化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2370-2385.DOI: 10.16085/j.issn.1000-6613.2024-0397
• 化石能源的清洁高效转化利用 • 上一篇
鄂尔多斯褐煤显微组分结构与其热解特性间的关系
- 1.西北大学化工学院,碳氢资源清洁利用国际科技合作基地,陕北能源先进化工利用技术教育部工程研究中心,陕西省洁净煤转化工程技术研究中心,陕北能源化工产业发展协同创新中心,陕西 西安 710127
2.陕西煤基特种燃料研究院有限公司,陕西 西安 710069
-
收稿日期:2024-03-11修回日期:2024-04-04出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:马晓迅 -
作者简介:吴琪(1999—),男,硕士研究生,研究方向为煤炭的清洁高效利用。E-mail:13546626853@163.com。 -
基金资助:国家科学技术基金(21536009);陕西省科技计划(2017ZDCXL-GY-10-03);陕西省教育部专项科研计划(19JK0854)
Relationship between the structure of macerals of Ordos lignite and its pyrolysis characteristics
WU Qi1( ), BAI Boyang2, YIN Yongjie1, MA Xiaoxun1(
), BAI Boyang2, YIN Yongjie1, MA Xiaoxun1( )
)
- 1.International Science & Technology Cooperation Base of MOST for Clean Utilization of Hydrocarbon Resources, Chemical Engineering Research Center of the Ministry of Education for Advanced Use Technology of Shanbei Energy, Shaanxi Research Center of Engineering Technology for Clean Coal Conversion, Collaborative Innovation Center for Development of Energy and Chemical Industry in Northern Shaanxi, School of Chemical Engineering, Northwest University, Xi’an 710127, Shaanxi, China
2.Shannxi Coal Based Special Fuel Research Institute Co. , Ltd. , Xi’an 710069, Shannxi, China
-
Received:2024-03-11Revised:2024-04-04Online:2024-05-15Published:2024-06-15 -
Contact:MA Xiaoxun
摘要:
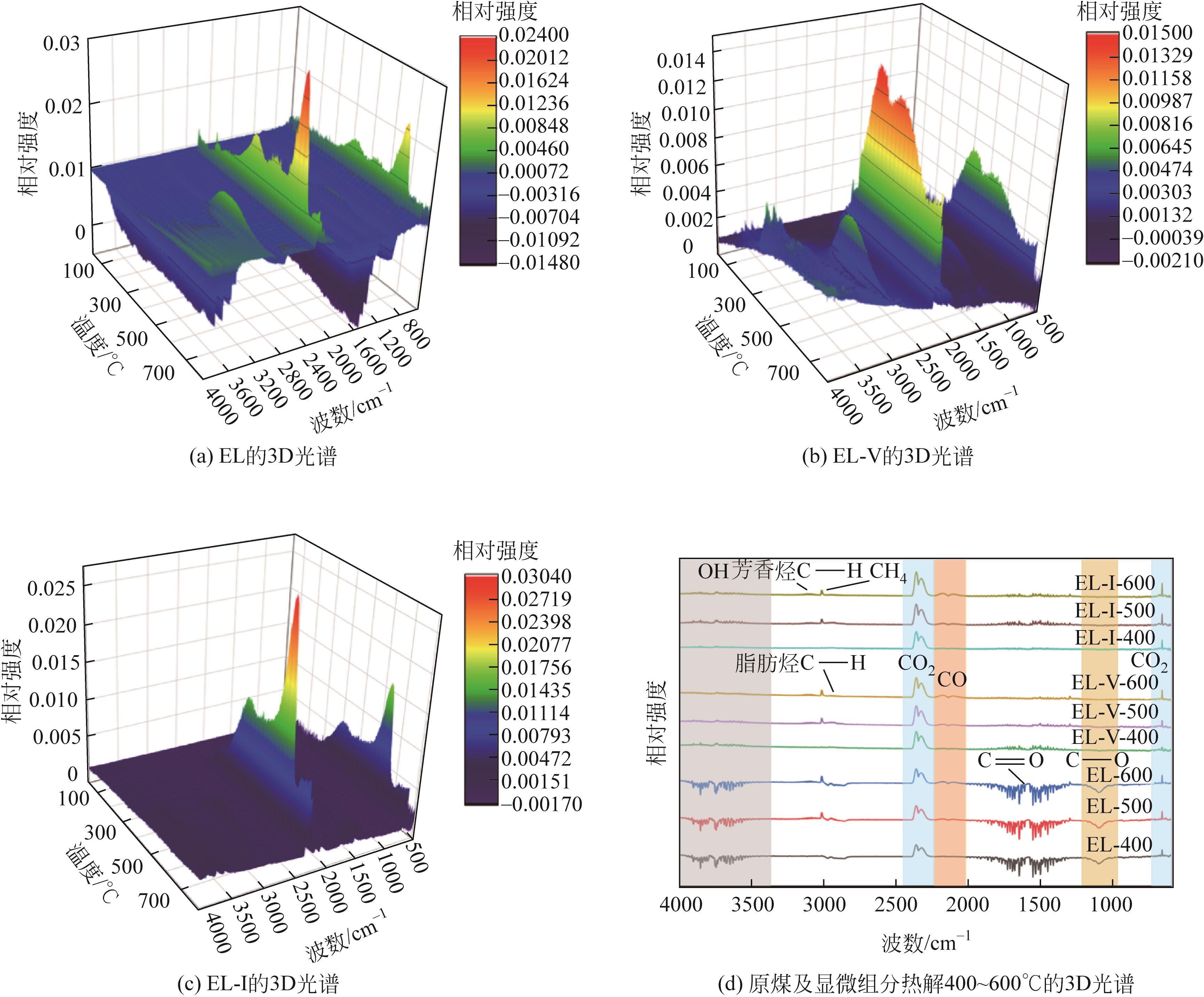
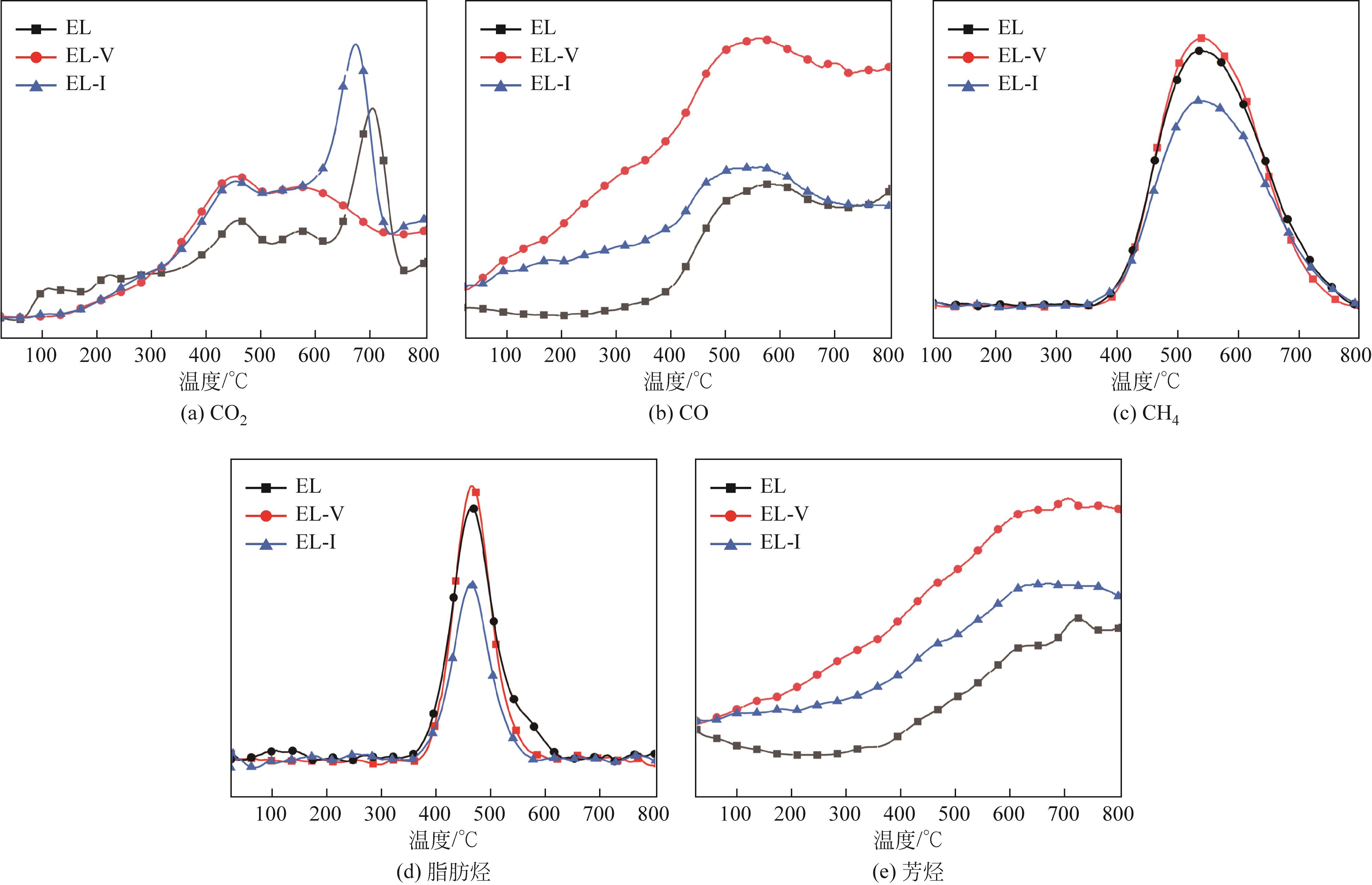
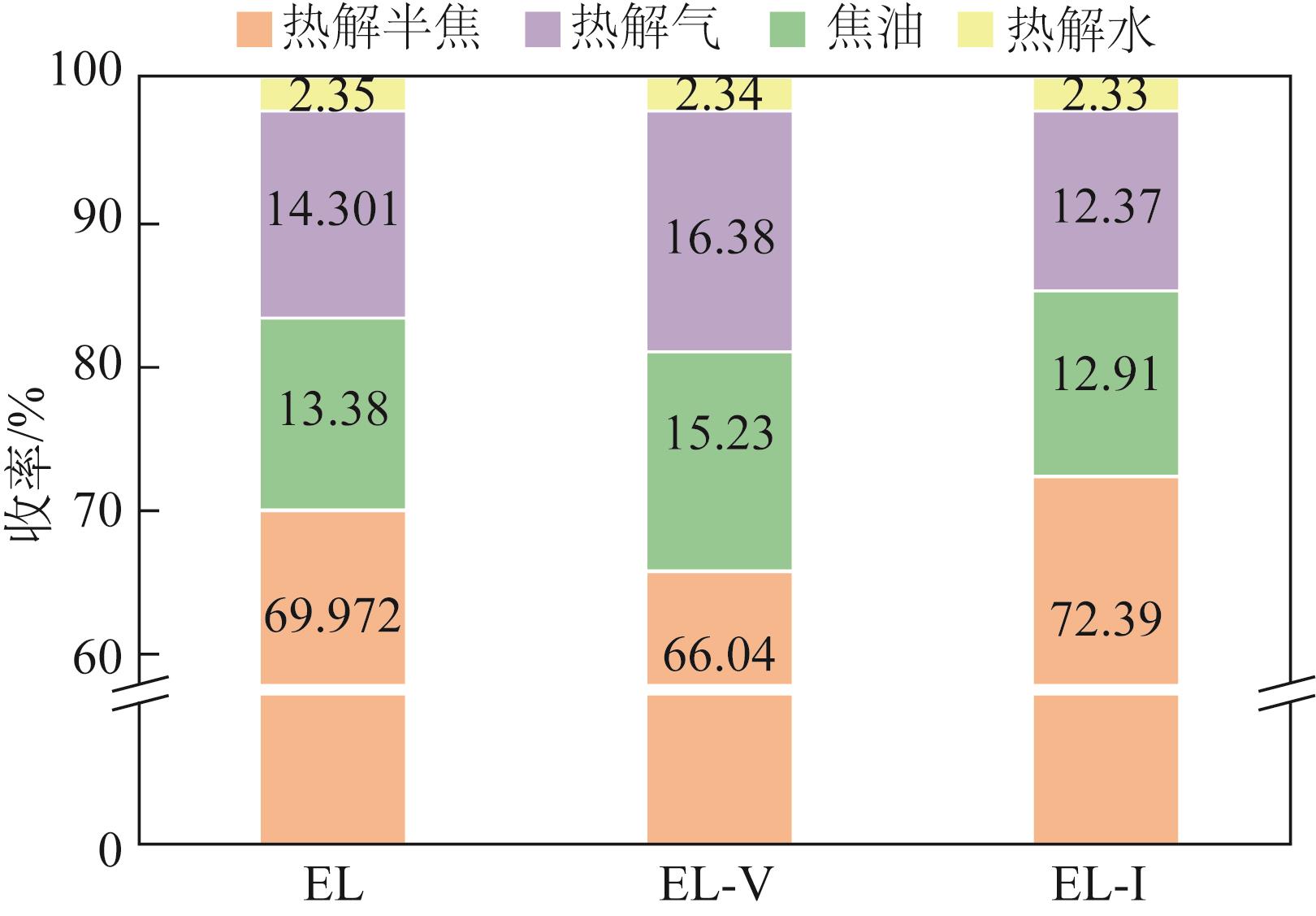
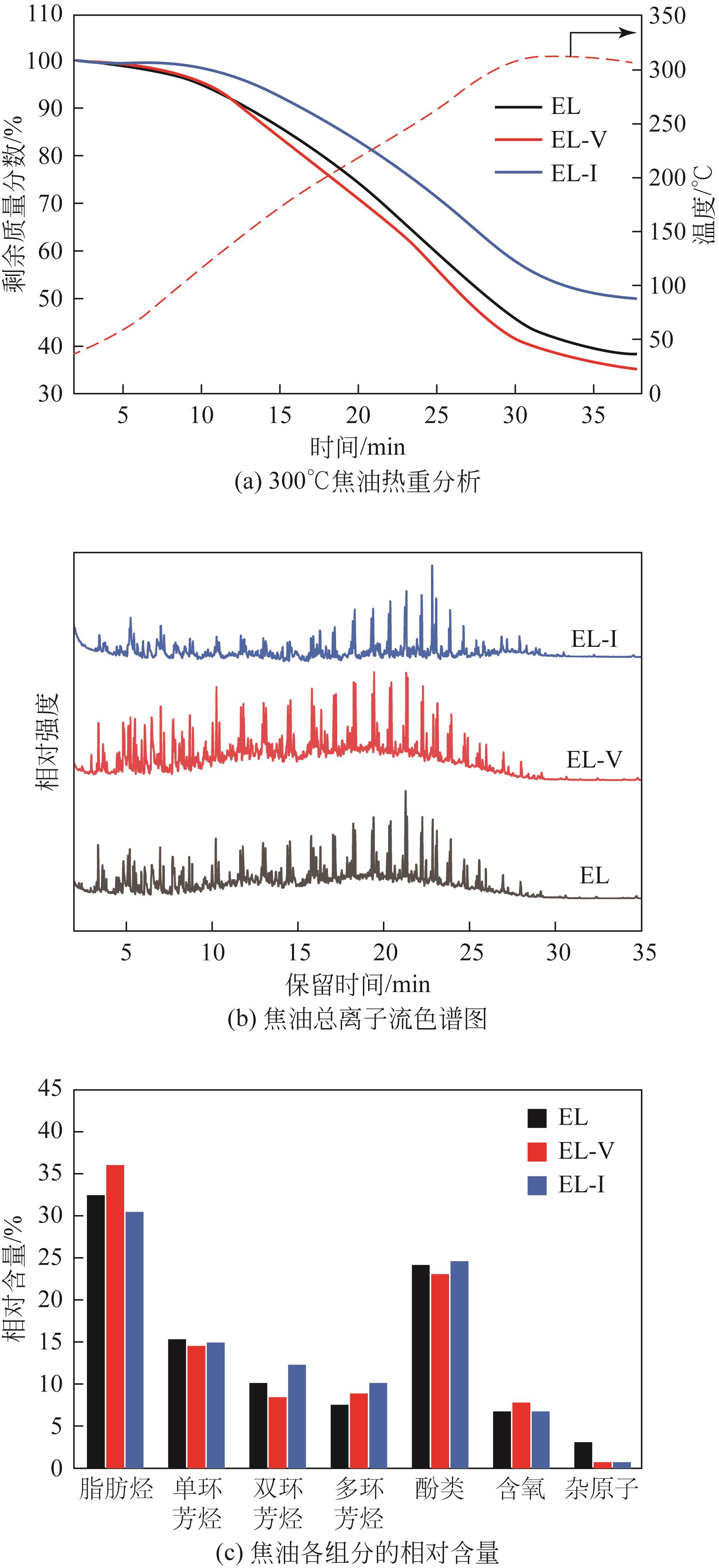
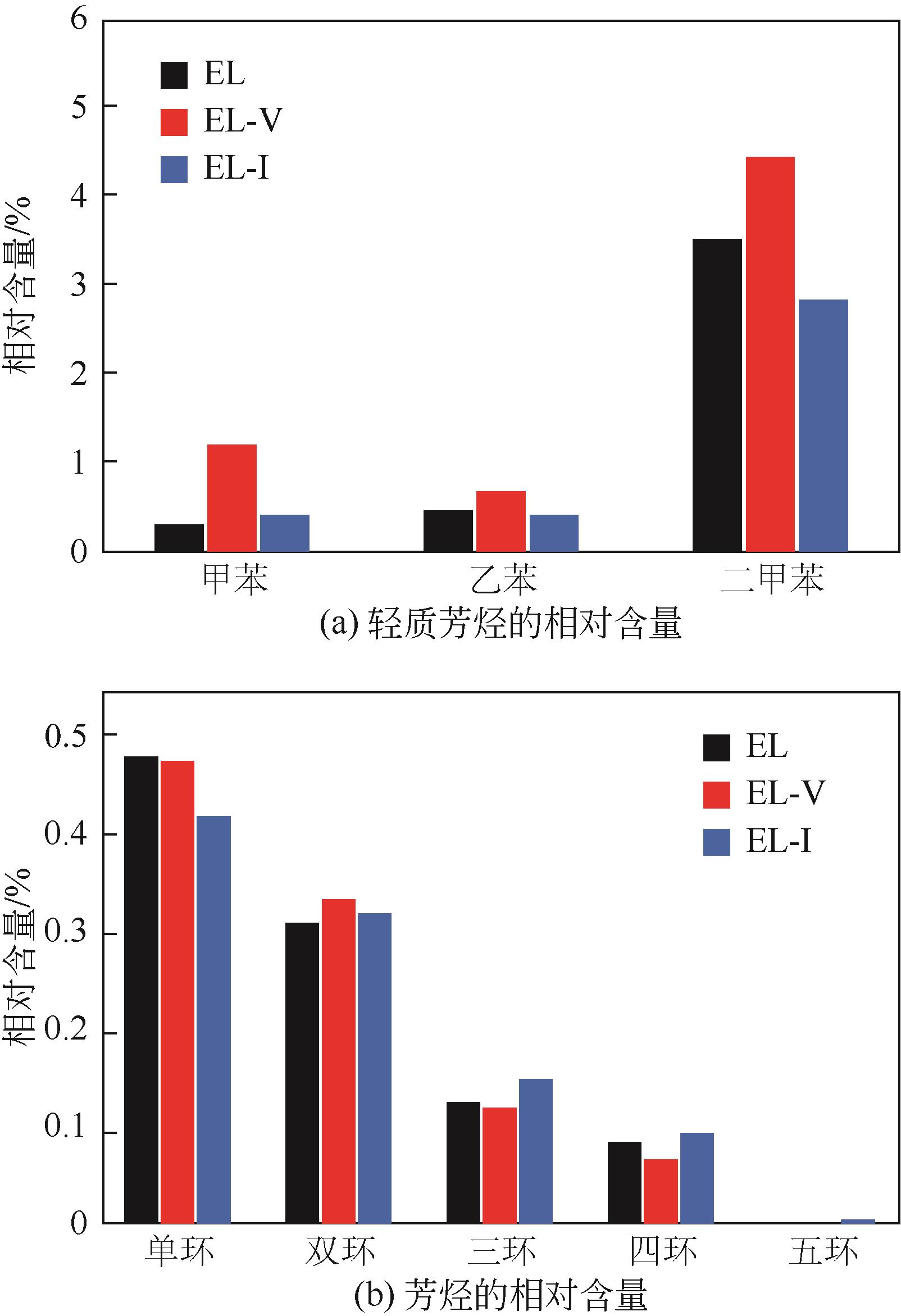
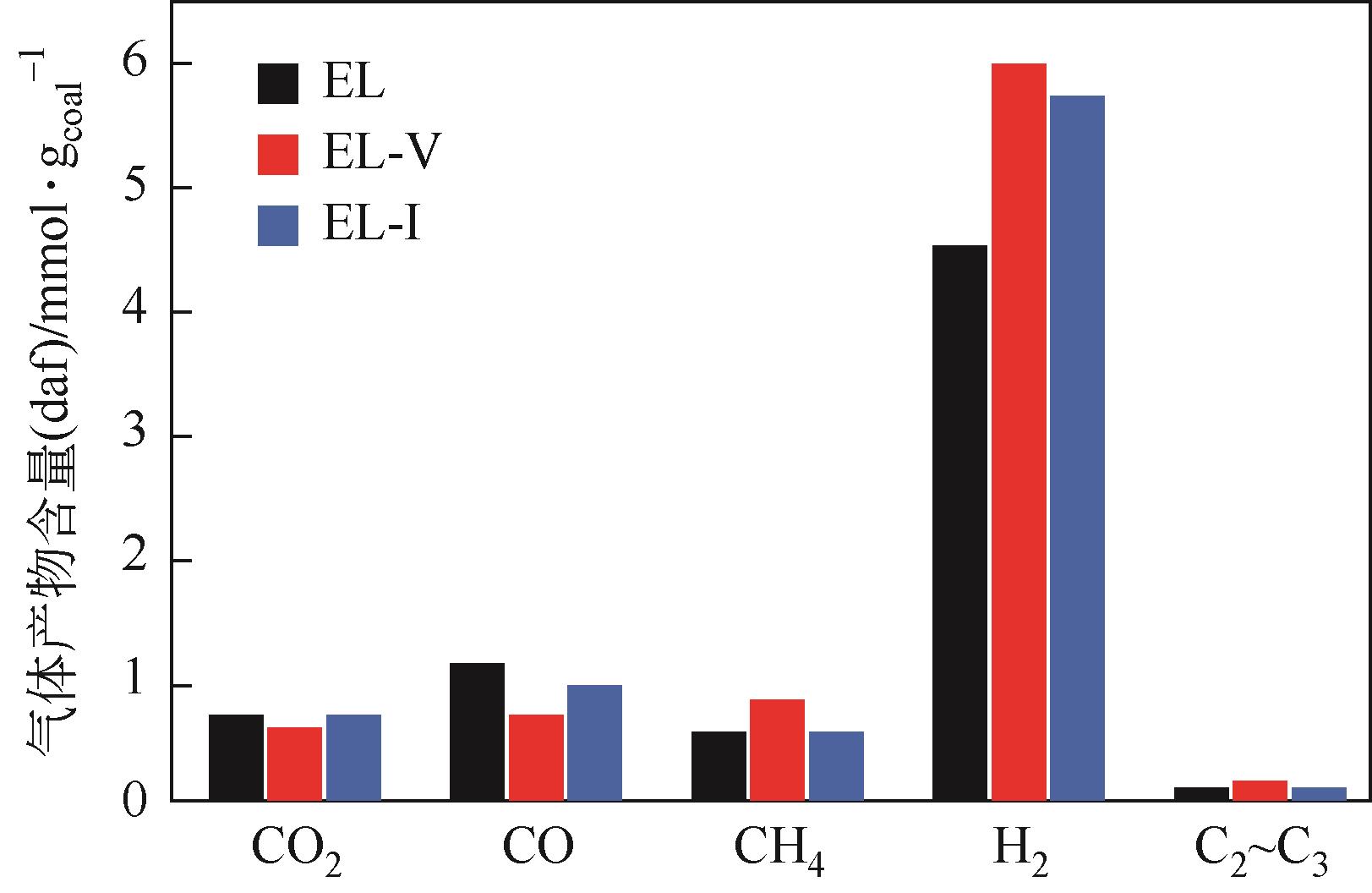
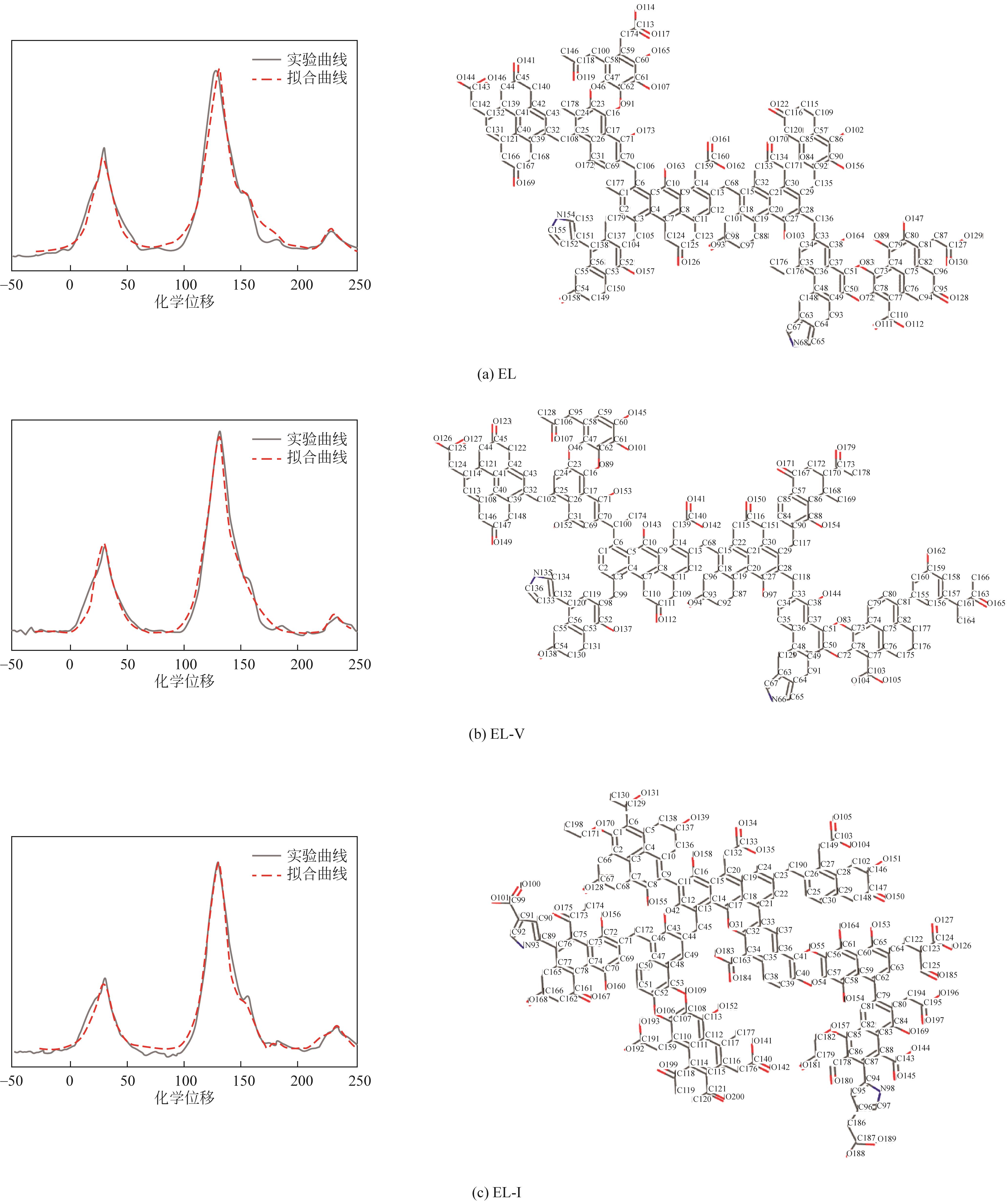
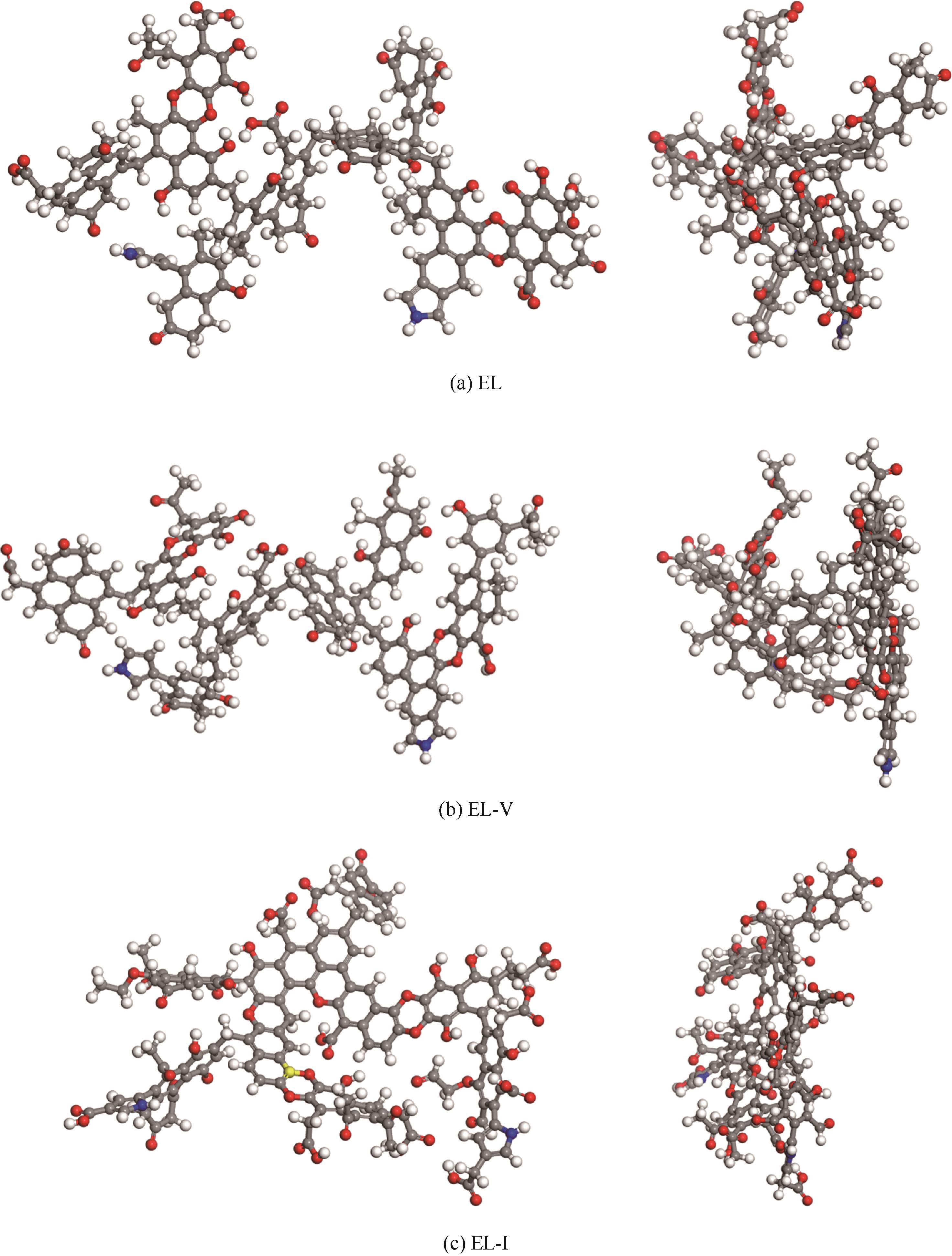
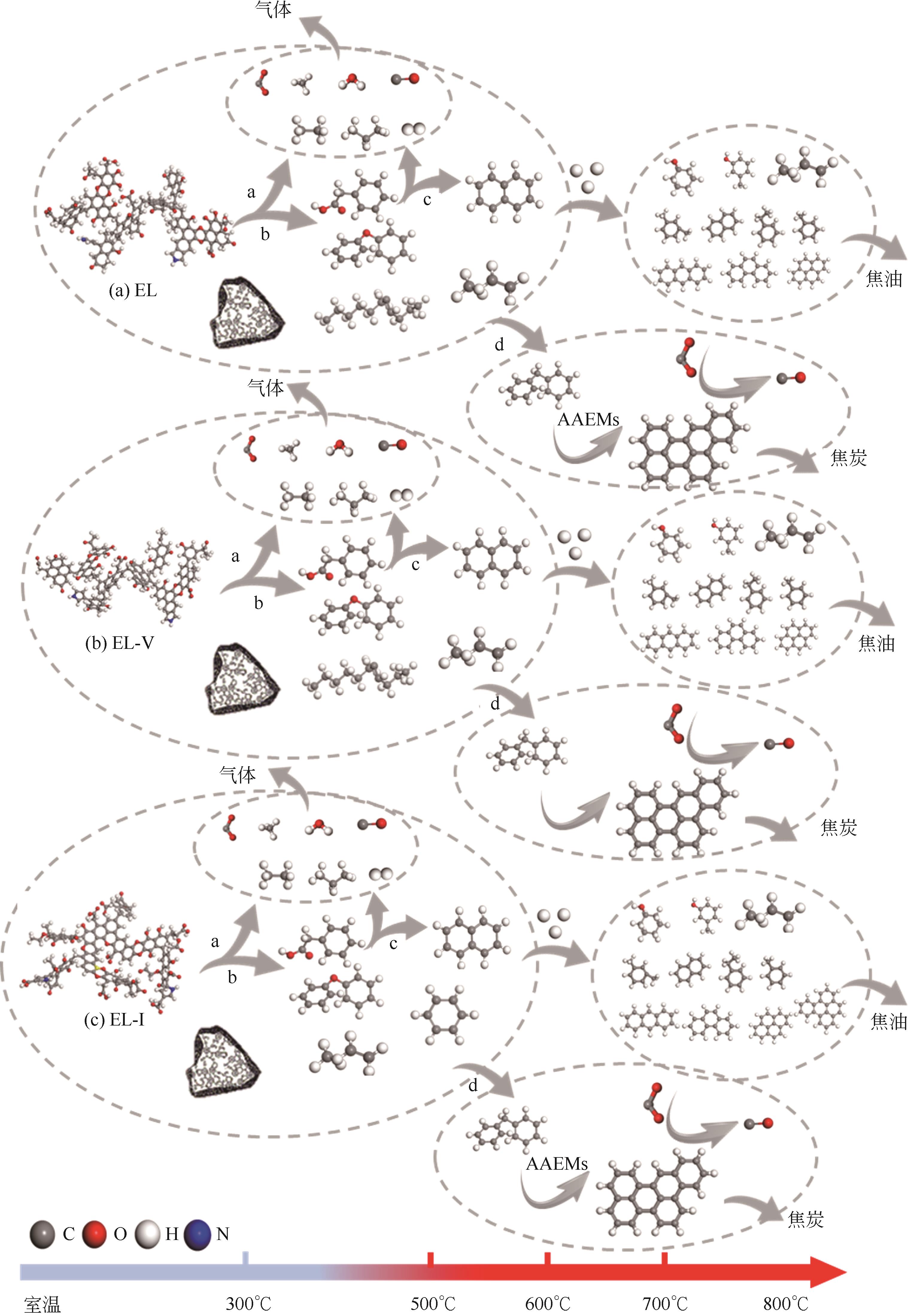
采用浮沉分离法将鄂尔多斯褐煤分离成富镜质组(EL-V)和富惰质组(EL-I),通过工业分析、元素分析、X射线衍射光谱(XRD)、傅里叶变换红外光谱(FTIR)和固体核磁(13C NMR)分析了原煤及显微组分的结构和组成。采用粉-粒流化床和TG-FTIR装置分别考察了原煤及显微组分的热解特性和气态挥发物的逸出规律,进一步构建原煤及显微组分的大分子结构模型,并进行了量子化学计算,认识了化学键的断裂规律。结果表明,富镜质组的挥发分含量较高,含有丰富的烷基侧链,热失重速率最大,表明镜质组的热解反应性较强。与原煤和惰质组相比,富镜质组热解气体含量和焦油含量较高,且热解焦油含有较多的脂肪烃和轻质芳烃(TXE)。富惰质组的H/C比较低,含氧量和芳环缩合程度较高,所得热解焦油中多环芳烃和酚类物质含量较高。此外,通过TG-FTIR重点分析了CO2、CO、CH4、脂肪烃和芳烃的析出行为。根据煤样大分子结构中化学键的断裂和气态挥发物的逸出规律,提出了煤热解过程中可能进行的反应路线。
中图分类号:
引用本文
吴琪, 白柏杨, 尹永杰, 马晓迅. 鄂尔多斯褐煤显微组分结构与其热解特性间的关系[J]. 化工进展, 2024, 43(5): 2370-2385.
WU Qi, BAI Boyang, YIN Yongjie, MA Xiaoxun. Relationship between the structure of macerals of Ordos lignite and its pyrolysis characteristics[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2370-2385.
工业分析(干燥无灰基) /%(质量分数) | 岩相分析/%(质量分数) | Rmax/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 水分 | 灰分 | 挥发分 | 固定碳* | 镜质组 | 惰质组 | 壳质组 | 矿物质 | ||
| 1.49 | 7.95 | 37.27 | 62.73 | 65.4 | 25.6 | 3.4 | 5.6 | 0.83 | |
表1 EL的工业分析和岩相分析
工业分析(干燥无灰基) /%(质量分数) | 岩相分析/%(质量分数) | Rmax/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 水分 | 灰分 | 挥发分 | 固定碳* | 镜质组 | 惰质组 | 壳质组 | 矿物质 | ||
| 1.49 | 7.95 | 37.27 | 62.73 | 65.4 | 25.6 | 3.4 | 5.6 | 0.83 | |
| 样品 | 工业分析(干燥无灰基)/%(质量分数) | 元素分析(干燥无灰基)/%(质量分数) | 原子比 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 水分 | 灰分 | 挥发分 | 固定碳① | C | H | N | S | O① | AO/C | AH/C | ||
| EL | 1.49 | 7.95 | 37.27 | 62.73 | 70.60 | 4.19 | 1.43 | 0.10 | 23.69 | 0.25 | 0.71 | |
| EL-V | 2.47 | 2.11 | 38.62 | 61.38 | 73.77 | 5.01 | 1.55 | 0.15 | 19.52 | 0.20 | 0.81 | |
| EL-I | 0.78 | 17.77 | 31.68 | 68.32 | 64.04 | 3.52 | 1.22 | 0.16 | 31.06 | 0.36 | 0.66 | |
表2 原煤及显微组分的工业分析和元素分析
| 样品 | 工业分析(干燥无灰基)/%(质量分数) | 元素分析(干燥无灰基)/%(质量分数) | 原子比 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 水分 | 灰分 | 挥发分 | 固定碳① | C | H | N | S | O① | AO/C | AH/C | ||
| EL | 1.49 | 7.95 | 37.27 | 62.73 | 70.60 | 4.19 | 1.43 | 0.10 | 23.69 | 0.25 | 0.71 | |
| EL-V | 2.47 | 2.11 | 38.62 | 61.38 | 73.77 | 5.01 | 1.55 | 0.15 | 19.52 | 0.20 | 0.81 | |
| EL-I | 0.78 | 17.77 | 31.68 | 68.32 | 64.04 | 3.52 | 1.22 | 0.16 | 31.06 | 0.36 | 0.66 | |
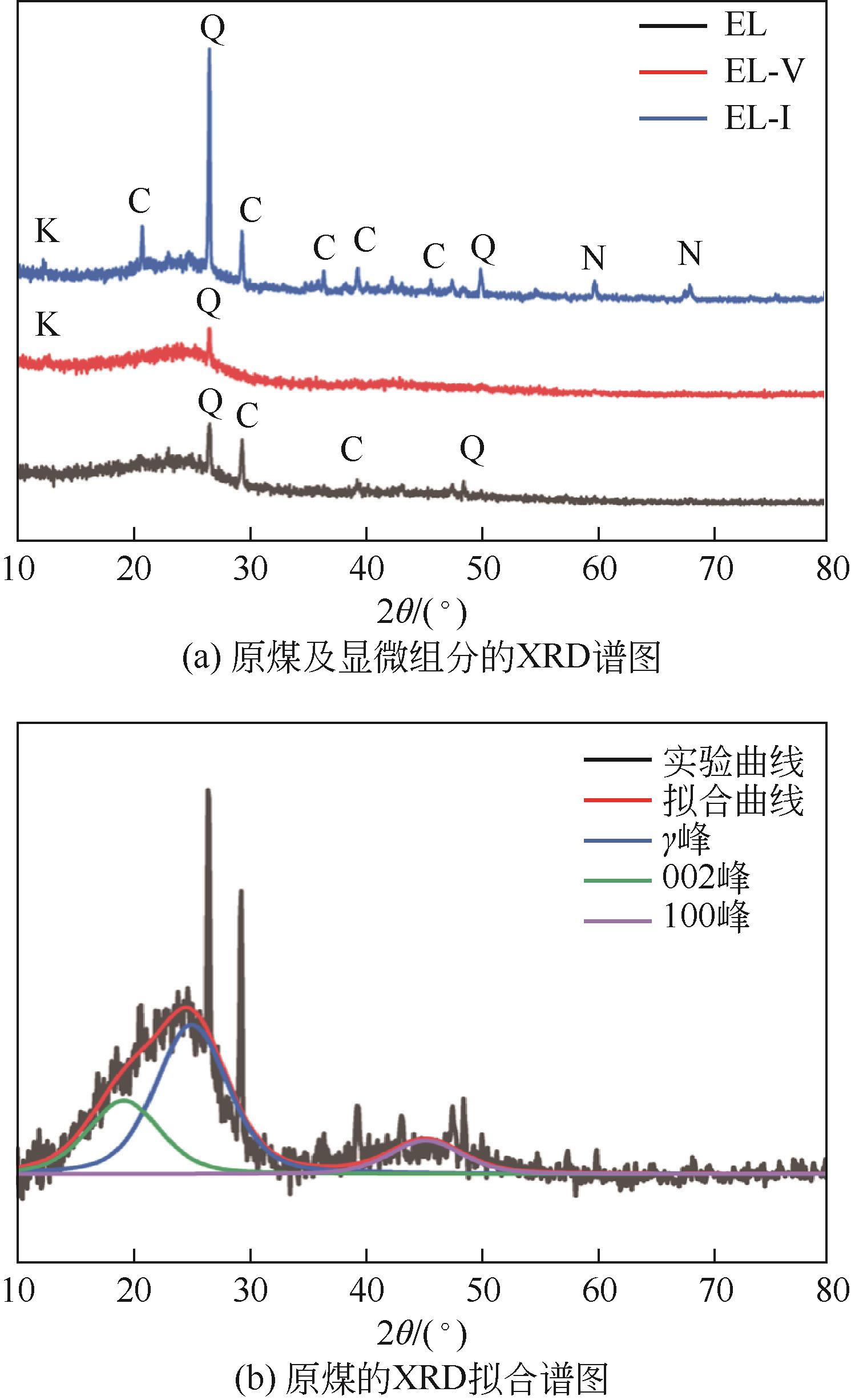
| 样品 | 2θ/(°) | d002/nm | Lc/nm | La/nm | fa |
|---|---|---|---|---|---|
| EL | 25.80 | 0.3452 | 1.21 | 1.36 | 0.60 |
| EL-V | 25.23 | 0.3528 | 1.55 | 0.98 | 0.51 |
| EL-I | 25.81 | 0.3450 | 1.67 | 1.04 | 0.63 |
表3 原煤及显微组分的碳微晶结构参数
| 样品 | 2θ/(°) | d002/nm | Lc/nm | La/nm | fa |
|---|---|---|---|---|---|
| EL | 25.80 | 0.3452 | 1.21 | 1.36 | 0.60 |
| EL-V | 25.23 | 0.3528 | 1.55 | 0.98 | 0.51 |
| EL-I | 25.81 | 0.3450 | 1.67 | 1.04 | 0.63 |
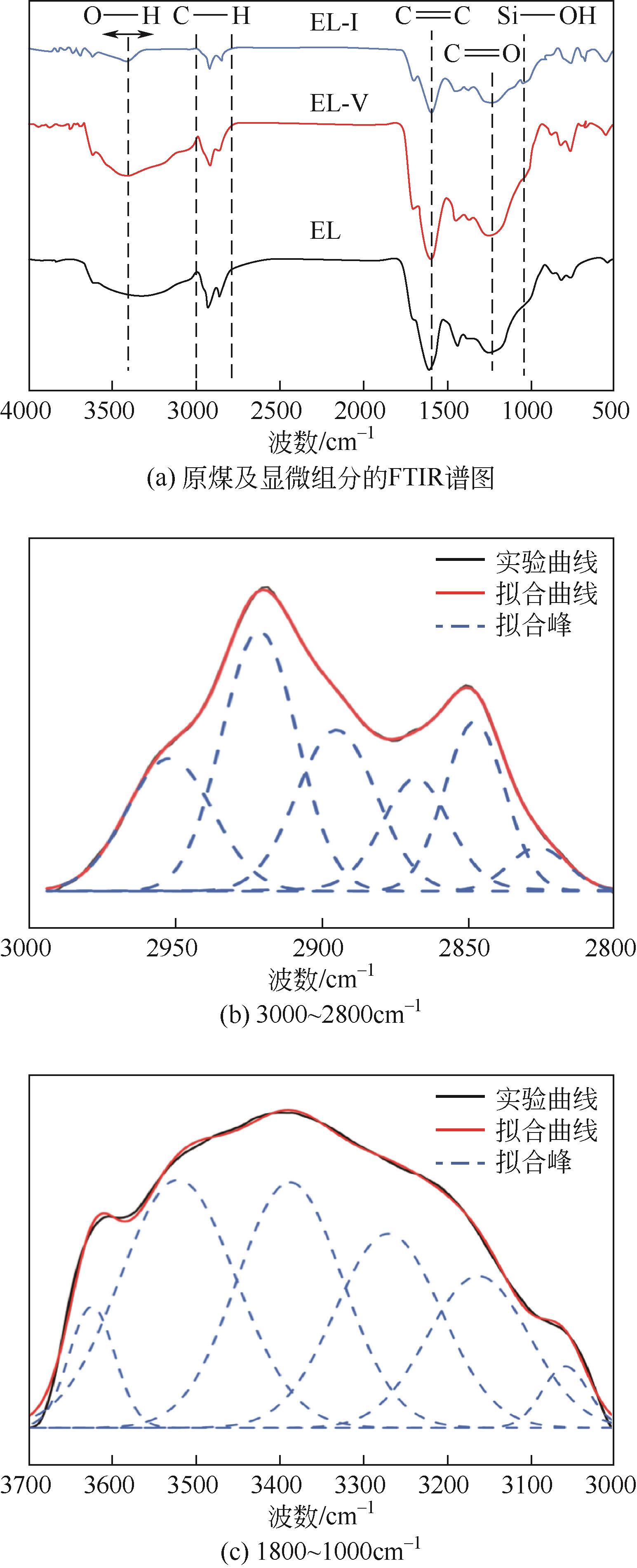
| 样品 | COOH/CAr | R—O—R/CAr | Ar—OH/CAr | CH2/CH3 |
|---|---|---|---|---|
| EL | 0.16 | 0.93 | 0.29 | 2.70 |
| EL-V | 0.20 | 1.01 | 0.17 | 3.63 |
| EL-I | 0.22 | 1.21 | 0.41 | 2.59 |
表4 原煤及显微组分的结构参数
| 样品 | COOH/CAr | R—O—R/CAr | Ar—OH/CAr | CH2/CH3 |
|---|---|---|---|---|
| EL | 0.16 | 0.93 | 0.29 | 2.70 |
| EL-V | 0.20 | 1.01 | 0.17 | 3.63 |
| EL-I | 0.22 | 1.21 | 0.41 | 2.59 |
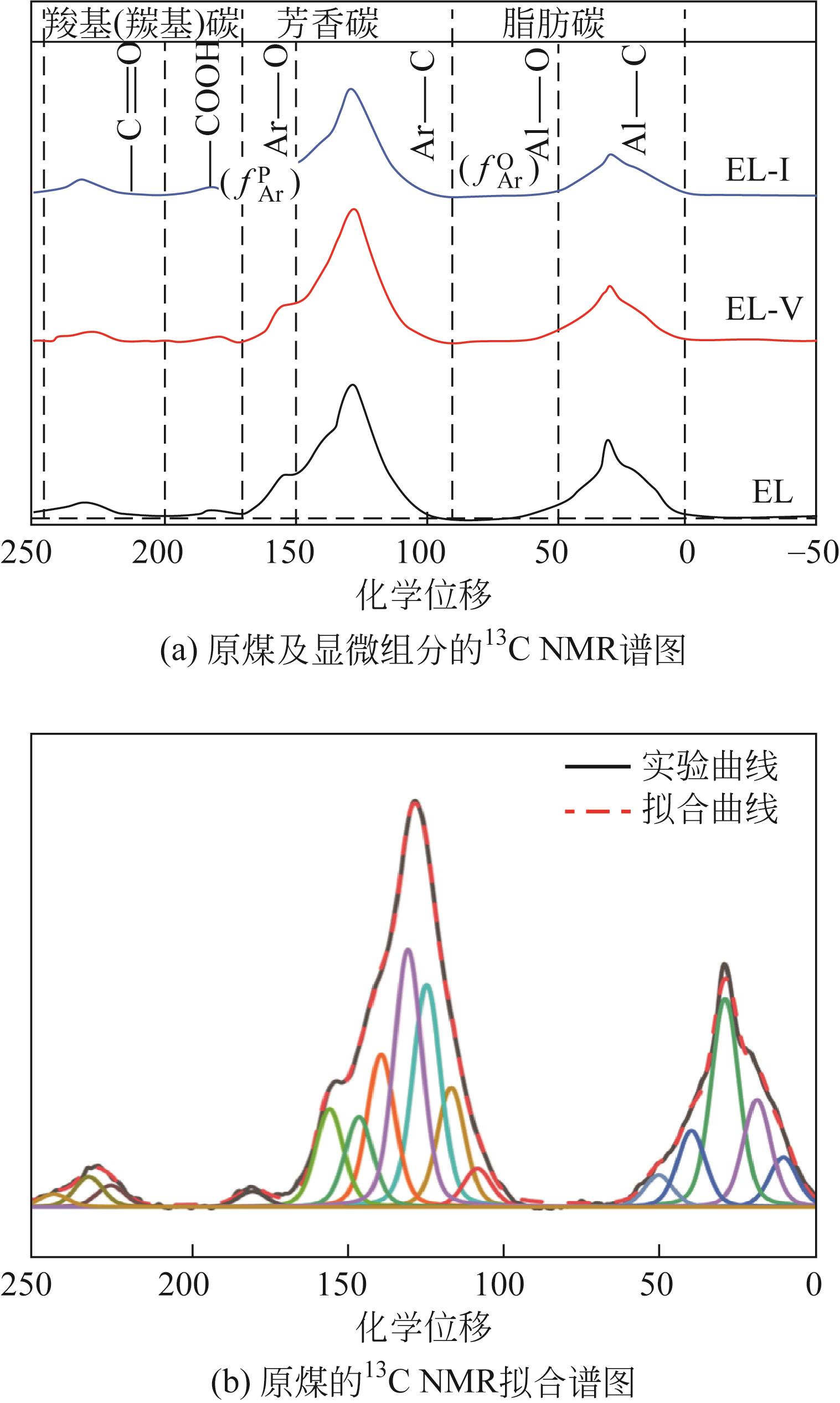
| 化学位移 | 归属 | 化学位移 | 归属 |
|---|---|---|---|
| 0~17 | 脂甲基R—CH3 | 60~90 | 环内氧接脂碳 |
| 17~22 | 芳甲基Ar—CH3 | 90~126 | 质子化碳 |
| 22~35 | 亚甲基—CH2 | 126~137 | 桥接芳碳 |
| 35~40 | 次亚甲基—CH | 137~148 | 侧支芳碳 |
| 40~50 | 季碳 | 148~165 | 氧取代芳碳 |
| 50~60 | 甲氧基 | 165~250 | 羧基(羰基碳) |
表5 核磁共振中碳原子化学位移归属
| 化学位移 | 归属 | 化学位移 | 归属 |
|---|---|---|---|
| 0~17 | 脂甲基R—CH3 | 60~90 | 环内氧接脂碳 |
| 17~22 | 芳甲基Ar—CH3 | 90~126 | 质子化碳 |
| 22~35 | 亚甲基—CH2 | 126~137 | 桥接芳碳 |
| 35~40 | 次亚甲基—CH | 137~148 | 侧支芳碳 |
| 40~50 | 季碳 | 148~165 | 氧取代芳碳 |
| 50~60 | 甲氧基 | 165~250 | 羧基(羰基碳) |
| 样品 | ƒa | ƒAl | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EL | 74.68 | 25.32 | 5.35 | 69.34 | 46.66 | 22.68 | 6.68 | 5.61 | 10.39 | 7.43 | 17.21 | 0.67 |
| EL-V | 69.10 | 30.90 | 5.21 | 63.88 | 41.64 | 22.25 | 6.40 | 5.88 | 9.96 | 10.23 | 18.59 | 2.08 |
| EL-I | 75.56 | 24.44 | 9.03 | 66.52 | 44.52 | 22.00 | 6.01 | 4.65 | 11.34 | 11.84 | 12.60 | 0.00 |
表6 原煤及显微组分的碳结构参数
| 样品 | ƒa | ƒAl | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ | ƒ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EL | 74.68 | 25.32 | 5.35 | 69.34 | 46.66 | 22.68 | 6.68 | 5.61 | 10.39 | 7.43 | 17.21 | 0.67 |
| EL-V | 69.10 | 30.90 | 5.21 | 63.88 | 41.64 | 22.25 | 6.40 | 5.88 | 9.96 | 10.23 | 18.59 | 2.08 |
| EL-I | 75.56 | 24.44 | 9.03 | 66.52 | 44.52 | 22.00 | 6.01 | 4.65 | 11.34 | 11.84 | 12.60 | 0.00 |
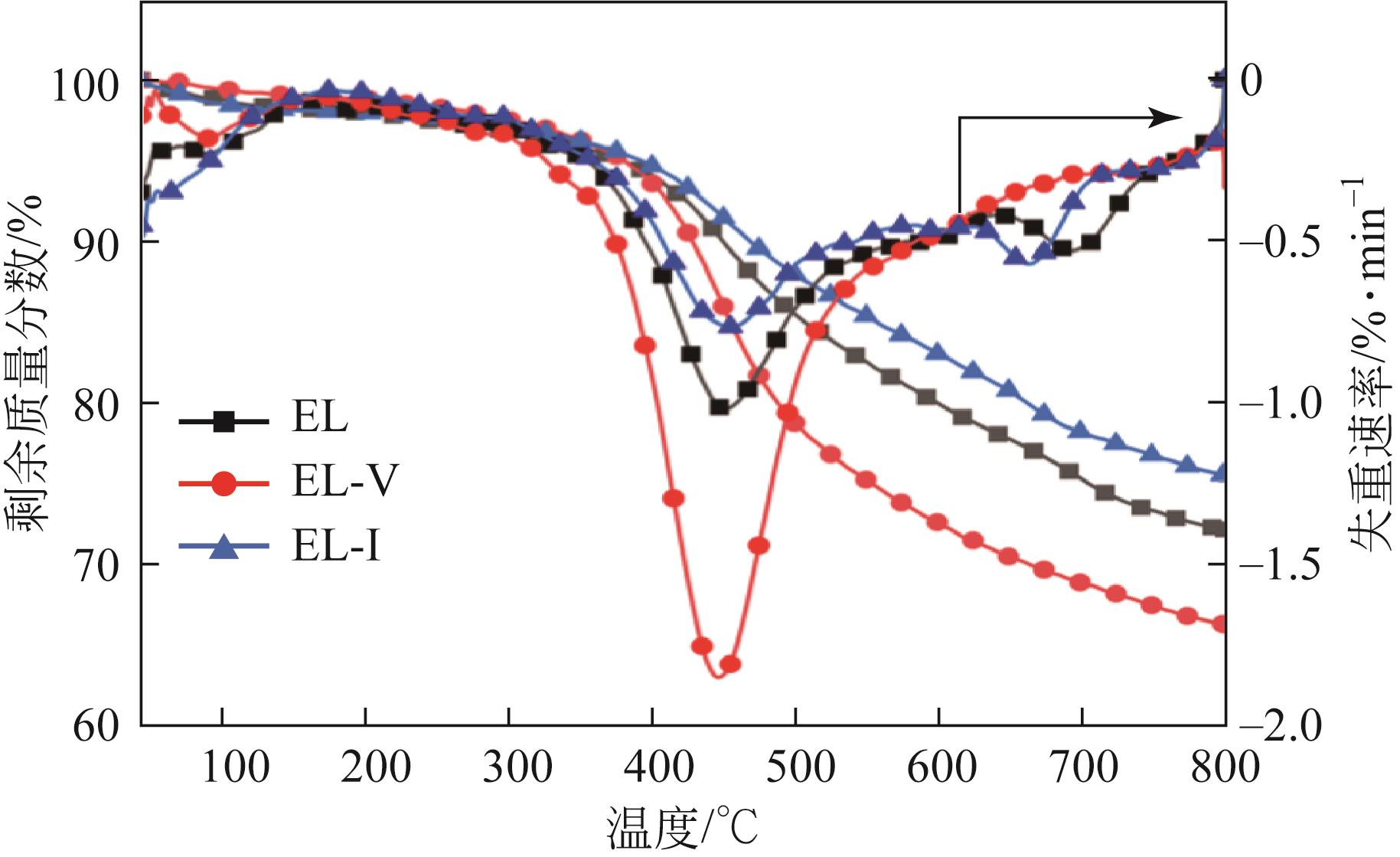
| 样品 | 质量损失范围(daf)/% | 净失重损失质量(daf)/% | 最大热失重峰温度/℃ | 最大热失重速率/%·min-1 |
|---|---|---|---|---|
| EL | 98.42~72.22 | 26.21 | 448.7 | -1.02 |
| EL-V | 99.21~66.32 | 32.89 | 444.1 | -1.85 |
| EL-I | 98.27~75.56 | 22.70 | 452.4 | -0.76 |
表7 原煤及显微组分的TG和DTG相关数据
| 样品 | 质量损失范围(daf)/% | 净失重损失质量(daf)/% | 最大热失重峰温度/℃ | 最大热失重速率/%·min-1 |
|---|---|---|---|---|
| EL | 98.42~72.22 | 26.21 | 448.7 | -1.02 |
| EL-V | 99.21~66.32 | 32.89 | 444.1 | -1.85 |
| EL-I | 98.27~75.56 | 22.70 | 452.4 | -0.76 |
| 样品 | 分子式 | 总能量/kcal·mol-1 | 元素含量/% | 分子量 | |||
|---|---|---|---|---|---|---|---|
| C | H | O | N | ||||
| EL | C142H112N2O35 | 448.37 | 70.87 | 4.69 | 23.27 | 1.16 | 2404 |
| EL-V | C147H116N2O30 | 459.48 | 73.86 | 4.89 | 20.08 | 1.17 | 2388 |
| EL-I | C148H100N2O50 | 446.52 | 65.68 | 3.72 | 29.56 | 1.04 | 2704 |
表8 原煤及显微组分煤样大分子结构模型参数
| 样品 | 分子式 | 总能量/kcal·mol-1 | 元素含量/% | 分子量 | |||
|---|---|---|---|---|---|---|---|
| C | H | O | N | ||||
| EL | C142H112N2O35 | 448.37 | 70.87 | 4.69 | 23.27 | 1.16 | 2404 |
| EL-V | C147H116N2O30 | 459.48 | 73.86 | 4.89 | 20.08 | 1.17 | 2388 |
| EL-I | C148H100N2O50 | 446.52 | 65.68 | 3.72 | 29.56 | 1.04 | 2704 |
| 1 | 范涛, 初茉, 畅志兵. 蒙东褐煤热解技术工业应用进展[J]. 化工进展, 2021, 40(3): 1362-1370. |
| FAN Tao, CHU Mo, CHANG Zhibing. Industrial application progress of lignite pyrolysis technology in eastern area of Inner Mongolia, China[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1362-1370. | |
| 2 | HE Qing, GUO Qinghua, UMEKI Kentaro, et al. Soot formation during biomass gasification: A critical review[J]. Renewable and Sustainable Energy Reviews, 2021, 139: 110710. |
| 3 | YAN Lunjing, WANG Wenmin, LIU Yan, et al. The roles of low molecular compounds on the light aromatics formation during different rank coal pyrolysis[J]. Journal of the Energy Institute, 2022, 100: 129-136. |
| 4 | FU Daqing, LI Xiaohong, LI Wenying, et al. Catalytic upgrading of coal pyrolysis products over bio-char[J]. Fuel Processing Technology, 2018, 176: 240-248. |
| 5 | ZHAO Xiaoyan, ZONG Zhimin, CAO Jingpei, et al. Difference in chemical composition of carbon disulfide-extractable fraction between vitrinite and inertinite from Shenfu-Dongsheng and Pingshuo coals[J]. Fuel, 2008, 87(4/5): 565-575. |
| 6 | DYRKACZ G R, BLOOMQUIST C A A, HORWITZ E P. Laboratory scale separation of coal macerals[J]. Separation Science and Technology, 1981, 16(10): 1571-1588. |
| 7 | QIN Rongfang, WANG Lu, CAO Daiyong, et al. Thermal simulation experimental study on the difference of molecular structure evolution between vitrinite and inertinite in low-rank coal[J]. Frontiers in Earth Science, 2022, 10: 992017. |
| 8 | ZHAO Yunpeng, HU Haoquan, JIN Lijun, et al. Pyrolysis behavior of vitrinite and inertinite from Chinese Pingshuo coal by TG-MS and in a fixed bed reactor[J]. Fuel Processing Technology, 2011, 92(4): 780-786. |
| 9 | ZHAO Meng, WANG Anmin, CAO Daiyong, et al. Differences in macromolecular structure evolution during the pyrolysis of vitrinite and inertinite based on in situ FTIR and XRD measurements[J]. Energies, 2022, 15(15): 5334. |
| 10 | WANG J-H, CHANG L-P. Pyrolysis and gasification reactivity of several typical Chinese coals and their macerals[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2015, 37(6): 670-678. |
| 11 | LEI Zhao, CHENG Zhanwang, LING Qiang, et al. Investigating the trigger mechanism of Shenfu bituminous coal pyrolysis[J]. Fuel, 2022, 313: 122995. |
| 12 | XU Fang, PAN Shuo, LIU Chunguang, et al. Construction and evaluation of chemical structure model of Huolinhe lignite using molecular modeling[J]. RSC Advances, 2017, 7(66): 41512-41519. |
| 13 | XU Fang, LIU Hui, WANG Qing, et al. Study of non-isothermal pyrolysis mechanism of lignite using ReaxFF molecular dynamics simulations[J]. Fuel, 2019, 256: 115884. |
| 14 | WANG Dong, PENG Zeyu, WANG Jun, et al. Study on pyrolysis behavior of the coal fractions based on macro maceral separation[J]. Fuel, 2021, 305: 121572. |
| 15 | BAI Boyang, QIANG Luyao, ZHANG Suisui, et al. Influence of coal structure change caused by different pretreatment methods on Shengli lignite pyrolysis[J]. Fuel, 2023, 332: 126089. |
| 16 | YIN Yanshan, WU Zihua, TAO Jianhang, et al. Investigation of the evolution of the chemical structure of bituminous coals and lignite during pyrolysis[J]. Crystals, 2022, 12(4): 444. |
| 17 | LIU Mengjie, BAI Jin, KONG Lingxue, et al. The correlation between coal char structure and reactivity at rapid heating condition in TGA and heating stage microscope[J]. Fuel, 2020, 260: 116318. |
| 18 | 王延君, 赵云飞, 何润霞, 等. 褐煤显微组分及碱处理对其结构和燃烧性能的影响[J]. 煤炭学报, 2023, 48(4): 1736-1746. |
| WANG Yanjun, ZHAO Yunfei, HE Runxia, et al. Macerals of lignite and the effect of alkali treatment on the structure and combustion performance of lignite[J]. Journal of China Coal Society, 2023, 48(4): 1736-1746. | |
| 19 | 李首毅, 林雄超, 鲁倍倍, 等. 矿物质对高碱煤显微组分热解特性的影响[J]. 化工进展, 2019, 38(8): 3650-3657. |
| LI Shouyi, LIN Xiongchao, LU Beibei, et al. Effects of minerals on pyrolysis characteristics of maceral in high-alkali coal[J]. Chemical Industry and Engineering Progress, 2019, 38(8): 3650-3657. | |
| 20 | SONG Huijuan, LIU Guangrui, ZHANG Jinzhi, et al. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Processing Technology, 2017, 156: 454-460. |
| 21 | Zhao Yan, QIU Penghua, CHEN Gang, et al. Selective enrichment of chemical structure during first grinding of Zhundong coal and its effect on pyrolysis reactivity[J]. Fuel, 2017, 189: 46-56. |
| 22 | MA Li, YU Wencong, REN Lifeng, et al. Micro-characteristics of low-temperature coal oxidation in CO2/O2 and N2/O2 atmospheres[J]. Fuel, 2019, 246: 259-267. |
| 23 | GENG Wenhua, NAKAJIMA Tsunenori, TAKANASHI Hirokazu, et al. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry[J]. Fuel, 2009, 88(1): 139-144. |
| 24 | Aldo D’ALESSIO, RASPOLLI-GALLETTI Anna Maria, LICURSI Domenico, et al. FT-IR investigation of the structural changes of Sulcis and South Africa coals under progressive heating in vacuum: Correlation with volatile matter[J]. Journal of Combustion, 2013, 2013: 134234. |
| 25 | YU Zunyi, GUO Wei, YANG Panxi, et al. In-situ infrared and kinetic characteristics analysis on pyrolysis of tar-rich coal and macerals[J]. Fuel, 2023, 348: 128601. |
| 26 | TIAN Bin, QIAO Yingyun, BAI Lei, et al. Separation and structural characterization of groups from a high-volatile bituminous coal based on multiple techniques[J]. Fuel Processing Technology, 2017, 159: 386-395. |
| 27 | 李耀高. 东曲2号煤大分子结构模型及其热反应性研究[D]. 太原: 太原理工大学, 2019. |
| LI Yaogao. Macromlecular structural model and thermal reactivity study of Dongqu No.2 coal[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 28 | 司加康. 马兰8号煤大分子结构模型构建及分子模拟[D]. 太原: 太原理工大学, 2014. |
| SI Jiakang. Macromlecular structure model construction and molecular simulation of Malan No.8 coal[D]. Taiyuan: Taiyuan University of Technology, 2014. | |
| 29 | 张怀青, 周安宁, 李振, 等. 神府煤显微组分大分子及聚集态结构模型构建[J]. 中国矿业大学学报, 2023, 52(4): 796-812. |
| ZHANG Huaiqing, ZHOU Anning, LI Zhen, et al. Construction of macromolecular and aggregate structure models of Shenfu coal macerals[J]. Journal of China University of Mining & Technology, 2023, 52(4): 796-812. | |
| 30 | LIU Jiaxun, YANG Xiuchao, JIANG Xue, et al. Pyrolysis mechanisms of coal extracts based on TG-FTIR and ReaxFF MD study[J]. Fuel Processing Technology, 2022, 227: 107124. |
| 31 | CHANG Haizhou, DENG Hongxiao, YANG Qun, et al. Interaction of vitrinite and inertinite of Bulianta coal in pyrolysis[J]. Fuel, 2017, 207: 643-649. |
| 32 | YAO Qiuxiang, MA Mingming, LIU Yongqi, et al. The structural and pyrolysis characteristics of vitrinite and inertinite from Shendong coal and the gasification performance of chars[J]. Journal of Analytical and Applied Pyrolysis, 2022, 164: 105519. |
| 33 | YAO Qiuxiang, MA Mingming, LIU Yongqi, et al. Pyrolysis characteristics of metal ion-exchanged Shendong coal and its char gasification performance[J]. Journal of Analytical and Applied Pyrolysis, 2021, 155: 105087. |
| 34 | BAI Yonghui, LV Peng, LI Fan, et al. Investigation into Ca/Na compounds catalyzed coal pyrolysis and char gasification with steam[J]. Energy Conversion and Management, 2019, 184(1): 172-179. |
| 35 | LIN Xiongchao, LUO Meng, LI Shouyi, et al. The evolutionary route of coal matrix during integrated cascade pyrolysis of a typical low-rank coal[J]. Applied Energy, 2017, 199: 335-346. |
| 36 | SUN Ming, ZHANG Dan, YAO Qiuxiang, et al. Separation and composition analysis of GC/MS analyzable and unanalyzable parts from coal tar[J]. Energy & Fuels, 2018, 32(7): 7404-7411. |
| 37 | REN Lei, WANG Fei, CHENG Fangqin, et al. Mechanisms of gas generation from conventional and microwave pyrolysis of coal slime[J]. Chemical Engineering Journal, 2023, 452: 139388. |
| 38 | LIU Jiaxun, JIANG Xiumin, SHEN Jun, et al. Influences of particle size, ultraviolet irradiation and pyrolysis temperature on stable free radicals in coal[J]. Powder Technology, 2015, 272: 64-74. |
| 39 | ZHANG Kang, LI Yan, WANG Zhihua, et al. Pyrolysis behavior of a typical Chinese sub-bituminous Zhundong coal from moderate to high temperatures[J]. Fuel, 2016, 185: 701-708. |
| 40 | CHONG Junkai, CHENG Xiang, XIAO Longheng, et al. Fine characterization of the macromolecular structure of Shanxi low-rank coal[J]. Journal of Molecular Structure, 2023, 1273: 134359. |
| 41 | 李焕同, 朱志蓉, 乔军伟, 等. 陕北侏罗纪富镜煤和富惰煤分子结构的FTIR, XPS和13C NMR表征[J]. 光谱学与光谱分析, 2022, 42(8): 2624-2630. |
| LI Huantong, ZHU Zhirong, QIAO Junwei, et al. Molecular representations of Jurassic-aged vitrinite-rich and inertinite-rich coals in northern shannxi province by FTIR, XPS and 13C NMR[J]. Spectroscopy and Spectral Analysis, 2022, 42(8): 2624-2630. | |
| 42 | SHI Lei, LIU Qingya, ZHOU Bin, et al. Interpretation of methane and hydrogen evolution in coal pyrolysis from the bond cleavage perspective[J]. Energy & Fuels, 2017, 31(1): 429-437. |
| [1] | 张海霞, 朱治平, 张思源. 循环流化床高碱煤气化技术研发及应用进展[J]. 化工进展, 2024, 43(5): 2254-2278. |
| [2] | 姚乃瑜, 曹景沛, 庞新博, 赵小燕, 蔡士杰, 徐敏, 赵静平, 冯晓博, 伊凤娇. 低阶煤热解挥发分热催化重整研究进展[J]. 化工进展, 2024, 43(5): 2279-2293. |
| [3] | 周安宁, 江雨寒, 刘墨宣, 赵伟, 李振. 电解煤浆制氢过程中煤阶及矿物的影响与煤结构演化研究进展[J]. 化工进展, 2024, 43(5): 2294-2310. |
| [4] | 张国卿, 宋舒波, 王兴瑞, 巩苗苗, 王旭, 许宇鸿, 冯继越, 张福扬, 陈汇勇. 煤固废基分子筛的制备及其应用进展[J]. 化工进展, 2024, 43(5): 2311-2323. |
| [5] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [6] | 王旭栋, 刘敦禹, 许开龙, 刘秋祺, 范昀培, 金晶. CeO2载氧体对煤化学链燃烧中汞迁移影响机制[J]. 化工进展, 2024, 43(4): 2191-2200. |
| [7] | 高增林, 张乾, 高晨明, 杨凯, 高志华, 黄伟. 水煤浆煤气化粗渣水流分级提炭分质[J]. 化工进展, 2024, 43(3): 1576-1583. |
| [8] | 张鑫, 汤吉昀, 陈娟, 宋占龙, 董勇, 姚洪. 高温烟气热解废轮胎过程中痕量金属Cu、Pb的迁移特性分析[J]. 化工进展, 2024, 43(3): 1606-1613. |
| [9] | 马赟, 付伟, 王昕, 杨如意, 钱相臣. 基于孪生Inception网络的燃烧器火焰状态监测[J]. 化工进展, 2024, 43(2): 760-767. |
| [10] | 陈国徽, 王君雷, 李世龙, 李金宇, 徐运飞, 罗俊潇, 王昆. 火焰喷雾热解制备锂离子电池三元正极材料研究进展[J]. 化工进展, 2024, 43(2): 971-983. |
| [11] | 杨梦茹, 彭琴, 常玉龙, 邱淑兴, 张溅波, 江霞. 生物炭替代煤粉/焦炭高炉炼铁碳减排技术研究进展[J]. 化工进展, 2024, 43(1): 490-500. |
| [12] | 任鹏锟, 仲兆平, 杨宇轩, 张杉, 杜浩然, 李骞. 改性海泡石对污泥热解过程中重金属的控制[J]. 化工进展, 2024, 43(1): 541-550. |
| [13] | 罗成, 范晓勇, 朱永红, 田丰, 崔楼伟, 杜崇鹏, 王飞利, 李冬, 郑化安. 中低温煤焦油加氢反应器不同分配器中液体分布的CFD模拟[J]. 化工进展, 2023, 42(9): 4538-4549. |
| [14] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [15] | 朱杰, 金晶, 丁正浩, 杨会盼, 侯封校. 化学链气化中准东煤灰对CaSO4载氧体改性及其作用机理[J]. 化工进展, 2023, 42(9): 4628-4635. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||