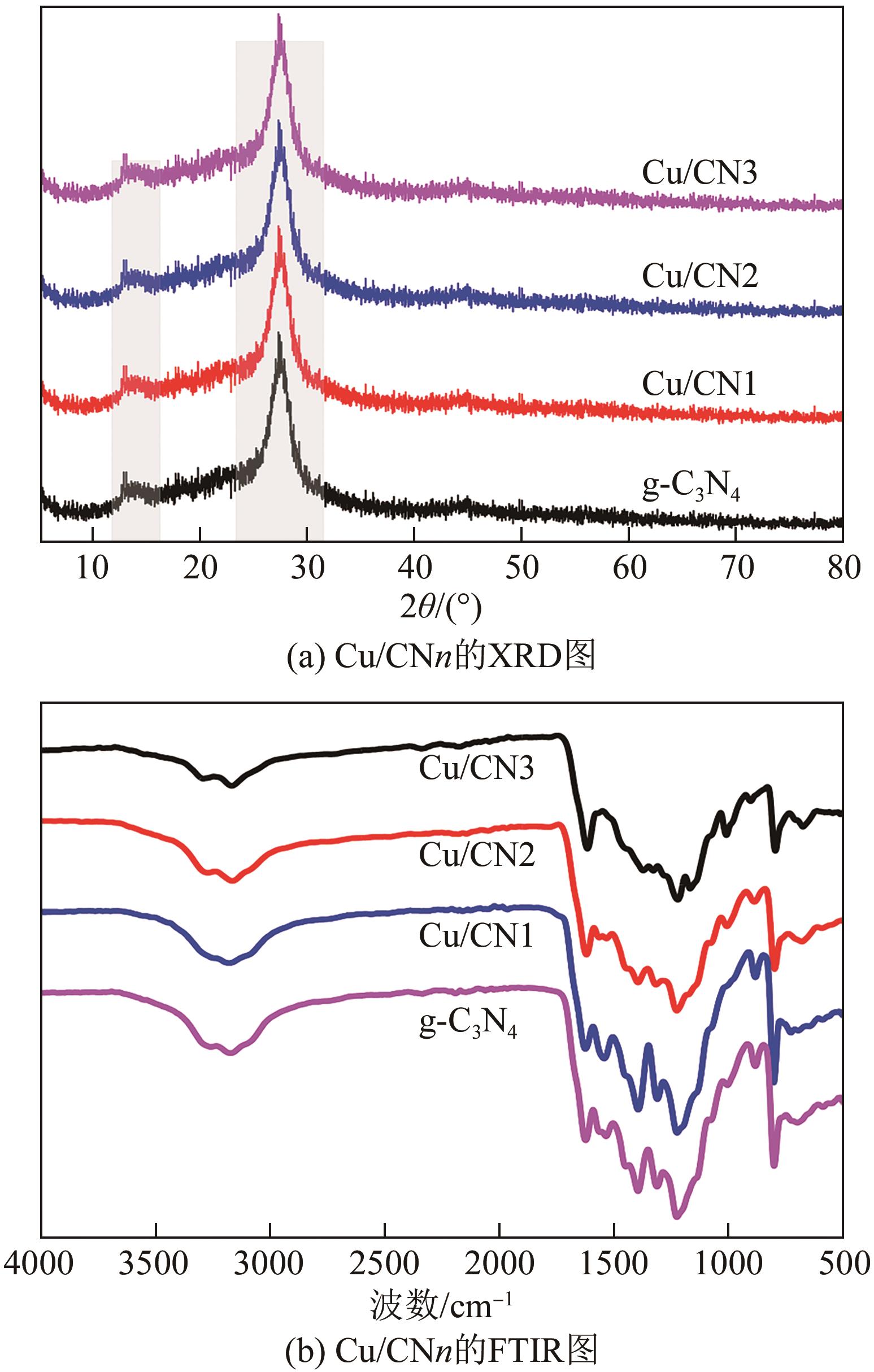
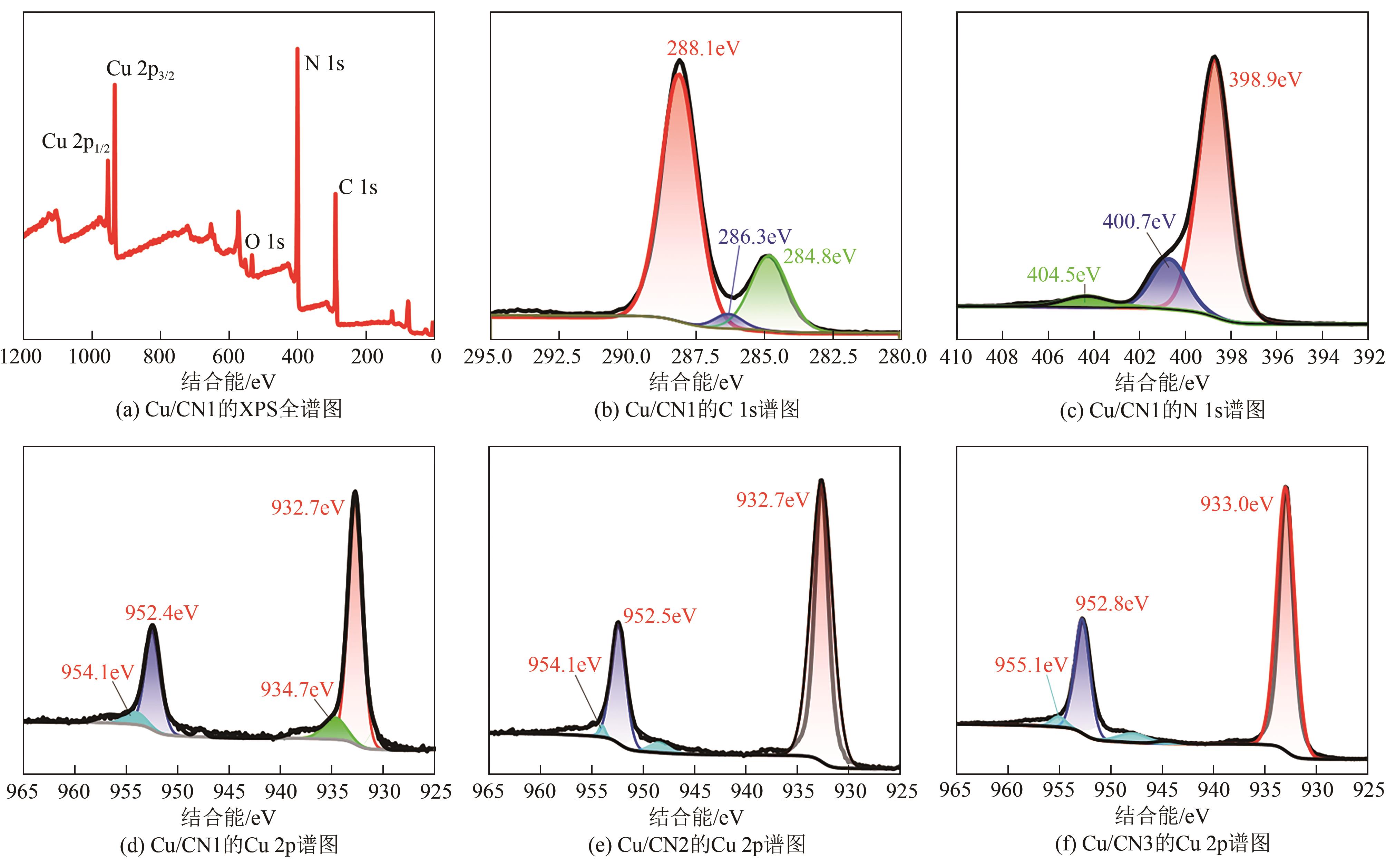
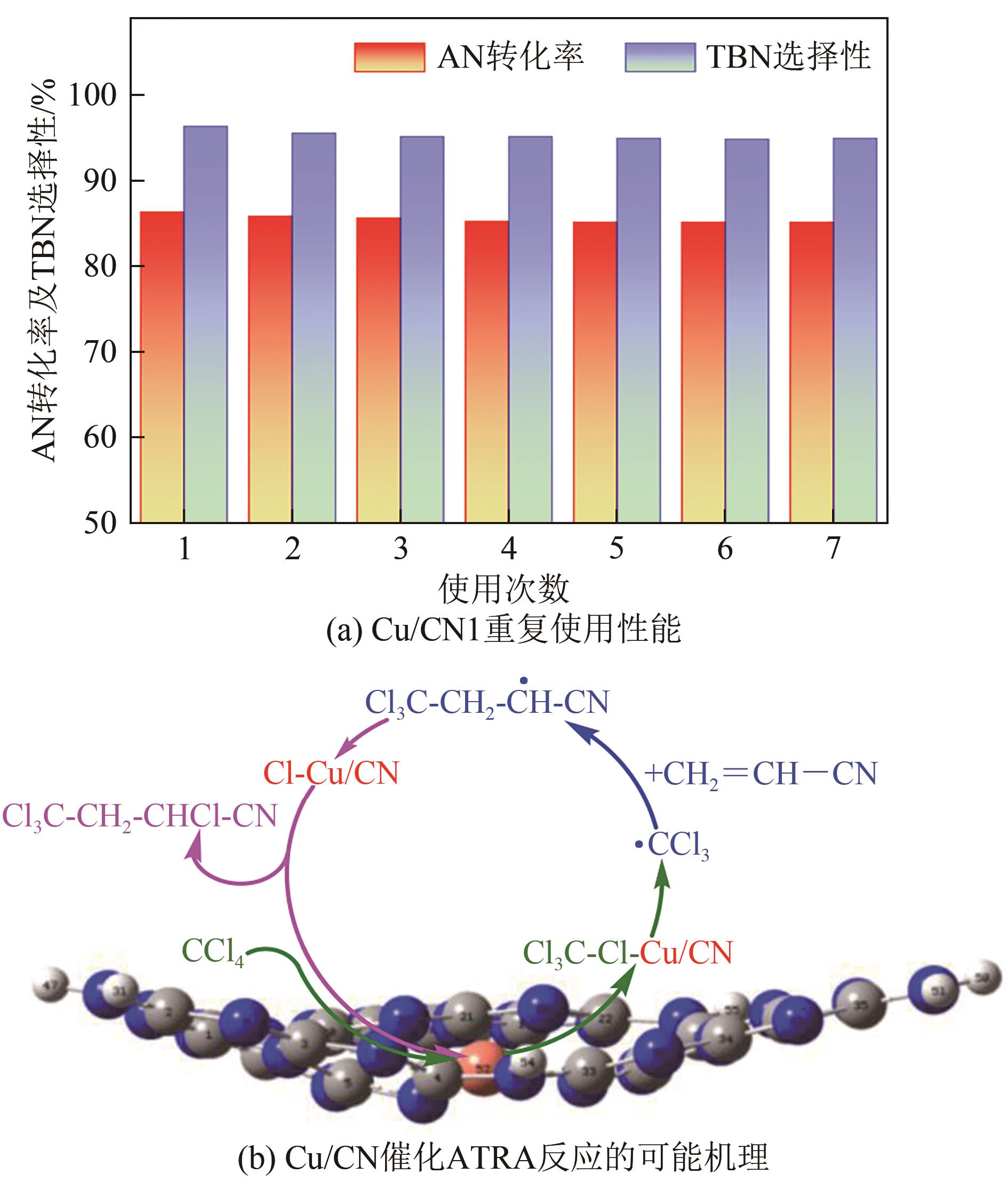
| 1 |
LIANG Yunyan, Guifen LYU, OUYANG Xuanhui, et al. Recent developments in the polychloroalkylation by use of simple alkyl chlorides[J]. Advanced Synthesis and Catalysis, 2021, 363(2): 290-304.
|
| 2 |
HUANG Gao, YU Jintao, PAN Changduo. Recent advances in polychloromethylation reactions[J]. Advanced Synthesis and Catalysis, 2021, 363(2): 305-327.
|
| 3 |
姜亚光, 王瑞康, 王倩, 等. 以三氯乙烯为供氢剂将四氯化碳转化为氯仿[J]. 化工进展, 2021, 40(2): 1114-1120.
|
|
JIANG Yaguang, WANG Ruikang, WANG Qian, et al. Converting carbon tetrachloride to chloroform by using trichloroethene as hydrogen donor[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1114-1120.
|
| 4 |
袁其亮, 姚焰生, 鲍张丰, 等. 一种 2,4,4,4-四氯丁腈的合成方法: CN104230751A[P]. 2016-05-25.
|
|
YUAN Qiliang, YAO Yansheng, BAO Zhangfeng, et al. Method for synthesizing 2,4,4,4-tetrachlorobutyronitrile: CN104230751A[P]. 2016-05-25.
|
| 5 |
肖自胜, 尹笃林, 兰支利, 等. 一种 2,4,4,4-四氯丁腈的合成方法: CN109535029B[P]. 2020-05-26.
|
|
XIAO Zisheng, YIN Dulin, LAN Zhili, et al. Synthesis method of 2,4,4,4-tetrachlorobutyronitrile: CN109535029B[P]. 2020-05-26.
|
| 6 |
LUNT M F, PARK S, LI S, et al. Continued emissions of the ozone-depleting substance carbon tetrachloride from eastern Asia[J].Geophysical Research Letters, 2018, 45(20): 11423-11430.
|
| 7 |
MOUSA Abdelrazek H, BENDIX Jesper, WENDT Ola F. Synthesis, characterization, and reactivity of PCN pincer nickel complexes[J]. Organometallics, 2018, 37(15): 2581-2593.
|
| 8 |
ENGL Sebastian, REISER Oliver. Copper-photocatalyzed ATRA reactions: Concepts, applications, and opportunities[J]. Chemical Society Reviews, 2022, 51(13): 5287-5299.
|
| 9 |
CHEN Bo, FANG Cheng, LIU Peng, et al. Rhodium-catalyzed enantioselective radical addition of CX4 reagents to olefins[J]. Angewandte Chemie International Edition, 2017, 56(30): 8780-8784.
|
| 10 |
Kanu DAS, DUTTA Moumita, Babulal DAS, et al. Efficient pincer-ruthenium catalysts for Kharasch addition of carbon tetrachloride to styrene[J]. Advanced Synthesis and Catalysis, 2019, 361(12): 2965-2980.
|
| 11 |
CHENG Wanmin, SHANG Rui. Transition metal-catalyzed organic reactions under visible light: Recent developments and future perspectives[J]. ACS Catalysis, 2020, 10(16): 9170-9196.
|
| 12 |
BUSSEY Katherine A, CAVALIER Annie R, MRAZ Margaret E, et al. Synthesis, characterization, X-ray crystallography analysis, and catalytic activity of bis(2-pyridylmethyl)amine copper complexes containing coupled pendent olefinic arms in atom transfer radical addition (ATRA) reactions[J]. Polyhedron, 2016, 114(1): 256-267.
|
| 13 |
CHAIBUTH Pawittra, CHUAYTANEE Nontakarn, HOJITSIRIYANONT Jutawat, et al. Copper(Ⅱ) complexes of quinoline-based ligands for efficient photoredox catalysis of atom transfer radical addition (ATRA) reaction[J]. New Journal of Chemistry, 2022, 46(25): 12158-12168.
|
| 14 |
张小玉, 薛冬萍, 杜宇, 等. MOF衍生碳基电催化剂限域催化O2还原和CO2还原反应[J]. 高等学校化学学报, 2022, 43(3): 12-31.
|
|
ZHANG Xiaoyu, XUE Dongping, DU Yu, et al. MOF-derived carbon-based electrocatalysts confinement catalyst on O2 reduction and CO2 reduction reactions[J]. Chemical Journal of Chinese Universities, 2022, 43(3): 12-31.
|
| 15 |
BAI Xiaowan, ZHAO Xunhua, ZHANG Yehui, et al. Dynamic stability of copper single-atom catalysts under working conditions[J]. Journal of the American Chemical Society, 2022, 144(37): 17140-17148.
|
| 16 |
YANG Zhengkun, CHEN Bingxu, CHEN Wenxing, et al. Directly transforming copper(Ⅰ) oxide bulk into isolated single-atom copper sites catalyst through gas-transport approach[J]. Nature Communications, 2019, 10: 3734.
|
| 17 |
LIU Xin, JIAO Yan, ZHENG Yao, et al. Building up a picture of the electrocatalytic nitrogen reduction activity of transition metal single-atom catalysts[J]. Journal of the American Chemical Society, 2019, 141(24): 9664-9672.
|
| 18 |
FEI Jia, PENG Xin, JIANG Longbo, et al. Recent advances in graphitic carbon nitride as a catalyst for heterogeneous Fenton-like reactions[J]. Dalton Transactions, 2021, 50(46): 16887-16908.
|
| 19 |
WU Qian, WEI Wei, Xingshuai LYU, et al. Cu@g-C3N4: An efficient single-atom electrocatalyst for NO electrochemical reduction with suppressed hydrogen evolution[J]. The Journal of Physical Chemistry C, 2019, 123(51): 31043-31049.
|
| 20 |
LE Shukun, JIANG Tingshun, ZHAO Qian, et al. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis[J]. RSC Advances, 2016, 6(45): 38811-38819.
|
| 21 |
CHEN Xiufang, ZHANG Jinshui, FU Xianzhi, et al. Fe-g-C3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light[J]. Journal of the American Chemical Society, 2009, 131(33): 11658-11659.
|
| 22 |
YANG Nating, ZHAO Yonghui, WU Ping, et al. Defective C3N4 frameworks coordinated diatomic copper catalyst: Towards mild oxidation of methane to C1 oxygenates[J]. Applied Catalysis B: Environmental, 2021, 299: 120682.
|
| 23 |
Julia BÜKER, HUANG Xiubing, BITZER Johannes, et al. Synthesis of Cu single atoms supported on mesoporous graphitic carbon nitride and their application in liquid-phase aerobic oxidation of cyclohexene[J]. ACS Catalysis, 2021, 11(13): 7863-7875.
|
| 24 |
IVANOV A V, MAKSIMOV A Y, TOMILOVA L G, et al. Metal phthalocyanine-catalyzed addition of polychlorine-containing organic compounds to C=C bonds[J]. Russian Chemical Bulletin, 2009, 58(11): 2393-2396.
|
| 25 |
BALILI Marielle Nicole C, PINTAUER Tomislav. Photoinitiated ambient temperature copper-catalyzed atom transfer radical addition (ATRA) and cyclization (ATRC) reactions in the presence of free-radical diazo initiator (AIBN) [J]. Dalton Transactions, 2011, 40(12): 3060-3066
|
| 26 |
肖自胜, 陈烨, 刘健, 等. CuCl2-N-甲基咪唑催化合成2,4,4,4-四氯丁腈[J]. 精细化工, 2022, 39(5): 927-932.
|
|
XIAO Zisheng, CHEN Ye, LIU Jian, et al. Synthesis of 2,4,4,4-tetrachlorobutyronitrile catalyzed by N-methylimidazole-CuCl2 complexes[J]. Fine Chemicals, 2022, 39(5): 927-932.
|
| 27 |
RICHOUX Gary M, YANG Liu, NORRIS Edmund J, et al. Structure-activity relationship analysis of potential new vapor-active insect repellents[J]. Journal of Agricultural and Food Chemistry, 2020, 68(47): 13960-13969.
|
| 28 |
MUKHOPADHYAY Titas Kumar, LEHERTE Laurence, DATTA Ayan. Molecular mechanism for the self-supported synthesis of graphitic carbon nitride from urea pyrolysis[J]. The Journal of Physical Chemistry Letters, 2021, 12(5): 1396-1406.
|
| 29 |
LI Qiulan, YANG Dezhi, YIN Qinhong, et al. Graphitic carbon nitride nanosheets decorated with Cu-doped carbon dots for the detection and degradation of phenolic pollutants[J]. ACS Applied Nano Materials, 2022, 5(2): 1925-1934.
|
| 30 |
WANG Danhao, AO Chengcheng, LIU Xiaokang, et al. Coordination-engineered Cu-N x single-site catalyst for enhancing oxygen reduction reaction[J]. ACS Applied Energy Materials, 2019, 2(9): 6497-6504.
|
| 31 |
SYAL Bindu, KUMAR Pawan, GUPTA Princy. Recent advancements in the preparation and application of copper single-atom catalysts[J]. ACS Applied Nano Materials, 2023, 6(7): 4987-5041.
|
| 32 |
DOU Hailong, CHEN Lu, ZHENG Shaohui, et al. Band structure engineering of graphitic carbon nitride via Cu2+/Cu+ doping for enhanced visible light photoactivity[J]. Materials Chemistry and Physics, 2018, 214: 482-488.
|
| 33 |
RAVICHANDRAN K, SHANTHA SEELAN K, KAVITHA P, et al. Influence of Cu+g-C3N4 incorporation on the photocatalytic dye decomposition of ZnO film coated on stainless steel wire meshes[J]. Journal of Materials Science: Materials in Electronics, 2019, 30(22): 19703-19717.
|
| 34 |
WOJTYLA S, BARAN T. Multi‐technical study of copper oxide on graphitic carbon nitride and its role in the photocatalytic reactions[J]. Nano Select, 2021, 2(2): 389-397.
|
| 35 |
SAMADI Saadi, ARVINNEZHAD Hamid, MANSOORI Sirwan, et al. Preparation and DFT studies of chiral Cu(Ⅰ)-complexes of biphenyl bisoxazolines and their application in enantioselective Kharasch-Sosnovsky reaction[J]. Scientific Reports, 2022, 12: 15038.
|
 ), 李金玲1,2, 陈伊睿1,2, 兰支利1,2(
), 李金玲1,2, 陈伊睿1,2, 兰支利1,2( ), 尹笃林1,2
), 尹笃林1,2
 ), LI Jinling1,2, CHEN Yirui1,2, LAN Zhili1,2(
), LI Jinling1,2, CHEN Yirui1,2, LAN Zhili1,2( ), YIN Dulin1,2
), YIN Dulin1,2