H31ON | 1 C39H25O7N2 | 1 C38H27N | 1 C32H13O4 | ||
| 1 C33H32O7N | 1 C38H29ON | 1 C33H17O3N | 1 C20H16O | |||
| 1 C33H26O4N2 | 1 C38H30ON | 1 C28H19O2N | ||||
| 1 C30H19ON2 | 1 C38H30N | 1 C28H16O | ||||
| 1 C29H19O2N | 1 C38H29O4N | 1 C27H20ON | ||||
| 1 C36H31O2N | 1 C27H12O2N | |||||
| 1 C35H21O3S | 1 C23H13O4 | |||||
| 1 C31H21O3N2 | 1 C20H12O2 | |||||
| 1 C31H20O3N | 1 C17H12O2 | |||||
| 1 C30H19O2N | ||||||
| 1 C17H15S | ||||||
| 1 C17H10O4 | ||||||
| L-tar | 1 C13H13O3 | 1 C13H6O5 | 1 C14H12O2 | 1 C12H5O3S | ||
| 1 C13H14O2 | 1 C11H8O2 | 1 C14H7O7S | 1 C12H5O3 | |||
| 1 C7H7O3 | 1 C11H7O4S | 1 C12H10 | 1 C11H6O2 | |||
| 1 C7H6O3 | 1 C9H8S2 | 1 C12H10O2 | 1 C8H7O | |||
| 1 C6H3O3 | 1 C9H8S | 1 C12H9O5 | 1 C8H4OS | |||
| 1 C5H3O2 | 1 C9H8 | 1 C11H9 | 1 C7H8 | |||
| 1 C4H6O3N | 1 C9H7O | 1 C11H8O | 1 C7H3O5 | |||
| 1 C9H7S | 1 C11H7O | 1 C6H8 | ||||
| 1 C9H7 | 1 C11H6O5S | 1 C5H6 | ||||
| 1 C7H6O | 1 C11H6O2 | |||||
| 1 C7H3O3 | 1 C11H5O4 | |||||
| 1 C6H5O | 1 C10H6O3 | |||||
| 1 C10H5O2 | ||||||
| 1 C9H6O2 | ||||||
| 1 C9H6OS | ||||||
| 2 C9H5O2 | ||||||
| 1 C8H5O2 | ||||||
| gas | 2 H2O | 1 C2H4O2N | 1 C3H6O2 | 1 C3H3O | 2 C3H3O | 1 C3HO2 |
| 6 CH2O | 1 C2H5O | 1 C3H4O | 1 C3H3 | 1 C2H4O2 | ||
| 6 H2O | 1 C2H3O2 | 1 C3O4 | 1 C3H2O3 | 2 C2H2O | ||
| 3 C2H3O | 2 C2H5O | 1 C2H3O2 | 1 C2O3 | |||
| 2 C2H2O2 | 1 C2H4O | 2 C2H3O | 1 C2O2S | |||
| 10 CH2O | 3 C2H3O2 | 2 C2H6 | 3 CH2O2 | |||
| 1 CH4O | 1 C2H3OS | 1 C2H4 | 7 CH2O | |||
| 1 CHO | 1 C2H3O | 5 C2H2O | 1 CH3O | |||
| 2 CH4 | 1 C2HO3 | 5 CH2O | 2 CHOS | |||
| 1 CH3 | 3 C2H2O | 10 CHO2 | 16 CHO | |||
| 1 CO2 | 1 CH6N | 1 CH4S | 1 CH3S | |||
| 2 NH3 | 1 CH3O | 2 CHOS | 1 CH2N | |||
| 18 H2O | 4 CH4O | 14 CHO | 19 CH4 | |||
| 3 H2 | 1 CH4N | 18 CH4 | 2 CH3 | |||
| 3 CH3N | 7 CO2 | 1 CH2N | ||||
| 5 CH2O | 1 NH4 | 15 NH3 | ||||
| 2 CHO2 | 9 NH3 | 1 NH2 | ||||
| 11 CHO | 22 H2O | 7 H2S | ||||
| 16 CH4 | 7 H2S | 18 CO2 | ||||
| 1 CH3 | 39 H2 | 1 CO | ||||
| 5 CO2 | 25 H2O | |||||
| 3 NH3 | 108 H2 | |||||
| 20 H2O | 1 H | |||||
| 10 H2 |
表2 不同温度下热解500ps后得到的产物组成
| 物质 | 800K | 1200K | 1600K | 2000K | 2400K | 2800K |
|---|---|---|---|---|---|---|
| char | 2 C225H182O36N4S3 | 1 C225H182O36N4S3 | 1 C217H161O28N3S3 | 1 C135H78O14S2 | 1 C388H211O25N5S2 | 1 C631H307O48N4S4 |
| 2 C225H181O36N4S3 | 1 C223H173O33N4S3 | 1 C187H139O23N3S3 | 1 C113H75O14NS | 1 C131H78O11N2 | 1 C229H103O18S | |
| 2 C225H181O35N4S3 | 1 C222H168O28N4S3 | 1 C186H146O24N3S3 | 1 C72H50O7NS | 1 C60H38O4N | 1 C189H85O13N | |
| 1 C222H177O33N3S3 | 1 C138H96O22S3 | 1 C69H46O4S | 1 C59H37O4 | 1 C48H27O5N | ||
| 1 C221H178O35N2S3 | 1 C137H99O8NS3 | 1 C65H42O8N | 1 C49H27O5S | |||
| 1 C163H142O22N2S3 | 1 C115H76O15N2 | 1 C64H42O10S2 | 1 C45H28O2S | |||
| 1 C66H44O15N2 | 1 C66H52O16N2 | 1 C62H38O6N2S | ||||
| 1 C52H33O2S3 | 1 C60H40O6S2 | |||||
| 1 C59H42O13 | ||||||
| 1 C52H30O4 | ||||||
| H-tar | 1 C39H31ON | 1 C39H25O7N2 | 1 C38H27N | 1 C32H13O4 | ||
| 1 C33H32O7N | 1 C38H29ON | 1 C33H17O3N | 1 C20H16O | |||
| 1 C33H26O4N2 | 1 C38H30ON | 1 C28H19O2N | ||||
| 1 C30H19ON2 | 1 C38H30N | 1 C28H16O | ||||
| 1 C29H19O2N | 1 C38H29O4N | 1 C27H20ON | ||||
| 1 C36H31O2N | 1 C27H12O2N | |||||
| 1 C35H21O3S | 1 C23H13O4 | |||||
| 1 C31H21O3N2 | 1 C20H12O2 | |||||
| 1 C31H20O3N | 1 C17H12O2 | |||||
| 1 C30H19O2N | ||||||
| 1 C17H15S | ||||||
| 1 C17H10O4 | ||||||
| L-tar | 1 C13H13O3 | 1 C13H6O5 | 1 C14H12O2 | 1 C12H5O3S | ||
| 1 C13H14O2 | 1 C11H8O2 | 1 C14H7O7S | 1 C12H5O3 | |||
| 1 C7H7O3 | 1 C11H7O4S | 1 C12H10 | 1 C11H6O2 | |||
| 1 C7H6O3 | 1 C9H8S2 | 1 C12H10O2 | 1 C8H7O | |||
| 1 C6H3O3 | 1 C9H8S | 1 C12H9O5 | 1 C8H4OS | |||
| 1 C5H3O2 | 1 C9H8 | 1 C11H9 | 1 C7H8 | |||
| 1 C4H6O3N | 1 C9H7O | 1 C11H8O | 1 C7H3O5 | |||
| 1 C9H7S | 1 C11H7O | 1 C6H8 | ||||
| 1 C9H7 | 1 C11H6O5S | 1 C5H6 | ||||
| 1 C7H6O | 1 C11H6O2 | |||||
| 1 C7H3O3 | 1 C11H5O4 | |||||
| 1 C6H5O | 1 C10H6O3 | |||||
| 1 C10H5O2 | ||||||
| 1 C9H6O2 | ||||||
| 1 C9H6OS | ||||||
| 2 C9H5O2 | ||||||
| 1 C8H5O2 | ||||||
| gas | 2 H2O | 1 C2H4O2N | 1 C3H6O2 | 1 C3H3O | 2 C3H3O | 1 C3HO2 |
| 6 CH2O | 1 C2H5O | 1 C3H4O | 1 C3H3 | 1 C2H4O2 | ||
| 6 H2O | 1 C2H3O2 | 1 C3O4 | 1 C3H2O3 | 2 C2H2O | ||
| 3 C2H3O | 2 C2H5O | 1 C2H3O2 | 1 C2O3 | |||
| 2 C2H2O2 | 1 C2H4O | 2 C2H3O | 1 C2O2S | |||
| 10 CH2O | 3 C2H3O2 | 2 C2H6 | 3 CH2O2 | |||
| 1 CH4O | 1 C2H3OS | 1 C2H4 | 7 CH2O | |||
| 1 CHO | 1 C2H3O | 5 C2H2O | 1 CH3O | |||
| 2 CH4 | 1 C2HO3 | 5 CH2O | 2 CHOS | |||
| 1 CH3 | 3 C2H2O | 10 CHO2 | 16 CHO | |||
| 1 CO2 | 1 CH6N | 1 CH4S | 1 CH3S | |||
| 2 NH3 | 1 CH3O | 2 CHOS | 1 CH2N | |||
| 18 H2O | 4 CH4O | 14 CHO | 19 CH4 | |||
| 3 H2 | 1 CH4N | 18 CH4 | 2 CH3 | |||
| 3 CH3N | 7 CO2 | 1 CH2N | ||||
| 5 CH2O | 1 NH4 | 15 NH3 | ||||
| 2 CHO2 | 9 NH3 | 1 NH2 | ||||
| 11 CHO | 22 H2O | 7 H2S | ||||
| 16 CH4 | 7 H2S | 18 CO2 | ||||
| 1 CH3 | 39 H2 | 1 CO | ||||
| 5 CO2 | 25 H2O | |||||
| 3 NH3 | 108 H2 | |||||
| 20 H2O | 1 H | |||||
| 10 H2 |
| 1 | YU Jiangdong, JIANG Chunyan, GUAN Qingqing, et al. Conversion of low-grade coals in sub- and supercritical water: A review[J]. Fuel, 2018, 217: 275-284. |
| 2 | WANG Chufan, FAN Xing, DONG Xueming, et al. Insights into the structural characteristics of four thermal dissolution extracts of a subbituminous coal by using higher-energy collisional dissociation[J]. Fuel, 2020, 282: 118844. |
| 3 | YANG Fang, DIAN Jie. Macro-economic impact of policies for controlling fossil energy consumption in China[J]. Energies, 2022, 15(3): 1051. |
| 4 | SOLOMON P R, FLETCHER T H, PUGMIRE R J. Progress in coal pyrolysis[J]. Fuel, 1993, 72(5): 587-597. |
| 5 | GAO Mingjie, LI Xiaoxia, GUO Li. Pyrolysis simulations of Fugu coal by large-scale ReaxFF molecular dynamics[J]. Fuel Processing Technology, 2018, 178: 197-205. |
| 6 | 靳立军, 李扬, 胡浩权. 甲烷活化与煤热解耦合过程提高焦油产率研究进展[J]. 化工学报, 2017, 68(10): 3669-3677. |
| JIN Lijun, LI Yang, HU Haoquan. Research progress of integrated methane activation with coal pyrolysis for improving coal tar yield[J]. CIESC Journal, 2017, 68(10): 3669-3677. | |
| 7 | HONG Dikun, GUO Xin. Molecular dynamics simulations of Zhundong coal pyrolysis using reactive force field[J]. Fuel, 2017, 210: 58-66. |
| 8 | 王芳, 曾玺, 王婷婷, 等. 基于过程强化与反应调控的煤定向热解制高品质油气产物基础研究及中试验证[J]. 化工学报, 2021, 72(12): 6131-6143. |
| WANG Fang, ZENG Xi, WANG Tingting, et al. Fundamentals and pilot demonstration of coal directional pyrolysis to high quality tar and gas products based on process intensification and reaction regulation[J]. CIESC Journal, 2021, 72(12): 6131-6143. | |
| 9 | SOLOMON Peter R, SERIO Michael A, CARANGELO Robert M, et al. Very rapid coal pyrolysis[J]. Fuel, 1986, 65(2): 182-194. |
| 10 | SOLOMON Peter R, SERIO Michael A, SUUBERG Eric M. Coal pyrolysis-experiments, kinetic rates and mechanisms[J]. Progress in Energy and Combustion Science, 1992, 18(2): 133—220. |
| 11 | YU Jianglong, TAHMASEBI Arash, HAN Yanna, et al. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization[J]. Fuel Processing Technology, 2013, 106: 9-20. |
| 12 | 刘方刚, 靳立军, 杨静, 等. 双电离源飞行时间质谱用于峰峰煤原位热解挥发分的表征[J]. 燃料化学学报, 2021, 49(5): 573-581, 564. |
| LIU Fanggang, JIN Lijun, YANG Jing, et al. In-situ characterization of volatiles from pyrolysis of Fengfeng coal by a double ionization time-of-flight mass spectrometer[J]. Journal of Fuel Chemistry and Technology, 2021, 49(5): 573-581, 564. | |
| 13 | FENG Dongdong, SHANG Qi, DONG Heming, et al. Catalytic mechanism of Na on coal pyrolysis-derived carbon black formation: Experiment and DFT simulation[J]. Fuel Processing Technology, 2021, 224: 107011. |
| 14 | VAN DUIN Adri C T, DASGUPTA Siddharth, LORANT Francois, et al. ReaxFF: A reactive force field for hydrocarbons[J]. The Journal of Physical Chemistry A, 2001, 105(41): 9396-9409. |
| 15 | SENFTLE Thomas P, HONG Sungwook, ISLAM Mahbubul Md, et al. The ReaxFF reactive force-field: Development, applications and future directions[J]. npj Computational Materials, 2016, 2(1): 1-14. |
| 16 | 张秀霞, 吕晓雪, 肖美华, 等. 典型烟煤热解机理的反应动力学模拟[J]. 燃料化学学报, 2020, 48(9): 1035-1046. |
| ZHANG Xiuxia, Xiaoxue LYU, XIAO Meihua, et al. Molecular reaction dynamics simulation of pyrolysis mechanism of typical bituminous coal via ReaxFF[J]. Journal of Fuel Chemistry and Technology, 2020, 48(9): 1035-1046. | |
| 17 | CHENOWETH Kimberly, VAN DUIN Adri C T, GODDARD William A. ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation[J]. The Journal of Physical Chemistry A, 2008, 112(5): 1040-1053. |
| 18 | SALMON Elodie, VAN DUIN Adri C T, LORANT François, et al. Early maturation processes in coal. Part 2: Reactive dynamics simulations using the ReaxFF reactive force field on Morwell Brown coal structures[J]. Organic Geochemistry, 2009, 40(12): 1195-1209. |
| 19 | BHOI Sanjukta, BANERJEE Tamal, MOHANTY Kaustubha. Molecular dynamic simulation of spontaneous combustion and pyrolysis of brown coal using ReaxFF[J]. Fuel, 2014, 136: 326-333. |
| 20 | Fidel CASTRO-MARCANO, KAMAT Amar M, RUSSO Michael F, et al. Combustion of an Illinois No. 6 coal char simulated using an atomistic char representation and the ReaxFF reactive force field[J]. Combustion and Flame, 2012, 159(3): 1272-1285. |
| 21 | 冯炜, 高红凤, 王贵, 等. 枣泉煤分子模型构建及热解的分子模拟[J]. 化工学报, 2019, 70(4): 1522-1531. |
| FENG Wei, GAO Hongfeng, WANG Gui, et al. Molecular model and pyrolysis simulation of Zaoquan coal[J]. CIESC Journal, 2019, 70(4): 1522-1531. | |
| 22 | 郑默, 李晓霞. ReaxFF MD模拟揭示的煤热解挥发分自由基反应的竞争与协调[J]. 化工学报, 2022, 73(6): 2732-2741. |
| ZHENG Mo, LI Xiaoxia. Revealing reaction compromise in competition for volatile radicals during coal pryolysis via ReaxFF MD simulation[J]. CIESC Journal, 2022, 73(6): 2732-2741. | |
| 23 | ZHAN Jinhui, WU Rongcheng, LIU Xiaoxing, et al. Preliminary understanding of initial reaction process for subbituminous coal pyrolysis with molecular dynamics simulation[J]. Fuel, 2014, 134: 283-292. |
| 24 | ZHENG Mo, LI Xiaoxia, LIU Jian, et al. Initial chemical reaction simulation of coal pyrolysis via ReaxFF molecular dynamics[J]. Energy & Fuels, 2013, 27(6): 2942-2951. |
| 25 | ZHOU Zhijun, GUO Longzhen, CHEN Liping, et al. Study of pyrolysis of brown coal and gasification of coal-water slurry using the ReaxFF reactive force field[J]. International Journal of Energy Research, 2018, 42(7): 2465-2480. |
| 26 | LIU Lianchi, Andres JARAMILLO-BOTERO, GODDARD William A Ⅲ, et al. Development of a ReaxFF reactive force field for ettringite and study of its mechanical failure modes from reactive dynamics simulations[J]. The Journal of Physical Chemistry A, 2012, 116(15): 3918-3925. |
| 27 | ZHENG Mo, PAN Yang, WANG Ze, et al. Capturing the dynamic profiles of products in Hailaer brown coal pyrolysis with reactive molecular simulations and experiments[J]. Fuel, 2020, 268: 117290. |
| 28 | WANG Jin, HOU Quanlin, ZENG Fangui, et al. Gas generation mechanisms of bituminous coal under shear stress based on ReaxFF molecular dynamics simulation[J]. Fuel, 2021, 298: 120240. |
| 29 | MATHEWS Jonathan P, CHAFFEE Alan L. The molecular representations of coal—A review[J]. Fuel, 2012, 96: 1-14. |
| 30 | JURKIEWICZ Antoni. Spatial system of the Wiser model of coal structure according to the second moment of the nuclear magnetic resonance line[J]. Journal of applied physics, 1987, 62(9): 3892-3897. |
| 31 | SHINN John H. From coal to single-stage and two-stage products: A reactive model of coal structure[J]. Fuel, 1984, 63(9): 1187-1196. |
| 32 | WOLFRUM E A. Correlations between petrographical properties, chemical structure, and technological behavior of Rhenish brown coal[M]. Washington, D.C.: American Chemical Society, 1984: 15-37. |
| 33 | LIANG Yinghua, WANG Feng, ZHANG Hang, et al. A Reax FF molecular dynamics study on the mechanism of organic sulfur transformation in the hydropyrolysis process of lignite[J]. Fuel Processing Technology, 2016, 147: 32-40. |
| 34 | ZHENG Mo, LI Xiaoxia, WANG Meijun, et al. Dynamic profiles of tar products during Naomaohu coal pyrolysis revealed by large-scale reactive molecular dynamic simulation[J]. Fuel, 2019, 253: 910-920. |
| 35 | MUELLER Jonathan E, VAN DUIN Adri C T, GODDARD William A Ⅲ. Application of the ReaxFF reactive force field to reactive dynamics of hydrocarbon chemisorption and decomposition[J]. The Journal of Physical Chemistry C, 2010, 114(12): 5675-5685. |
| 36 | Malte DÖNTGEN, Marie-Dominique PRZYBYLSKI-FREUND, KRÖGER Leif C, et al. Automated discovery of reaction pathways, rate constants, and transition states using reactive molecular dynamics simulations[J]. Journal of Chemical Theory and Computation, 2015, 11(6): 2517-2524. |
| 37 | FLETCHER Thomas H, KERSTEIN Alan R, PUGMIRE Ronald J, et al. Chemical percolation model for devolatilization. 3. Direct use of carbon-13 NMR data to predict effects of coal type[J]. Energy & Fuels, 1992, 6(4): 414-431. |
| 38 | SATHE Chirag, PANG Yanyuan, LI Chunzhu. Effects of heating rate and ion-exchangeable cations on the pyrolysis yields from a Victorian brown coal[J]. Energy & Fuels, 1999, 13(3): 748-755. |
| 39 | HAYASHI Jun-ichiro, TAKAHASHI Hiroshi, Satoshi DOI, et al. Reactions in brown coal pyrolysis responsible for heating rate effect on tar yield[J]. Energy & Fuels, 2000, 14(2): 400-408. |
| 40 | LEI Zhao, LIANG Qijun, LING Qiang, et al. Investigating the reaction mechanism of light tar for Shenfu bituminous coal pyrolysis[J]. Energy, 2023, 263: 125731. |
| [1] | 姚乃瑜, 曹景沛, 庞新博, 赵小燕, 蔡士杰, 徐敏, 赵静平, 冯晓博, 伊凤娇. 低阶煤热解挥发分热催化重整研究进展[J]. 化工进展, 2024, 43(5): 2279-2293. |
| [2] | 吴琪, 白柏杨, 尹永杰, 马晓迅. 鄂尔多斯褐煤显微组分结构与其热解特性间的关系[J]. 化工进展, 2024, 43(5): 2370-2385. |
| [3] | 张鹏飞, 严张艳, 任亮, 张奎, 梁家林, 赵广乐, 张璠玢, 胡志海. C |
| [4] | 张鑫, 汤吉昀, 陈娟, 宋占龙, 董勇, 姚洪. 高温烟气热解废轮胎过程中痕量金属Cu、Pb的迁移特性分析[J]. 化工进展, 2024, 43(3): 1606-1613. |
| [5] | 陈国徽, 王君雷, 李世龙, 李金宇, 徐运飞, 罗俊潇, 王昆. 火焰喷雾热解制备锂离子电池三元正极材料研究进展[J]. 化工进展, 2024, 43(2): 971-983. |
| [6] | 任鹏锟, 仲兆平, 杨宇轩, 张杉, 杜浩然, 李骞. 改性海泡石对污泥热解过程中重金属的控制[J]. 化工进展, 2024, 43(1): 541-550. |
| [7] | 成昊霖, 年瑶, 韩优. CH4和CO2共转化反应机理研究进展[J]. 化工进展, 2024, 43(1): 60-75. |
| [8] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [9] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [10] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [11] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [12] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [13] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [14] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [15] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||