化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4204-4212.DOI: 10.16085/j.issn.1000-6613.2021-2197
氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑
唑
潘杰1( ), 王明新1, 高生旺2(
), 王明新1, 高生旺2( ), 夏训峰2, 韩雪2
), 夏训峰2, 韩雪2
- 1.常州大学环境与安全工程学院,江苏 常州 213164
2.中国环境科学研究院,北京 100012
-
收稿日期:2021-10-27修回日期:2022-03-13出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:高生旺 -
作者简介:潘杰(1997—),女,硕士研究生,研究方向为水污染控制。E-mail:526313107@qq.com 。 -
基金资助:国家重点研发计划(2019YFD1100201)
Nitrogen-sulfur doped biochar/permonosulfate for degradation of sulfisoxazole in water
PAN Jie1( ), WANG Mingxin1, GAO Shengwang2(
), WANG Mingxin1, GAO Shengwang2( ), XIA Xunfeng2, HAN Xue2
), XIA Xunfeng2, HAN Xue2
- 1.College of Environmental and Safety Engineering, Changzhou University, Changzhou 213164, Jiangsu, China
2.Chinese Research Academy of Environmental Sciences, Beijing 100012, China
-
Received:2021-10-27Revised:2022-03-13Online:2022-08-25Published:2022-08-22 -
Contact:GAO Shengwang
摘要:
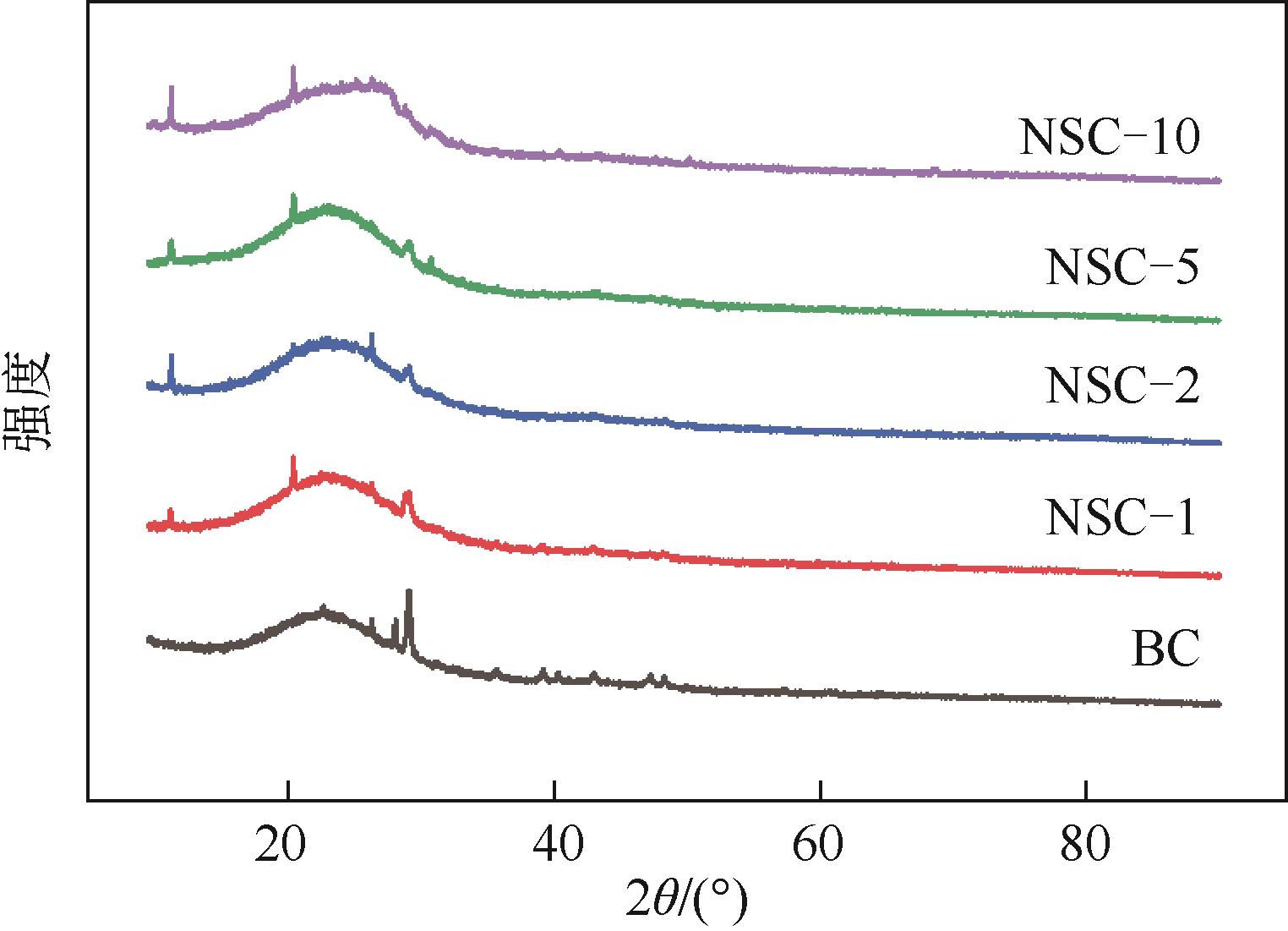
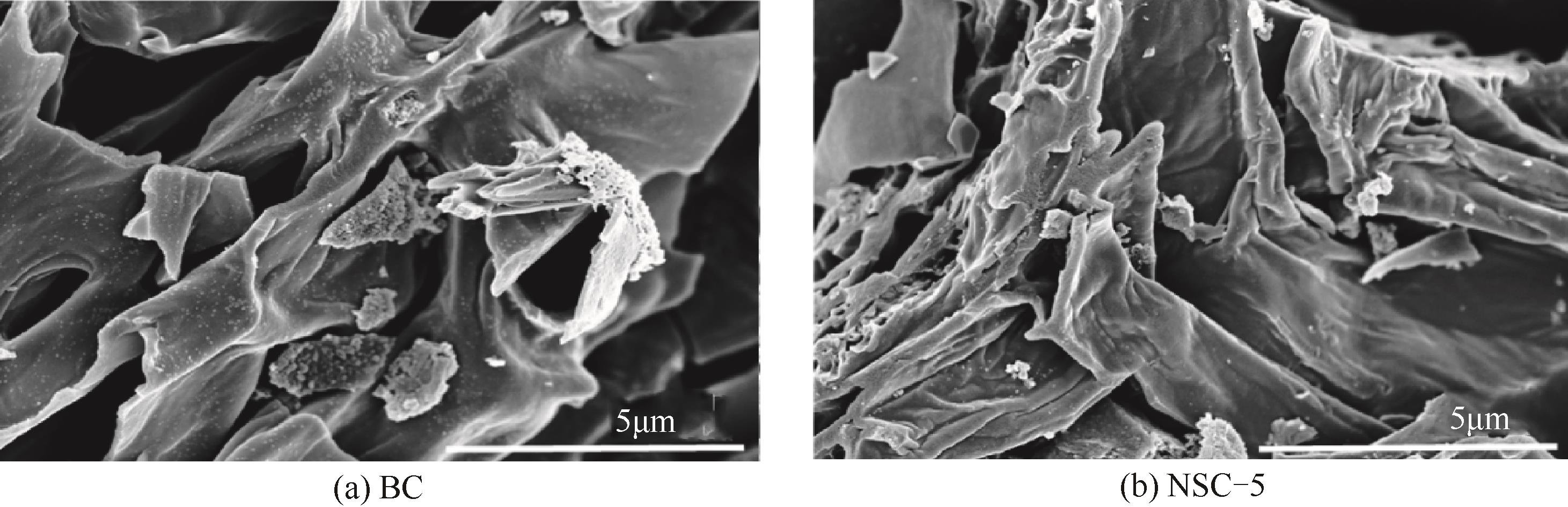
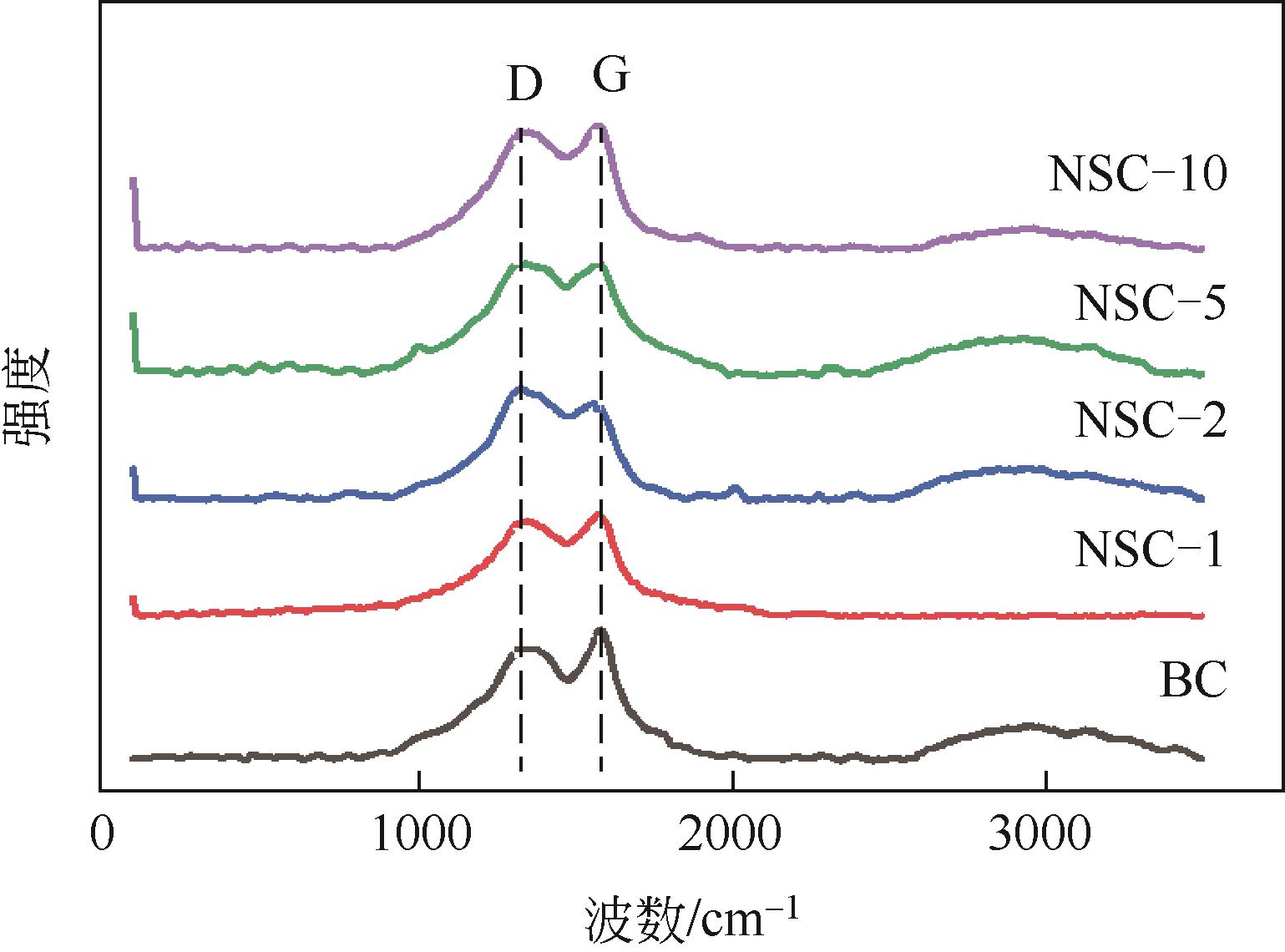
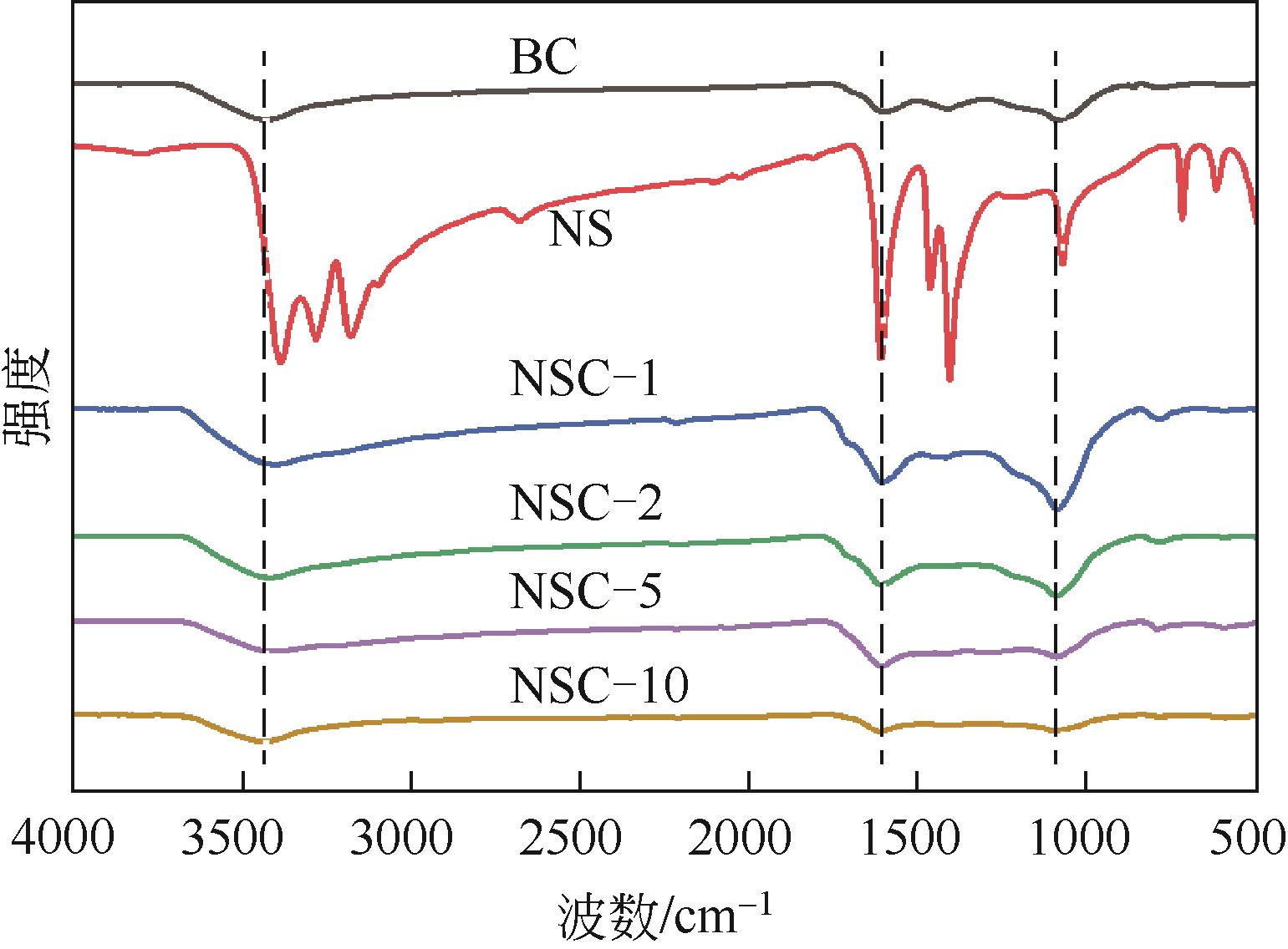
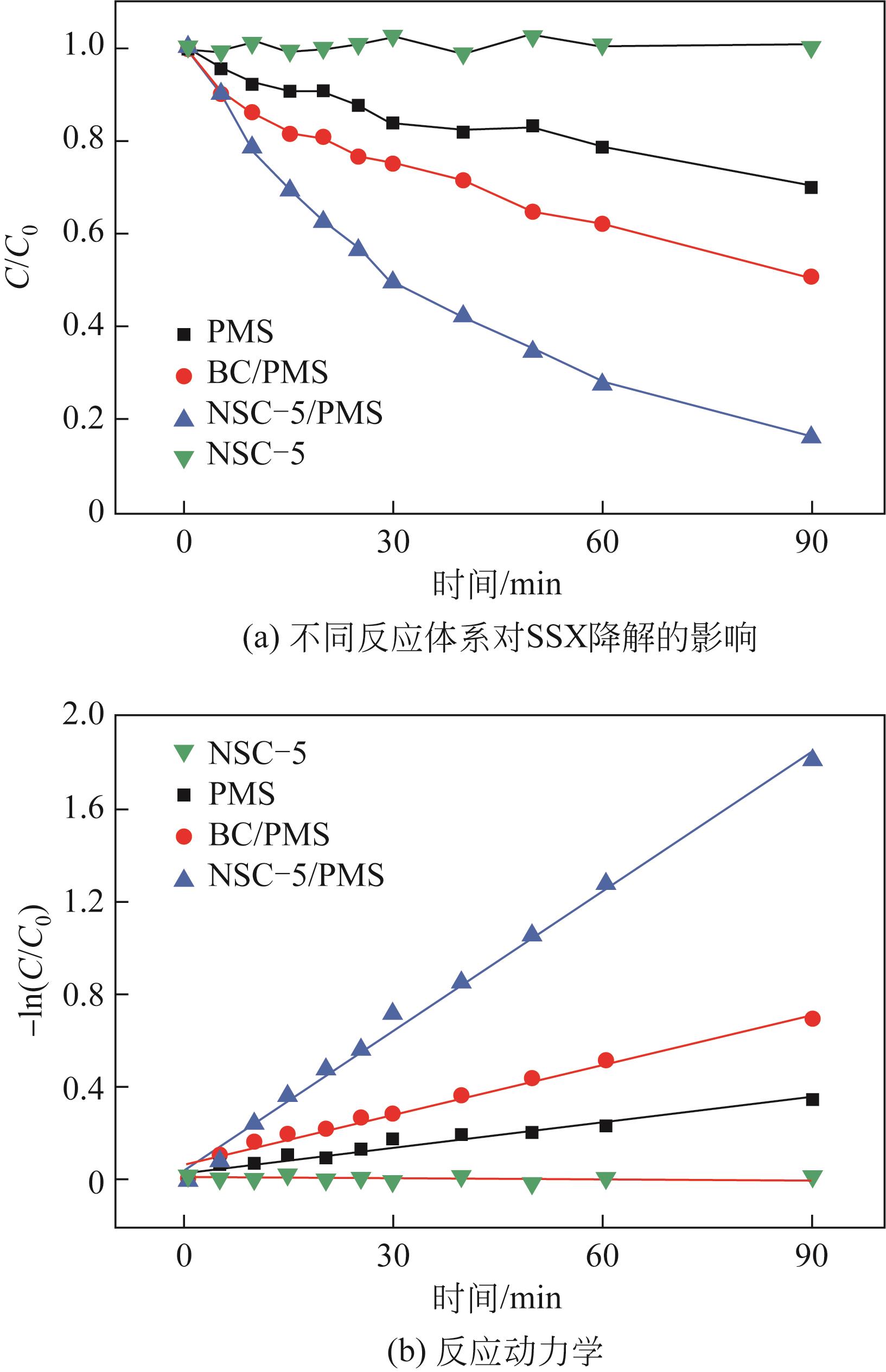
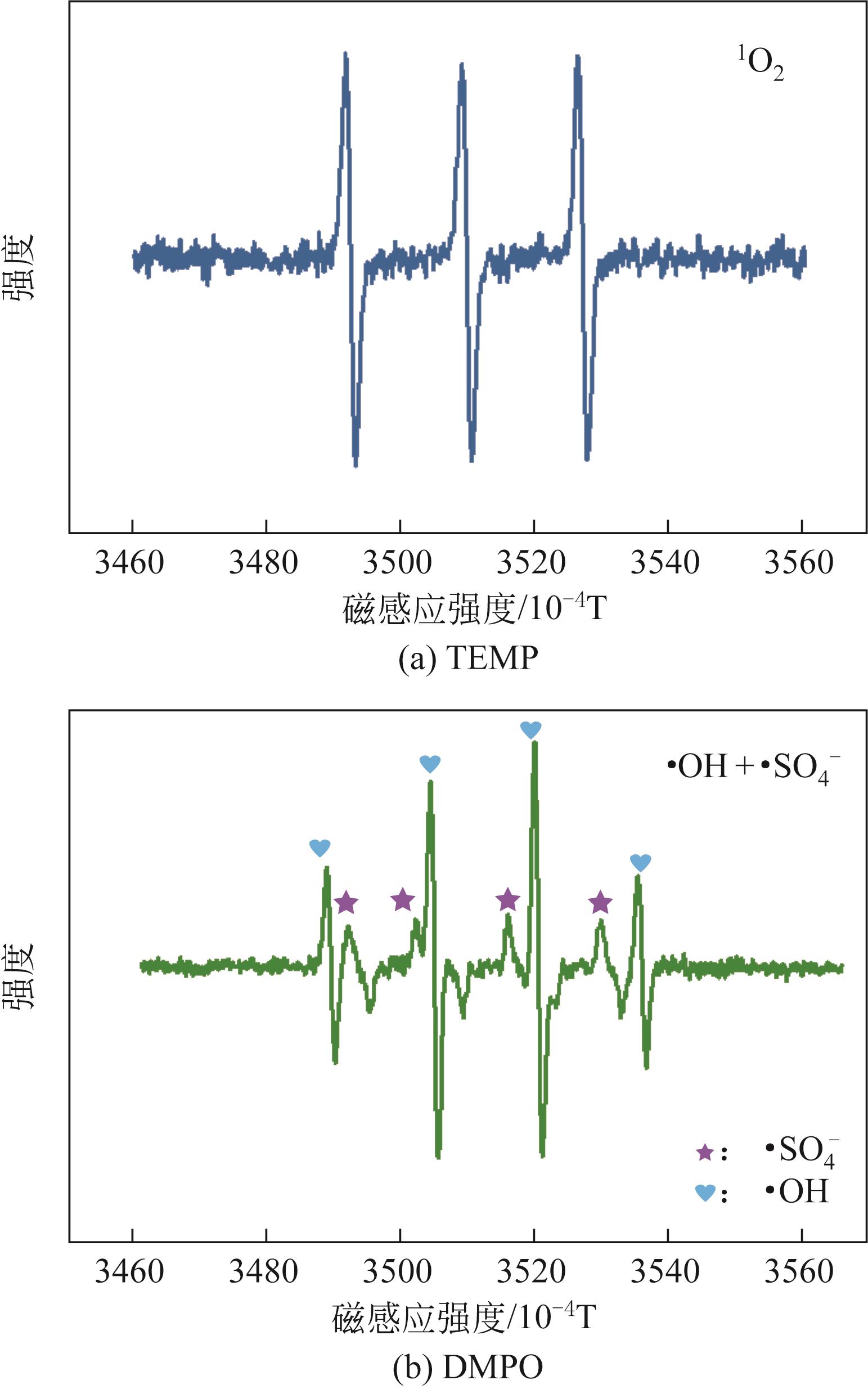
利用高温热解的方式制备由氮、硫元素掺杂改性的生物碳质纤维材料,并借用扫描电镜(SEM)、X射线衍射(XRD)、X射线光电子能谱(XPS)等多种技术对材料性质进行分析。实验以制备材料为催化剂活化过一硫酸盐(PMS)降解水中的磺胺异 唑(SSX),研究其降解效果,探讨材料活化PMS的机理。结果表明:N、S的掺杂显著提升了材料活化PMS降解SSX的性能,其中NSC-5的催化性能最佳,当NSC-5投加量为0.4g/L、PMS浓度为0.25mmol/L、SSX浓度为10mg/L时,反应90min后可去除80%以上,反应速率是生物碳质材料(BC)参与进行反应的2.7倍,这与其表面增加的官能团相关。电子顺磁共振(EPR)结果表明,SSX降解过程中起主要作用的组分是单线态氧(1O2)、硫酸根自由基(·SO
唑(SSX),研究其降解效果,探讨材料活化PMS的机理。结果表明:N、S的掺杂显著提升了材料活化PMS降解SSX的性能,其中NSC-5的催化性能最佳,当NSC-5投加量为0.4g/L、PMS浓度为0.25mmol/L、SSX浓度为10mg/L时,反应90min后可去除80%以上,反应速率是生物碳质材料(BC)参与进行反应的2.7倍,这与其表面增加的官能团相关。电子顺磁共振(EPR)结果表明,SSX降解过程中起主要作用的组分是单线态氧(1O2)、硫酸根自由基(·SO
中图分类号:
引用本文
潘杰, 王明新, 高生旺, 夏训峰, 韩雪. 氮硫掺杂生物炭/过一硫酸盐体系降解水中磺胺异 唑[J]. 化工进展, 2022, 41(8): 4204-4212.
唑[J]. 化工进展, 2022, 41(8): 4204-4212.
PAN Jie, WANG Mingxin, GAO Shengwang, XIA Xunfeng, HAN Xue. Nitrogen-sulfur doped biochar/permonosulfate for degradation of sulfisoxazole in water[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4204-4212.
| 1 | 朱洁, 孟香港. 抗生素在我国水生环境中的研究进展[J]. 科技风, 2019(34): 119-120. |
| ZHU Jie, MENG Xianggang. Research progress of antibiotics in aquatic environment in my country[J]. Technology Wind, 2019(34): 119-120. | |
| 2 | 李秀文, 何益得, 张巍, 等. 磺胺类抗生素对水环境的污染及生态毒理效应[J]. 环境科学与技术, 2018, 41(S1): 62-67. |
| LI Xiuwen, HE Yide, ZHANG Wei, et al. Pollution status of sulfonamides in aquatic environment and its ecotoxicological effects on aquatic organisms[J]. Environmental Science & Technology, 2018, 41(S1): 62-67. | |
| 3 | 颜平平, 隋倩, 吕树光, 等. 二价铁活化过碳酸钠对磺胺甲𫫇唑的去除[J]. 化工进展, 2018, 37(9): 3635-3639. |
| YAN Pingping, SUI Qian, Shuguang LYU, et al. Removal of sulfamethoxazole by ferrous-activated sodium percarbonate in the aqueous phase[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3635-3639. | |
| 4 | 刘一清, 苏冰琴, 陶艳, 等. 磁性纳米Fe3O4活化过硫酸盐降解水中磺胺甲𫫇唑[J]. 环境工程学报, 2020, 14(9): 2515-2526. |
| LIU Yiqing, SU Bingqin, TAO Yan, et al. Degradation of sulfamethoxazole in water by magnetic nano-Fe3O4 activated persulfate[J]. Chinese Journal of Environmental Engineering, 2020, 14(9): 2515-2526. | |
| 5 | 李峰, 刘桂芳, 梁涛, 等. Fe2+/NH2OH联合活化过硫酸盐降解水中磺胺甲𫫇唑[J]. 高校化学工程学报, 2017, 31(5): 1210-1216. |
| LI Feng, LIU Guifang, LIANG Tao, et al. Degradation of sulfamethoxazole in aqueous solution by ferrous/hydroxylamine activated peroxymonosulfate[J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(5): 1210-1216. | |
| 6 | GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 7 | ANIPSITAKIS G P, DIONYSIOU D D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt[J]. Environmental Science & Technology, 2003, 37(20): 4790-4797. |
| 8 | GHAUCH A, TUQAN A M. Oxidation of bisoprolol in heated persulfate/H2O systems: kinetics and products[J]. Chemical Engineering Journal, 2012, 183: 162-171. |
| 9 | QI C D, LIU X T, MA J, et al. Activation of peroxymonosulfate by base: implications for the degradation of organic pollutants[J]. Chemosphere, 2016, 151: 280-288. |
| 10 | GUO H G, GAO N Y, YANG Y, et al. Kinetics and transformation pathways on oxidation of fluoroquinolones with thermally activated persulfate[J]. Chemical Engineering Journal, 2016, 292: 82-91. |
| 11 | LIU J, ZHOU J H, DING Z X, et al. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye[J]. Ultrasonics Sonochemistry, 2017, 34: 953-959. |
| 12 | SHARMA J, MISHRA I M, DIONYSIOU D D, et al. Oxidative removal of bisphenol A by UV-C/peroxymonosulfate (PMS): kinetics, influence of co-existing chemicals and degradation pathway[J]. Chemical Engineering Journal, 2015, 276: 193-204. |
| 13 | ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. |
| 14 | LIU H, SUN P, FENG M B, et al. Nitrogen and sulfur co-doped CNT-COOH as an efficient metal-free catalyst for the degradation of UV filter BP-4 based on sulfate radicals[J]. Applied Catalysis B: Environmental, 2016, 187: 1-10. |
| 15 | 王郑, 王佳豪, 田湉, 等. 改性活化过硫酸盐应用及机理研究进展[J]. 精细化工, 2021, 38(7): 1305-1313. |
| WANG Zheng, WANG Jiahao, TIAN Tian, et al. Research progress on the application and mechanism of modified biochar activated persulfate[J]. Fine Chemicals, 2021, 38(7): 1305-1313. | |
| 16 | LIU Z, ZHANG L H, SHENG L Z, et al. Edge-nitrogen-rich carbon dots pillared graphene blocks with ultrahigh volumetric/gravimetric capacities and ultralong life for sodium-ion storage[J]. Advanced Energy Materials, 2018, 8(30): 1802042. |
| 17 | ZHU S S, HUANG X C, MA F, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms[J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. |
| 18 | JIANG J J, WANG X Y, YUE C L, et al. Nitrogen vacancies induce sustainable redox of iron-cobalt bimetals for efficient peroxymonosulfate activation: dual-path electron transfer[J]. Chemical Engineering Journal, 2022, 427: 131702. |
| 19 | CHEN X, DUAN X G, OH W D, et al. Insights into nitrogen and boron-co-doped graphene toward high-performance peroxymonosulfate activation: maneuverable N-B bonding configurations and oxidation pathways[J]. Applied Catalysis B: Environmental, 2019, 253: 419-432. |
| 20 | BAG S, MONDAL B, DAS A K, et al. Nitrogen and sulfur dual-doped reduced graphene oxide: synergistic effect of dopants towards oxygen reduction reaction[J]. Electrochimica Acta, 2015, 163: 16-23. |
| 21 | SUN W, PANG K F, YE F, et al. Efficient persulfate activation catalyzed by pyridinic N, COH, and thiophene S on N, S-co-doped carbon for nonradical sulfamethoxazole degradation: identification of active sites and mechanisms[J]. Separation and Purification Technology, 2022, 284: 120197. |
| 22 | GADISA B T, BAYE A F, APPIAH-NTIAMOAH R, et al. ZnO@Ni foam photoelectrode modified with heteroatom doped graphitic carbon for enhanced photoelectrochemical water splitting under solar light[J]. International Journal of Hydrogen Energy, 2021, 46(2): 2075-2085. |
| 23 | KARAMAN C. Orange peel derived-nitrogen and sulfur co-doped carbon dots: a nano-booster for enhancing ORR electrocatalytic performance of 3D graphene networks[J]. Electroanalysis, 2021, 33(5): 1356-1369. |
| 24 | 姚淑华, 马锡春, 李士凤. 秸秆生物炭活化过硫酸盐氧化降解苯酚[J]. 中国环境科学, 2018, 38(11): 4166-4172. |
| YAO Shuhua, MA Xichun, LI Shifeng. Straw biochar activated persulfate oxidation and degradation of phenol[J]. China Environmental Science, 2018, 38(11): 4166-4172. | |
| 25 | SHI C F, LI Y M, FENG H Y, et al. Removal of p-nitrophenol using persulfate activated by biochars prepared from different biomass materials[J]. Chemical Research in Chinese Universities, 2018, 34(1): 39-43. |
| 26 | SUN W, PANG K F, YE F, et al. Carbonization of camphor sulfonic acid and melamine to N,S-co-doped carbon for sulfamethoxazole degradation via persulfate activation: nonradical dominant pathway[J]. Separation and Purification Technology, 2021, 279: 119723. |
| 27 | 熊玲, 张敏, 陈绍华. 氮掺杂碳纳米管活化过硫酸盐降解丁基黄药[J]. 化工环保, 2021, 41(3): 296-302. |
| XIONG Ling, ZHANG Min, CHEN Shaohua. Degradation of butyl xanthate using persulfate activated with nitrogen-doped carbon nanotube[J]. Environmental Protection of Chemical Industry, 2021, 41(3): 296-302. | |
| 28 | LI Y Q, NI B, LI X D, et al. High-performance Na-ion storage of S-doped porous carbon derived from conjugated microporous polymers[J]. Nano-Micro Letters, 2019, 11(1): 1-13. |
| 29 | MIAO Y L, MA Y L, WANG Q. Plasma-assisted simultaneous reduction and nitrogen/sulfur codoping of graphene oxide for high-performance supercapacitors[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(8): 7597-7608. |
| 30 | 杨成海, 宁寻安, 赖晓君, 等. 硫氮共掺杂碳基材料活化过一硫酸盐降解2,4-二氯苯酚的效能及机理[J]. 环境科学学报, 2021, 41(7): 2785-2795. |
| YANG Chenghai, NING Xun’an, LAI Xiaojun,et al. Degradation efficiency and mechanism of 2,4-dichlorophenol by activation of peroxymonosulfate with sulfur and nitrogen co-doped carbocatalysts[J]. Acta Scientiae Circumstantiae, 2021, 41(7): 2785-2795. | |
| 31 | SARKAR R, KAR M, HABIB M, et al. Common defects accelerate charge separation and reduce recombination in CNT/molecule composites: atomistic quantum dynamics[J]. Journal of the American Chemical Society, 2021, 143(17): 6649-6656. |
| 32 | 郭明帅, 王菲, 张学良, 等.改性生物炭活化过硫酸盐对水中苯和氯苯的去除机制[J]. 中国环境科学, 2020, 40: 5280-5289. |
| GUO Mingshuai, WANG Fei, ZHANG Xueliang, et al. Removal mechanism of benzene and chlorobenzene in water by modified biochar activates persulfate[J]. China Environmental Science, 2020, 40(12): 5280-5289. | |
| 33 | 郭锐税. 改性生物炭的制备及其对有机污染物去除的研究[D]. 上海: 上海工程技术大学, 2020. |
| GUO Ruishui. The preparation of modified biochar and its removal of organic pollutants[D]. Shanghai: Shanghai University of Engineering Science, 2020. | |
| 34 | PAN C, FU L B, DING Y B, et al. Homogeneous catalytic activation of peroxymonosulfate and heterogeneous reductive regeneration of Co2+ by MoS2: the pivotal role of pH[J]. Science of the Total Environment, 2020, 712: 136447. |
| 35 | ANDREW LIN K Y, HSU F K, LEE W D. Magnetic cobalt-graphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate[J]. Journal of Materials Chemistry A, 2015, 3(18): 9480-9490. |
| 36 | 姚怡媛, 王超海, 晏鑫, 等. 硼-氮共掺杂中空碳纳米纤维的制备及其活化过一硫酸盐降解双酚A的性能研究[J]. 环境科学学报, 2021, 41(7): 2774-2784. |
| YAO Yiyuan, WANG Chaohai, YAN Xin, et al. The fabrication of B-N co-doping hollow carbon nanofibers and peroxymonosulfate activation for the degradation of bisphenol A [J]. Acta Scientiae Circumstantiae, 2021, 41(7): 2774-2784. | |
| 37 | WANG H Z, GUO W Q, LIU B H, et al. Sludge-derived biochar as efficient persulfate activators: sulfurization-induced electronic structure modulation and disparate nonradical mechanisms[J]. Applied Catalysis B: Environmental, 2020, 279: 119361. |
| 38 | DU W Y, ZHANG Q Z, SHANG Y N, et al. Sulfate saturated biosorbent-derived Co-S@NC nanoarchitecture as an efficient catalyst for peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2020, 262: 118302. |
| 39 | LIU S Y, LAI C, LI B S, et al. Heteroatom doping in metal-free carbonaceous materials for the enhancement of persulfate activation[J]. Chemical Engineering Journal, 2022, 427: 131655. |
| 40 | HE Z M, ZHENG W D, LI M X, et al. Fe2P/biocarbon composite derived from a phosphorus-containing biomass for levofloxacin removal through peroxymonosulfate activation[J]. Chemical Engineering Journal, 2022, 427: 130928. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 白志华, 张军. 二乙烯三胺五亚甲基膦酸/Fenton体系氧化脱除NO[J]. 化工进展, 2023, 42(9): 4967-4973. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||