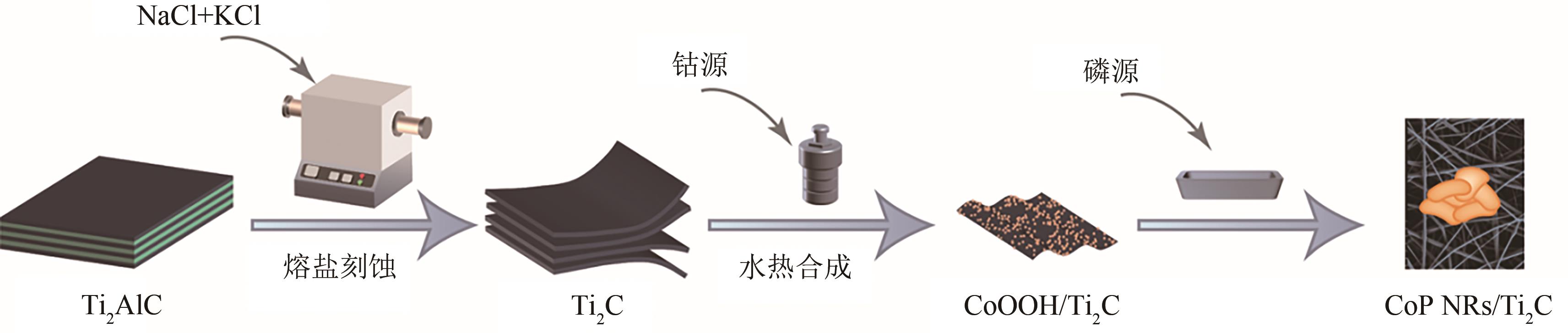
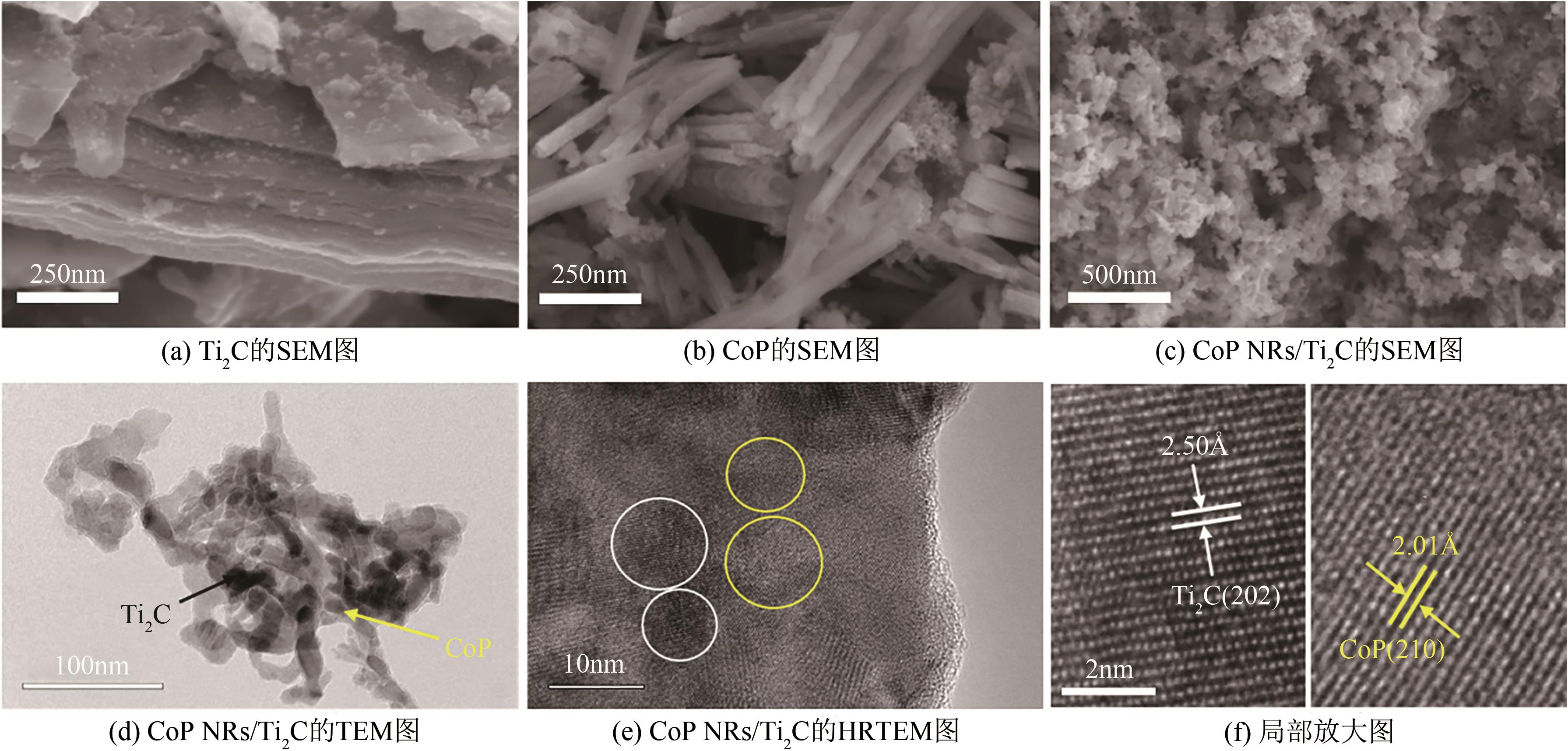
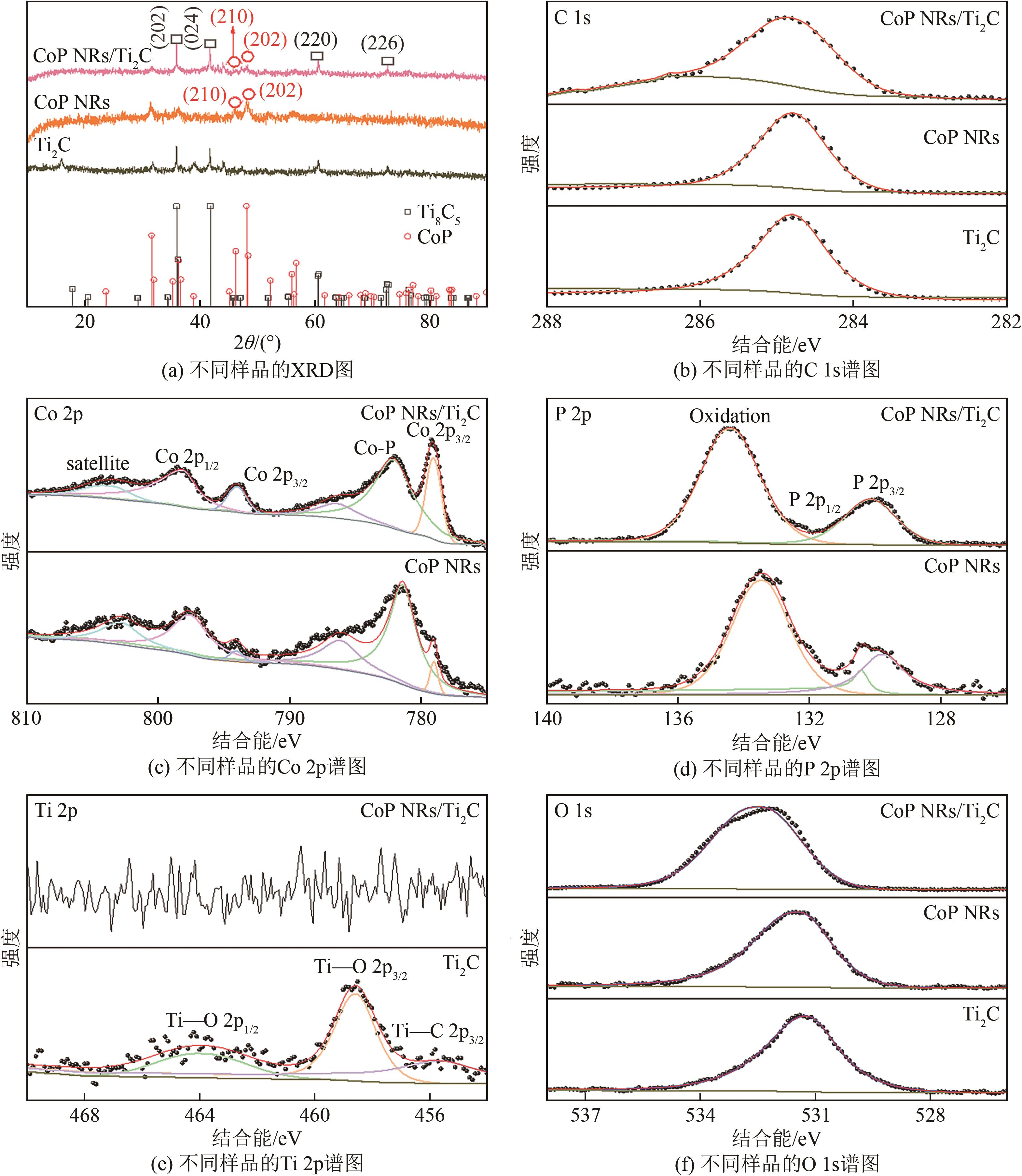
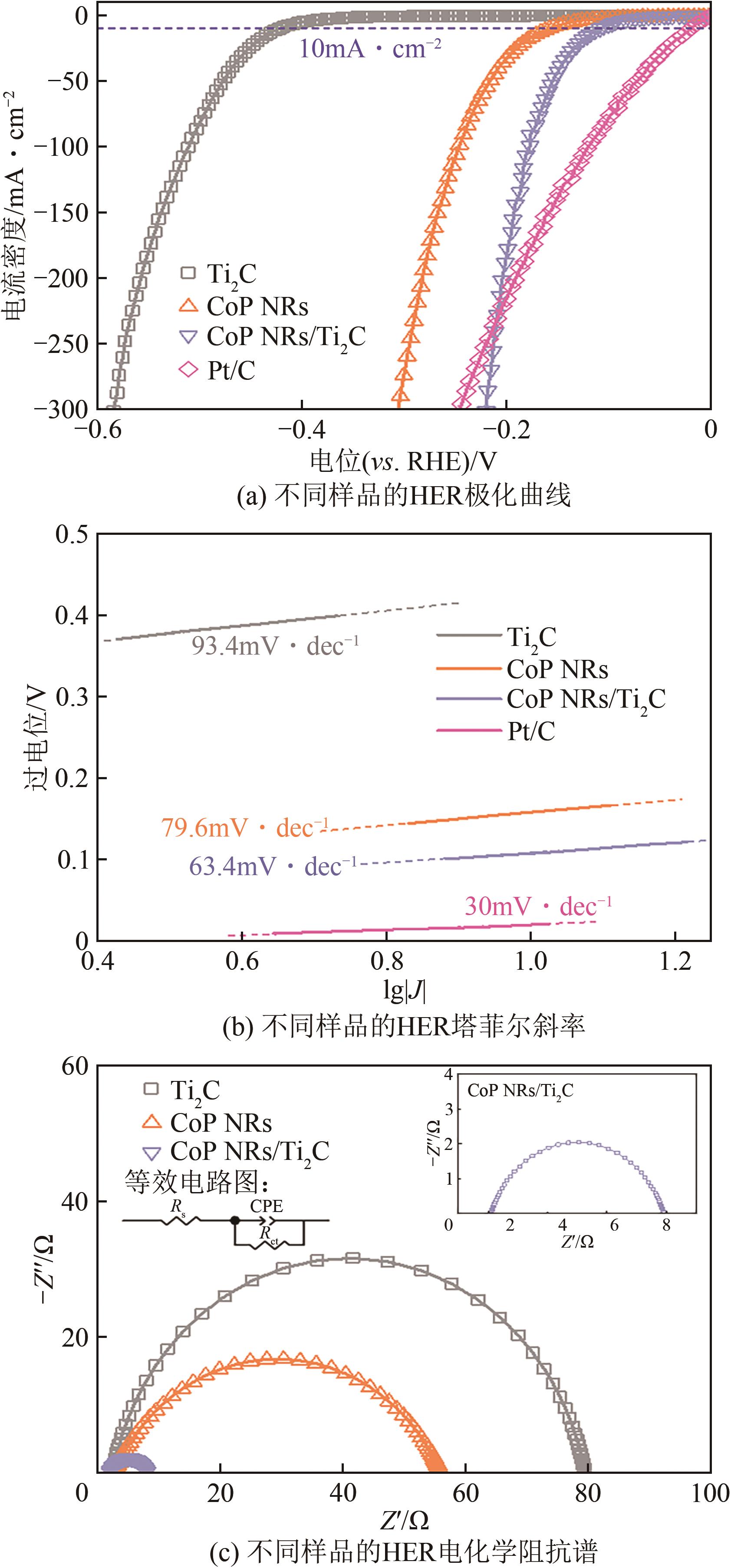
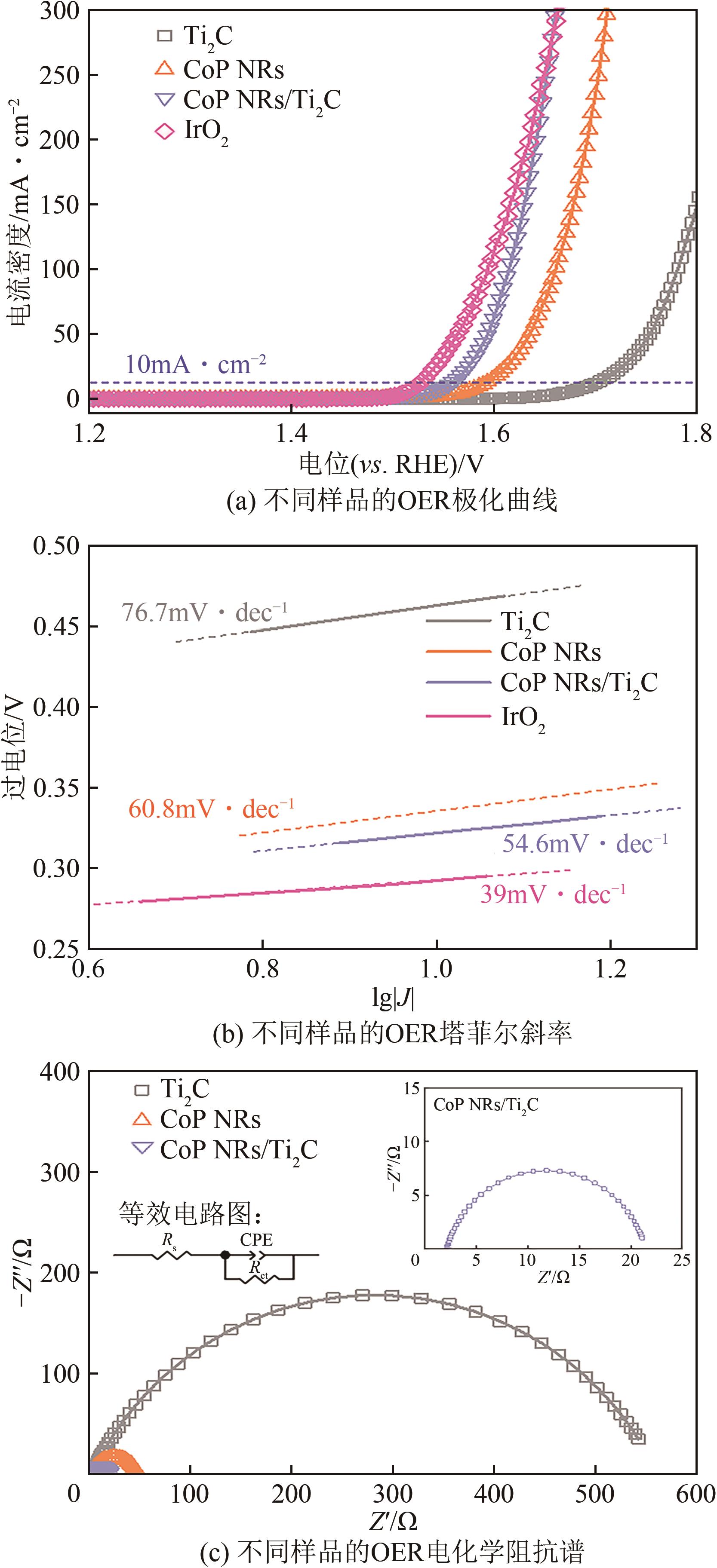
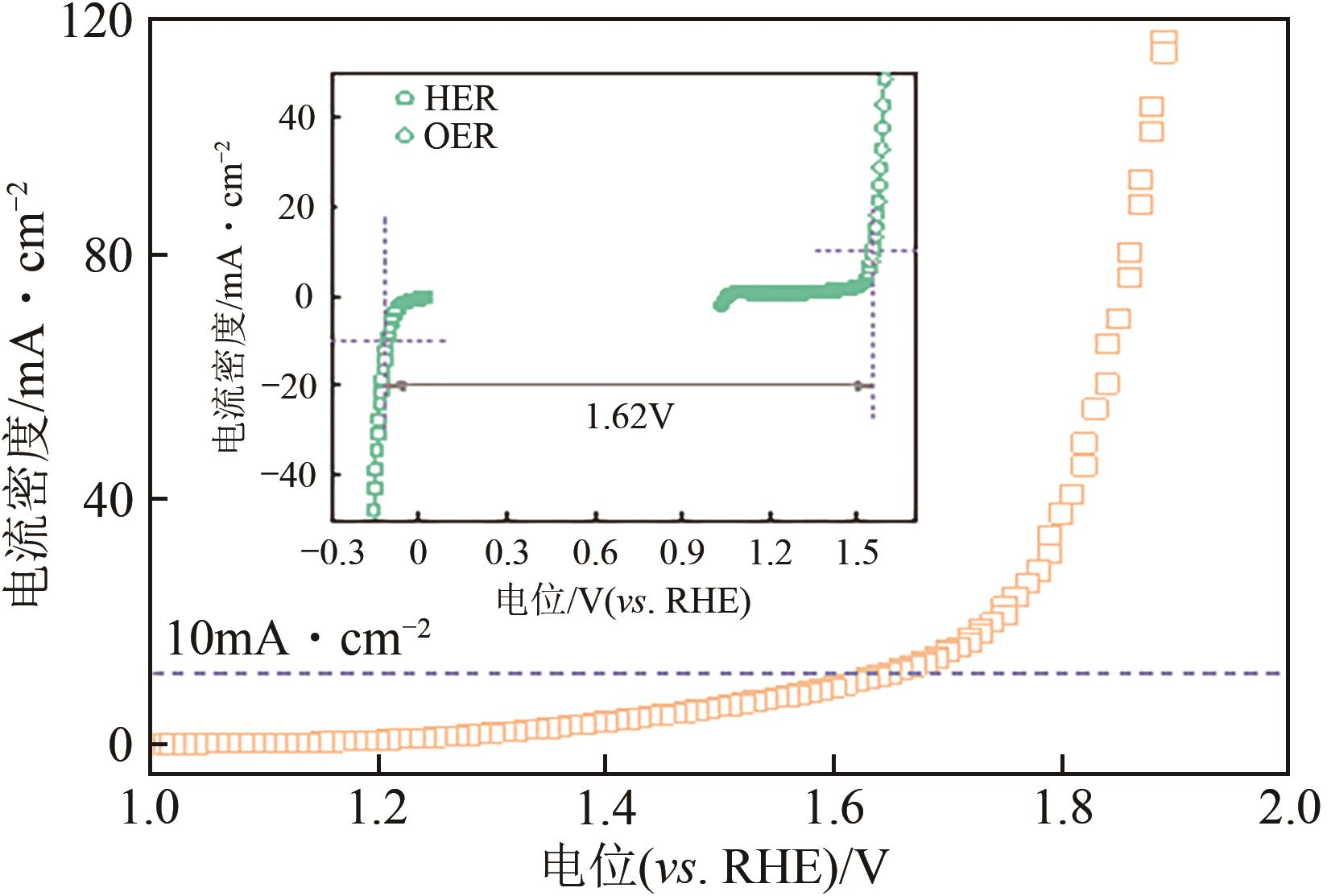
| 1 |
BARRETO L, MAKIHIRA A, RIAHI K. The hydrogen economy in the 21st century: A sustainable development scenario[J]. International Journal of Hydrogen Energy, 2003, 28(3): 267-284.
|
| 2 |
DINCER Ibrahim. Environmental impacts of energy[J]. Energy Policy, 1999, 27(14): 845-854.
|
| 3 |
MIDILLI A, AY M, DINCER I, et al. On hydrogen and hydrogen energy strategies Ⅰ: Current status and needs[J]. Renewable and Sustainable Energy Reviews, 2005, 9(3): 255-271.
|
| 4 |
SAZALI Norazlianie. Emerging technologies by hydrogen: A review[J]. International Journal of Hydrogen Energy, 2020, 45(38): 18753-18771.
|
| 5 |
YU Xiaowen, ZHAO Jun, ZHENG Lirong, et al. Hydrogen evolution reaction in alkaline media: alpha- or beta-nickel hydroxide on the surface of platinum?[J]. ACS Energy Letters, 2018, 3(1): 237-244.
|
| 6 |
WANG Jing, LIAO Ting, WEI Zhongzhe, et al. Heteroatom-doping of non-noble metal-based catalysts for electrocatalytic hydrogen evolution: An electronic structure tuning strategy[J]. Small Methods, 2021, 5(4): e2000988.
|
| 7 |
SU Jinzhan, ZHOU Jinglan, WANG Lu, et al. Synthesis and application of transition metal phosphides as electrocatalyst for water splitting[J]. Science Bulletin, 2017, 62(9): 633-644.
|
| 8 |
WU Rui, XIAO Bing, GAO Qiang, et al. A Janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting[J]. Angewandte Chemie International Edition, 2018, 57(47): 15445-15449.
|
| 9 |
CHEN Peirong, YE Jianshan, WANG Hui, et al. Recent progress of transition metal carbides/nitrides for electrocatalytic water splitting[J]. Journal of Alloys and Compounds, 2021, 883: 160833.
|
| 10 |
YU Yadong, ZHOU Jian, SUN Zhimei. 2D transition-metal carbides: Novel 2D transition-metal carbides: Ultrahigh performance electrocatalysts for overall water splitting and oxygen reduction[J]. Advanced Functional Materials, 2020, 30(47): 2070311.
|
| 11 |
HUANG Xiaokang, GONG Li, XU Hui, et al. Hierarchical iron-doped CoP heterostructures self-assembled on copper foam as a bifunctional electrocatalyst for efficient overall water splitting[J]. Journal of Colloid and Interface Science, 2020, 569: 140-149.
|
| 12 |
ZONG Quan, YANG Hui, WANG Qianqian, et al. Three-dimensional coral-like NiCoP@C@Ni(OH)2 core-shell nanoarrays as battery-type electrodes to enhance cycle stability and energy density for hybrid supercapacitors[J]. Chemical Engineering Journal, 2019, 361: 1-11.
|
| 13 |
YAN Liang, ZHANG Bing, WU Shangyou, et al. A general approach to the synthesis of transition metal phosphide nanoarrays on MXene nanosheets for pH-universal hydrogen evolution and alkaline overall water splitting[J]. Journal of Materials Chemistry A, 2020, 8(28): 14234-14242.
|
| 14 |
WANG Jian, CIUCCI Francesco. In-situ synthesis of bimetallic phosphide with carbon tubes as an active electrocatalyst for oxygen evolution reaction[J]. Applied Catalysis B: Environmental, 2019, 254: 292-299.
|
| 15 |
YUE Qin, SUN Jiao, CHEN Shan, et al. Hierarchical mesoporous MXene-NiCoP electrocatalyst for water-splitting[J]. ACS Applied Materials & Interfaces, 2020, 12(16): 18570-18577.
|
| 16 |
QIAO Jingyuan, KONG Lingqiao, XU Shaokang, Research progress of MXene-based catalysts for electrochemical water-splitting and metal-air batteries[J]. Energy Storage Materials, 2021, 43: 509-530.
|
| 17 |
MICHAEL Naguib, MURAT Kurtoglu, VOLKER Presser, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2 [J]. Advanced Materials, 2011, 23(37): 4248-4253.
|
| 18 |
HU Mei, XIN Jin, LIU Zhenyu, et al. Preparation of TiO2 supported mxene catalyst for high efficiency hydrogen production[J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2023, 38(2): 286-291.
|
| 19 |
WANG Yuwei, DU Rong, LI Zhuo, et al. Rationally designed CdS/Ti3C2 MXene electrocatalysts for efficient CO2 reduction in aqueous electrolyte[J]. Ceramics International, 2021, 47(20): 28321-28327.
|
| 20 |
GHIDIU Michael, LUKATSKAYA Maria R, ZHAO Mengqiang, et al. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance[J]. Nature, 2014, 516(7529): 78-81.
|
| 21 |
YU Hong, WANG Yonghui, JING Yao, et al. Surface modified MXene-based nanocomposites for electrochemical energy conversion and storage[J]. Small, 2019, 15(25): 1901503.
|
| 22 |
PANG Sin-Yi, WONG Yuen-Ting, YUAN Shuoguo, et al. Universal strategy for HF-free facile and rapid synthesis of two-dimensional MXenes as multifunctional energy materials[J]. Journal of the American Chemical Society, 2019, 141(24): 9610-9616.
|
| 23 |
XU Chuan, WANG Libin, LIU Zhibo, et al. Large-area high-quality 2D ultrathin Mo2C superconducting crystals[J]. Nature Materials, 2015, 14(11): 1135-1141.
|
| 24 |
YAN Ming, ZHANG Hao, DENG Yuxiao, et al. Preparation of fluorine-free MXene Ti3C2T x and its electrical properties[J]. Journal of Wuhan University of Technology-Mater Sci Ed, 2023, 38(2): 304-307.
|
| 25 |
ZHANG Wei, HAN Ning, LUO Jiangshui, et al. Critical role of phosphorus in hollow structures cobalt-based phosphides as bifunctional catalysts for water splitting[J]. Small, 2022, 18(4): e2103561.
|
| 26 |
LIU Yunxiu, TIAN Yu, HAN Qiuyang, et al. Synergism of 2D/1D MXene/cobalt nanowire heterojunctions for boosted photo-activated antibacterial application[J]. Chemical Engineering Journal, 2021, 410: 128209.
|
| 27 |
DENG Jiao, REN Pengju, DENG Dehui, et al. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction[J]. Energy & Environmental Science, 2014, 7(6): 1919-1923.
|
| 28 |
FU Huai qin, ZHOU Min, LIU Peng fei, et al. Hydrogen spillover-bridged volmer/tafel processes enabling ampere-level current density alkaline hydrogen evolution reaction under low overpotential[J]. Journal of the American Chemical Society, 2022, 144(13): 6028-6039.
|
| 29 |
SURENDRAN Subramani, JESUDASS Sebastian Cyril, JANANI Gnanaprakasam, et al. Sulphur assisted nitrogen-rich CNF for improving electronic interactions in Co-NiO heterostructures toward accelerated overall water splitting[J]. Advanced Materials Technologies, 2023, 8(2): 2200572.
|
| 30 |
MANDAL Jyoti Ranjan, SINGH Khushboo, SHAHI Vinod K. Amphoteric membrane loaded with a noble metal-free hollow spherical NiCoP@rGO bifunctional electrocatalyst for alkaline water electrolyzers[J]. ACS Applied Energy Materials, 2022, 5(7): 8611-8620.
|
| 31 |
JI Yuyao, YANG Li, REN Xiang, et al. Full water splitting electrocatalyzed by NiWO4 nanowire array[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9555-9559.
|
| 32 |
SARMAD Qassam, KHAN Uneeb Masood, BAIG Mutawara Mahmood, et al. Praseodymium-doped Sr2TiFeO6- δ double perovskite as a bi-functional electrocatalyst for hydrogen production through water splitting[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107609.
|
| 33 |
WANG Xixi, SUNARSO Jaka, LU Qian, et al. High-performance platinum-perovskite composite bifunctional oxygen electrocatalyst for rechargeable Zn-air battery[J]. Advanced Energy Materials, 2020, 10(5): 1903271.
|
| 34 |
Jincheng HEI, XU Guancheng, WEI Bei, et al. NiFeP nanosheets on N-doped carbon sponge as a hierarchically structured bifunctional electrocatalyst for efficient overall water splitting[J]. Applied Surface Science, 2021, 549: 149297.
|
| 35 |
PAN Yuan, SUN Kaian, LIU Shoujie, et al. Core-shell ZIF-8@ZIF-67-derived CoP nanoparticle-embedded N-doped carbon nanotube hollow polyhedron for efficient overall water splitting[J]. Journal of the American Chemical Society, 2018, 140(7): 2610-2618.
|
| 36 |
WANG Jianmei, YANG Wenrong, LIU Jingquan. CoP2 nanoparticles on reduced graphene oxide sheets as a super-efficient bifunctional electrocatalyst for full water splitting[J]. Journal of Materials Chemistry A, 2016, 4(13): 4686-4690.
|
| 37 |
LI Zhida, ZHANG Chunyue, YANG Yang, et al. Molten-salt-induced phosphorus vacancy defect engineering of heterostructured cobalt phosphides for efficient overall water splitting[J]. Inorganic Chemistry Frontiers, 2023, 10(1): 325-334.
|
 ), LI Zhida1, ZHANG Chunyue1, LU Lu1,2(
), LI Zhida1, ZHANG Chunyue1, LU Lu1,2( )
)