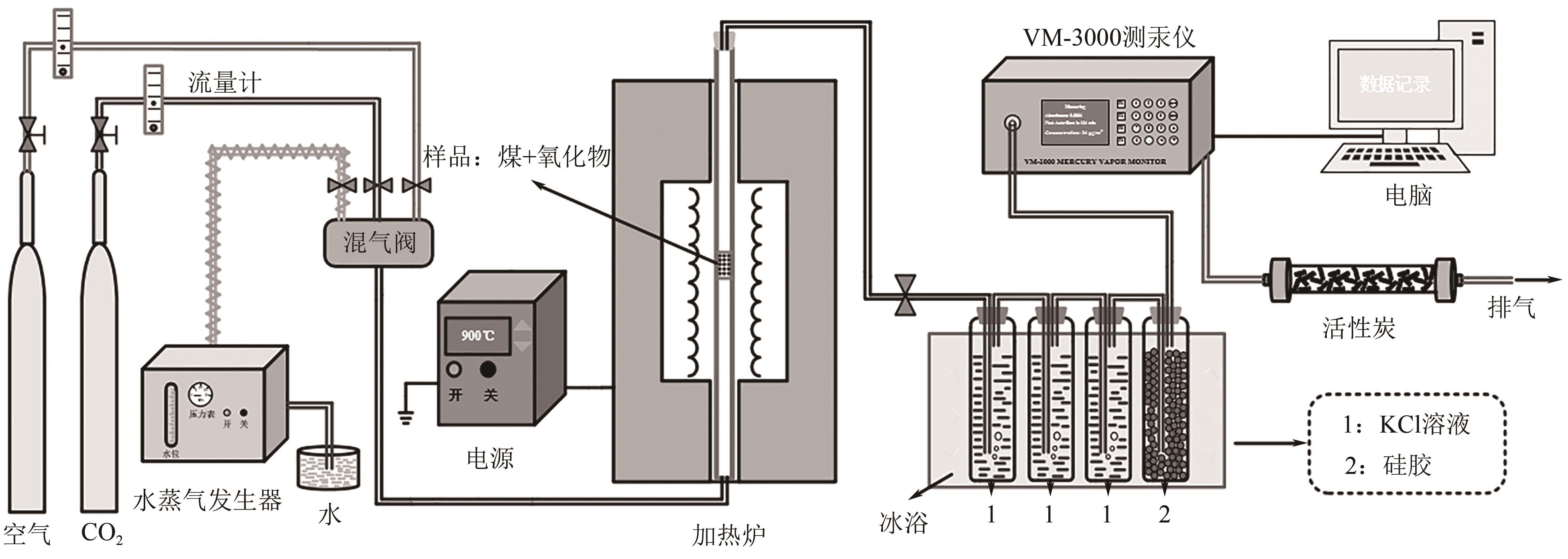
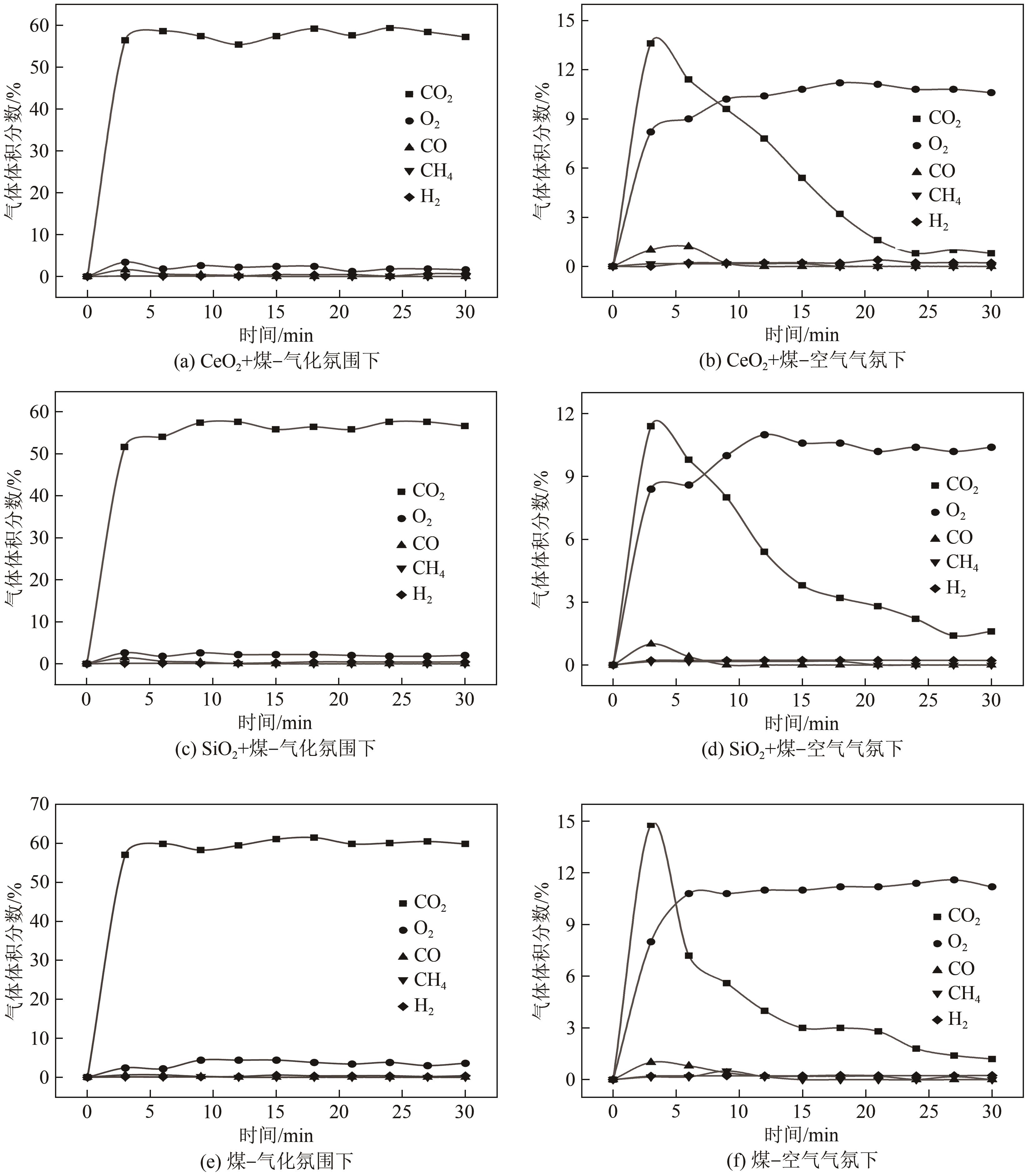
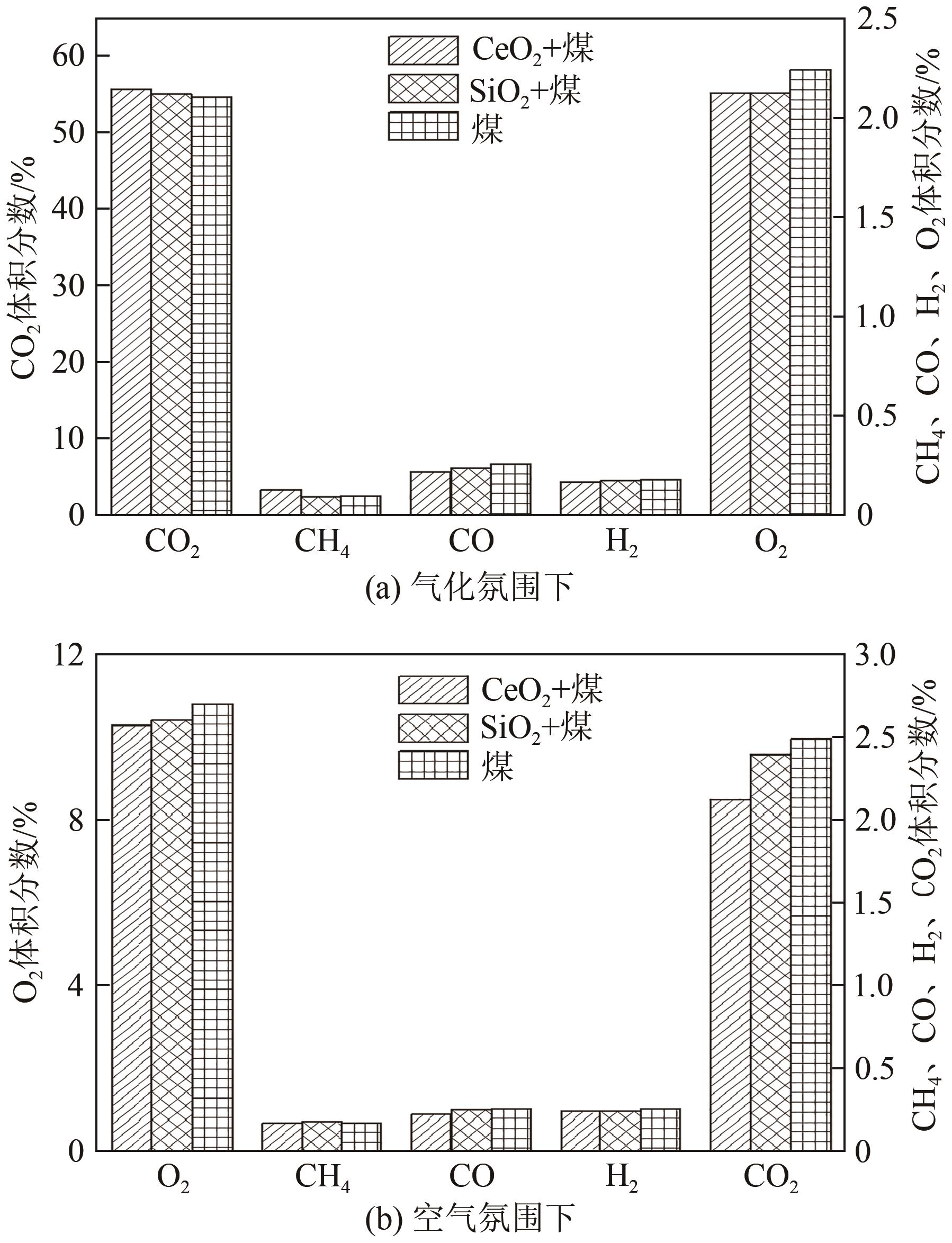
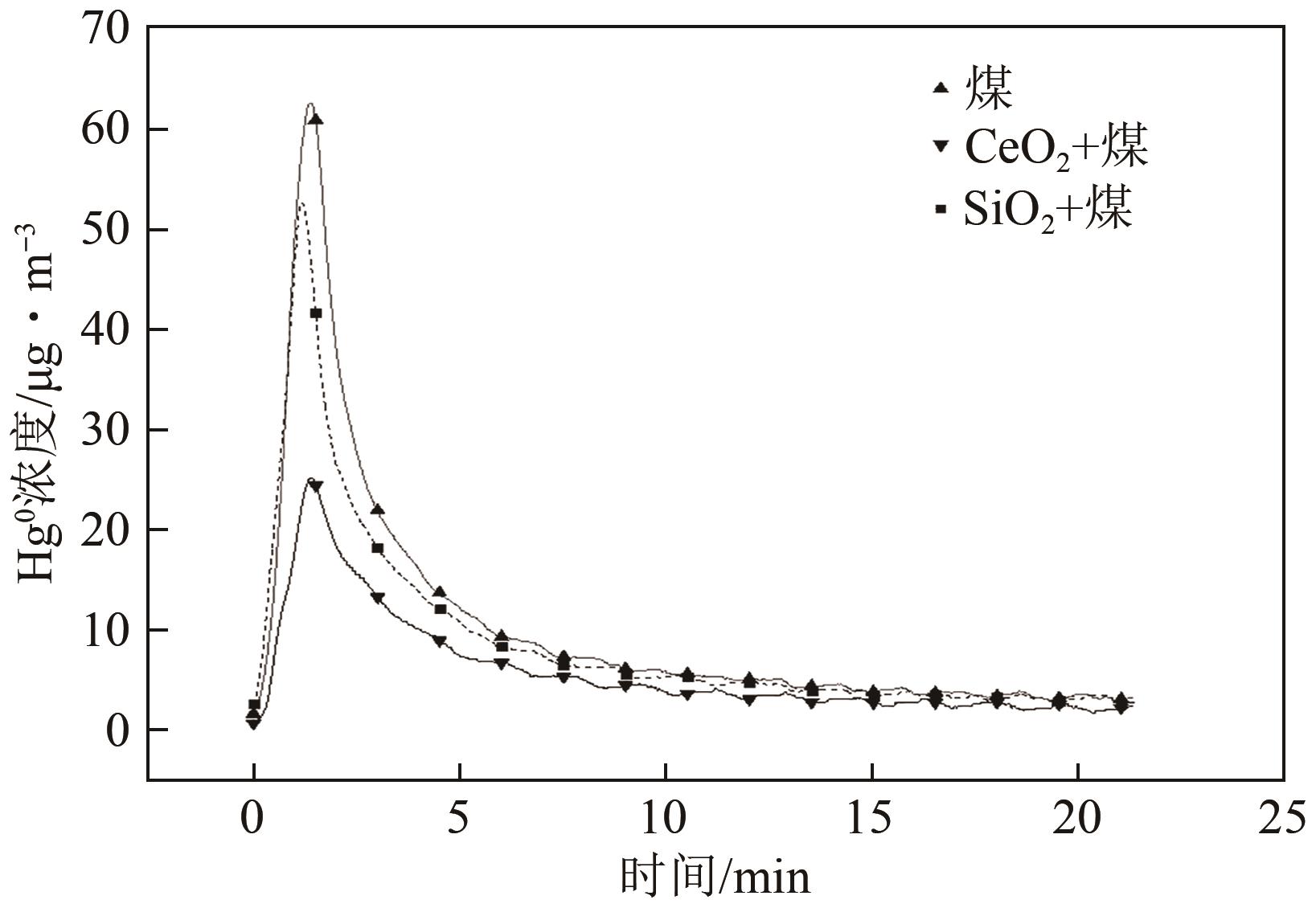
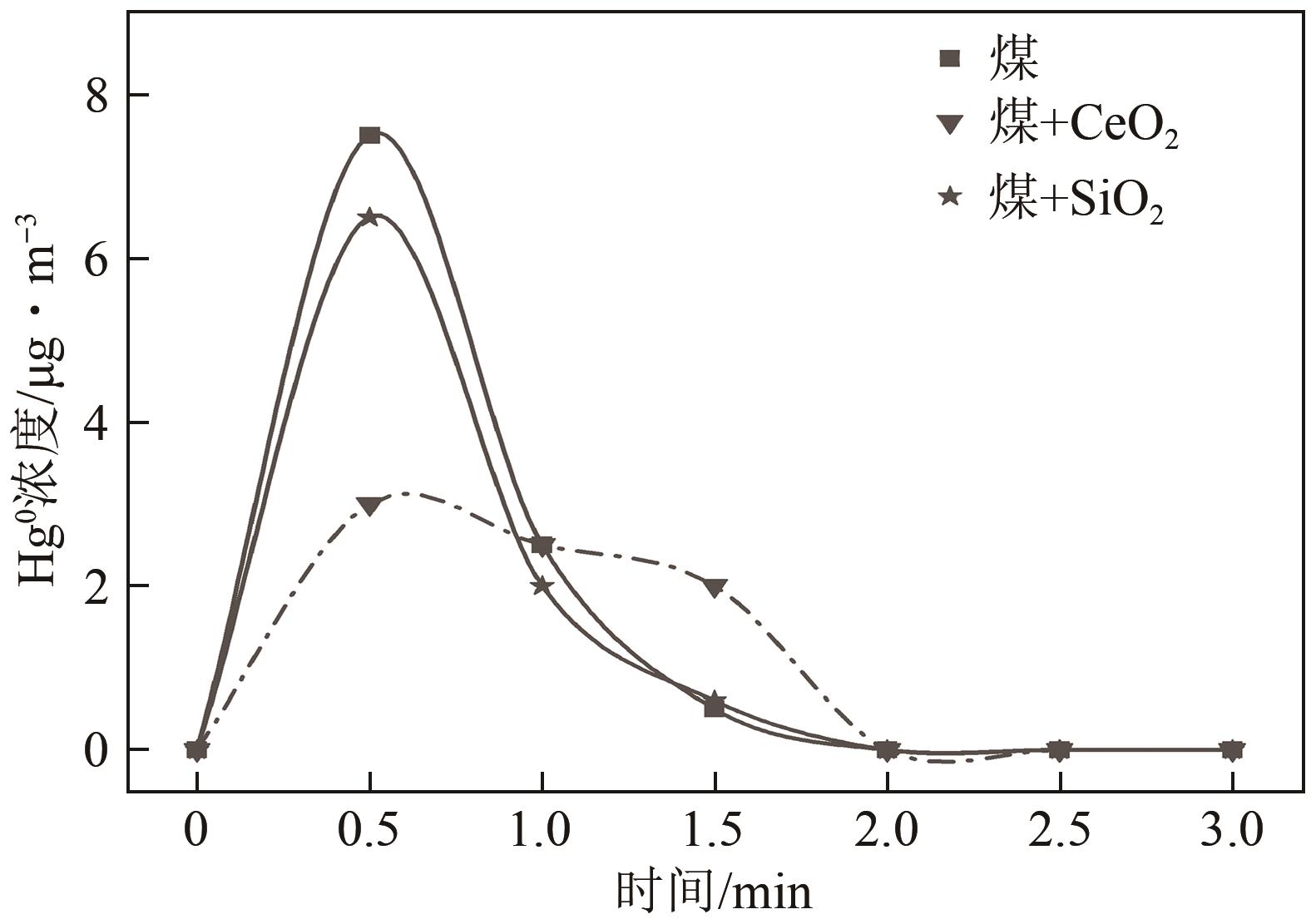
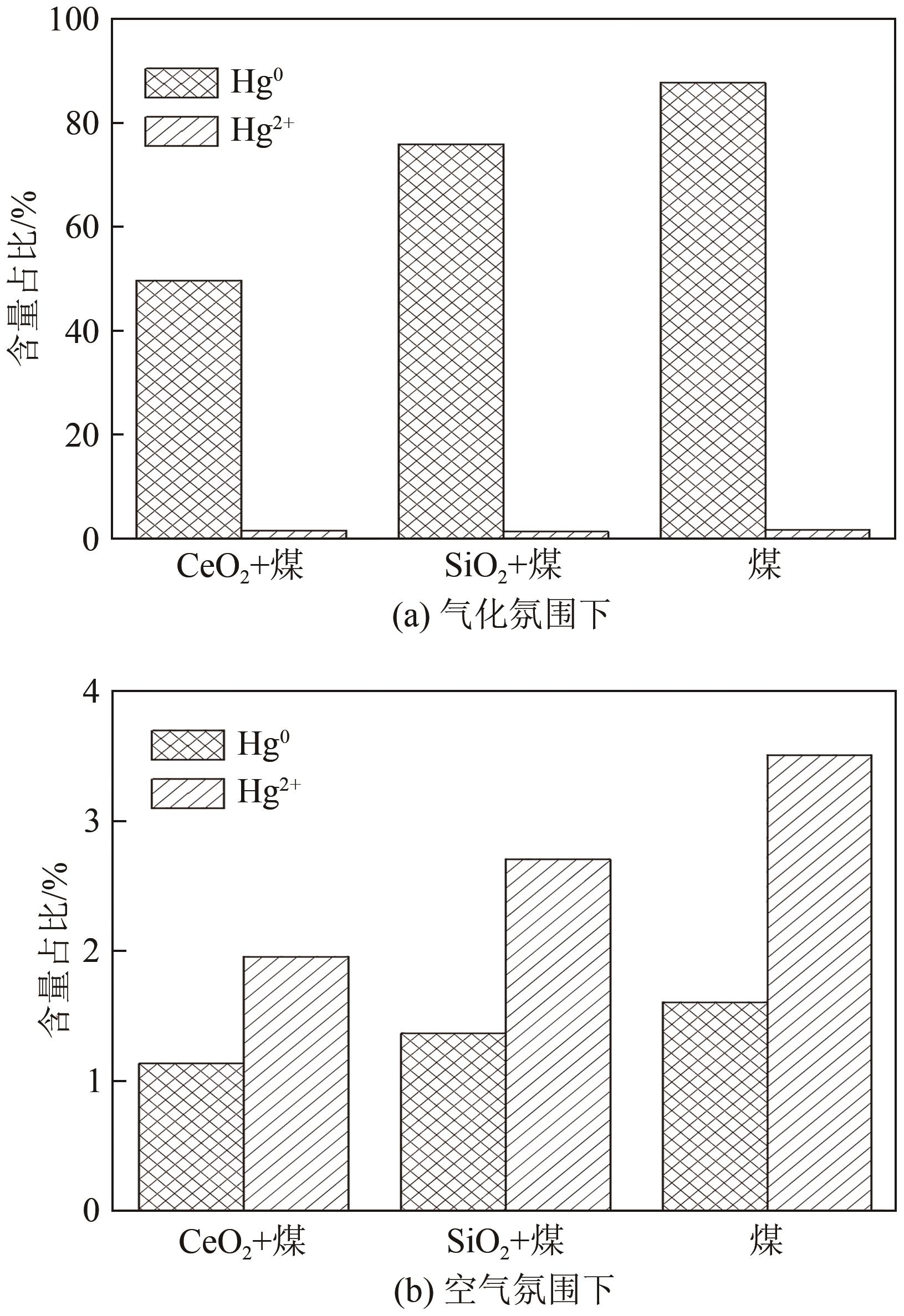
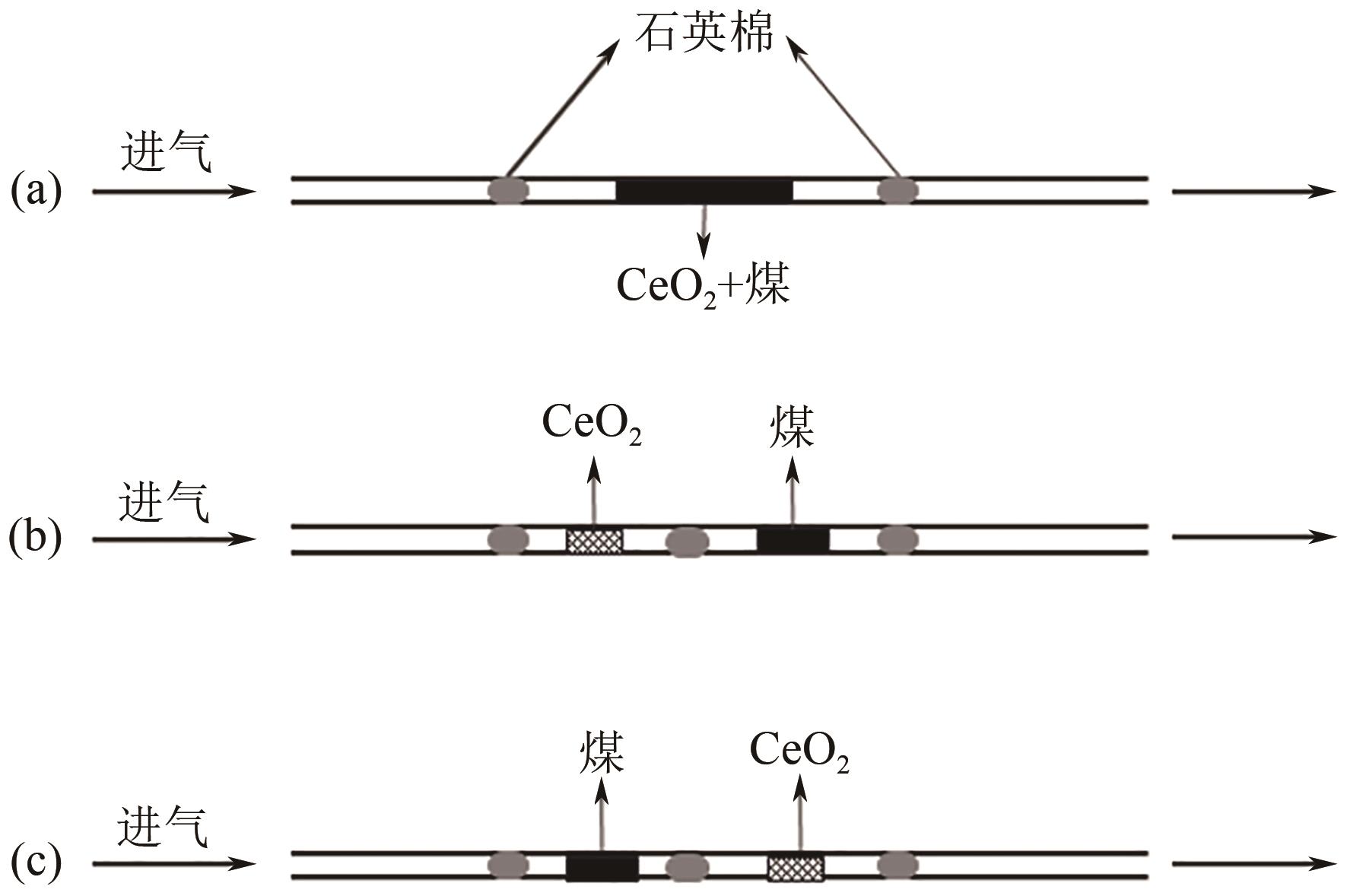
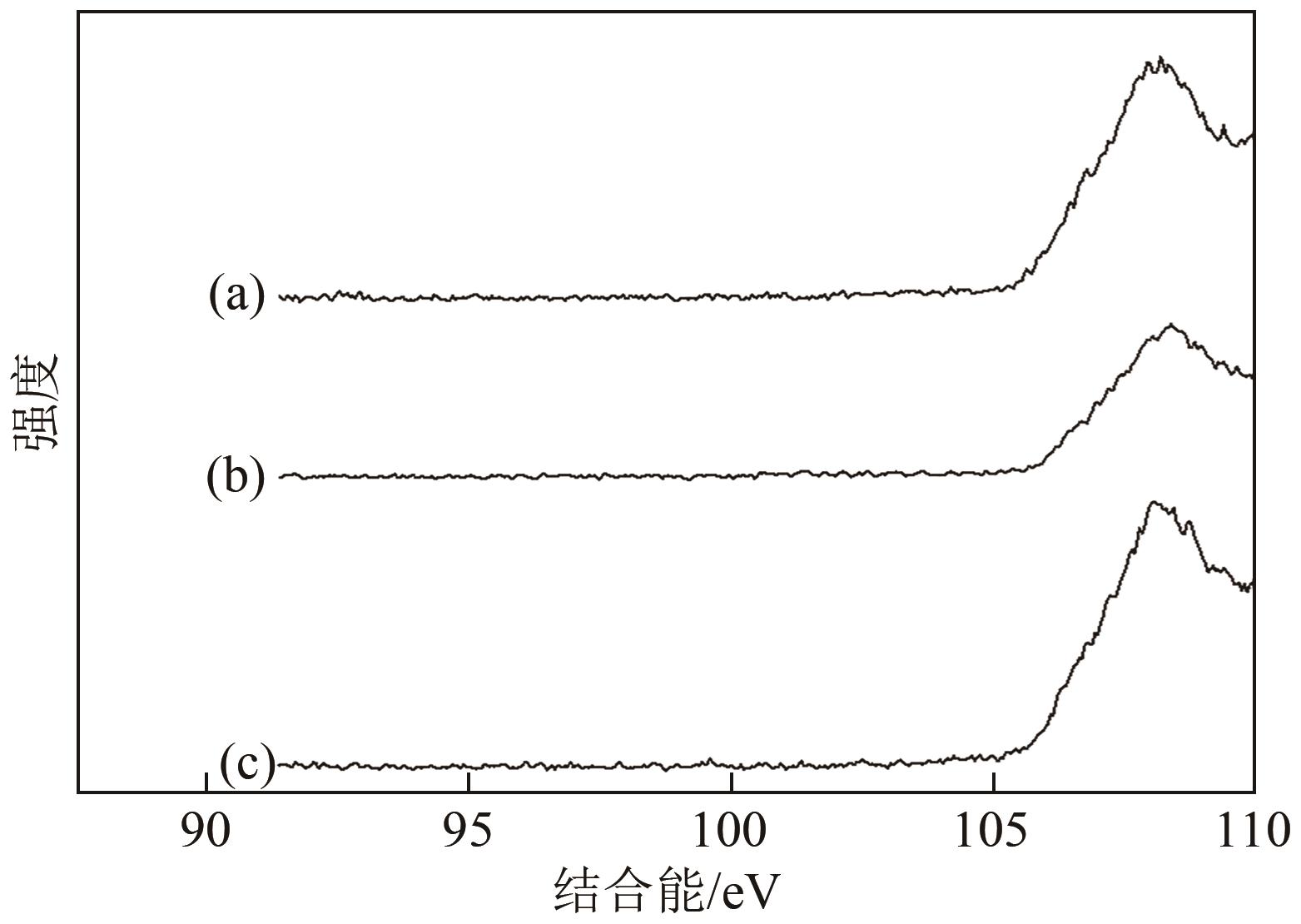
| 1 |
王毅, 顾佰和. 中国可持续发展新进程: 探索迈向碳中和之路[J]. 可持续发展经济导刊, 2021(S2): 15-20.
|
|
WANG Yi, GU Baihe. A new process of sustainable development in China: exploring the road to carbon neutrality[J]. China Sustainability Tribune, 2021(S2): 15-20.
|
| 2 |
林伯强, 李江龙. 环境治理约束下的中国能源结构转变——基于煤炭和二氧化碳峰值的分析[J]. 中国社会科学, 2015(9): 84-107, 205.
|
|
LIN Boqiang, LI Jianglong. China’s energy structure transformation under the constraints of environmental governance——Based on the analysis of coal and carbon dioxide peak[J]. Social Sciences in China, 2015(9): 84-107, 205.
|
| 3 |
BANDILLA Karl W. Carbon capture and storage[M]//Future Energy. Amsterdam: Elsevier, 2020: 669-692.
|
| 4 |
ADANEZ Juan, ABAD Alberto, Francisco GARCIA-LABIANO, et al. Progress in chemical-looping combustion and reforming technologies[J]. Progress in Energy and Combustion Science, 2012, 38(2): 215-282.
|
| 5 |
ABUELGASIM Siddig, WANG Wenju, ABDALAZEEZ Atif. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress[J]. Science of the Total Environment, 2021, 764: 142892.
|
| 6 |
JI Ling, WANG Qianwen, ZHANG Zhiyue, et al. Release characteristics of mercury in chemical looping combustion of bituminous coal[J]. Journal of Environmental Sciences, 2020, 94: 197-203.
|
| 7 |
LIU Zhuang, LIU Dunyu, ZHAO Bingtao, et al. Mercury removal based on adsorption and oxidation by fly ash: A review[J]. Energy & Fuels, 2020, 34(10): 11840-11866.
|
| 8 |
XU Yang, ZENG Xiaobo, LUO Guangqian, et al. Chlorine-char composite synthesized by co-pyrolysis of biomass wastes and polyvinyl chloride for elemental mercury removal[J]. Fuel, 2016, 183: 73-79.
|
| 9 |
WANG Yunjun, DUAN Yufeng, YANG Liguo, et al. Comparison of mercury removal characteristic between fabric filter and electrostatic precipitators of coal-fired power plants[J]. Journal of Fuel Chemistry and Technology, 2008, 36(1): 23-29.
|
| 10 |
WU Hao, SUN Jiaxing, QI Dongxu, et al. Photocatalytic removal of elemental mercury from flue gas using multi-walled carbon nanotubes impregnated with titanium dioxide[J]. Fuel, 2018, 230: 218-225.
|
| 11 |
LI Guoliang, WU Qingru, WANG Shuxiao, et al. The influence of flue gas components and activated carbon injection on mercury capture of municipal solid waste incineration in China[J]. Chemical Engineering Journal, 2017, 326: 561-569.
|
| 12 |
ZHAO Bo, LIU Xiaowei, ZHOU Zijian, et al. Catalytic oxidation of elemental mercury by Mn-Mo/CNT at low temperature[J]. Chemical Engineering Journal, 2016, 284: 1233-1241.
|
| 13 |
LIU Xi, JIANG Shaojian, LI Hailong, et al. Elemental mercury oxidation over manganese oxide octahedral molecular sieve catalyst at low flue gas temperature[J]. Chemical Engineering Journal, 2019, 356: 142-150.
|
| 14 |
LIU Yangxian, ADEWUYI Yusuf G. A review on removal of elemental mercury from flue gas using advanced oxidation process: Chemistry and process[J]. Chemical Engineering Research and Design, 2016, 112: 199-250.
|
| 15 |
NI Mingguo, LIU Dunyu, JIN Jing, et al. Ranking oxygen carriers for elemental mercury oxidation in coal-fired chemical-looping combustion: A thermodynamic approach[J]. Energy & Fuels, 2020, 34(2): 2355-2365.
|
| 16 |
张志越, 毛琳, 孙佳兴, 等. 温度对载氧体还原过程中汞的析出特性及形态分布的影响[J]. 化工进展, 2018, 37(3): 1187-1193.
|
|
ZHANG Zhiyue, MAO Lin, SUN Jiaxing, et al. Characterization of mercury releasing during reduction of oxygen carriers with coal in chemical looping combustion[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 1187-1193.
|
| 17 |
高明刚, 刘永卓, 张欣涛, 等. 铁基载氧体对煤中汞释放行为的影响[J]. 化工进展, 2019, 38(S1): 240-246.
|
|
GAO Minggang, LIU Yongzhuo, ZHANG Xintao, et al. Effects of Fe-based oxygen carriers on mercury release behavior from coal[J]. Chemical Industry and Engineering Progress, 2019, 38(S1): 240-246.
|
| 18 |
AN Mei, MA Jingjing, GUO Qingjie. Transformation and migration of mercury during chemical-looping gasification of coal[J]. Industrial & Engineering Chemistry Research, 2019, 58(44): 20481-20490.
|
| 19 |
KANG Kyoung-Soo, KIM Chang-Hee, Ki-Kwang BAE, et al. Oxygen-carrier selection and thermal analysis of the chemical-looping process for hydrogen production[J]. International Journal of Hydrogen Energy, 2010, 35(22): 12246-12254.
|
| 20 |
CHEN Ching-Shiun, CHEN Tse-Ching, WU Hung-Chi, et al. The influence of ceria on Cu/TiO2 catalysts to produce abundant oxygen vacancies and induce highly efficient CO oxidation[J]. Catalysis Science & Technology, 2020, 10(13): 4271-4281.
|
| 21 |
MOHAMED Shamseldin A, QUDDUS Mohammad R, RAZZAK Shaikh A, et al. Fluidizable NiO/Ce-γAl2O3 oxygen carrier for chemical looping combustion[J]. Energy & Fuels, 2015, 29(9): 6095-6103.
|
| 22 |
MA Shiwei, CHEN Shiyi, ZHU Min, et al. Enhanced sintering resistance of Fe2O3/CeO2 oxygen carrier for chemical looping hydrogen generation using core-shell structure[J]. International Journal of Hydrogen Energy, 2019, 44(13): 6491-6504.
|
| 23 |
ASTM Standards. Standard test method for elemental, oxidized, particle-bound and total mercury in flue gas generated from coal-fired stationary sources(Ontario Hydro Method): D6784-16 [S]. West Conshohocken: ASTM International, 2016.
|
| 24 |
MA Jinchen, TIAN Xin, ZHAO Bo, et al. Behavior of mercury in chemical looping with oxygen uncoupling of coal[J]. Fuel Processing Technology, 2021, 216: 106747.
|
| 25 |
MENDIARA T, IZQUIERDO M T, ABAD A, et al. Mercury release and speciation in chemical looping combustion of coal[J]. Energy & Fuels, 2014, 28(4): 2786-2794.
|
| 26 |
刘壮, 刘敦禹, 金晶, 等. CO2对高铁高钙煤灰载氧体炉内脱汞的影响[J]. 石油学报(石油加工), 2020, 36(6): 1148-1160.
|
|
LIU Zhuang, LIU Dunyu, JIN Jing, et al. Effects of CO2 on mercury removal in furnace with high iron and calcium coal ash oxygen carrier[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(6): 1148-1160.
|
| 27 |
DIAMANTOPOULOU Ir, SKODRAS G, SAKELLAROPOULOS G P. Sorption of mercury by activated carbon in the presence of flue gas components[J]. Fuel Processing Technology, 2010, 91(2): 158-163.
|
| 28 |
YANG Shijian, GUO Yongfu, YAN Naiqiang, et al. Nanosized cation-deficient Fe-Ti spinel: A novel magnetic sorbent for elemental mercury capture from flue gas[J]. ACS Applied Materials & Interfaces, 2011, 3(2): 209-217.
|
| 29 |
LIU Zhuang, LIU Dunyu, JIN Jing, et al. Impact of gas impurities on the Hg0 oxidation on high iron and calcium coal ash for chemical looping combustion[J]. Environmental Science and Pollution Research, 2021, 28(34): 46130-46146.
|
| 30 |
YE Dong, WANG Runxian, WANG Xiaoxiang, et al. Improvement in the Hg0 removal performance of CeO2 by modifying with CuO[J]. Applied Surface Science, 2022, 579: 152200.
|
| 31 |
AL-DOGHACHI F A J, RASHID U, ZAINAL Z, et al. Influence of Ce2O3 and CeO2 promoters on Pd/MgO catalysts in the dry-reforming of methane[J]. RSC Advances, 2015, 5(99): 81739-81752.
|
| 32 |
KAMBOLIS A, MATRALIS H, TROVARELLI A, et al. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane[J]. Applied Catalysis A: General, 2010, 377(1/2): 16-26.
|
| 33 |
LIU Dunyu, WANG Chaoran, FAN Yunpei, et al. Mercury transformation and removal in chemical looping combustion of coal: A review[J]. Fuel, 2023, 347: 128440.
|
| 34 |
YANG Shijian, YAN Naiqiang, GUO Yongfu, et al. Gaseous elemental mercury capture from flue gas using magnetic nanosized (Fe3- x Mn x )1- δ O4 [J]. Environmental Science & Technology, 2011, 45(4): 1540-1546.
|
| 35 |
GAO Xiang, JIANG Ye, ZHONG Yi, et al. The activity and characterization of CeO2-TiO2 catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3 [J]. Journal of Hazardous Materials, 2010, 174(1/2/3): 734-739.
|
 ), 刘敦禹1,2(
), 刘敦禹1,2( ), 许开龙1,2, 刘秋祺1,2, 范昀培1,2, 金晶1,2
), 许开龙1,2, 刘秋祺1,2, 范昀培1,2, 金晶1,2
 ), LIU Dunyu1,2(
), LIU Dunyu1,2( ), XU Kailong1,2, LIU Qiuqi1,2, FAN Yunpei1,2, JIN Jing1,2
), XU Kailong1,2, LIU Qiuqi1,2, FAN Yunpei1,2, JIN Jing1,2