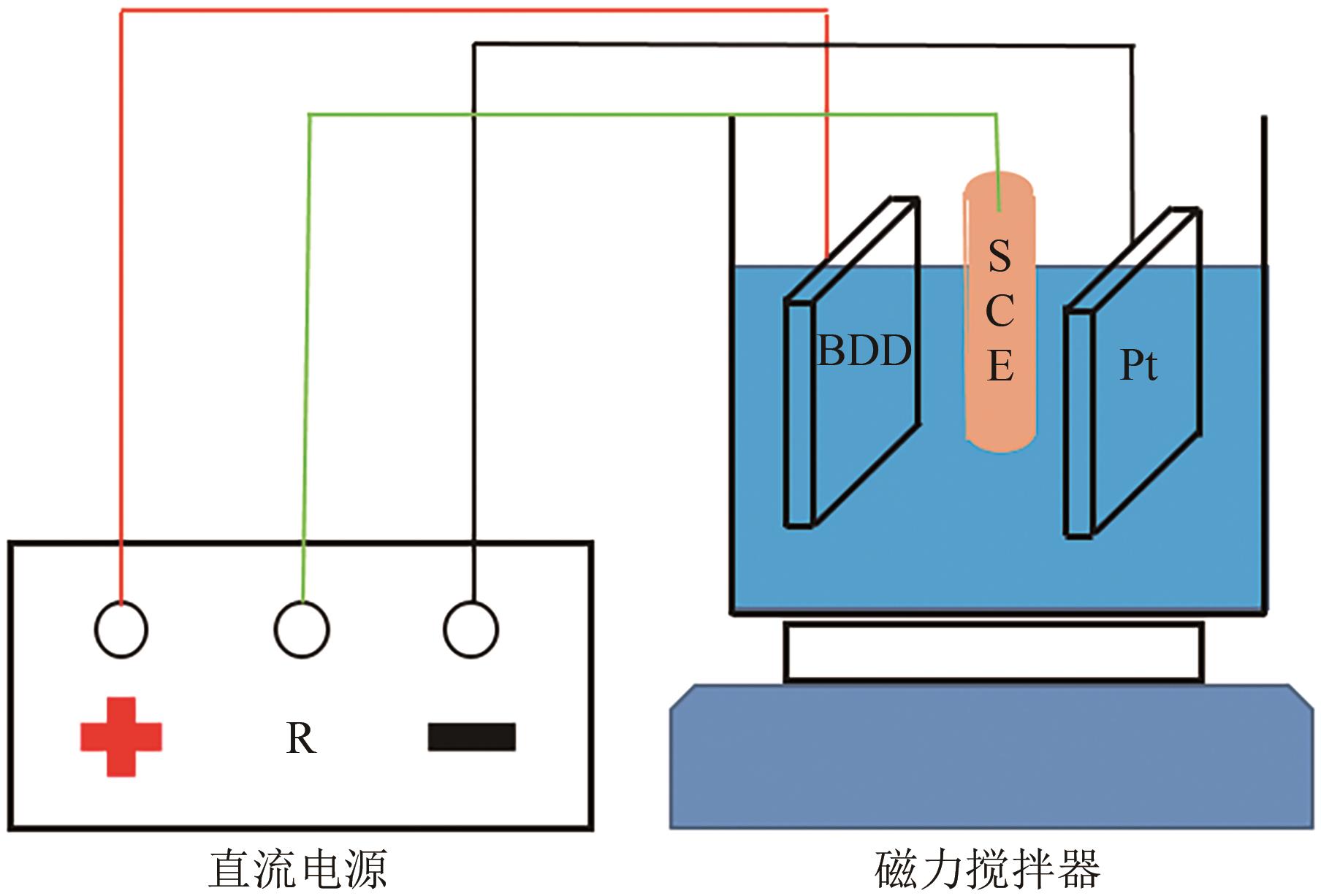
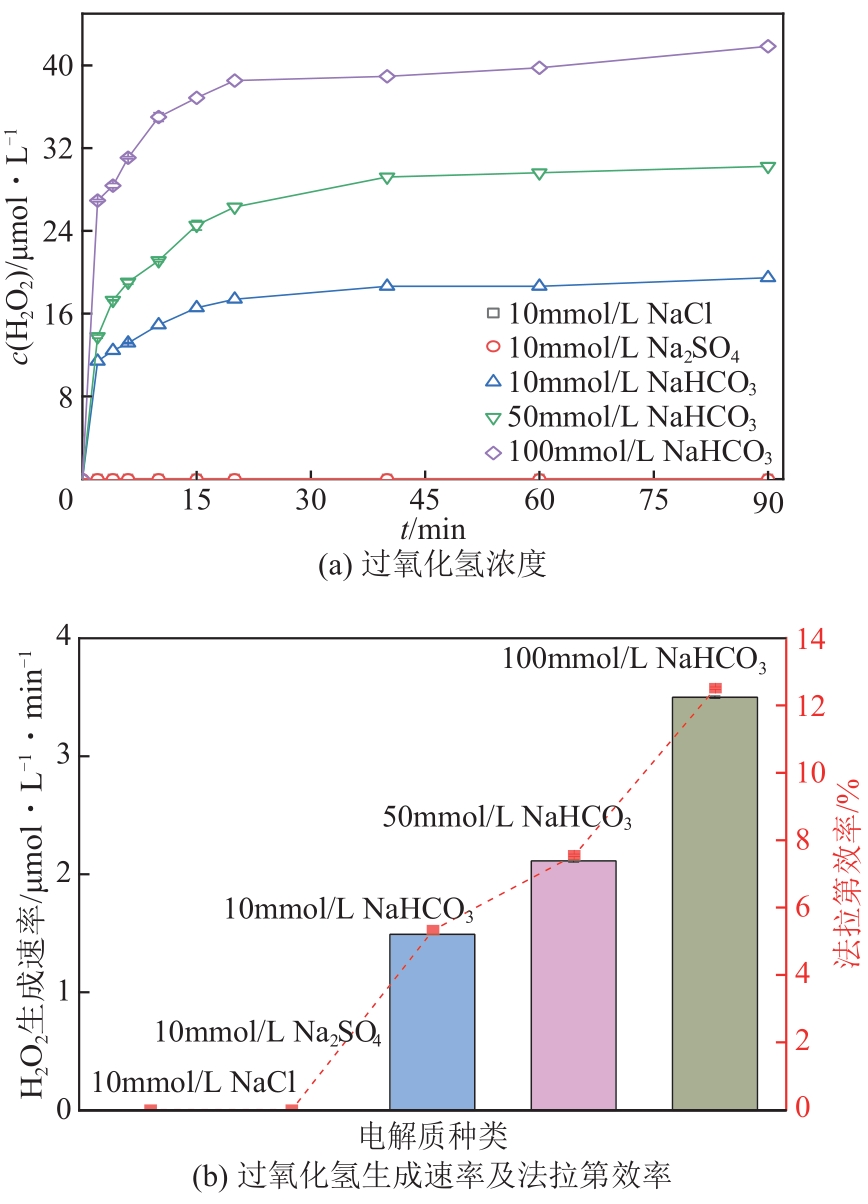
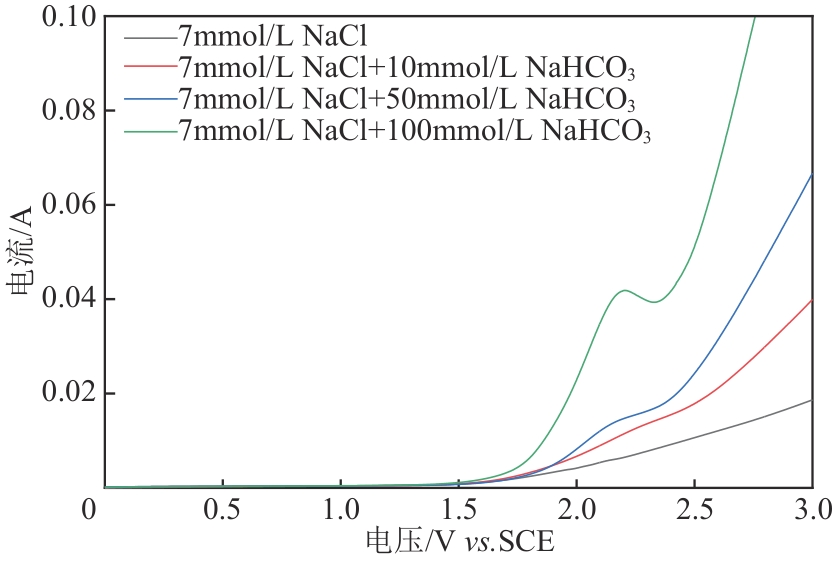
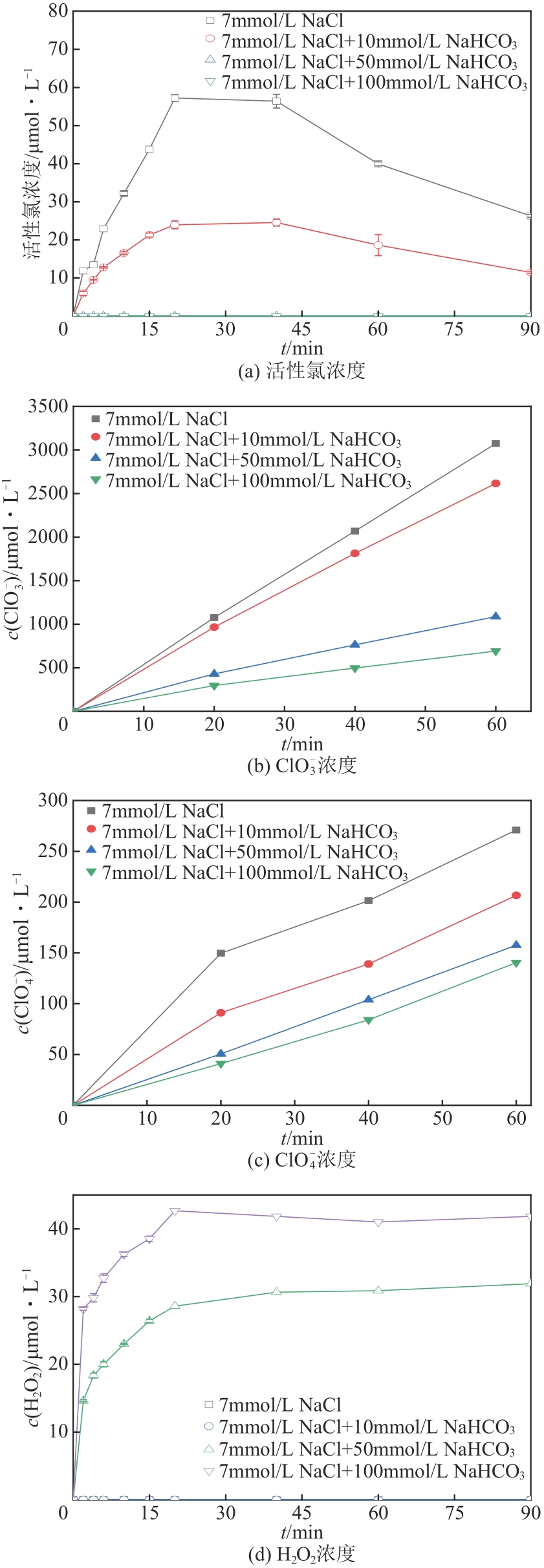
| 1 |
翟重渊, 赵丹荻, 何亚鹏, 等. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626.
|
|
ZHAI Chongyuan, ZHAO Dandi, HE Yapeng, et al. Recent development on boron-doped diamond anodes in electrochemical degradation of emerging antibiotic pollutants[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6615-6626.
|
| 2 |
YANG Nianjun, YU Siyu, MACPHERSON Julie V, et al. Conductive diamond: Synthesis, properties, and electrochemical applications[J]. Chemical Society Reviews, 2019, 48(1): 157-204.
|
| 3 |
Agnieszka KAPAŁKA, LANOVA Barbora, BALTRUSCHAT Helmut, et al. Electrochemically induced mineralization of organics by molecular oxygen on boron-doped diamond electrode[J]. Electrochemistry Communications, 2008, 10(9): 1215-1218.
|
| 4 |
F-L SOUZA, SÁEZ C, M-R-V LANZA, et al. Removal of pesticide 2, 4-D by conductive-diamond photoelectrochemical oxidation[J]. Applied Catalysis B: Environmental, 2016, 180: 733-739.
|
| 5 |
GUINEA Elena, CENTELLAS Francesc, BRILLAS Enric, et al. Electrocatalytic properties of diamond in the oxidation of a persistant pollutant[J]. Applied Catalysis B: Environmental, 2009, 89(3/4): 645-650.
|
| 6 |
COTILLAS Salvador, LACASA Engracia, Cristina SÁEZ, et al. Disinfection of urine by conductive-diamond electrochemical oxidation[J]. Applied Catalysis B: Environmental, 2018, 229: 63-70.
|
| 7 |
MOUSSET Emmanuel, OTURAN Nihal, OTURAN Mehmet A. An unprecedented route of OH radical reactivity evidenced by an electrocatalytical process: Ipso-substitution with perhalogenocarbon compounds[J]. Applied Catalysis B: Environmental, 2018, 226: 135-146.
|
| 8 |
Hanspeter ZÖLLIG, REMMELE Annette, FRITZSCHE Cristina, et al. Formation of chlorination byproducts and their emission pathways in chlorine mediated electro-oxidation of urine on active and nonactive type anodes[J]. Environmental Science & Technology, 2015, 49(18): 11062-11069.
|
| 9 |
CHAPLIN Brian P. The prospect of electrochemical technologies advancing worldwide water treatment[J]. Accounts of Chemical Research, 2019, 52(3): 596-604.
|
| 10 |
CAO Feifei, JAUNAT Jessy, STURCHIO Neil, et al. Worldwide occurrence and origin of perchlorate ion in waters: A review[J]. The Science of the Total Environment, 2019, 661: 737-749.
|
| 11 |
KUMARATHILAKA Prasanna, Christopher OZE, INDRARATNE S P, et al. Perchlorate as an emerging contaminant in soil, water and food[J]. Chemosphere, 2016, 150: 667-677.
|
| 12 |
SHI Yali, ZHANG Ping, WANG Yawei, et al. Perchlorate in sewage sludge, rice, bottled water and milk collected from different areas in China[J]. Environment International, 2007, 33(7): 955-962.
|
| 13 |
国家市场监督管理总局, 国家标准化管理委员会. 生活饮用水卫生标准: [S]. 北京: 中国标准出版社, 2022.
|
|
State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Standards for drinking water quality: [S]. Beijing: Standards Press of China, 2022.
|
| 14 |
RADJENOVIC Jelena, SEDLAK David L. Challenges and opportunities for electrochemical processes as next-generation technologies for the treatment of contaminated water[J]. Environmental Science & Technology, 2015, 49(19): 11292-11302.
|
| 15 |
JUNG Yeon Jung, BAEK Ko Woon, Byung Soo OH, et al. An investigation of the formation of chlorate and perchlorate during electrolysis using Pt/Ti electrodes: The effects of pH and reactive oxygen species and the results of kinetic studies[J]. Water Research, 2010, 44(18): 5345-5355.
|
| 16 |
HUBLER D K, BAYGENTS J C, CHAPLIN B P, et al. Understanding chlorite and chlorate formation associated with hypochlorite generation at boron doped diamond film anodes[J]. Journal of the Electrochemical Society, 2014, 161(12): E182-E189.
|
| 17 |
WANG Lu, LU Junhe, LI Lei, et al. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes[J]. Water Research, 2020, 170: 115254.
|
| 18 |
BERGMANN M E Henry, ROLLIN Johanna, IOURTCHOUK Tatiana. The occurrence of perchlorate during drinking water electrolysis using BDD anodes[J]. Electrochimica Acta, 2009, 54(7): 2102-2107.
|
| 19 |
BERGMANN M E Henry, ROLLIN Johanna. Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes[J]. Catalysis Today, 2007, 124(3): 198-203.
|
| 20 |
GAYEN Pralay, CHAPLIN Brian P. Fluorination of boron-doped diamond film electrodes for minimization of perchlorate formation[J]. ACS Applied Materials & Interfaces, 2017, 9(33): 27638-27648.
|
| 21 |
YANG Shasha, FERNANDO Sujan, HOLSEN Thomas M, et al. Inhibition of perchlorate formation during the electrochemical oxidation of perfluoroalkyl acid in groundwater[J]. Environmental Science & Technology Letters, 2019, 6(12): 775-780.
|
| 22 |
MAVRIKIS Sotirios, Maximilian GÖLTZ, ROSIWAL Stefan, et al. Boron-doped diamond electrocatalyst for enhanced anodic H2O2 production[J]. ACS Applied Energy Materials, 2020, 3(4): 3169-3173.
|
| 23 |
SHI Xinjian, BACK Seoin, GILL Thomas Mark, et al. Electrochemical synthesis of H2O2 by two-electron water oxidation reaction[J]. Chem, 2021, 7(1): 38-63.
|
| 24 |
SOUZA F L, SAÉZ C, LANZA M R V, et al. The effect of the sp3/sp2 carbon ratio on the electrochemical oxidation of 2, 4-D with p-Si BDD anodes[J]. Electrochimica Acta, 2016, 187: 119-124.
|
| 25 |
SONG Haoran, YAN Linxia, JIANG Jin, et al. Electrochemical activation of persulfates at BDD anode: Radical or nonradical oxidation?[J]. Water Research, 2018, 128: 393-401.
|
| 26 |
XIA Chuan, BACK Seoin, RINGE Stefan, et al. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide[J]. Nature Catalysis, 2020, 3: 125-134.
|
| 27 |
JIANG Yuanyuan, NI Pengjuan, CHEN Chuanxia, et al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry[J]. Advanced Energy Materials, 2018, 8(31): 1801909.
|
| 28 |
RICHARDSON David E, YAO Huirong, FRANK Karen M, et al. Equilibria, kinetics, and mechanism in the bicarbonate activation of hydrogen peroxide: oxidation of sulfides by peroxymonocarbonate[J]. Journal of the American Chemical Society, 2000, 122(8): 1729-1739.
|
| 29 |
BARAZESH James M, PRASSE Carsten, SEDLAK David L. Electrochemical transformation of trace organic contaminants in the presence of halide and carbonate ions[J]. Environmental Science & Technology, 2016, 50(18): 10143-10152.
|
| 30 |
GORDON Gilbert, TACHIYASHIKI Satoshi. Kinetics and mechanism of formation of chlorate ion from the hypochlorous acid/chlorite ion reaction at pH 6-10[J]. Environmental Science and Technology, 1991, 25(3): 468-474.
|
| 31 |
SIDDIQUI Mohamed S. Chlorine-ozone interactions: Formation of chlorate[J]. Water Research, 1996, 30(9): 2160-2170.
|
| 32 |
AZIZI Orchideh, HUBLER David, SCHRADER Glenn, et al. Mechanism of perchlorate formation on boron-doped diamond film anodes[J]. Environmental Science & Technology, 2011, 45(24): 10582-10590.
|
| 33 |
PI Liu, YANG Nuo, HAN Wei, et al. Heterogeneous activation of peroxymonocarbonate by Co-Mn oxides for the efficient degradation of chlorophenols in the presence of a naturally occurring level of bicarbonate[J]. Chemical Engineering Journal, 2018, 334: 1297-1308.
|
| 34 |
LEE Minyong, WANG Wenlong, DU Ye, et al. Applications of UV/H2O2, UV/persulfate, and UV/persulfate/Cu2+ for the elimination of reverse osmosis concentrate generated from municipal wastewater reclamation treatment plant: Toxicity, transformation products, and disinfection byproducts[J]. Science of the Total Environment, 2021, 762: 144161.
|
| 35 |
GALLARD H, DE LAAT J. Kinetic modelling of Fe(III)/H2O2 oxidation reactions in dilute aqueous solution using atrazine as a model organic compound[J]. Water Research, 2000, 34(12): 3107-3116.
|
| 36 |
丁嘉, 李钰, 官宝红. 聚四氟乙烯改性掺硼金刚石电极强化降解阿特拉津[J]. 浙江大学学报(农业与生命科学版), 2022, 48(3): 369-376.
|
|
DING Jia, LI Yu, GUAN Baohong. Boron-doped diamond electrode modified by polytetrafluoroethylene to enhance the degradation of atrazine[J]. Journal of Zhejiang University (Agriculture and Life Sciences), 2022, 48(3): 369-376.
|
| 37 |
JAWAD Ali, LU Xiaoyan, CHEN Zhuqi, et al. Degradation of chlorophenols by supported Co-Mg-Al layered double hydrotalcite with bicarbonate activated hydrogen peroxide[J]. The Journal of Physical Chemistry A, 2014, 118(43): 10028-10035.
|
| 38 |
JASPER Justin T, SEDLAK David L. Phototransformation of wastewater-derived trace organic contaminants in open-water unit process treatment wetlands[J]. Environmental Science & Technology, 2013, 47(19): 10781-10790.
|
 ), WU Wenqi3, LI Pengcheng1(
), WU Wenqi3, LI Pengcheng1( )
)