化工进展 ›› 2021, Vol. 40 ›› Issue (6): 3389-3400.DOI: 10.16085/j.issn.1000-6613.2020-1305
二次等温热缩聚改性对g-C3N4光催化剂性能的影响
段丽媛1,2( ), 李国强1,2(
), 李国强1,2( ), 张舒婷1,2, 王宏宇1,2, 赵永乐1,2, 张永发1,2
), 张舒婷1,2, 王宏宇1,2, 赵永乐1,2, 张永发1,2
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学煤科学与 技术教育部重点实验室,山西 太原 030024
-
收稿日期:2020-07-10修回日期:2020-11-12出版日期:2021-06-06发布日期:2021-06-22 -
通讯作者:李国强 -
作者简介:段丽媛(1996—),女,硕士研究生,研究方向为光催化。E-mail:136487953@qq.com 。 -
基金资助:山西省煤基重点攻关项目(MJH2014-09);山西省重点研发项目(201703D321006);太原理工大学青年基金(2015QN093)
Effect of secondary isothermal condensation modification on the performance of g-C3N4 photocatalyst
DUAN Liyuan1,2( ), LI Guoqiang1,2(
), LI Guoqiang1,2( ), ZHANG Shuting1,2, WANG Hongyu1,2, ZHAO Yongle1,2, ZHANG Yongfa1,2
), ZHANG Shuting1,2, WANG Hongyu1,2, ZHAO Yongle1,2, ZHANG Yongfa1,2
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.Key Laboratory of Coal Science and Technology, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2020-07-10Revised:2020-11-12Online:2021-06-06Published:2021-06-22 -
Contact:LI Guoqiang
摘要:
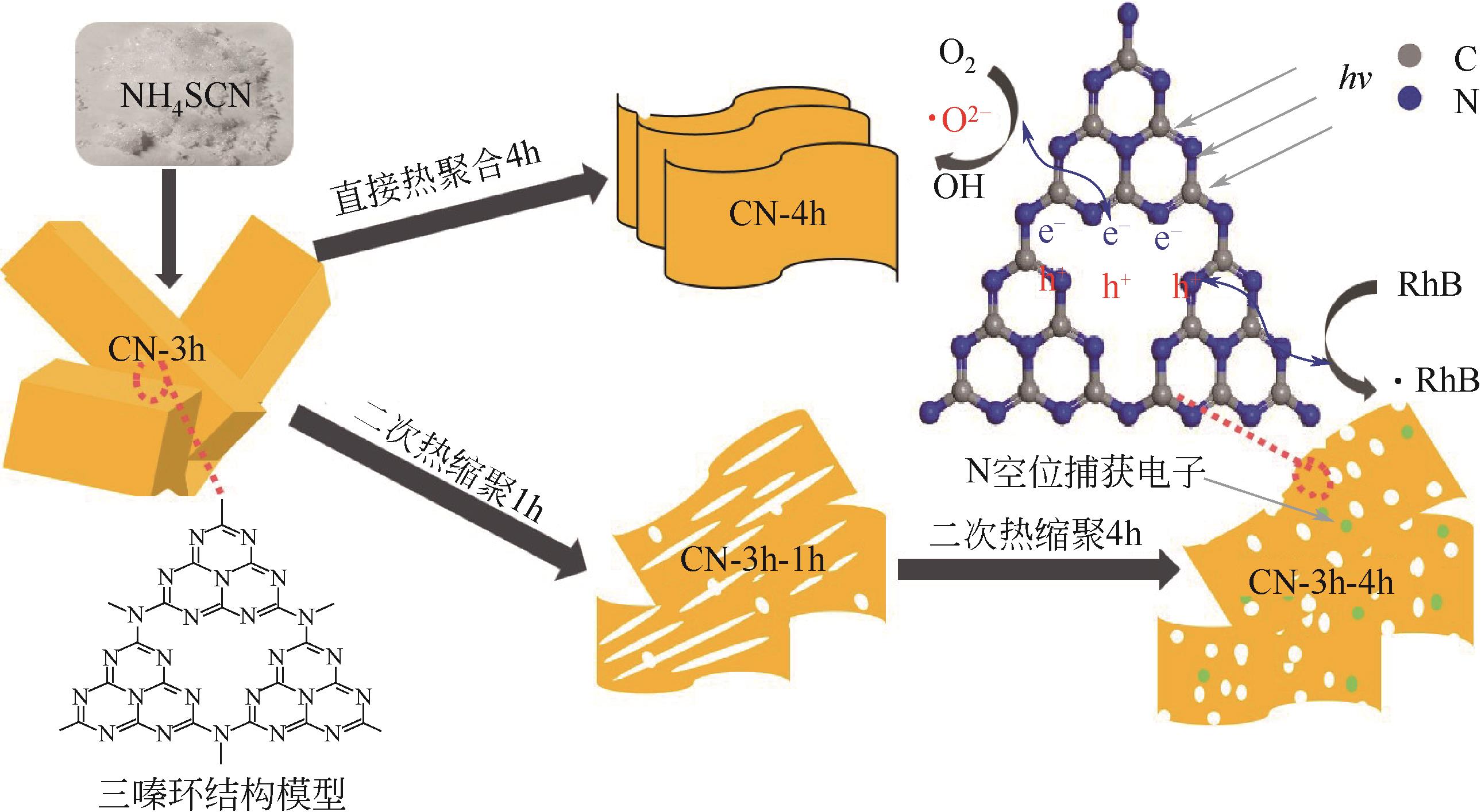
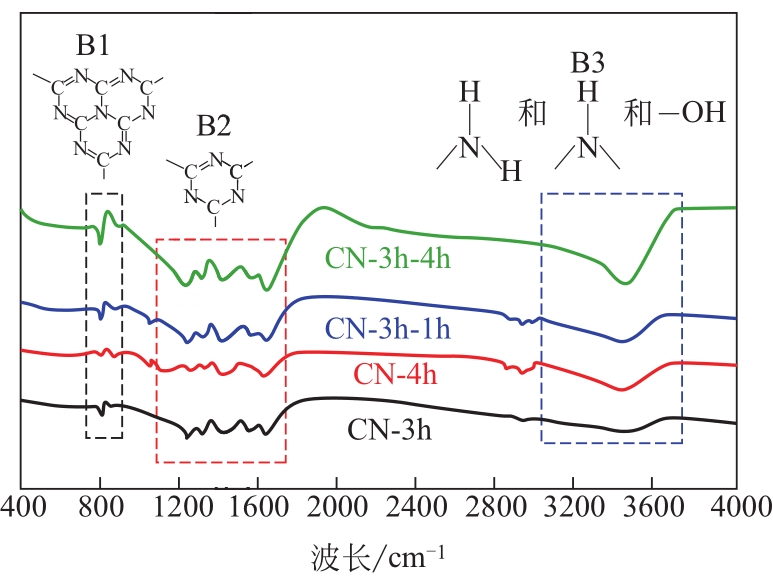
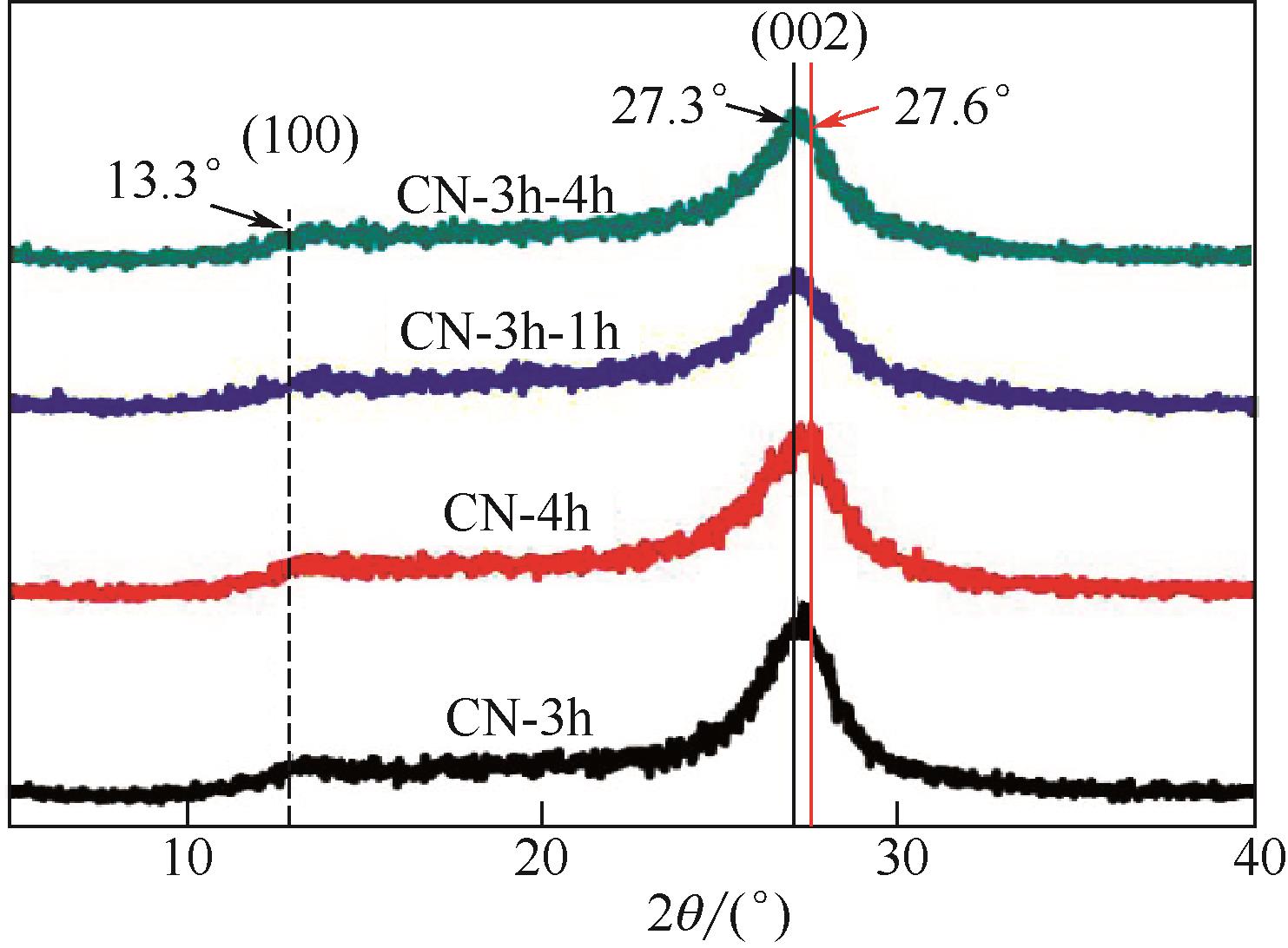
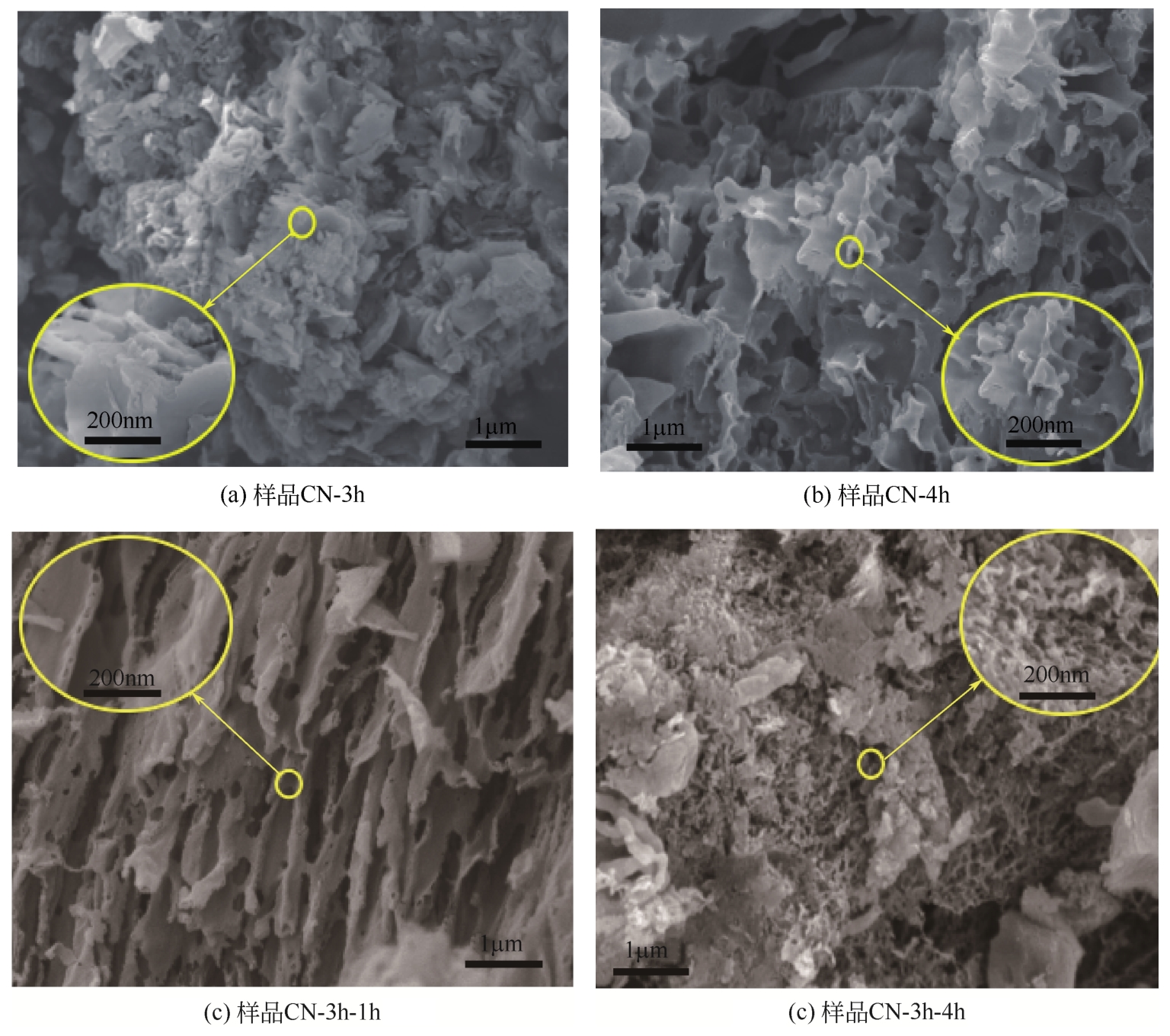
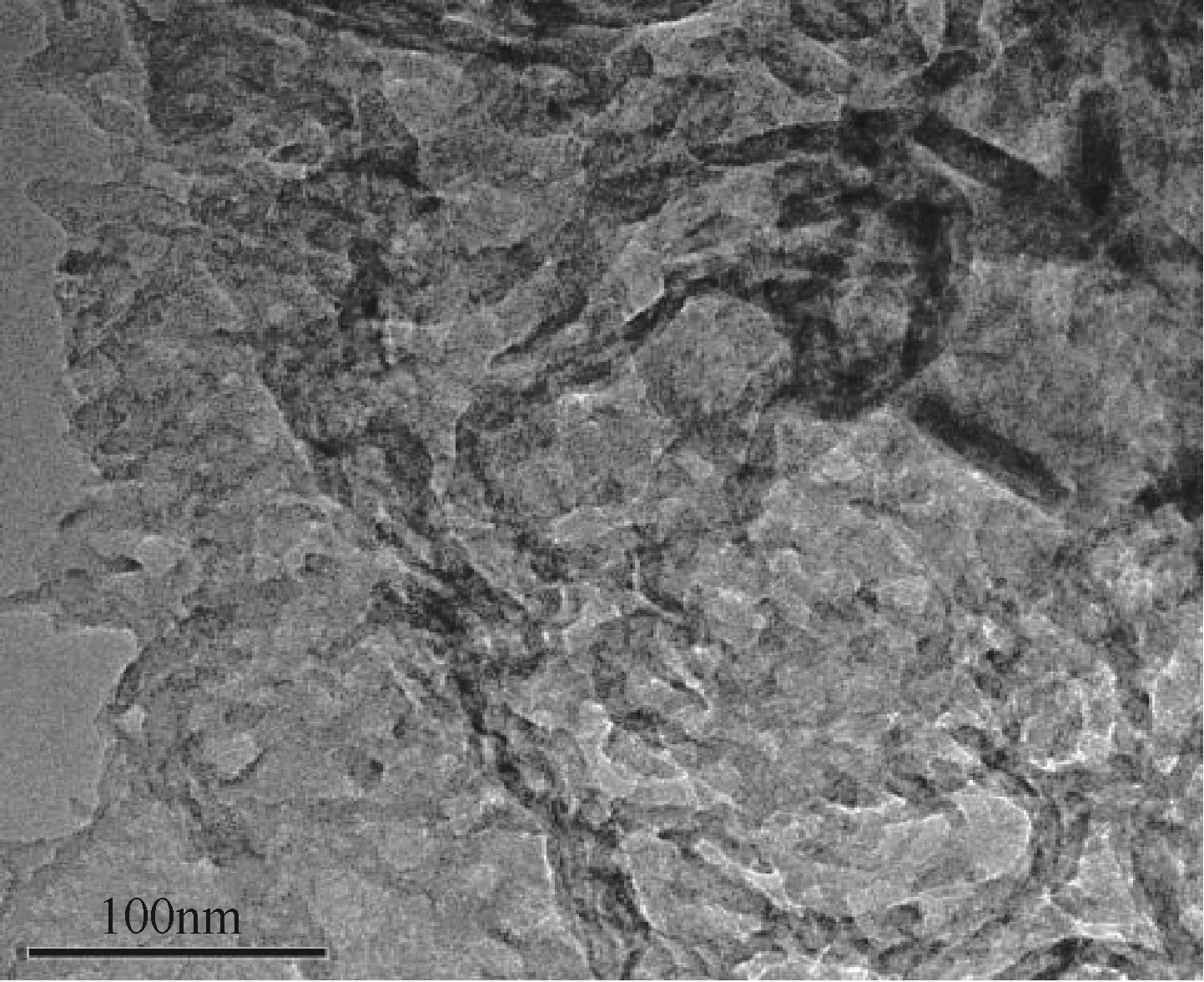
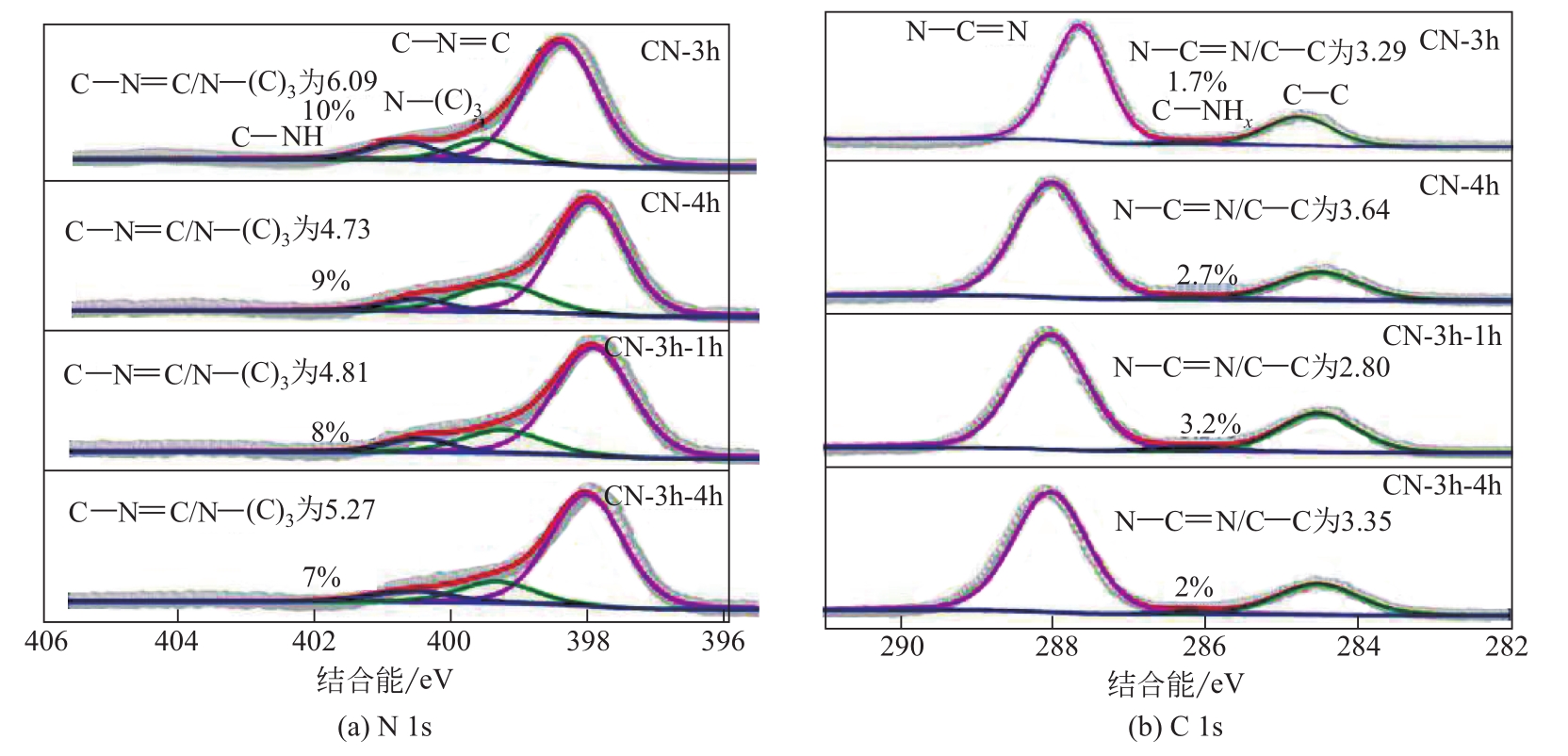
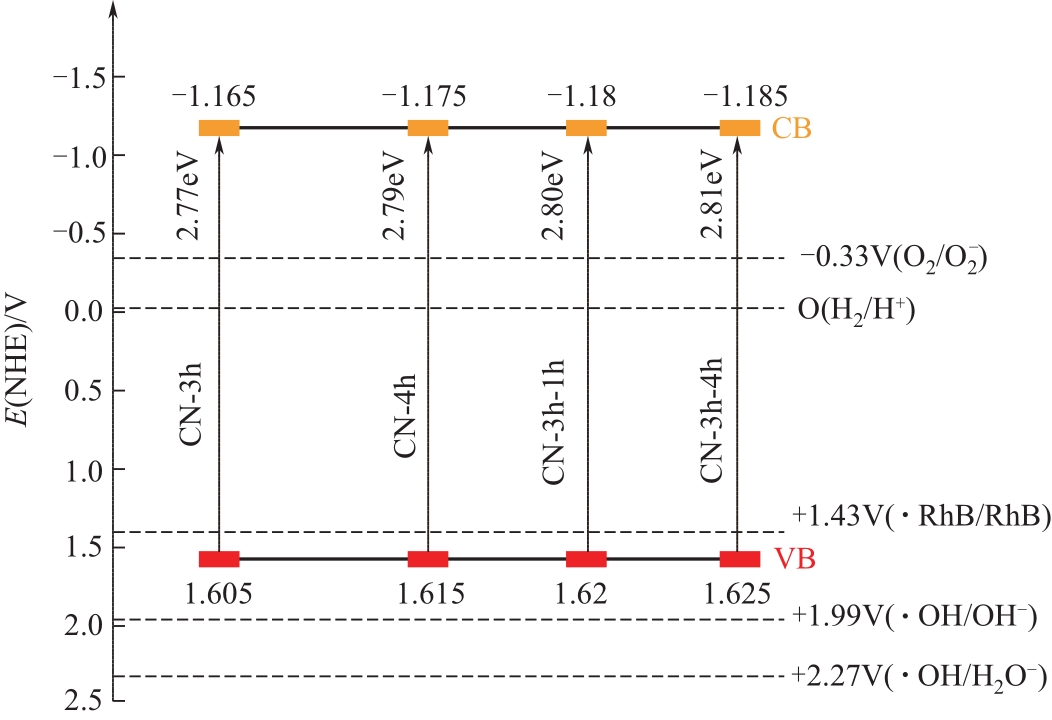
采用二次等温热缩聚方式对通过NH4SCN制得的g-C3N4进行处理,通过XRD和SEM/TEM等手段对所制备样品的物相结构、表面形貌等结构特性及有机染料RhB的降解效果进行了研究。结果表明,二次等温热缩聚改性使催化剂聚合度增加,层间距缩短,通过调节等温热缩聚处理时间构建了蜂窝孔洞薄片层表面结构,比表面积和孔容积分别增加了2.3倍和4.7倍;大量—NH—、—NH2等氨基基团的产生,带来了更高的吸附氧含量;带隙宽度由2.77eV拓宽至2.81eV,且价带位置增至+1.625eV,使光生空穴拥有更强的氧化降解能力;边缘三嗪环结构单元中C—N
中图分类号:
引用本文
段丽媛, 李国强, 张舒婷, 王宏宇, 赵永乐, 张永发. 二次等温热缩聚改性对g-C3N4光催化剂性能的影响[J]. 化工进展, 2021, 40(6): 3389-3400.
DUAN Liyuan, LI Guoqiang, ZHANG Shuting, WANG Hongyu, ZHAO Yongle, ZHANG Yongfa. Effect of secondary isothermal condensation modification on the performance of g-C3N4 photocatalyst[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3389-3400.
| 样品 | 比表面积 /m2·g-1 | 孔容积 /mL·g-1 | 平均孔径 /nm | 罗丹明B降解率 /% |
|---|---|---|---|---|
| CN-3h | 12.95 | 0.072 | 22.12 | 42 |
| CN-4h | 13.40 | 0.071 | 21.28 | 58 |
| CN-3h-1h | 15.42 | 0.094 | 24.42 | 71 |
| CN-3h-4h | 29.83 | 0.334 | 44.82 | 98 |
表1 CN-3h、CN-4h、CN-3h-1h和CN-3h-4h样品的比表面积、孔容积、平均孔径和RhB降解率
| 样品 | 比表面积 /m2·g-1 | 孔容积 /mL·g-1 | 平均孔径 /nm | 罗丹明B降解率 /% |
|---|---|---|---|---|
| CN-3h | 12.95 | 0.072 | 22.12 | 42 |
| CN-4h | 13.40 | 0.071 | 21.28 | 58 |
| CN-3h-1h | 15.42 | 0.094 | 24.42 | 71 |
| CN-3h-4h | 29.83 | 0.334 | 44.82 | 98 |
| 样品 | N元素摩尔分数 /%① | C元素摩尔分数 /%① | 三种N拟合峰含量与总N含量比/%② | 三种C拟合峰含量与总C含量比/%② | ||||
|---|---|---|---|---|---|---|---|---|
| C—N | N—(C)3 | C—NH | N—C | C—C | C—NHx | |||
| CN-3h | 48.48 | 32.61 | 77 | 19 | 10 | 76 | 22 | 2 |
| CN-4h | 48.68 | 32.31 | 75 | 16 | 9 | 77 | 21 | 2 |
| CN-3h-1h | 47.85 | 32.85 | 76 | 16 | 8 | 71 | 26 | 3 |
| CN-3h-4h | 47.86 | 32.90 | 78 | 15 | 7 | 76 | 22 | 2 |
表2 CN-3h、CN-4h、CN-3h-1h和CN-3h-4h催化剂表面C、N组成
| 样品 | N元素摩尔分数 /%① | C元素摩尔分数 /%① | 三种N拟合峰含量与总N含量比/%② | 三种C拟合峰含量与总C含量比/%② | ||||
|---|---|---|---|---|---|---|---|---|
| C—N | N—(C)3 | C—NH | N—C | C—C | C—NHx | |||
| CN-3h | 48.48 | 32.61 | 77 | 19 | 10 | 76 | 22 | 2 |
| CN-4h | 48.68 | 32.31 | 75 | 16 | 9 | 77 | 21 | 2 |
| CN-3h-1h | 47.85 | 32.85 | 76 | 16 | 8 | 71 | 26 | 3 |
| CN-3h-4h | 47.86 | 32.90 | 78 | 15 | 7 | 76 | 22 | 2 |
| 1 | TORRES-PINTO A, SAMPAIO M J, SILVA C G, et al. Metal-free carbon nitride photocatalysis with in situ hydrogen peroxide generation for the degradation of aromatic compounds[J]. Applied Catalysis B: Environmental, 2019, 252: 128-137. |
| 2 | 李成伟, 张安超, 宋军, 等. Ag/BiOI光催化剂湿法脱除烟气中气态单质汞性能及机理[J]. 化工进展, 2018, 37(4): 1442-1450. |
| LI Chengwei, ZHANG Anchao, SONG Jun, et al. Mechanism and performance of wet process to remove gaseous elemental mercury from flue gas using Ag/BiOI photocatalyst[J]. Chemical Industry and Engineering Progress, 2018, 37(4): 1442-1450. | |
| 3 | 白照杲, 胡芸, 游素珍, 等. Bi2WO6-TiO2复合光催化剂对Cu-EDTA复合污染的高效光催化协同处理[J]. 化工进展, 2017, 36(6): 2164-2170. |
| BAI Zhaogao, HU Yun, YOU Suzhen, et al. Synergetic treatment of Cu-EDTA on Bi2WO6-TiO2 composite photocatalysts[J]. Chemical Industry and Engineering Progress, 2017, 36(6): 2164-2170. | |
| 4 | HU K, WEI Z Y, YANG Z X, et al. One-step synthesis of few layers g-C3N4 with suitable band structure and enhanced photocatalytic activities[J]. Chemical Physics Letters, 2019, 732: 136613. |
| 5 | LIU J H, ZHANG T K, WANG Z C, et al. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity[J]. Journal of Materials Chemistry, 2011, 21(38): 14398. |
| 6 | XIN G, MENG Y L. Pyrolysis synthesized g-C3N4 for photocatalytic degradation of methylene blue[J]. Journal of Chemistry, 2013, 2013: 1-5. |
| 7 | XU J, WANG Y, ZHU Y. Nanoporous graphitic carbon nitride with enhanced photocatalytic performance[J]. Langmuir, 2013, 29(33): 10566-10572. |
| 8 | FAN X Q, XING Z, SHU Z, et al. Improved photocatalytic activity of g-C3N4 derived from cyanamide-urea solution[J]. RSC Advances, 2015, 5(11): 8323-8328. |
| 9 | CUI Y J, WANG Y X, WANG H, et al. Polycondensation of ammonium thiocyanate into novel porous g-C3N4 nanosheets as photocatalysts for enhanced hydrogen evolution under visible light irradiation[J]. Chinese Journal of Catalysis, 2016, 37(11): 1899-1906. |
| 10 | HONG J D, XIA X Y, WANG Y S, et al. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light[J]. Journal of Materials Chemistry, 2012, 22(30): 15006. |
| 11 | CUI Y J, WANG Y X, WANG H, et al. Polycondensation of ammonium thiocyanate into novel porous g-C3N4 nanosheets as photocatalysts for enhanced hydrogen evolution under visible light irradiation[J]. Chinese Journal of Catalysis, 2016, 37(11): 1899-1906. |
| 12 | ZHANG S T, LI G Q, WANG H Y, et al. Study on the pyrolysis of ammonium thiocyanate and its product formation characteristics in H2[J]. Journal of Analytical and Applied Pyrolysis, 2018, 134: 427-438. |
| 13 | LARSEN F K, HASEN MAMAKHEL A, OVERGAARD J, et al. Accessing the rich carbon nitride materials chemistry by heat treatments of ammonium thiocyanate, NH4SCN[J]. Acta Crystallographica Section B, 2019, 75(4): 621-633. |
| 14 | 李宇涵. 石墨型碳化氮的微/纳观结构优化及可见光催化净化气相NO的性能增强机制[D]. 重庆: 重庆工商大学, 2015. |
| LI Yuhan. Optimization of the micro/nano structure of graphitic carbon nitride and enhancement mechanism of visible light photocatalytic removal of gaseous NO[D]. Chongqing: Chongqing Technology and Business University, 2015. | |
| 15 | 张晓君, 李佳乐, 刘一儒, 等. 固相研磨法制备AgI/Ag3PO4复合光催化剂及其光催化性能[J]. 化工进展, 2019, 38(2): 892-898. |
| ZHANG Xiaojun, LI Jiale, LIU Yiru, et al. Synthesis of AgI/Ag3PO4composite photocatalysts using solid state grinding method and their photocatalytic activities[J]. Chemical Industry and Engineering Progress, 2019, 38(2): 892-898. | |
| 16 | 严平, 占昌朝, 曹小华, 等. 原位合成H4SiW12O40@C协同UV/H2O2降解罗丹明B模拟废水[J]. 化工进展, 2015, 34(3): 872-878. |
| YAN Ping, ZHAN Changchao, CAO Xiaohua, et al. Synergetic degradation of Rhodamine B in simulated wastewater using ultraviolet and hydrogen peroxide catalyzed by H4SiW12O40@C synthesized in situ[J]. Chemical Industry and Engineering Progress, 2015, 34(3): 872-878. | |
| 17 | CUI Y J, ZHANG J S, ZHANG G G, et al. Synthesis of bulk and nanoporous carbon nitride polymers from ammonium thiocyanate for photocatalytic hydrogen evolution[J]. Journal of Materials Chemistry, 2011, 21(34): 13032. |
| 18 | ZHANG X Z, CHUN Y. Preparation of MgO/g-C3N4 composite and it enhanced photocatalytic activity[C]//Proceedings of the 2015 2nd International Workshop on Materials Engineering and Computer Sciences. October10-11, 2015. |
| Jinan, China. Paris: Atlantis Press, 2015: 509-521. | |
| 19 | CHAI B, YAN J T, WANG C L, et al. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride[J]. Applied Surface Science, 2017, 391: 376-383. |
| 20 | 郭志伟, 许亮. H.P.F法脱硫工艺[J]. 硅谷, 2011(6): 40. |
| GUO Zhiwei, XU Liang. H.P.F desulfurization process[J]. Silicon Valley, 2011(6): 40. | |
| 21 | 张翠翠. 脱硫废液提盐回收技术[D]. 太原: 太原理工大学, 2013. |
| ZHANG Cuicui. Study on reusing the technology of waste liquid generated desulfrization[D]. Taiyuan: Taiyuan University of Technology, 2013. | |
| 22 | LIU Q, WANG X L, YANG Q, et al. Mesoporous g-C3N4 nanosheets prepared by calcining a novel supramolecular precursor for high-efficiency photocatalytic hydrogen evolution[J]. Applied Surface Science, 2018, 450: 46-56. |
| 23 | KANG X D, KANG Y Y, HONG X X, et al. Improving the photocatalytic activity of graphitic carbon nitride by thermal treatment in a high-pressure hydrogen atmosphere[J]. Progress in Natural Science: Materials International, 2018, 28(2): 183-188. |
| 24 | DING W, LIU S Q, HE Z. One-step synthesis of graphitic carbon nitride nanosheets for efficient catalysis of phenol removal under visible light[J]. Chinese Journal of Catalysis, 2017, 38(10): 1711-1718. |
| 25 | CHEN Q Y, DU F, CHENG C, et al. Enhancing hydrogen evolution of g-C3N4 with nitrogen vacancies by ethanol thermal treatment[J]. Journal of Nanoparticle Research, 2018, 20(4): 1-9. |
| 26 | XU Q L, ZHU B C, CHENG B, et al. Photocatalytic H2 evolution on graphdiyne/g-C3N4 hybrid nanocomposites[J]. Applied Catalysis B: Environmental, 2019, 255: 117770. |
| 27 | HONG Y Z, LIU E L, SHI J Y, et al. A direct one-step synthesis of ultrathin g-C3N4 nanosheets from thiourea for boosting solar photocatalytic H2 evolution[J]. International Journal of Hydrogen Energy, 2019, 44(14): 7194-7204. |
| 28 | SUN S, FAN E, XU H, et al. Enhancement of photocatalytic activity of g-C3N4 by hydrochloric acid treatment of melamine[J]. Nanotechnology, 2019, 30(31): 315601. |
| 29 | HAN D Y, LIU J, CAI H, et al. High-yield and low-cost method to synthesize large-area porous g-C3N4 nanosheets with improved photocatalytic activity for gaseous nitric oxide and 2-propanol photodegradation[J]. Applied Surface Science, 2019, 464: 577-585. |
| 30 | SHAN X, GE G F, ZHAO Z K. Facile and scalable fabrication of porous g-C3N4 nanosheets with nitrogen defects and oxygen-doping for synergistically promoted visible light photocatalytic H2 evolution[J]. Energy Technology, 2019, 7(5): 1800886. |
| 31 | SHI J H, FENG S T, CHEN T, et al. Effect of porous modification on the synthesis and photocatalytic activity of graphitic carbon nitride/carbon quantum dot nanocomposite[J]. Journal of Materials Science: Materials in Electronics, 2018, 29(20): 17454-17462. |
| 32 | LIU J Y, YAN J, JI H Y, et al. Controlled synthesis of ordered mesoporous g-C3N4 with a confined space effect on its photocatalytic activity[J]. Materials Science in Semiconductor Processing, 2016, 46: 59-68. |
| 33 | HO W, ZHANG Z Z, XU M K, et al. Enhanced visible-light-driven photocatalytic removal of NO: effect on layer distortion on g-C3N4 by H2 heating[J]. Applied Catalysis B: Environmental, 2015, 179: 106-112. |
| 34 | YANG L R, LIU X Y, LIU Z G, et al. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor[J]. Ceramics International, 2018, 44(17): 20613-20619. |
| 35 | BOORBOOR AZIMI E, BADIEI A, HOSSAINI SADR M, et al. A template-free method to synthesize porous G-C3N4 with efficient visible light photodegradation of organic pollutants in water[J]. Advanced Powder Technology, 2018, 29(11): 2785-2791. |
| 36 | LIAO J Z, CUI W, LI J Y, et al. Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4[J]. Chemical Engineering Journal, 2020, 379: 122282. |
| 37 | YU W W, SHAN X, ZHAO Z K. Unique nitrogen-deficient carbon nitride homojunction prepared by a facile inserting-removing strategy as an efficient photocatalyst for visible light-driven hydrogen evolution[J]. Applied Catalysis B: Environmental, 2020, 269: 118778. |
| 38 | LIU M J, ZHANG D P, HAN J L, et al. Adsorption enhanced photocatalytic degradation sulfadiazine antibiotic using porous carbon nitride nanosheets with carbon vacancies[J]. Chemical Engineering Journal, 2020, 382: 123017. |
| 39 | ZHOU B, WAQAS M, YANG B, et al. Convenient one-step fabrication and morphology evolution of thin-shelled honeycomb-like structured g-C3N4 to significantly enhance photocatalytic hydrogen evolution[J]. Applied Surface Science, 2020, 506: 145004. |
| 40 | ANDRYUSHINA N, SHVALAGIN V, KORZHAK G, et al. Photocatalytic evolution of H2 from aqueous solutions of two-component electron-donor substrates in the presence of g-C3N4 activated by heat treatment in the KCl + LiCl melt[J]. Applied Surface Science, 2019, 475: 348-354. |
| 41 | HUANG Q, YU J G, CAO S W, et al. Efficient photocatalytic reduction of CO2 by amine-functionalized g-C3N4[J]. Applied Surface Science, 2015, 358: 350-355. |
| 42 | LAN Y L, LI Z S, LI D H, et al. Graphitic carbon nitride synthesized at different temperatures for enhanced visible-light photodegradation of 2-naphthol[J]. Applied Surface Science, 2019, 467/468: 411-422. |
| 43 | YI J J, LIAO J Z, XIA K X, et al. Integrating the merits of two-dimensional structure and heteroatom modification into semiconductor photocatalyst to boost NO removal[J]. Chemical Engineering Journal, 2019, 370: 944-951. |
| 44 | XU Y G, GE F Y, CHEN Z G, et al. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance[J]. Applied Surface Science, 2019, 469: 739-746. |
| 45 | SADIQ M M J, SHENOY U S, BHAT D K. Synthesis of BaWO4/NRGO-g-C3N4 nanocomposites with excellent multifunctional catalytic performance via microwave approach[J]. Frontiers of Materials Science, 2018, 12(3): 247-263. |
| 46 | LI H L, JIN C, WANG Z Y, et al. Effect of the intra- and inter-triazine N-vacancies on the photocatalytic hydrogen evolution of graphitic carbon nitride[J]. Chemical Engineering Journal, 2019, 369: 263-271. |
| 47 | KANG S F, ZHANG L, HE M F, et al. “Alternated cooling and heating” strategy enables rapid fabrication of highly-crystalline g-C3N4 nanosheets for efficient photocatalytic water purification under visible light irradiation[J]. Carbon, 2018, 137: 19-30. |
| 48 | BAI J, SUN Y Z, LI M Y, et al. The effect of phosphate modification on the photocatalytic H2O2 production ability of g-C3N4 catalyst prepared via acid-hydrothermal post-treatment[J]. Diamond and Related Materials, 2018, 87: 1-9. |
| 49 | ZHU K X, LV Y, LIU J, et al. Explosive thermal exfoliation of intercalated graphitic carbon nitride for enhanced photocatalytic degradation properties[J]. Ceramics International, 2019, 45(3): 3643-3647. |
| 50 | ZHONG Y J, WANG Z Q, FENG J Y, et al. Improvement in photocatalytic H2 evolution over g-C3N4 prepared from protonated melamine[J]. Applied Surface Science, 2014, 295: 253-259. |
| 51 | LI X X, WAN T, QIU J Y, et al. In-situ photocalorimetry-fluorescence spectroscopy studies of RhB photocatalysis over Z-scheme g-C3N4@Ag@Ag3PO4 nanocomposites: a pseudo-zero-order rather than a first-order process[J]. Applied Catalysis B: Environmental, 2017, 217: 591-602. |
| 52 | BO L L, HU Y S, ZHANG Z X, et al. Efficient photocatalytic degradation of Rhodamine B catalyzed by SrFe2O4/g-C3N4 composite under visible light[J]. Polyhedron, 2019, 168: 94-100. |
| 53 | AKHUNDI A, HABIBI-YANGJEH A. Novel g-C3N4/Ag2SO4 nanocomposites: fast microwave-assisted preparation and enhanced photocatalytic performance towards degradation of organic pollutants under visible light[J]. Journal of Colloid and Interface Science, 2016, 482: 165-174. |
| 54 | ZHOU M, DONG G H, MA J L, et al. Photocatalytic removal of NO by intercalated carbon nitride: the effect of group IIA element ions[J]. Applied Catalysis B: Environmental, 2020, 273: 119007. |
| 55 | SUN Z C, ZHU M S, LYU X, et al. Insight into iron group transition metal phosphides (Fe2P, Co2P, Ni2P) for improving photocatalytic hydrogen generation[J]. Applied Catalysis B: Environmental, 2019, 246: 330-336. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [10] | 许友好, 王维, 鲁波娜, 徐惠, 何鸣元. 中国炼油创新技术MIP的开发策略及启示[J]. 化工进展, 2023, 42(9): 4465-4470. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [14] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [15] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||