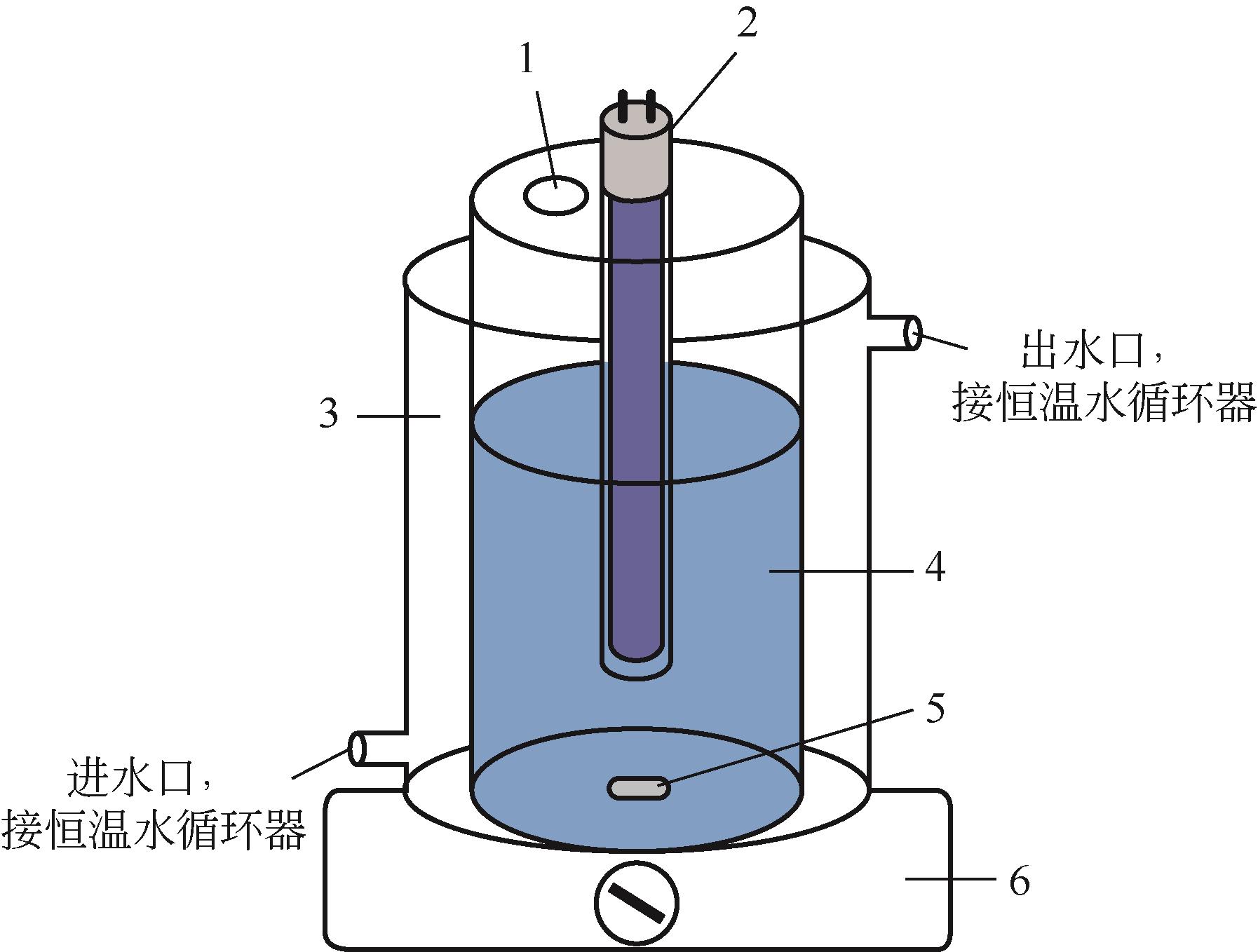
化工进展 ›› 2023, Vol. 42 ›› Issue (11): 6102-6112.DOI: 10.16085/j.issn.1000-6613.2023-0009
• 资源与环境化工 • 上一篇
UV/H2O2和UV/NaClO工艺降解吉非罗齐的比较
闫博引( ), 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成(
), 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成( )
)
- 青岛理工大学环境与市政工程学院,山东 青岛 266033
-
收稿日期:2023-01-04修回日期:2023-02-09出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:李金成 -
作者简介:闫博引(1992—),女,博士,副教授,研究方向为水质净化技术。Email:hit_yanby@126.com。 -
基金资助:山东省自然科学基金(ZR2021QE234);国家自然科学基金(52100010);河海大学浅水湖泊综合治理与资源开发教育部重点实验室开放项目
Comparative investigation of gemfibrozil degradation by UV/H2O2 and UV/NaClO processes
YAN Boyin( ), HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng(
), HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng( )
)
- School of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266033, Shandong, China
-
Received:2023-01-04Revised:2023-02-09Online:2023-11-20Published:2023-12-15 -
Contact:LI Jincheng
摘要:
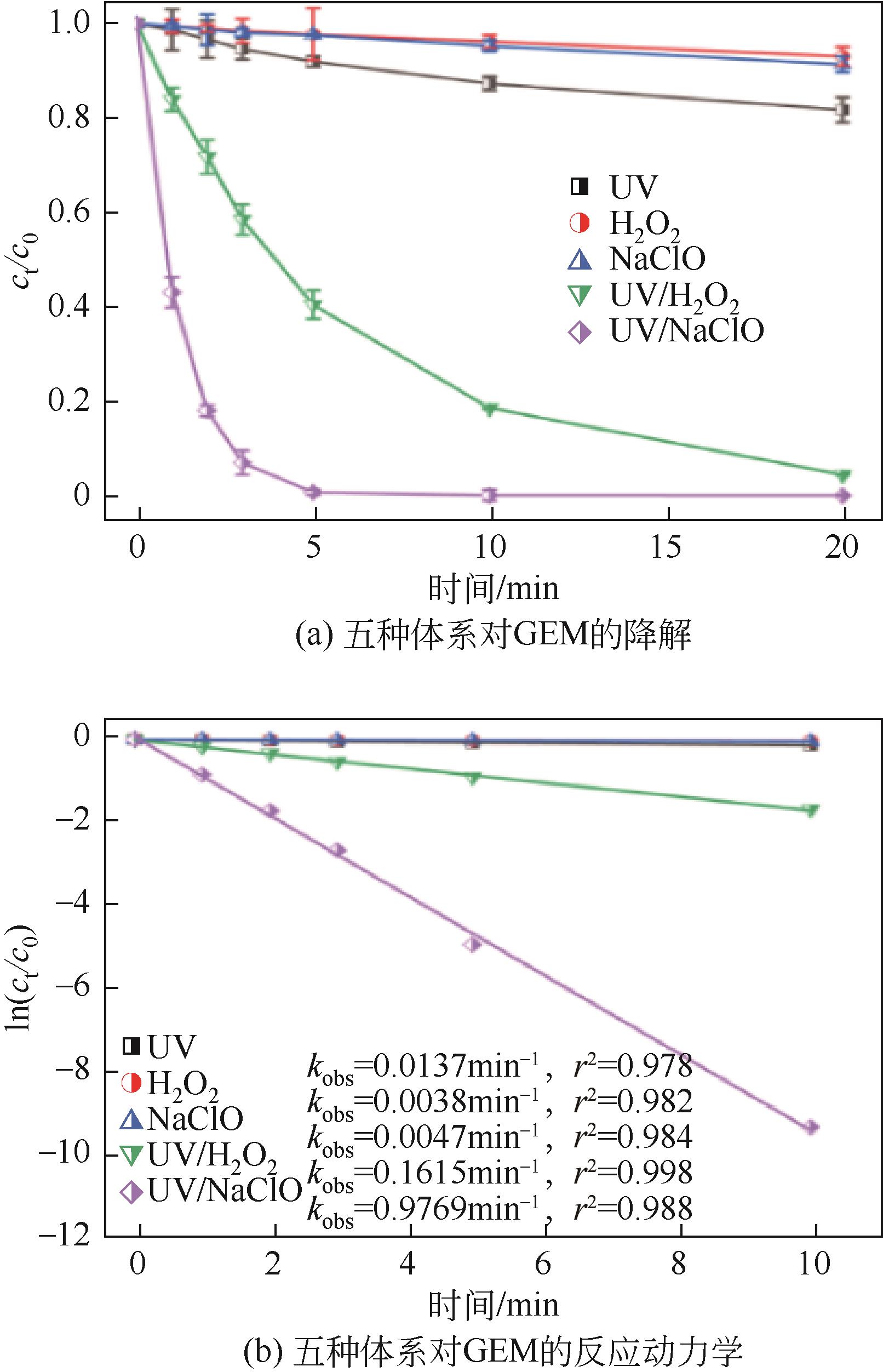
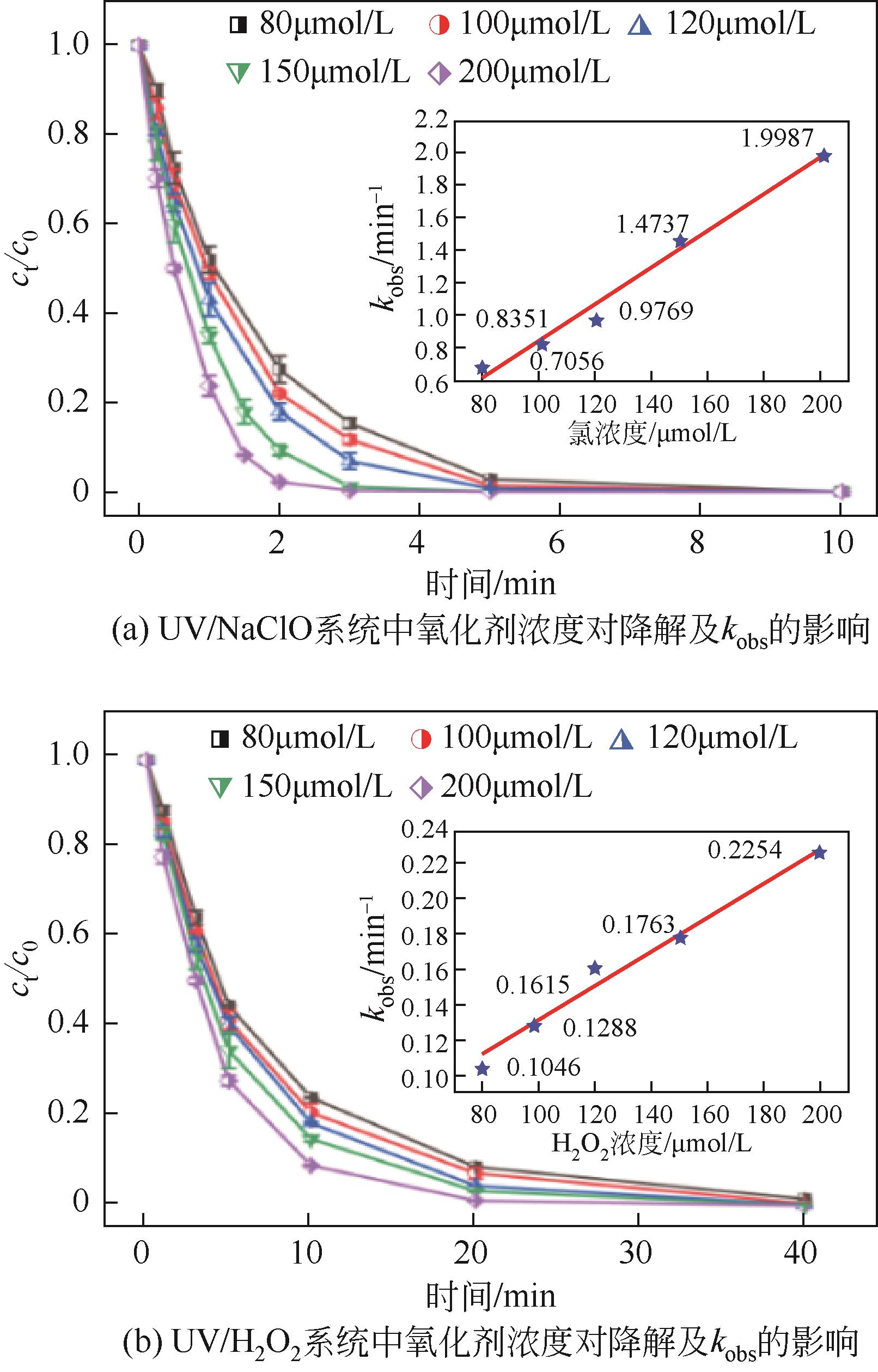
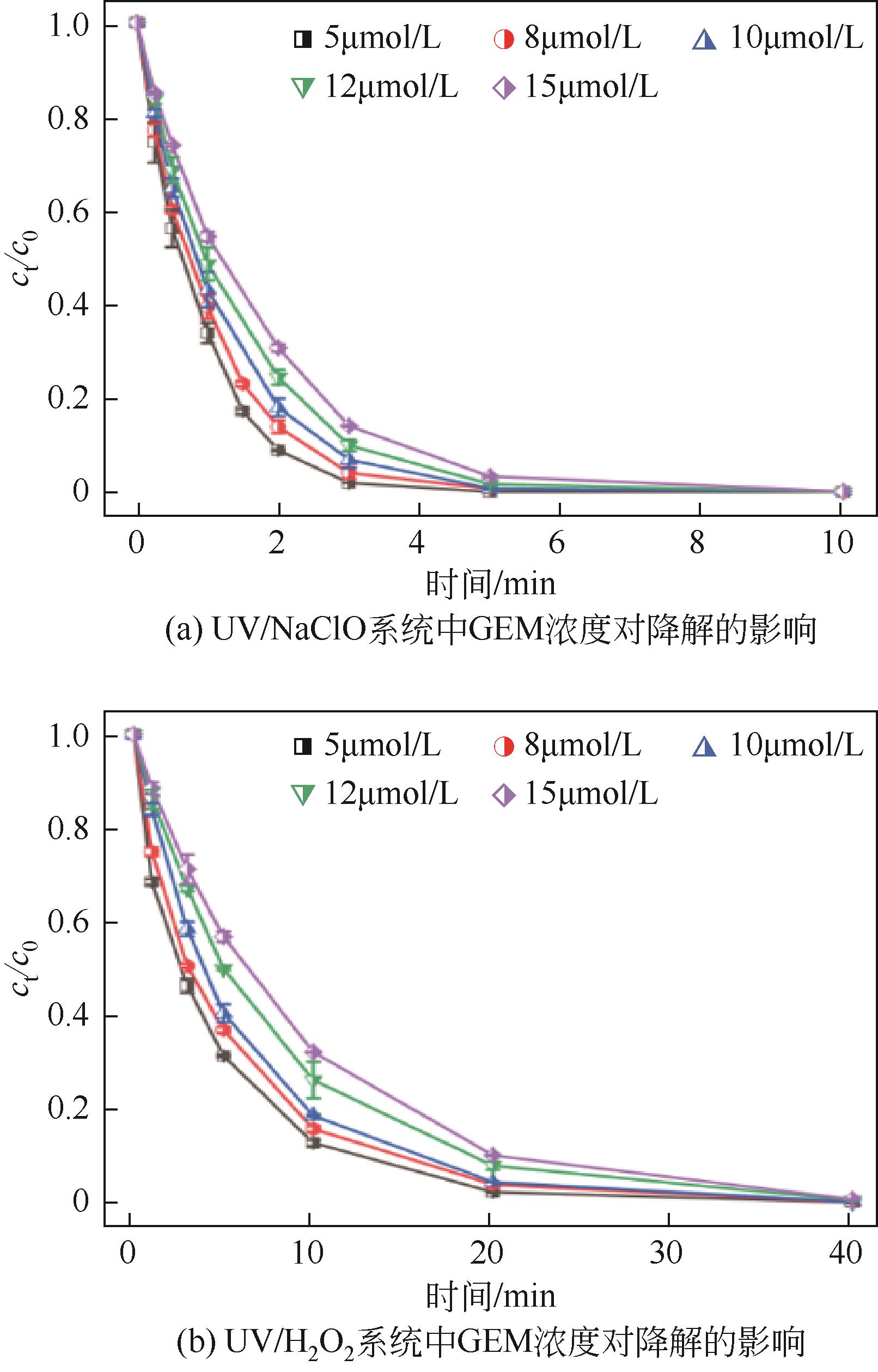
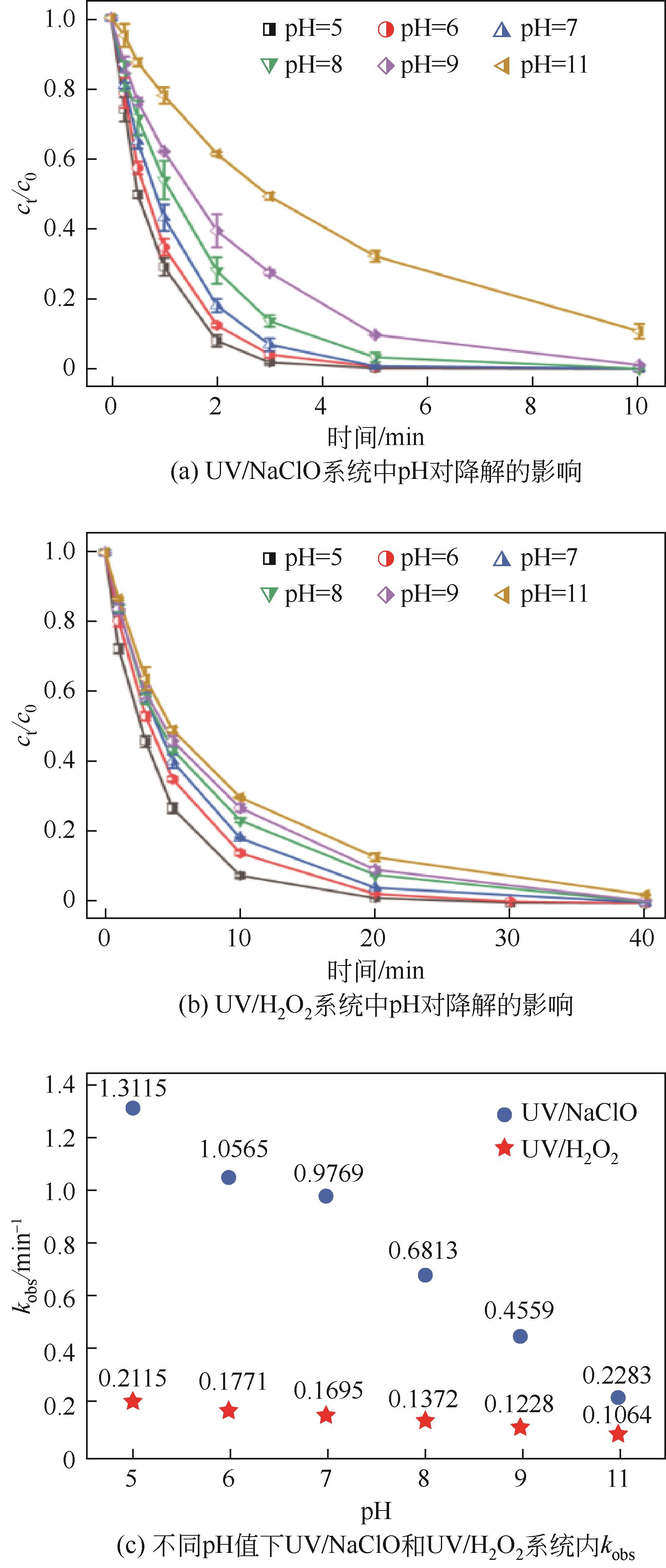
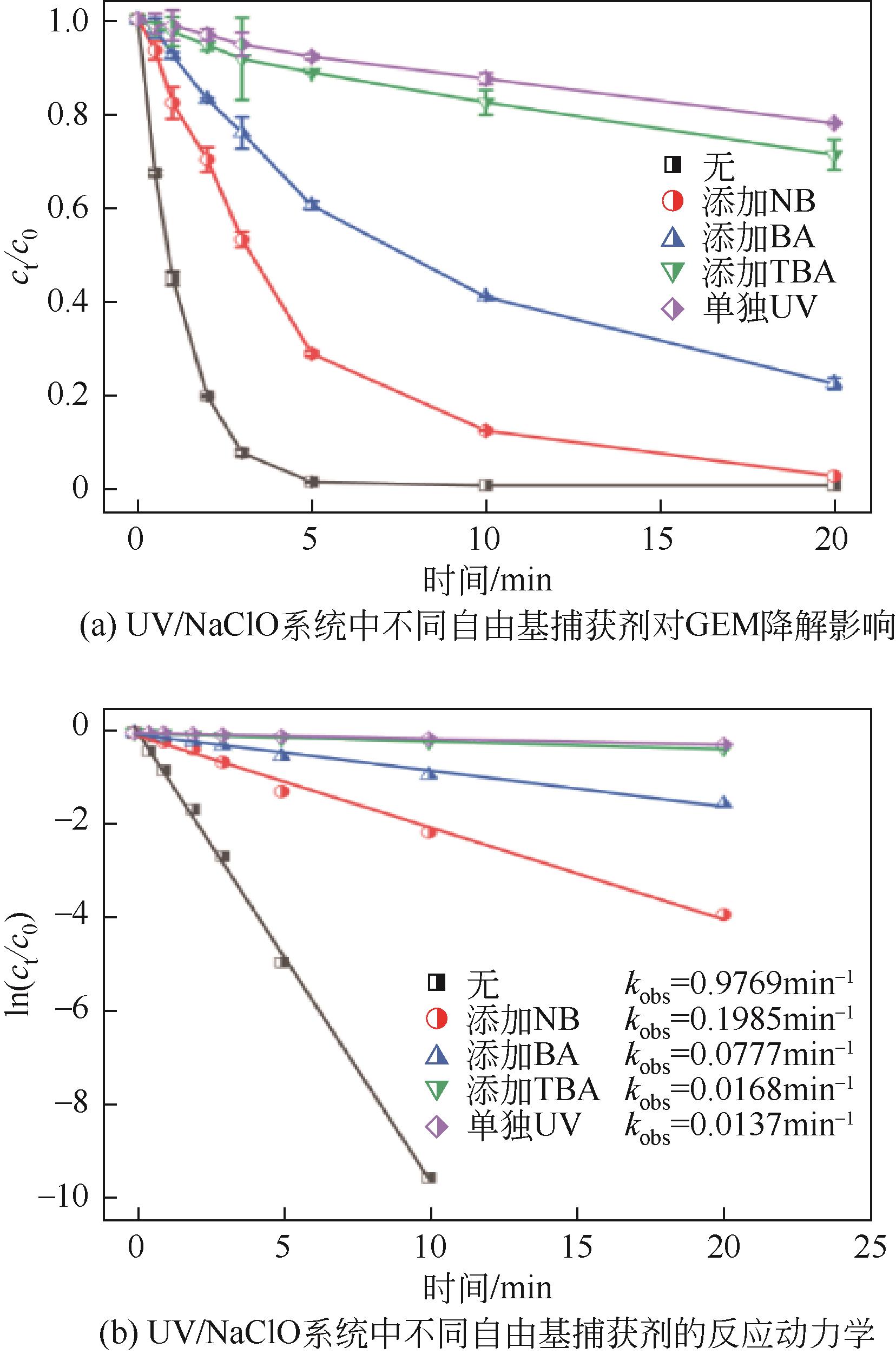
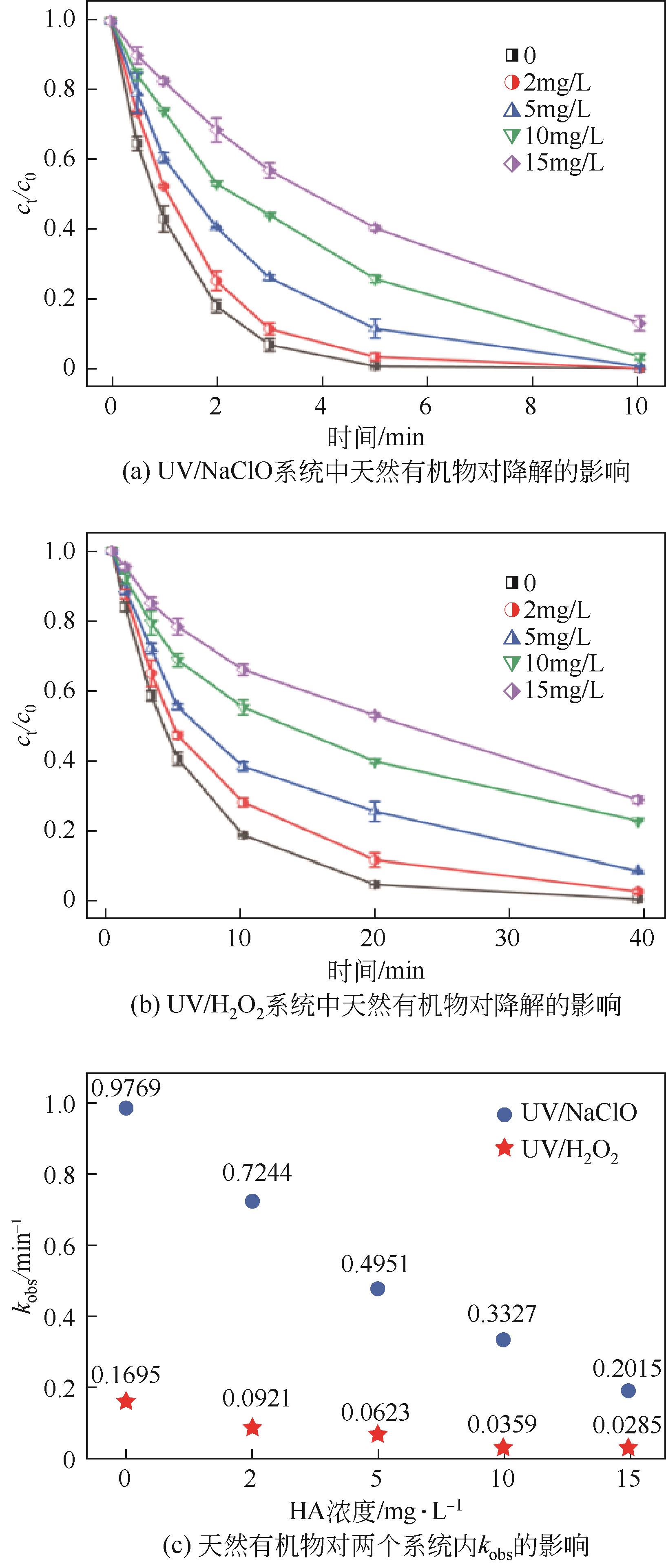
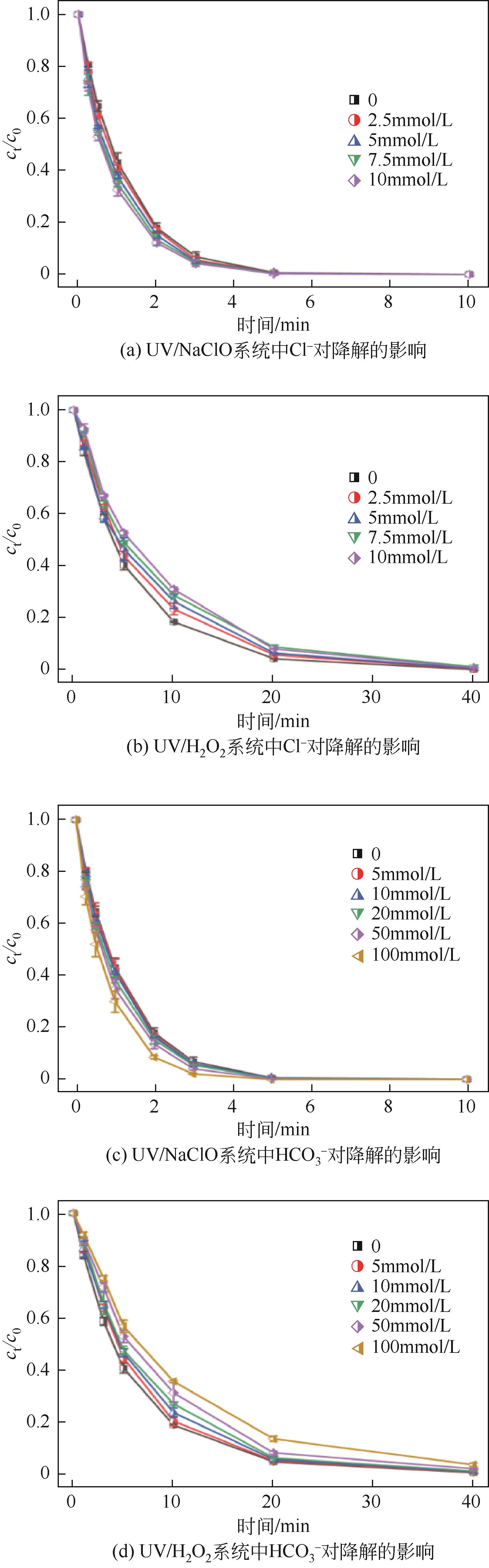
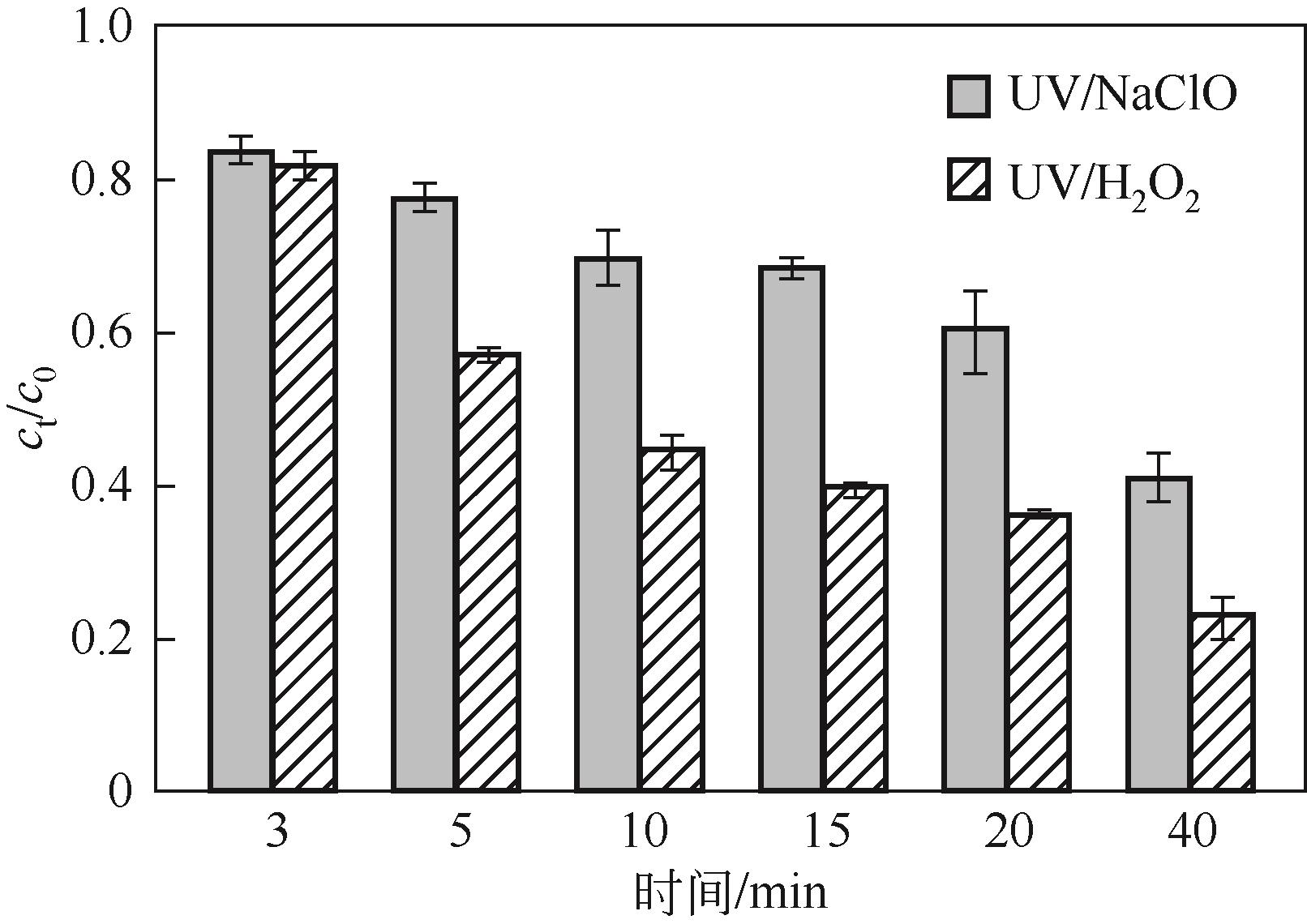
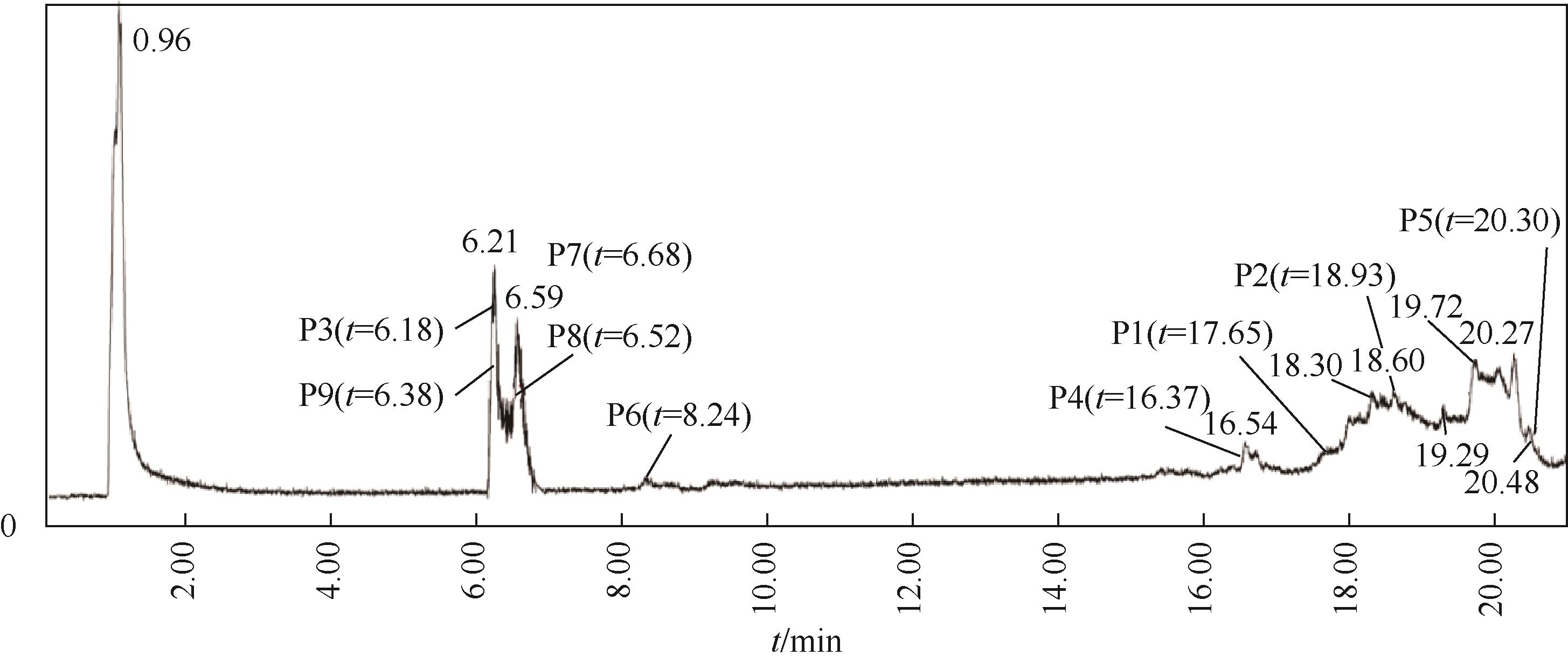
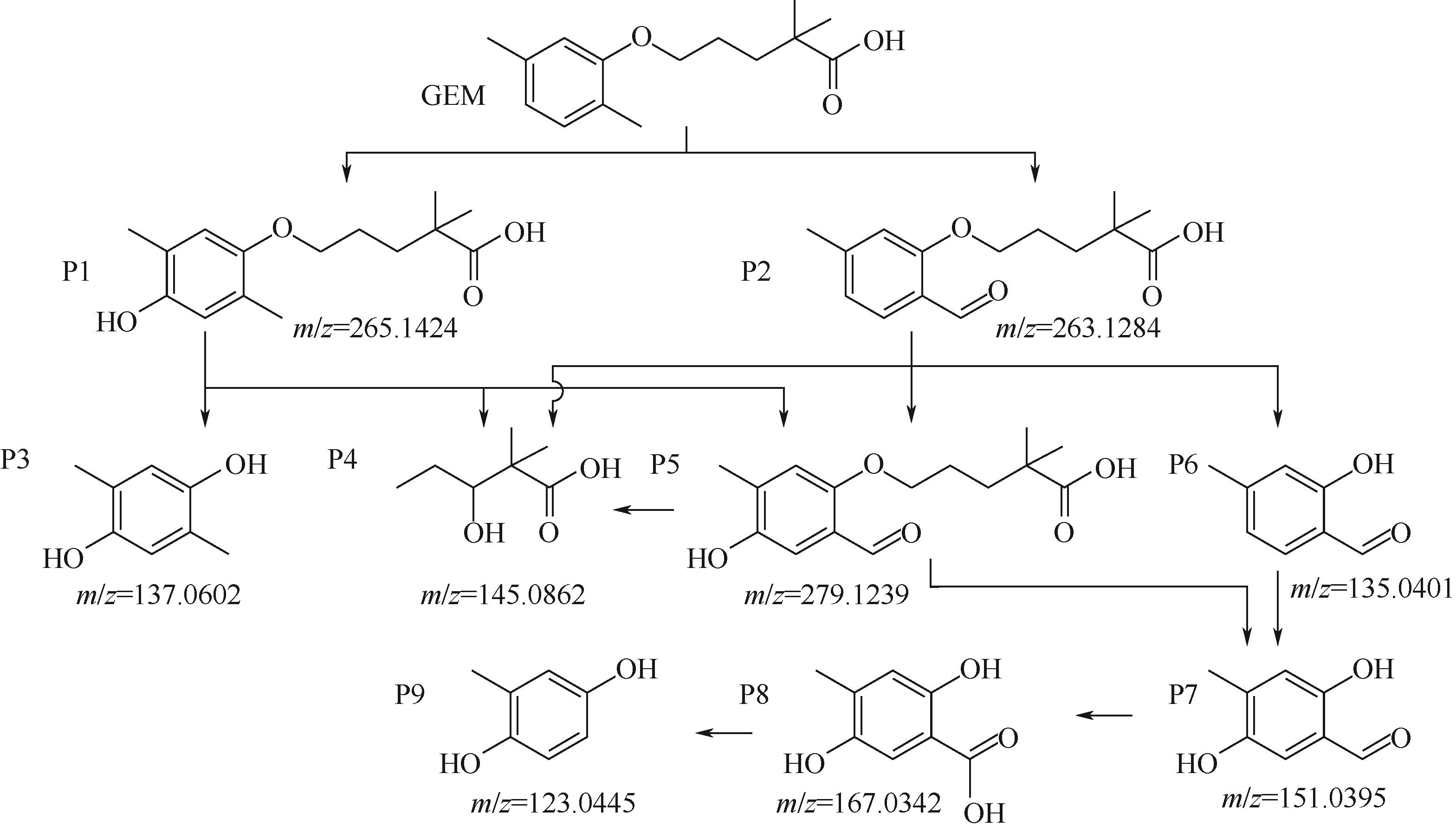
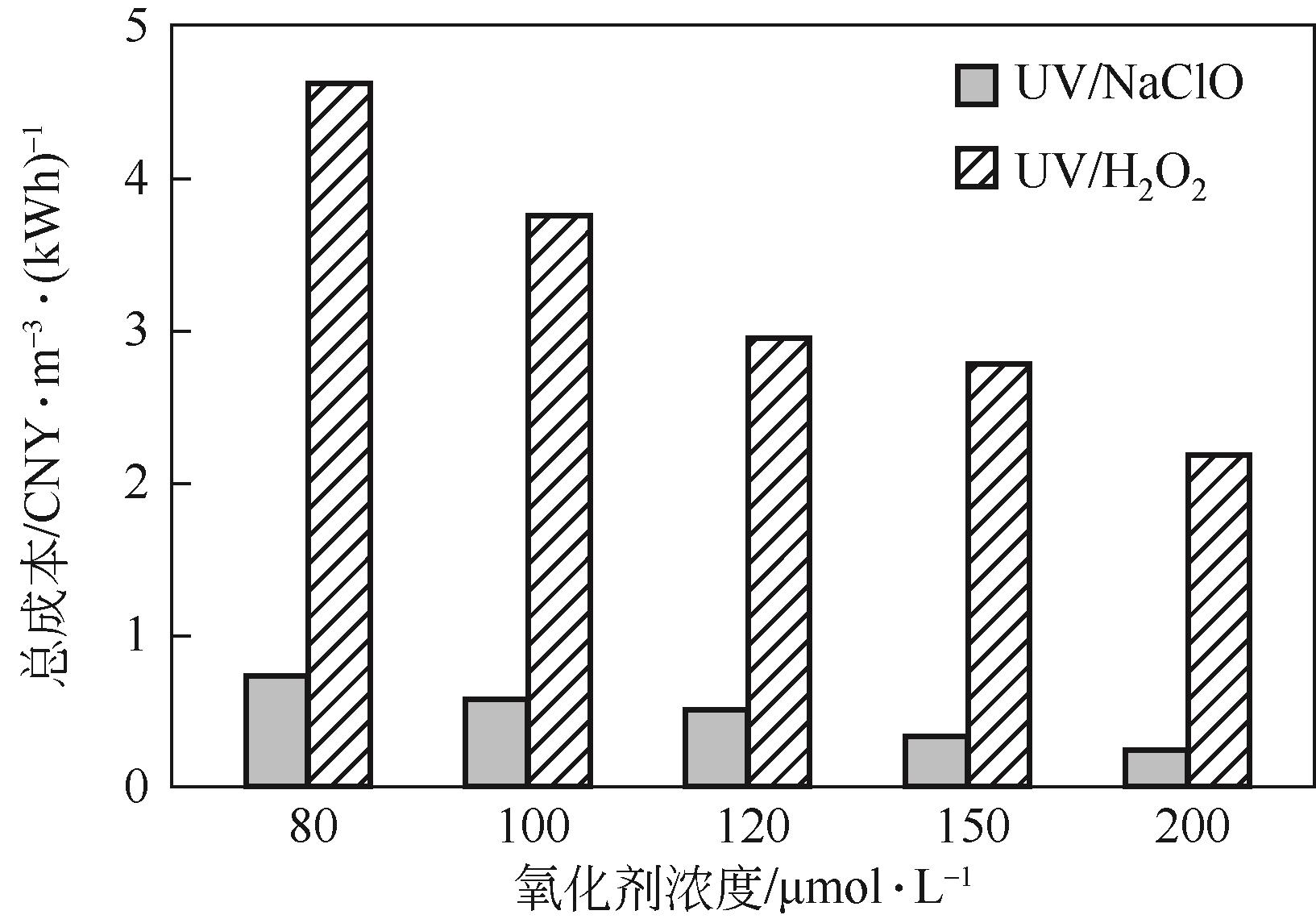
对比了吉非罗齐(GEM)在UV/H2O2和UV/NaClO系统中的降解,两个高级氧化系统均可以有效降解GEM,与UV/H2O2相比,UV/NaClO对GEM的去除速率更快。在UV/H2O2和UV/NaClO系统内,目标物的降解速率随着氧化剂浓度的增加而加快,由于pH会影响‧OH的氧化能力和UV/NaClO系统内自由基的组成比例,因此溶液的pH对两个系统均有显著的影响,当溶液pH从5增加到11时,GEM在UV/H2O2系统和UV/NaClO系统内的kobs分别从0.2115min-1和1.3115min-1降低到0.1064min-1和0.2283min-1。Cl-和HCO
中图分类号:
引用本文
闫博引, 韩春宇, 夏晶晶, 王松雪, 武桂芝, 夏文香, 李金成. UV/H2O2和UV/NaClO工艺降解吉非罗齐的比较[J]. 化工进展, 2023, 42(11): 6102-6112.
YAN Boyin, HAN Chunyu, XIA Jingjing, WANG Songxue, WU Guizhi, XIA Wenxiang, LI Jincheng. Comparative investigation of gemfibrozil degradation by UV/H2O2 and UV/NaClO processes[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6102-6112.
| 1 | ZHANG Ruochun, MENG Tan, HUANG Chinghua, et al. PPCP degradation by chlorine-UV processes in ammoniacal water: New reaction insights, kinetic modeling, and DBP formation[J]. Environmental Science & Technology, 2018, 52(14): 7833-7841. |
| 2 | KÜMMERER K, DIONYSIOU D D, OLSSON O, et al. A path to clean water[J]. Science, 2018, 361(6399): 222-224. |
| 3 | ZHANG Panwei, ZHOU Huaidong, LI Kun, et al. Occurrence of pharmaceuticals and personal care products, and their associated environmental risks in a large shallow lake in North China[J]. Environmental Geochemistry and Health, 2018, 40(4): 1525-1539. |
| 4 | YIN Haoran, ZHANG Qizhan, SU Yi, et al. A novel UV based advanced oxidation process with electrochemical co-generation of chlorine and H2O2 for carbamazepine abatement: Better performance, lower energy consumption and less DBPs formation[J]. Chemical Engineering Journal, 2021, 425: 131857. |
| 5 | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| YI Xuenong, LI Jingmei, GAO Yuqiong. Oxidative degradation of naproxen in water by UV-Fe(Ⅵ) process[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4562-4570. | |
| 6 | MIKLOS D B, REMY C, JEKEL M, et al. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review[J]. Water Research, 2018, 139: 118-131. |
| 7 | DAS D, BORDOLOI A, ACHARY M P, et al. Degradation and inactivation of chromosomal and plasmid encoded resistance genes/ARBs and the impact of different matrices on UV and UV/H2O2 based advanced oxidation process[J]. Science of the Total Environment, 2022, 833: 155205. |
| 8 | WANG Pin, BU Lingjun, WU Yangtao, et al. Mechanistic insight into the degradation of ibuprofen in UV/H2O2 process via a combined experimental and DFT study[J]. Chemosphere, 2021, 267: 128883. |
| 9 | 胡晋博, 李梦凯, 蔡恒文, 等. 不同光源UV/H2O2工艺降解四环素动力学[J]. 环境工程学报, 2021, 15(8): 2618-2626. |
| HU Jinbo, LI Mengkai, CAI Hengwen, et al. Degradation kinetics of tetracycline by UV/H2O2 process with various light sources[J]. Chinese Journal of Environmental Engineering, 2021, 15(8): 2618-2626. | |
| 10 | LU Gang, HU Jiangyong. Effect of alpha-hydroxy acids on transformation products formation and degradation mechanisms of carbamazepine by UV/H2O2 process[J]. Science of the Total Environment, 2019, 689: 70-78. |
| 11 | DHAWLE Rebecca, MANTZAVINOS Dionissios, LIANOS Panagiotis. UV/H2O2 degradation of diclofenac in a photocatalytic fuel cell[J]. Applied Catalysis B: Environmental, 2021, 299: 120706. |
| 12 | YAN Boyin, WANG Songxue, LIU Zhiquan, et al. Degradation mechanisms of cyanobacteria neurotoxin β-N-methylamino-l-alanine (BMAA) during UV254/H2O2 process: Kinetics and pathways[J]. Chemosphere, 2022, 302: 134939. |
| 13 | ZHAN Lumeng, LI Wentao, LIU Li, et al. Degradation of micropolluants in flow-through VUV/UV/H2O2 reactors: Effects of H2O2 dosage and reactor internal diameter[J]. Journal of Environmental Sciences, 2021, 110: 28-37. |
| 14 | PARK J A, YANG B, JANG M, et al. Oxidation and molecular properties of microcystin-LR, microcystin-RR and anatoxin-a using UV-light-emitting diodes at 255nm in combination with H2O2 [J]. Chemical Engineering Journal, 2019, 366: 423-432. |
| 15 | HAN Tao, LI Wentao, LI Jin, et al. Degradation of micropollutants in flow-through UV/chlorine reactors: Kinetics, mechanism, energy requirement and toxicity evaluation[J]. Chemosphere, 2022, 307: 135890. |
| 16 | CAI Wenwen, PENG Tao, YANG Bin, et al. Kinetics and mechanism of reactive radical mediated fluconazole degradation by the UV/chlorine process: Experimental and theoretical studies[J]. Chemical Engineering Journal, 2020, 402: 126224. |
| 17 | GUO Kaiheng, WU Zihao, SHANG Chi, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water[J]. Environmental Science & Technology, 2017, 51(18): 10431-10439. |
| 18 | NIKRAVESH Behnaz, SHOMALNASAB Asma, NAYYER Aliyehsadat, et al. UV/Chlorine process for dye degradation in aqueous solution: Mechanism, affecting factors and toxicity evaluation for textile wastewater[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104244. |
| 19 | GAO Yuqiong, ZHANG Jia, LI Cong, et al. Comparative evaluation of metoprolol degradation by UV/chlorine and UV/H2O2 processes[J]. Chemosphere, 2020, 243: 125325. |
| 20 | DENG Jia, WU Guangxue, YUAN Shoujun, et al. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 371: 151-158. |
| 21 | YIN Kai, HE Qunying, LIU Chengbin, et al. Prednisolone degradation by UV/chlorine process: Influence factors, transformation products and mechanism[J]. Chemosphere, 2018, 212: 56-66. |
| 22 | GUO Kaiheng, WU Zihao, YAN Shuwen, et al. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements[J]. Water Research, 2018, 147: 184-194. |
| 23 | WAN Ying, XIE Pengchao, WANG Zongping, et al. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling[J]. Water Research, 2019, 158: 213-226. |
| 24 | GHANBARI Farshid, Ali YAGHOOT-NEZHAD, Stanisław WACŁAWEK, et al. Comparative investigation of acetaminophen degradation in aqueous solution by UV/chlorine and UV/H2O2 processes: Kinetics and toxicity assessment, process feasibility and products identification[J]. Chemosphere, 2021, 285: 131455. |
| 25 | 马京帅, 吕文英, 刘国光, 等. 吉非罗齐在热活化过硫酸盐体系中的降解机制研究[J]. 环境科学学报, 2016, 36(10): 3774-3783. |
| MA Jingshuai, Wenying LYU, LIU Guoguang, et al. Degradation mechanism of gemfibrozil in heat-activated persulfate oxidation process[J]. Acta Scientiae Circumstantiae, 2016, 36(10): 3774-3783. | |
| 26 | LUO Congwei, JIANG Jin, MA Jun, et al. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways[J]. Water Research, 2016, 96: 12-21. |
| 27 | YAN Boyin, XU Dongchuan, LIU Zhiquan, et al. Degradation of neurotoxin β-N-methylamino-L-alanine by UV254 activated persulfate: Kinetic model and reaction pathways[J]. Chemical Engineering Journal, 2021, 404: 127041. |
| 28 | XIANG Yingying, FANG Jingyun, SHANG Chii. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process[J]. Water Research, 2016, 90: 301-308. |
| 29 | LI Boqiang, MA Xiaoyan, LI Qingsong, et al. Factor affecting the role of radicals contribution at different wavelengths, degradation pathways and toxicity during UV-LED/chlorine process[J]. Chemical Engineering Journal, 2020, 392: 124552. |
| 30 | CAO Y, YAO J, KNUDSEN T Š, et al. Radical chemistry, degradation mechanism and toxicity evolution of BPA in the UV/chlorine and UV/H2O2 [J]. Chemosphere, 2023, 312: 137169. |
| 31 | LIU Huaying, HOU Zhichao, LI Yingjie, et al. Modeling degradation kinetics of gemfibrozil and naproxen in the UV/chlorine system: Roles of reactive species and effects of water matrix[J]. Water Research, 2021, 202: 117445. |
| 32 | REMUCAL C K, MANLEY D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment[J]. Environmental Science: Water Research & Technology, 2016, 2(4): 565-579. |
| 33 | WU Zihao, GUO Kaiheng, FANG Jingyun, et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process[J]. Water Research, 2017, 126: 351-360. |
| 34 | CHEN Chunyan, WU Zihao, HOU Shaodong, et al. Transformation of gemfibrozil by the interaction of chloride with sulfate radicals: Radical chemistry, transient intermediates and pathways[J]. Water Research, 2022, 209: 117944. |
| 35 | FANG Jingyun, FU Yun, SHANG Chii. The roles of reactive species in micropollutant degradation in the UV/free chlorine system[J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. |
| 36 | LI Tong, JIANG Yan, AN Xiaoqiang, et al. Transformation of humic acid and halogenated byproduct formation in UV-chlorine processes[J]. Water Research, 2016, 102: 421-427. |
| [1] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [2] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [3] | 徐沛瑶, 陈标奇, KANKALA Ranjith Kumar, 王士斌, 陈爱政. 纳米材料用于铁死亡联合治疗的研究进展[J]. 化工进展, 2023, 42(7): 3684-3694. |
| [4] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [5] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [6] | 孙千千, 刘阵, 李瑞, 张溪, 杨明德, 吴玉龙. 低温水热耦合亚铁离子活化过硫酸盐提高剩余污泥的脱水性能[J]. 化工进展, 2023, 42(2): 595-602. |
| [7] | 王雨晴, 段钰锋, 王睿, 刘晓硕, 申镇. 乙醇改性钙基脱氯剂实验及动力学分析[J]. 化工进展, 2023, 42(11): 6053-6063. |
| [8] | 王庆宏, 姜晨旭, 王鑫, 余美琪, 朱帅, 李一鸣, 陈春茂. 天然矿物催化氧化水中难降解有机污染物研究进展[J]. 化工进展, 2023, 42(1): 417-434. |
| [9] | 段毅, 邹烨, 周书葵, 杨柳. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158. |
| [10] | 潘杰, 王明新, 高生旺, 夏训峰, 韩雪. 氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑[J]. 化工进展, 2022, 41(8): 4204-4212. 唑[J]. 化工进展, 2022, 41(8): 4204-4212. |
| [11] | 季炫宇, 林伟坚, 周雄, 柏继松, 杨宇, 孔杰, 廖重阳. 废轮胎热裂解技术研究现状与进展[J]. 化工进展, 2022, 41(8): 4498-4512. |
| [12] | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| [13] | 庄雨婷, 王建华, 向智艳, 赵娟, 徐琼, 刘贤响, 尹笃林. 半纤维素及其衍生物转化为γ-戊内酯及其动力学研究进展[J]. 化工进展, 2022, 41(7): 3519-3533. |
| [14] | 李贵贤, 张军强, 杨勇, 范学英, 王东亮. 基于PX选择性强化的短流程甲苯甲醇甲基化PX生产新工艺[J]. 化工进展, 2022, 41(6): 2939-2947. |
| [15] | 廖兵, 胥雯, 叶秋月. 活化过碳酸盐及过氧碳酸氢盐在水处理领域中的研究进展[J]. 化工进展, 2022, 41(6): 3235-3248. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||