化工进展 ›› 2024, Vol. 43 ›› Issue (7): 4072-4088.DOI: 10.16085/j.issn.1000-6613.2023-1229
过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展
- 1.北京林业大学环境科学与工程学院,北京 100083
2.北京市第八十中学,北京 100020
-
收稿日期:2023-07-19修回日期:2023-09-16出版日期:2024-07-25发布日期:2024-08-14 -
通讯作者:马伟芳 -
作者简介:何弈雪(2000—),女,硕士研究生,研究方向为过硫酸盐高级氧化。E-mail:793612252@qq.com。 -
基金资助:国家自然科学基金(51678052)
Research progress on in situ remediation of halogenated hydrocarbon contamination in groundwater by persulfate-based advanced oxidation process
HE Yixue1( ), QIN Xianchao2, MA Weifang1(
), QIN Xianchao2, MA Weifang1( )
)
- 1.Beijing Forestry University, College of Environmental Science and Engineering, Beijing 100091, China
2.Beijing 80th Middle School, Beijing 100020, China
-
Received:2023-07-19Revised:2023-09-16Online:2024-07-25Published:2024-08-14 -
Contact:MA Weifang
摘要:
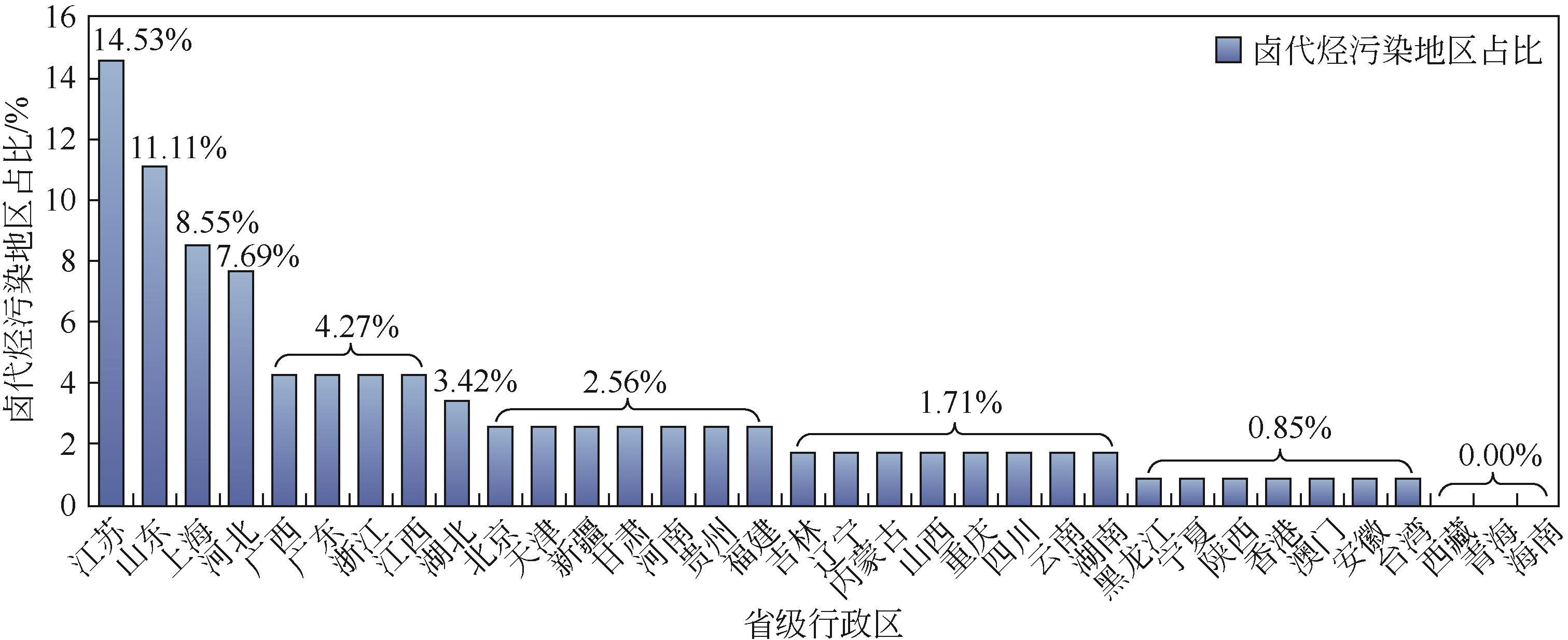
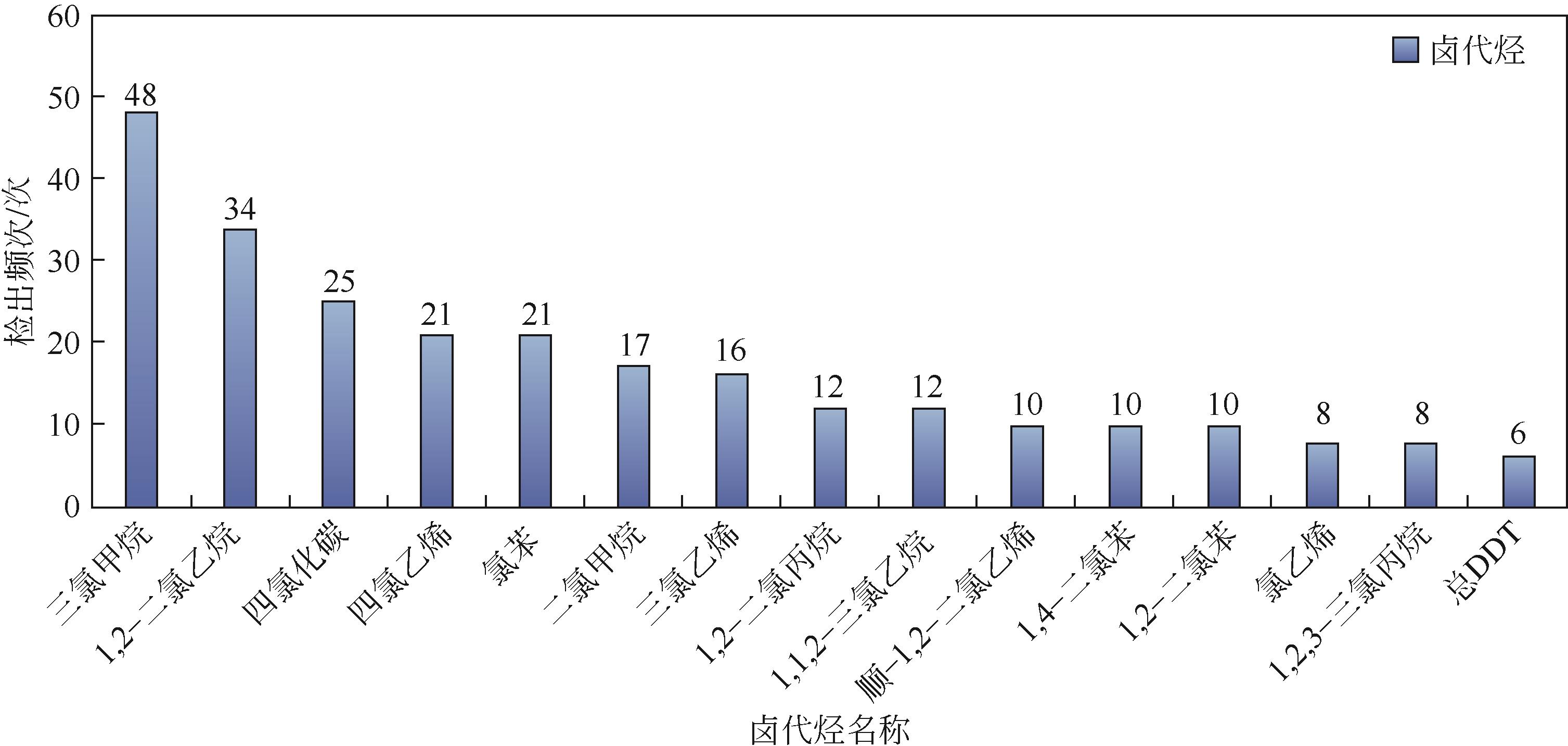
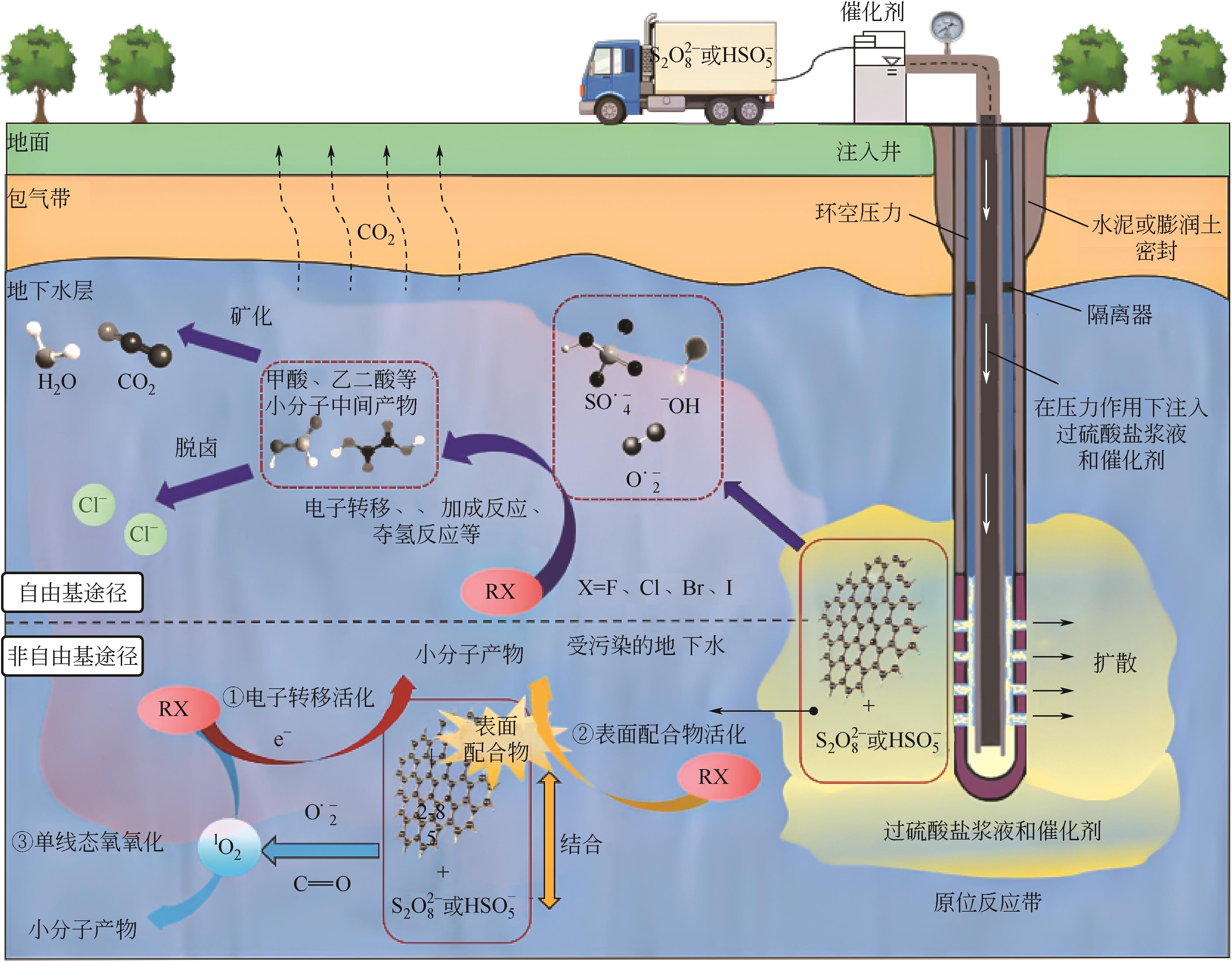
近年来,地下水卤代烃污染问题日益突出,在多种修复技术中,过硫酸盐(PS)高级氧化技术由于其氧化能力强、反应适用pH范围广、半衰期长且氧化范围广,在水处理界引起了广泛关注。本文先对地下水卤代烃的赋存类型进行了分类,指出国内污染场地检出的卤代烃大部分为重非水相液体(DNAPLs),然后对收集的155个污染地区的卤代烃检出类型和频次进行了统计。对于PS高级氧化技术对卤代烃的降解机制,本文作了详细的分析,大体上分为自由基途径和非自由基途径。由于单一的PS活性不高,因此需要将其活化才能起到较好的氧化效果。在此,总结了金属基催化剂和碳基催化剂的种类和特点。最后讨论了具体应用时PS浓度、无机阴离子、反应初始pH和地下水中天然有机物对反应的影响。与传统的地下水卤代烃修复技术相比,过硫酸盐高级氧化技术可以成为高效清洁的替代技术。
中图分类号:
引用本文
何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088.
HE Yixue, QIN Xianchao, MA Weifang. Research progress on in situ remediation of halogenated hydrocarbon contamination in groundwater by persulfate-based advanced oxidation process[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4072-4088.
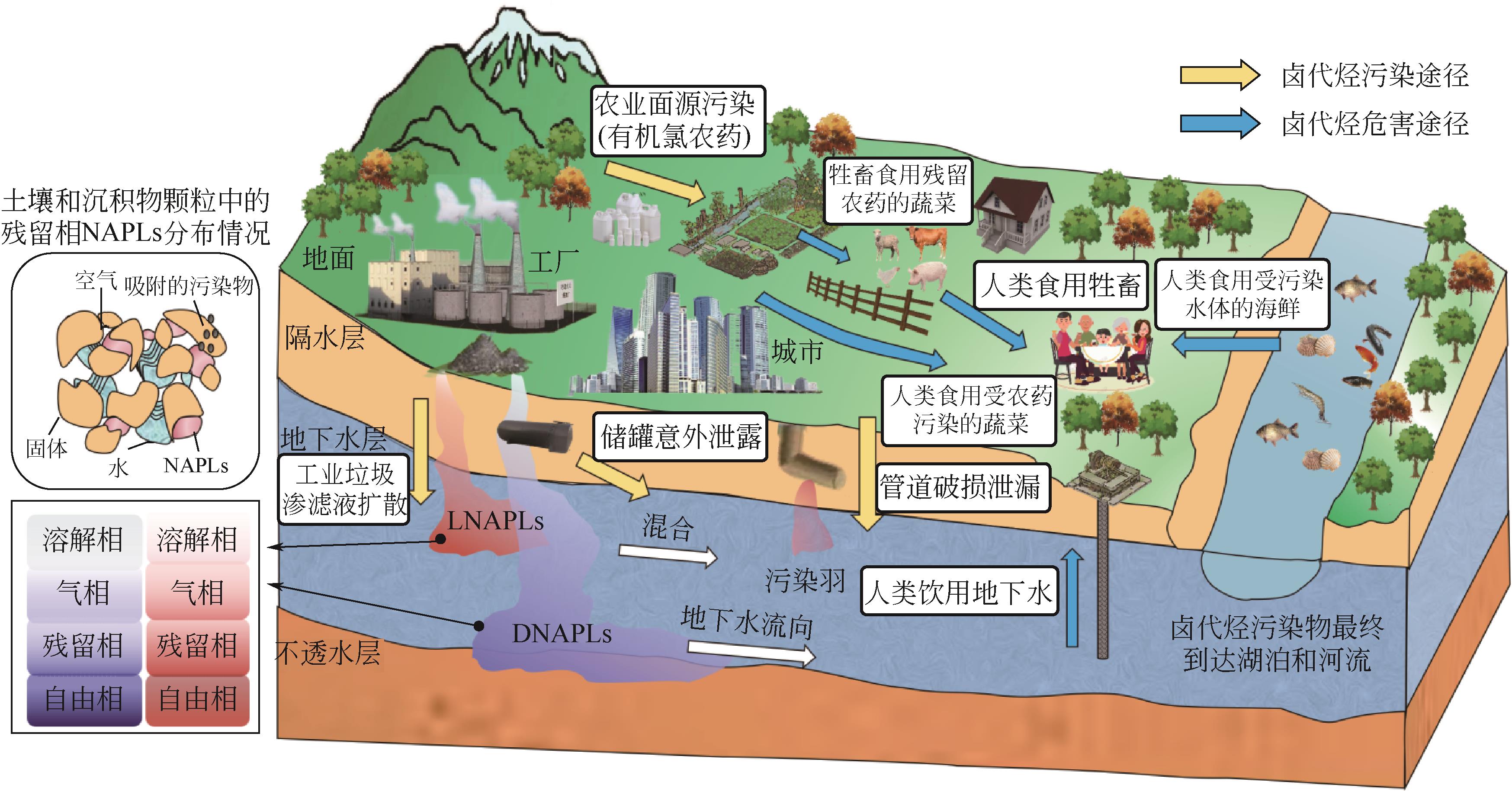
| 类型 | 相对密度 | 主要迁移运动 | 主要存在区域 | 常见有机物 |
|---|---|---|---|---|
| LNAPLs | >1.0 | 垂向迁移 | 包气带 | 苯、甲苯、原油、汽油、柴油、煤油、热煤油、一氟代烷烃、一氯代烷烃等 |
| DNAPLs | <S1.0 | 水平迁移 | 包气带、地下水层 | 氯代溶剂、煤焦油、重矿物油、杂酚油、多环芳烃、多氯联苯等 |
表1 两类非水相液体对比表
| 类型 | 相对密度 | 主要迁移运动 | 主要存在区域 | 常见有机物 |
|---|---|---|---|---|
| LNAPLs | >1.0 | 垂向迁移 | 包气带 | 苯、甲苯、原油、汽油、柴油、煤油、热煤油、一氟代烷烃、一氯代烷烃等 |
| DNAPLs | <S1.0 | 水平迁移 | 包气带、地下水层 | 氯代溶剂、煤焦油、重矿物油、杂酚油、多环芳烃、多氯联苯等 |
| 相态 | LNAPLs | DNAPLs |
|---|---|---|
| 自由相 | (1)受重力、毛细作用垂直迁移; (2)在水力梯度下水平运移; (3)受水力梯度影响更大; (4)主要污染源 | (1)受重力、毛细作用垂直迁移; (2)在水力梯度下水平运移; (3)主要污染源 |
| 残留相 | (1)残留于运移路径; (2)受吸附和毛细作用以薄膜或液滴赋存于孔隙,难以在重力作用下继续迁移 | (1)残留于运移路径; (2)受吸附和毛细作用以薄膜或液滴赋存于孔隙,难以在重力作用下继续迁移; (3)可在雨水冲刷或外力作用下垂向迁移 |
| 气相 | 极少量LNAPLs卤代烃挥发进入地下水,并在浓度梯度下迁移形成污染羽 | (1)一些DNAPLs卤代烃挥发进入地下水,并在浓度梯度下迁移形成污染羽; (2)包气带中的气相卤代烃,可在雨水冲刷或外力作用下垂向迁移 |
| 溶解相 | (1)溶于地下水并随地下水流动形成污染羽; (2)泄漏量足够大时,部分能够缓慢向下迁移到达地下水层,积聚后向周边水平运移 | (1)溶于地下水并随地下水流动形成污染羽; (2)缓慢向下迁移,到达低渗层后,少量垂直迁移,大部分积聚后向周边水平运移 |
表2 两类非水相液体各相存在特征表
| 相态 | LNAPLs | DNAPLs |
|---|---|---|
| 自由相 | (1)受重力、毛细作用垂直迁移; (2)在水力梯度下水平运移; (3)受水力梯度影响更大; (4)主要污染源 | (1)受重力、毛细作用垂直迁移; (2)在水力梯度下水平运移; (3)主要污染源 |
| 残留相 | (1)残留于运移路径; (2)受吸附和毛细作用以薄膜或液滴赋存于孔隙,难以在重力作用下继续迁移 | (1)残留于运移路径; (2)受吸附和毛细作用以薄膜或液滴赋存于孔隙,难以在重力作用下继续迁移; (3)可在雨水冲刷或外力作用下垂向迁移 |
| 气相 | 极少量LNAPLs卤代烃挥发进入地下水,并在浓度梯度下迁移形成污染羽 | (1)一些DNAPLs卤代烃挥发进入地下水,并在浓度梯度下迁移形成污染羽; (2)包气带中的气相卤代烃,可在雨水冲刷或外力作用下垂向迁移 |
| 溶解相 | (1)溶于地下水并随地下水流动形成污染羽; (2)泄漏量足够大时,部分能够缓慢向下迁移到达地下水层,积聚后向周边水平运移 | (1)溶于地下水并随地下水流动形成污染羽; (2)缓慢向下迁移,到达低渗层后,少量垂直迁移,大部分积聚后向周边水平运移 |
| 污染物种类及浓度 | 催化剂种类及浓度 | 氧化剂种类及浓度 | 反应条件 | 反应活性物种 | 污染物 降解率/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|
| 时间 | 温度 | pH | ||||||
| TCE,0.15mmol/L | 螯合Fe2+,0.3mmol/L | 未区分,2.25mmol/L | 3h | 20℃ | 3 | ·SO | 97.5 | [ |
| 氯苯,300mg/L | nZVI,6g/L | PDS,6g/L | 20h | (20.5±4)℃ | 6.5 | ·SO | >97 | [ |
| TCE,9.4mmol/L | nZVI,376mmol/L | PDS,376mmol/L | 16h | (20.5±4)℃ | 6.5 | ·SO | 96.3 | |
| TCE,0.1mmol/L | CuO,0.1g/L | PDS,2.5mmol/L | 3h | 25℃ | 11 | PDS(电子转移) | 57.42 | [ |
| TCE,5μmol/L | CuO,200mg/L | PDS,40μmol/L | 40min | 20℃ | 5.8 | 未提及 | 约66 | [ |
| 三氯乙烷,0.15mmol/L | CuO,0.2g/L | PMS,0.4mmol/L | 2h | 25℃ | 7 | ·SO | 43 | [ |
| Co3O4,0.2g/L | 47 | |||||||
| 片状CuO,0.25g/L | PMS,0.5mmol/L | 5 | 97.5 | |||||
| TCE,20mg/L | S-Fe2MnO4,0.2g/L | PMS,0.2g/L | 10min | — | 7.2 | ·SO metal-PMS表面配合物 | 92.4 | [ |
| TCE,0.15mmol/L | 负载生物炭的nZVI,5mmol/L | PDS,4.5mmol/L | 5min | 25℃ | 6.2 | ·SO | 100 | [ |
| TCE,0.16mmol/L | 负载沸石的nZVI,84mg/L | PDS,1.5mmol/L | 2h | (22±2)℃ | 7 | ·SO | 98.8 | [ |
表3 不同方式活化过硫酸盐降解卤代烃总结表
| 污染物种类及浓度 | 催化剂种类及浓度 | 氧化剂种类及浓度 | 反应条件 | 反应活性物种 | 污染物 降解率/% | 参考文献 | ||
|---|---|---|---|---|---|---|---|---|
| 时间 | 温度 | pH | ||||||
| TCE,0.15mmol/L | 螯合Fe2+,0.3mmol/L | 未区分,2.25mmol/L | 3h | 20℃ | 3 | ·SO | 97.5 | [ |
| 氯苯,300mg/L | nZVI,6g/L | PDS,6g/L | 20h | (20.5±4)℃ | 6.5 | ·SO | >97 | [ |
| TCE,9.4mmol/L | nZVI,376mmol/L | PDS,376mmol/L | 16h | (20.5±4)℃ | 6.5 | ·SO | 96.3 | |
| TCE,0.1mmol/L | CuO,0.1g/L | PDS,2.5mmol/L | 3h | 25℃ | 11 | PDS(电子转移) | 57.42 | [ |
| TCE,5μmol/L | CuO,200mg/L | PDS,40μmol/L | 40min | 20℃ | 5.8 | 未提及 | 约66 | [ |
| 三氯乙烷,0.15mmol/L | CuO,0.2g/L | PMS,0.4mmol/L | 2h | 25℃ | 7 | ·SO | 43 | [ |
| Co3O4,0.2g/L | 47 | |||||||
| 片状CuO,0.25g/L | PMS,0.5mmol/L | 5 | 97.5 | |||||
| TCE,20mg/L | S-Fe2MnO4,0.2g/L | PMS,0.2g/L | 10min | — | 7.2 | ·SO metal-PMS表面配合物 | 92.4 | [ |
| TCE,0.15mmol/L | 负载生物炭的nZVI,5mmol/L | PDS,4.5mmol/L | 5min | 25℃ | 6.2 | ·SO | 100 | [ |
| TCE,0.16mmol/L | 负载沸石的nZVI,84mg/L | PDS,1.5mmol/L | 2h | (22±2)℃ | 7 | ·SO | 98.8 | [ |
| 卤代烃种类 | 活化方式 /催化剂类型 | 反应时间 /min | 反应结果 | 原因 | 参考文献 |
|---|---|---|---|---|---|
| TCE | 螯合-Fe(Ⅱ) | 180 | 当pH从3增加至11时,TCE降解率从97.5%逐步下降至40.3% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,螯合-Fe(Ⅱ)形态变得更加稳定,活性降低; ③pH升高,螯合-Fe(Ⅱ)生成沉淀 | [ |
| TCE | 天然沸石负载nZVI | 120 | ①pH为4和7时降解率几乎达到100%; ②pH为10时降解率下降至77.5% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,nZVI生成铁氧化物、氢氧化铁和氢氧化亚铁,活性降低 | [ |
| TCE | 生物炭负载S-FeNi | 60 | 反应降解率为pH 9.2(57.2%)<pH 7.2(75.3%)<pH 6.39(82.4%)<pH 5.2(88.6%)<pH 3.2(98.4%) | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,S-FeNi@BC中的Fe(Ⅱ)释放量减少; ③pH升高,催化剂生成Fe(Ⅲ)沉淀; ④pH升高,S-FeNi@BC表面发生钝化 | [ |
| 三氯乙烷(TCA) | (片状)Sh-CuO | 120 | ①pH为3、4、9、11时降解率均低于80%; ②pH为5、7、8时降解率大于80%,且pH=5时达到最大降解率,为92.1% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,Sh-CuO结构变得更加稳定,活性降低; ③pH为3、4时,加剧了Sh-CuO的腐蚀 | [ |
| 1,2-二氯乙烷(1,2-DCA) | 碱活化 | 120 | ①pH为10和11时降解率低于采用未活化的PS参与反应时的降解率; ②pH为12和13时降解率几乎达到100% | ①pH为10和11时,·OH占主导地位,反应活性降低; ②高pH下碱活化产生·OH和·SO4-以外的活性氧化剂 | [ |
| 四氯乙烯(PCE) | Fe(Ⅱ) | 30 | ①pH为3时降解率最高,为97.6%; ②pH为6.25~11时,降解率从80.4%下降至2.1% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,Fe(Ⅱ)生成铁氧化物、氢氧化铁和氢氧化亚铁,活性降低 | [ |
表4 不同反应条件下初始pH对反应结果的影响及原因表
| 卤代烃种类 | 活化方式 /催化剂类型 | 反应时间 /min | 反应结果 | 原因 | 参考文献 |
|---|---|---|---|---|---|
| TCE | 螯合-Fe(Ⅱ) | 180 | 当pH从3增加至11时,TCE降解率从97.5%逐步下降至40.3% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,螯合-Fe(Ⅱ)形态变得更加稳定,活性降低; ③pH升高,螯合-Fe(Ⅱ)生成沉淀 | [ |
| TCE | 天然沸石负载nZVI | 120 | ①pH为4和7时降解率几乎达到100%; ②pH为10时降解率下降至77.5% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,nZVI生成铁氧化物、氢氧化铁和氢氧化亚铁,活性降低 | [ |
| TCE | 生物炭负载S-FeNi | 60 | 反应降解率为pH 9.2(57.2%)<pH 7.2(75.3%)<pH 6.39(82.4%)<pH 5.2(88.6%)<pH 3.2(98.4%) | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,S-FeNi@BC中的Fe(Ⅱ)释放量减少; ③pH升高,催化剂生成Fe(Ⅲ)沉淀; ④pH升高,S-FeNi@BC表面发生钝化 | [ |
| 三氯乙烷(TCA) | (片状)Sh-CuO | 120 | ①pH为3、4、9、11时降解率均低于80%; ②pH为5、7、8时降解率大于80%,且pH=5时达到最大降解率,为92.1% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,Sh-CuO结构变得更加稳定,活性降低; ③pH为3、4时,加剧了Sh-CuO的腐蚀 | [ |
| 1,2-二氯乙烷(1,2-DCA) | 碱活化 | 120 | ①pH为10和11时降解率低于采用未活化的PS参与反应时的降解率; ②pH为12和13时降解率几乎达到100% | ①pH为10和11时,·OH占主导地位,反应活性降低; ②高pH下碱活化产生·OH和·SO4-以外的活性氧化剂 | [ |
| 四氯乙烯(PCE) | Fe(Ⅱ) | 30 | ①pH为3时降解率最高,为97.6%; ②pH为6.25~11时,降解率从80.4%下降至2.1% | ①pH升高,·OH占主导地位,反应活性降低; ②pH升高,Fe(Ⅱ)生成铁氧化物、氢氧化铁和氢氧化亚铁,活性降低 | [ |
| 1 | 水利部水资源管理司. 2021年中国水资源公报[EB/OL]. (2022-06-15)[2023-07-01]. . |
| Ministry of Water Resource of the People’s Republic of China. China Water Resources Bulletin in 2021[EB/OL]. (2022-06-15)[2023-07-01]. . | |
| 2 | 乔斐, 王锦国, 郑诗钰, 等. 重点区域建设用地污染地块特征分析[J]. 中国环境科学, 2022, 42(11): 5265-5275. |
| Qiao Fei, Wang Jinguo, Zheng Shiyu, et al. Characterization of contaminated construction sites in key regions[J].China Environmental Science, 2022, 42(11): 5265-5275. | |
| 3 | ZHANG Yunhui, CAO Benyi, YIN Hailong, et al. Application of zeolites in permeable reactive barriers (PRBs) for in situ groundwater remediation: A critical review[J]. Chemosphere, 2022, 308: 136290. |
| 4 | USHANI Uthirakrishnan, LU Xueqin, WANG Jianhui, et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the-art review[J]. Chemical Engineering Journal, 2020, 402: 126232. |
| 5 | YU Jiangfang, FENG Haopeng, TANG Lin, et al. Metal-free carbon materials for persulfate-based advanced oxidation process: Microstructure, property and tailoring[J]. Progress in Materials Science, 2020, 111: 100654. |
| 6 | LIU Tianhao, YAO Bin, LUO Zirui, et al. Applications and influencing factors of the biochar-persulfate based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds[J]. Science of the Total Environment, 2022, 836: 155421. |
| 7 | TOMLINSON Derek W, RIVETT Michael O, WEALTHALL Gary P, et al. Understanding complex LNAPL sites: Illustrated handbook of LNAPL transport and fate in the subsurface[J]. Journal of Environmental Management, 2017, 204: 748-756. |
| 8 | HE Zhennan, LIANG Fachun, MENG Jia, et al. Effects of groundwater fluctuation on migration characteristics and representative elementary volume of entrapped LNAPL[J]. Journal of Hydrology, 2022, 610: 127833. |
| 9 | ENGELMANN Christian, Falk HÄNDEL, BINDER Martin, et al. The fate of DNAPL contaminants in non-consolidated subsurface systems—Discussion on the relevance of effective source zone geometries for plume propagation[J]. Journal of Hazardous Materials, 2019, 375: 233-240. |
| 10 | 陶佳辉, 施小清, 康学远, 等. 轻非水相液体污染源区结构的影响因素数值分析[J]. 水文地质工程地质, 2018, 45(6): 132-140. |
| TAO Jiahui, SHI Xiaoqing, KANG Xueyuan, et al. Numerical analyses of factors affecting the LNAPL source-zone architecture[J]. Hydrogeology & Engineering Geology, 2018, 45(6): 132-140. | |
| 11 | 宋美钰, 施小清, 马春龙, 等. 复杂DNAPL污染源区溶解相污染通量的升尺度计算[J]. 中国环境科学, 2022, 42(5): 2095-2104. |
| SONG Meiyu, SHI Xiaoqing, MA Chunlong, et al. Upscaling dissolved phase mass flux for complex DNAPL source zones[J]. China Environmental Science, 2022, 42(5): 2095-2104. | |
| 12 | 徐耀宽. 从“退二进三”到价值创新园区——广州市工业用地的转型与回归[J]. 规划师, 2018, 34(12): 54-62. |
| XU Yaokuan. From “third industry priority” to “value park”: Transition and regression of Guangzhou industrial land use[J]. Planners, 2018, 34(12): 54-62. | |
| 13 | 朱国繁, 应蓉蓉, 叶茂, 等. 我国农药生产场地污染土壤修复技术研究进展[J]. 土壤通报, 2021, 52(2): 462-473. |
| ZHU Guofan, YING Rongrong, YE Mao, et al. Research progress on remediation technology of contaminated soil in pesticide production sites in China[J]. Chinese Journal of Soil Science, 2021, 52(2): 462-473. | |
| 14 | CHEN Zhiguo, CAO Wenqing, BAI He, et al. Review on the degradation of chlorinated hydrocarbons by persulfate activated with zero-valent iron-based materials[J]. Water Science and Technology, 2023, 87(3): 761-782. |
| 15 | ZHANG Hao, JI Yuanyuan, WU Zhenhai, et al. Atmospheric volatile halogenated hydrocarbons in air pollution episodes in an urban area of Beijing: Characterization, health risk assessment and sources apportionment[J]. Science of the Total Environment, 2022, 806: 150283. |
| 16 | LEE Jaesang, VON GUNTEN Urs, KIM Jae-Hong. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. |
| 17 | HOUSE D A. Kinetics and mechanism of oxidations by peroxydisulfate[J]. Chemical Reviews, 1962, 62(3): 185-203. |
| 18 | LI Bingzhi, LI Lin, LIN Kuangfei, et al. Removal of 1,1 , 1 - trichloroethane from aqueous solution by a sono-activated persulfate process[J]. Ultrasonics Sonochemistry, 2013, 20(3): 855-863. |
| 19 | FENG Meiyun, XU Zhiqiang, BAI Xue, et al. Exploration the mechanisms underlying peroxymonosulfate activation by nano-cubic spinel M2MnO4 nanoparticles for degrading trichloroethylene[J]. Chemical Engineering Journal, 2022, 446: 137394. |
| 20 | XIE Tian, DANG Zhi, ZHANG Qian, et al. Kinetics, mechanism, and application of sodium persulfate activated by sodium hydroxide for removing 1,2-dichloroethane from groundwater[J]. Environmental Research, 2023, 216: 114694. |
| 21 | 张俊涛. 天然含铁矿物激活过硫酸盐修复1,1,2-三氯乙烷污染的地下水[D]. 长沙: 湖南大学, 2018. |
| ZHANG Juntao. Activation of persulfate by naturally occurring iron minerals for degradation of 1,1,2-TCA in groundwater[D]. Changsha: Hunan University, 2018. | |
| 22 | 杨程明. 臭氧/过氧化氢氧化修复氟氯苯类复合污染场地研究[D]. 合肥: 合肥工业大学, 2022. |
| YANG Chengming. Study on remediation of complex contaminated sites containing fluorinated or chlorinated benzene by ozone/hydrogen peroxide oxidation[D]. Hefei: Hefei University of Technology, 2022. | |
| 23 | 姜凤成. 半胱氨酸强化亚铁/过硫酸钠修复氯苯污染地下水[D]. 武汉: 中国地质大学, 2021. |
| JIANG Fengcheng. Cysteine enhanced ferrous iron/persulfate system for the remediation of monochlorobenzene-contaminated groundwater[D]. Wuhan: China University of Geosciences, 2021. | |
| 24 | WANG Jianlong, WANG Shizong. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism[J]. Chemical Engineering Journal, 2020, 401: 126158. |
| 25 | Omneya EL-SHARNOUBY, BOPARAI Hardiljeet K, HERRERA Jose, et al. Aqueous-phase catalytic hydrodechlorination of 1,2-dichloroethane over palladium nanoparticles (nPd) with residual borohydride from nPd synthesis[J]. Chemical Engineering Journal, 2018, 342: 281-292. |
| 26 | FENG Yong, WU Deli, DENG Yu, et al. Sulfate radical-mediated degradation of sulfadiazine by CuFeO2 rhombohedral crystal-catalyzed peroxymonosulfate: Synergistic effects and mechanisms[J].Environmental Science & Technology, 2016, 50(6): 3119-3127. |
| 27 | CHEN Guanyi, WU Guanyun, LI Ning, et al. Landfill leachate treatment by persulphate related advanced oxidation technologies[J]. Journal of Hazardous Materials, 2021, 418: 126355. |
| 28 | LUO Shuang, WEI Zongsu, DIONYSIOU Dionysios D, et al. Mechanistic insight into reactivity of sulfate radical with aromatic contaminants through single-electron transfer pathway[J]. Chemical Engineering Journal, 2017, 327: 1056-1065. |
| 29 | CAREGNATO Paula, DAVID GARA Pedro M, BOSIO Gabriela N, et al. Theoretical and experimental investigation on the oxidation of Gallic acid by sulfate radical anions[J]. The Journal of Physical Chemistry A, 2008, 112(6): 1188-1194. |
| 30 | LI Yang, NIU Junfeng, YIN Lifeng, et al. Photocatalytic degradation kinetics and mechanism of pentachlorophenol based on Superoxide radicals[J]. Journal of Environmental Sciences, 2011, 23(11): 1911-1918. |
| 31 | BAUM Rudy M. Superoxide theory of oxygen toxicity is center of heated debate[J]. Chemical & Engineering News Archive, 1984, 62(15): 20-26. |
| 32 | Suthersan Suthan S.. Monitored natural attenuation[M]//Natural and Enhanced Remediation Systems. CRC Press, 2001: 83-150. |
| 33 | WANG Bing, GAO Chunyang, LI Xingchun, et al. Remediation of groundwater pollution by in situ reactive zone: A review[J]. Process Safety and Environmental Protection, 2022, 168: 858-871. |
| 34 | KARN Barbara, KUIKEN Todd, OTTO Martha. Nanotechnology and in situ remediation: A review of the benefits and potential risks[J]. Environmental Health Perspectives, 2009, 117(12): 1813-1831. |
| 35 | 李书鹏, 刘鹏, 杜晓明, 等. 采用零价铁-缓释碳修复氯代烃污染地下水的中试研究[J]. 环境工程, 2013, 31(4): 53-58. |
| LI Shupeng, LIU Peng, DU Xiaoming, et al. A field pilot test for restoring chlorohydrocarbon contaminated groundwater using ZVI & controlled releasing carbon material[J]. Environmental Engineering, 2013, 31(4): 53-58. | |
| 36 | 孟梁, 郭琳, 杨洁, 等. 原位修复氯代烃污染场地地下水的现场中试研究[J]. 工业水处理, 2014, 34(8): 18-21. |
| MENG Liang, GUO Lin, YANG Jie, et al. Research on the pilot scale in situ remediation of groundwater polluted by chlorinated hydrocarbons in a contaminated site[J]. Industrial Water Treatment, 2014, 34(8): 18-21. | |
| 37 | WANG Bing, DENG Chaoxiao, MA Wei, et al. Modified nanoscale zero-valent iron in persulfate activation for organic pollution remediation: A review[J]. Environmental Science and Pollution Research International, 2021, 28(26): 34229-34247. |
| 38 | ZHANG Tao, CHEN Yin, WANG Yuru, et al. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation[J]. Environmental Science & Technology, 2014, 48(10): 5868-5875. |
| 39 | DU Xiaodong, ZHANG Yongqing, SI Fan, et al. Persulfate non-radical activation by nano-CuO for efficient removal of chlorinated organic compounds: Reduced graphene oxide-assisted and CuO (001) facet-dependent[J]. Chemical Engineering Journal, 2019, 356: 178-189. |
| 40 | GUAN Zeyu, ZUO Shiyu, YANG Fan, et al. The p and d hybridization interaction in Fe-N-C boosts peroxymonosulfate non-radical activation[J]. Separation and Purification Technology, 2021, 258: 118025. |
| 41 | DING Yichen, LI Dongya, ZUO Shiyu, et al. Boron-doping accelerated Cu(Ⅱ)/Cu(Ⅰ) cycle for enhancing peroxymonosulfate activation[J]. Separation and Purification Technology, 2022, 282: 120086. |
| 42 | DUAN Xiaoguang, SUN Hongqi, SHAO Zongping, et al. Nonradical reactions in environmental remediation processes: Uncertainty and challenges[J]. Applied Catalysis B: Environmental, 2018, 224: 973-982. |
| 43 | PENG Wenya, DONG Yongxia, FU Yu, et al. Non-radical reactions in persulfate-based homogeneous degradation processes: A review[J]. Chemical Engineering Journal, 2021, 421: 127818. |
| 44 | DING Yaobin, FU Libin, PENG Xueqin, et al. Copper catalysts for radical and nonradical persulfate based advanced oxidation processes: Certainties and uncertainties[J]. Chemical Engineering Journal, 2022, 427: 131776. |
| 45 | SU Hanrui, WEI Yan, QU Xiaolei, et al. Mechanistic inference on the reaction kinetics of phenols and anilines in carbon nanotubes-activated peroxydisulfate systems: Pp-LFERs and QSARs analyses[J]. Chemical Engineering Journal, 2020, 385: 123923. |
| 46 | DING Yaobin, WANG Xueru, FU Libin, et al. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective[J]. Science of the Total Environment, 2021, 765: 142794. |
| 47 | HUANG Danlian, XU Wenbo, LEI Lei, et al. Promoted generation strategies and corresponding roles of singlet oxygen in activation of persulfate by nanoscale zero-valent iron systems[J]. Chemical Engineering Journal, 2022, 449: 137493. |
| 48 | ZHU Shishu, HUANG Xiaochen, MA Fang, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms[J].Environmental Science & Technology, 2018, 52(15): 8649-8658. |
| 49 | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: Activation performance and structure-function relationship[J]. Water Research, 2019, 157: 406-414. |
| 50 | LI Huarui, TIAN Jiayu, XIAO Feng, et al. Structure-dependent catalysis of cuprous oxides in peroxymonosulfate activation via nonradical pathway with a high oxidation capacity[J]. Journal of Hazardous Materials, 2020, 385: 121518. |
| 51 | TAN Weihua, REN Wei, WANG Chanjuan, et al. Peroxymonosulfate activated with waste battery-based Mn-Fe oxides for pollutant removal: Electron transfer mechanism, selective oxidation and LFER analysis[J]. Chemical Engineering Journal, 2020, 394: 124864. |
| 52 | REN Mingzhu, SUN Sihan, WU Yiqiu, et al. The structure-activity relationship of aromatic compounds in advanced oxidation processes: A review[J]. Chemosphere, 2022, 296: 134071. |
| 53 | LEE Hongshin, KIM Hyoung-Il, WEON Seunghyun, et al. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds[J]. Environmental Science & Technology, 2016, 50(18): 10134-10142. |
| 54 | XU Xiaomin, ZHANG Yongqing, ZHOU Shaoqi, et al. Activation of persulfate by MnOOH: Degradation of organic compounds by nonradical mechanism[J]. Chemosphere, 2021, 272: 129629. |
| 55 | WANG Chen, KANG Jian, LIANG Ping, et al. Ferric carbide nanocrystals encapsulated in nitrogen-doped carbon nanotubes as an outstanding environmental catalyst[J]. Environmental Science: Nano, 2017, 4(1): 170-179. |
| 56 | Yong-Yoon AHN, Hyokwan BAE, KIM Hyoung-Il, et al. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance[J]. Applied Catalysis B: Environmental, 2018, 241: 561-569. |
| 57 | Jawad ALI, JIANG Wang, SHAHZAD Ajmal, et al. Isolated copper ions and surface hydroxyl groups as a function of non-redox metals to modulate the reactivity and persulfate activation mechanism of spinel oxides[J]. Chemical Engineering Journal, 2021, 425: 130679. |
| 58 | LIU Junqin, WU Pingxiao, YANG Shanshan, et al. A photo-switch for peroxydisulfate non-radical/radical activation over layered CuFe oxide: Rational degradation pathway choice for pollutants[J]. Applied Catalysis B: Environmental, 2020, 261: 118232. |
| 59 | LUO Haoyu, FU Hengyi, YIN Hua, et al. Carbon materials in persulfate-based advanced oxidation processes: The roles and construction of active sites[J]. Journal of Hazardous Materials, 2022, 426: 128044. |
| 60 | TIAN Ke, HU Limin, LI Letian, et al. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment[J]. Chinese Chemical Letters, 2022, 33(10): 4461-4477. |
| 61 | ZHENG Xiaoxian, NIU Xiaojun, ZHANG Dongqing, et al. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review[J]. Chemical Engineering Journal, 2022, 429: 132323. |
| 62 | ZHU Xiurong, ZHANG Yue, YAN Wei, et al. Peroxymonosulfate activation by mesoporous CuO nanocage for organic pollutants degradation via a singlet oxygen-dominated pathway[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106757. |
| 63 | FENG Meiyun, XU Zhiqiang, LIN Kuangfei, et al. Heterogeneous activation of peroxymonosulfate by sulfur-doped Fe x Mn3- x O4 (x=1,2) for trichloroethylene degradation: Non-radical and radical mechanisms[J]. Chemical Engineering Journal, 2023, 459: 141627. |
| 64 | XIONG Zhaokun, JIANG Yanni, WU Zelin, et al. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review[J]. Chemical Engineering Journal, 2021, 421: 127863. |
| 65 | ZOU Jiajing, YU Jiangfang, TANG Lin, et al. Analysis of reaction pathways and catalytic sites on metal-free porous biochar for persulfate activation process[J]. Chemosphere, 2020, 261: 127747. |
| 66 | GAO Yaowen, CHEN Zhenhuan, ZHU Yue, et al. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: Important role of electron-deficient carbon atoms[J]. Environmental Science & Technology, 2020, 54(2): 1232-1241. |
| 67 | JIANG Mengdi, ZHANG Qingyue, JI Yuefei, et al. Transformation of antimicrobial agent sulfamethazine by peroxymonosulfate: Radical vs. nonradical mechanisms[J]. Science of the Total Environment, 2018, 636: 864-871. |
| 68 | YANG Xueying, CAI Jingsheng, WANG Xiaoning, et al. A bimetallic Fe-Mn oxide-activated oxone for in situ chemical oxidation (ISCO) of trichloroethylene in groundwater: Efficiency, sustained activity, and mechanism investigation[J]. Environmental Science & Technology, 2020, 54(6): 3714-3724. |
| 69 | ZHU Shishu, LI Xiaojie, KANG Jian, et al. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science & Technology, 2019, 53(1): 307-315. |
| 70 | FAN Jinhong, QIN Hehe, JIANG Simin. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen[J]. Chemical Engineering Journal, 2019, 359: 723-732. |
| 71 | WANG Jianlong, WANG Shizong. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 72 | YAN Jingchun, HAN Lu, GAO Weiguo, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene[J]. Bioresource Technology, 2015, 175: 269-274. |
| 73 | AL-SHAMSI Mohammed A, THOMSON Neil R. Treatment of organic compounds by activated persulfate using nanoscale zerovalent iron[J]. Industrial & Engineering Chemistry Research, 2013, 52(38): 13564-13571. |
| 74 | SUN Yong, LI Ming, GU Xiaogang, et al. Mechanism of surfactant in trichloroethene degradation in aqueous solution by sodium persulfate activated with chelated-Fe(Ⅱ)[J]. Journal of Hazardous Materials, 2021, 407: 124814. |
| 75 | WU Shaohua, SHEN Leyuan, LIN Yan, et al. Sulfite-based advanced oxidation and reduction processes for water treatment[J]. Chemical Engineering Journal, 2021, 414: 128872. |
| 76 | ZHOU Yaoyu, XIANG Yujia, HE Yangzhuo, et al. Applications and factors influencing of the persulfate-based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds[J]. Journal of Hazardous Materials, 2018, 359: 396-407. |
| 77 | SHAN Ali, IDREES Ayesha, ZAMAN Waqas Qamar, et al. Enhancement in reactivity via sulfidation of FeNi@BC for efficient removal of trichloroethylene: Insight mechanism and the role of reactive oxygen species[J]. The Science of the Total Environment, 2021, 794: 148674. |
| 78 | FAROOQ Usman, WANG Fei, ASLAM Malik Muhammad Arslan, et al. How do CuO sheets (sh-CuO) enable efficient chlorinated hydrocarbon removal?[J]. Journal of Water Process Engineering, 2022, 50: 103204. |
| 79 | LI Xingyu, Borui JIE, LIN Huidong, et al. Application of sulfate radicals-based advanced oxidation technology in degradation of trace organic contaminants (TrOCs): Recent advances and prospects[J]. Journal of Environmental Management, 2022, 308: 114664. |
| 80 | SUN Hongqi, LIU Shizhen, ZHOU Guanliang, et al. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants[J]. ACS Applied Materials & Interfaces, 2012, 4(10): 5466-5471. |
| 81 | SUN Ping, LIU Hui, FENG Mingbao, et al. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants[J]. Applied Catalysis B: Environmental, 2019, 251: 335-345. |
| 82 | HUANG Junyi, YI Shuping, ZHENG Chunmiao, et al. Persulfate activation by natural zeolite supported nanoscale zero-valent iron for trichloroethylene degradation in groundwater[J]. The Science of the Total Environment, 2019, 684: 351-359. |
| 83 | GUAN Chaoting, JIANG Jin, LUO Congwei, et al. Oxidation of bromophenols by carbon nanotube activated peroxymonosulfate (PMS) and formation of brominated products: Comparison to peroxydisulfate (PDS)[J]. Chemical Engineering Journal, 2018, 337: 40-50. |
| 84 | WU Xiaoliang, GU Xiaogang, LU Shuguang, et al. Strong enhancement of trichloroethylene degradation in ferrous ion activated persulfate system by promoting ferric and ferrous ion cycles with hydroxylamine[J]. Separation and Purification Technology, 2015, 147: 186-193. |
| 85 | YANG Qi, MA Yinghao, CHEN Fei, et al. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water[J]. Chemical Engineering Journal, 2019, 378: 122149. |
| 86 | PENG Libin, DENG Dayi, GUAN Mengyun, et al. Remediation HCHs POPs-contaminated soil by activated persulfate technologies: Feasibility, impact of activation methods and mechanistic implications[J]. Separation and Purification Technology, 2015, 150: 215-222. |
| 87 | RAYAROTH Manoj P, MARCHEL Mateusz, BOCZKAJ Grzegorz. Advanced oxidation processes for the removal of mono and polycyclic aromatic hydrocarbons—A review[J]. The Science of the Total Environment, 2023, 857: 159043. |
| 88 | SHIELDS Benjamin J, DOYLE Abigail G. Direct C(sp3)-H cross coupling enabled by catalytic generation of chlorine radicals[J]. Journal of the American Chemical Society, 2016, 138(39): 12719-12722. |
| 89 | WINTER J, ILBERT M, Graf P C F, et al. Bleach activates a redox-regulated chaperone by oxidative protein unfolding[J]. Cell, 2008, 135(4): 691-701. |
| 90 | HUIE Robert E, CLIFTON Carol L, NETA Pedatsur. Electron transfer reaction rates and equilibria of the carbonate and sulfate radical anions[J]. International Journal of Radiation Applications and Instrumentation Part C Radiation Physics and Chemistry, 1991, 38(5): 477-481. |
| 91 | SHAN Ali, IDREES Ayesha, ZAMAN Waqas Qamar, et al. Synthesis of nZVI-Ni@BC composite as a stable catalyst to activate persulfate: Trichloroethylene degradation and insight mechanism[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104808. |
| 92 | GERASIMOV Oleg V, LYMAR Sergei V. The yield of hydroxyl radical from the decomposition of peroxynitrous acid[J]. Inorganic Chemistry, 1999, 38(19): 4317-4321. |
| 93 | LI Huarui, TIAN Jiayu, ZHU Zhigao, et al. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application[J]. Chemical Engineering Journal, 2018, 354: 507-516. |
| 94 | YANG Yi, BANERJEE Gourab, BRUDVIG Gary W, et al. Oxidation of organic compounds in water by unactivated peroxymonosulfate[J]. Environmental Science & Technology, 2018, 52(10): 5911-5919. |
| 95 | ZHANG Huixuan, XIE Chenghan, CHEN Long, et al. Different reaction mechanisms of S O 4 - · and •OH with organic compound interpreted at molecular orbital level in C o ( Ⅱ ) /peroxymonosulfate catalytic activation system[J]. Water Research, 2023, 229: 119392. |
| 96 | DUAN Xiaodi, YANG Shanshan, Stanisław WACŁAWEK, et al. Limitations and prospects of sulfate-radical based advanced oxidation processes[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103849. |
| 97 | CHEN Kufan, CHANG Yuchen, LIU Kuanyu. A kinetic and mechanistic study of the degradation of 1,2-dichloroethane and methyl tert-butyl ether using alkaline-activated persulfate oxidation[J]. RSC Advances, 2016, 6(79): 75578-75587. |
| 98 | WU Xiaoliang, GU Xiaogang, LU Shuguang, et al. Accelerated degradation of tetrachloroethylene by Fe(Ⅱ) activated persulfate process with hydroxylamine for enhancing Fe(Ⅱ) regeneration[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(5): 1280-1289. |
| 99 | MATILAINEN Anu, Mikko VEPSÄLÄINEN, Mika SILLANPÄÄ. Natural organic matter removal by coagulation during drinking water treatment: A review[J]. Advances in Colloid and Interface Science, 2010, 159(2): 189-197. |
| 100 | HADI Sousan, TAHERI Ensiyeh, AMIN Mohammad Mehdi, et al. Advanced oxidation of 4-chlorophenol via combined pulsed light and sulfate radicals methods: Effect of co-existing anions[J]. Journal of Environmental Management, 2021, 291: 112595. |
| 101 | HOANG Nguyen Tien, NGUYEN Vo Thang, TUAN Nguyen Dinh Minh, et al. Degradation of dyes by UV/Persulfate and comparison with other UV-based advanced oxidation processes: Kinetics and role of radicals[J]. Chemosphere, 2022, 298: 134197. |
| 102 | MATILAINEN Anu, GJESSING Egil T, LAHTINEN Tanja, et al. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment[J]. Chemosphere, 2011, 83(11): 1431-1442. |
| 103 | LI Xiaodong, WU Bin, ZHANG Qian, et al. Mechanisms on the impacts of humic acids on persulfate/Fe2+-based groundwater remediation[J]. Chemical Engineering Journal, 2019, 378: 122142. |
| [1] | 赵雨龙, 蔡凯, 于善青. 氧化铝孔结构对催化裂化烃类分子吸附扩散及反应性能的影响[J]. 化工进展, 2025, 44(S1): 213-221. |
| [2] | 李军良, 李悦, 孙道来. Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇[J]. 化工进展, 2025, 44(S1): 222-231. |
| [3] | 刘超, 丁承奥, 吴宝顺, 雷欣宇, 王光应, 余正伟. TiO2载体粒度对RuO x -V2O5-WO3/TiO2催化剂脱硝及抗水硫中毒性能的影响[J]. 化工进展, 2025, 44(S1): 232-242. |
| [4] | 张涵林, 岳学海, 刘俊希, 殷逢俊. 钌锶铱电沉积构筑高稳定性析氧反应电催化剂[J]. 化工进展, 2025, 44(S1): 243-251. |
| [5] | 刘哲, 周顺利, 李永祥, 张成喜, 刘宜鹏. 烷基萘合成催化剂研究进展[J]. 化工进展, 2025, 44(S1): 144-158. |
| [6] | 林已杰, 乔鹏, 李心睿, 张宏斌, 王雪芹. TiO2纳米光催化剂的异质结构建策略与应用研究进展[J]. 化工进展, 2025, 44(S1): 159-177. |
| [7] | 王涛, 张雪冰, 张琪, 陈强, 张魁, 门卓武. 还原碳化温度和CO浓度对工业级费托合成沉淀铁催化剂性能的影响[J]. 化工进展, 2025, 44(S1): 178-184. |
| [8] | 包新德, 刘必烨, 黄仁伟, 洪宇豪, 关鑫, 林金国. 生物质基@CuNiOS复合催化剂的制备及其在有机染料还原中的应用[J]. 化工进展, 2025, 44(S1): 185-196. |
| [9] | 赵思阳, 李陈冉, 刘洋. 副产C4预积炭调控MTO再生催化剂双烯选择性的工艺优化[J]. 化工进展, 2025, 44(S1): 205-212. |
| [10] | 孙梦圆, 陆诗建, 刘玲, 薛艳阳, 张云蓉, 董琦, 康国俊. 金属有机框架及衍生物在碳捕集领域的研究进展[J]. 化工进展, 2025, 44(9): 5339-5350. |
| [11] | 王文君, 刘瑞鑫, 王军, 张庆磊, 侯立安. 浅析二氧化钛材料可见光降解室内VOCs的研究进展[J]. 化工进展, 2025, 44(9): 5351-5362. |
| [12] | 曾金, 高艳, 王赵鹏, 谢雨芸, 刘俊, 梁旗, 王春英. NaYF4:Yb,Tm复合TiO2/Bi2WO6光催化降解2,4-二氯苯氧乙酸机制及产物毒性评价[J]. 化工进展, 2025, 44(9): 5416-5431. |
| [13] | 陈子朝, 何方书, 胡强, 杨扬, 陈汉平, 杨海平. 甲烷干重整抗积炭Ni基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4968-4978. |
| [14] | 王振, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 甲烷干重整用Ni/Al2O3基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4979-4998. |
| [15] | 张海鹏, 秦珊珊, 王俣萱, 于海彪. 3.0F-Ag x Co催化剂的制备及其催化分解N2O[J]. 化工进展, 2025, 44(9): 4999-5005. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||