化工进展 ›› 2023, Vol. 42 ›› Issue (4): 1885-1894.DOI: 10.16085/j.issn.1000-6613.2022-1112
Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学
- 昆明理工大学冶金与能源工程学院,云南 昆明 650093
-
收稿日期:2022-06-14修回日期:2022-09-12出版日期:2023-04-25发布日期:2023-05-08 -
通讯作者:廖亚龙 -
作者简介:葛伟童(1995—),男,硕士研究生,研究方向为纳米金属催化材料制备。E-mail:793749752@qq.com。 -
基金资助:国家自然科学基金(21978112);昆明理工大学分析测试基金(20192202030)
Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst
GE Weitong( ), LIAO Yalong(
), LIAO Yalong( ), LI Mingyuan, JI Guangxiong, XI Jiajun
), LI Mingyuan, JI Guangxiong, XI Jiajun
- Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, Yunnan, China
-
Received:2022-06-14Revised:2022-09-12Online:2023-04-25Published:2023-05-08 -
Contact:LIAO Yalong
摘要:
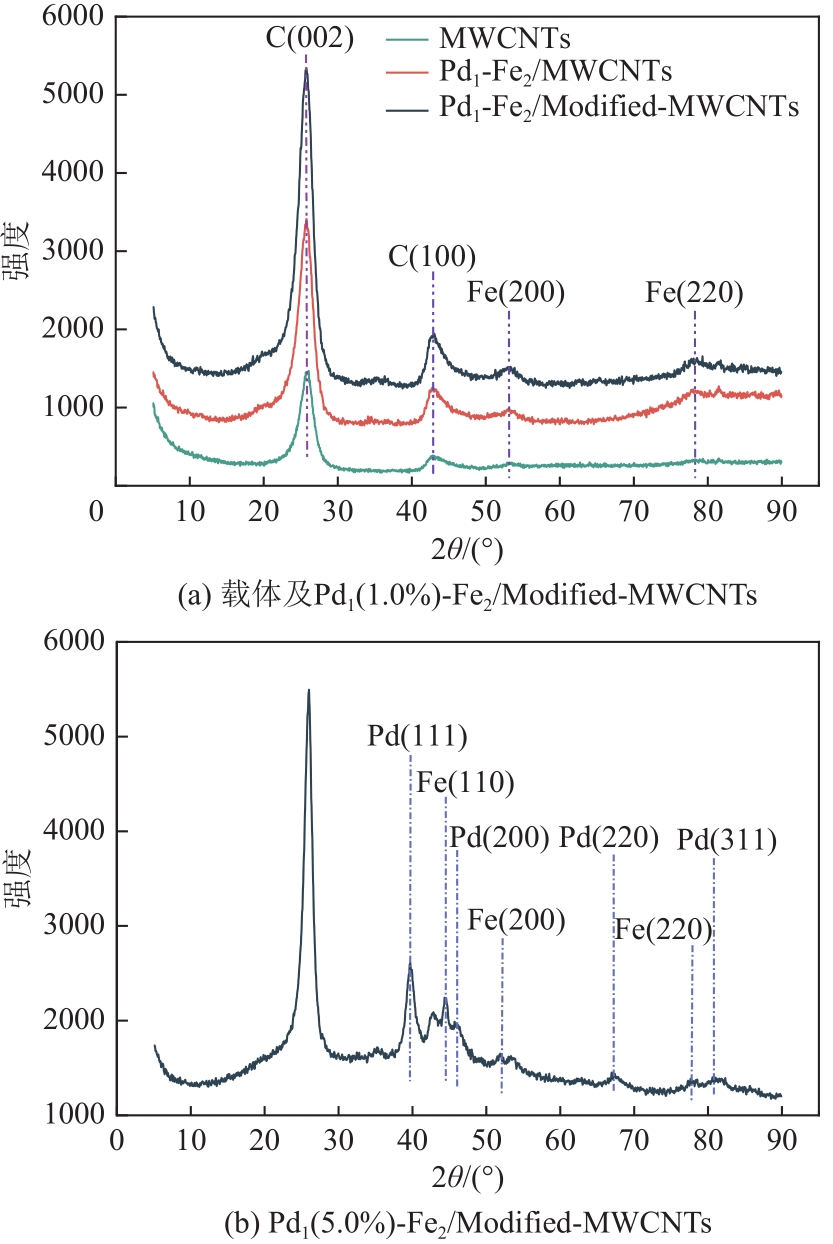
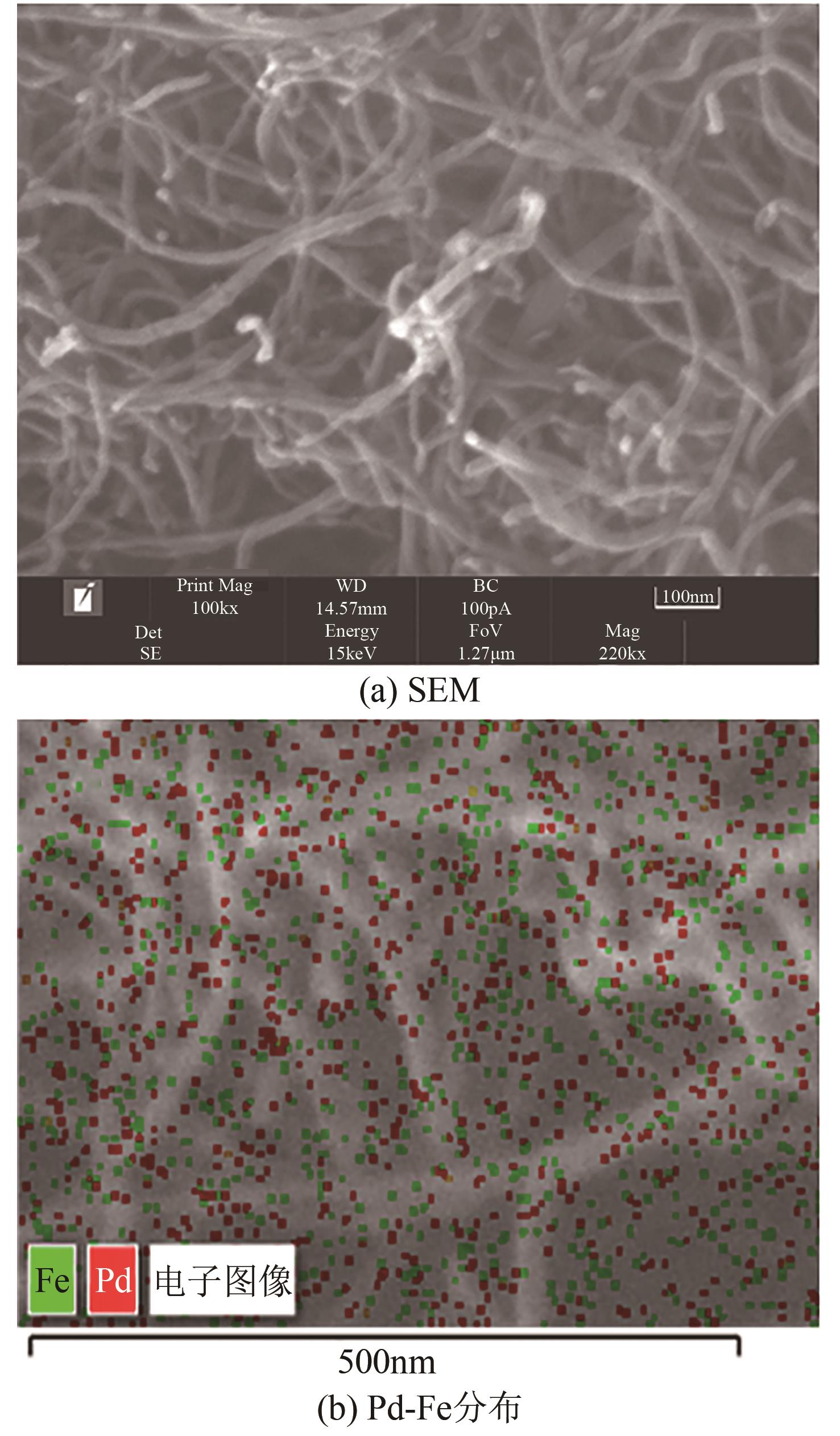
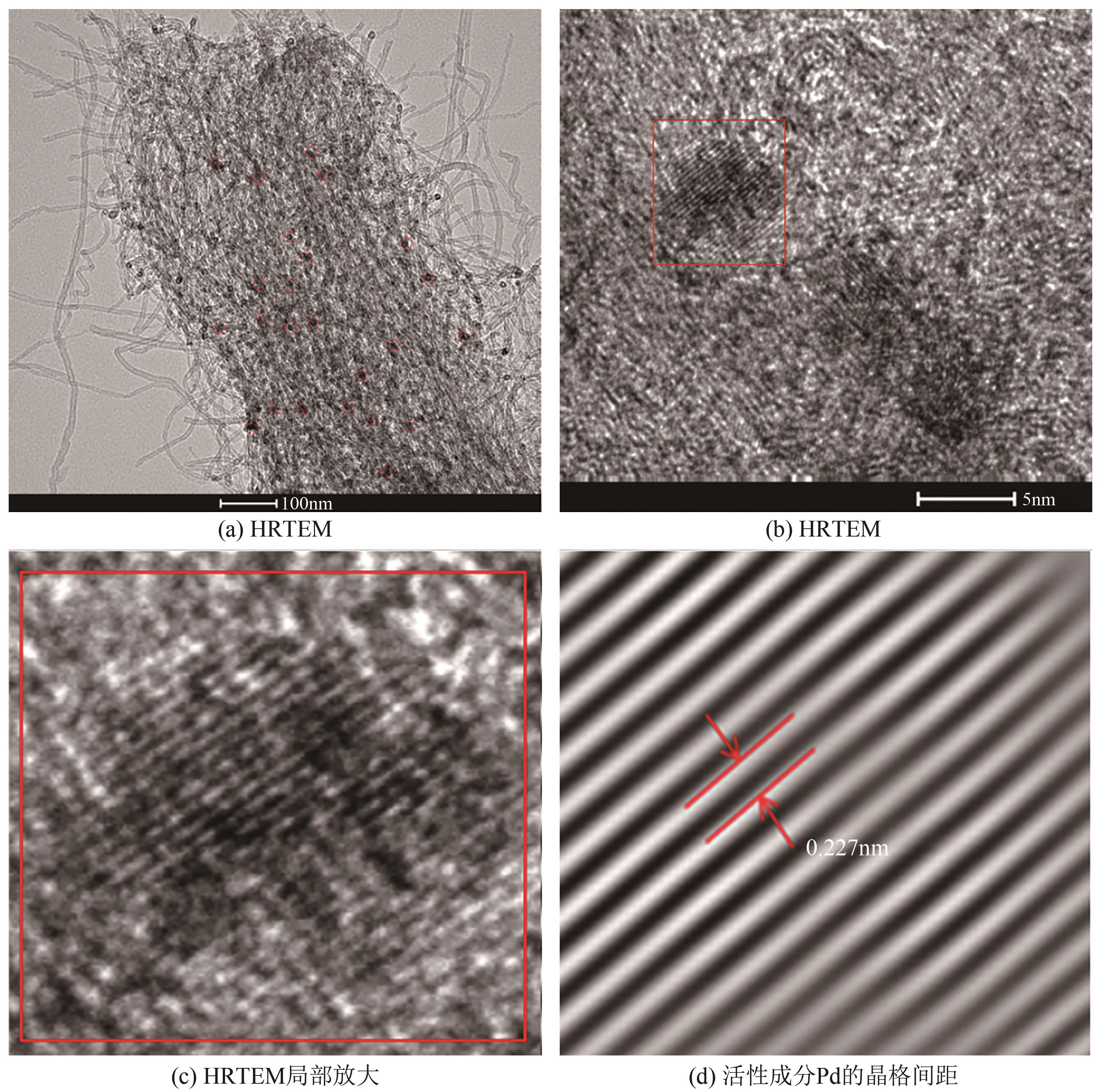
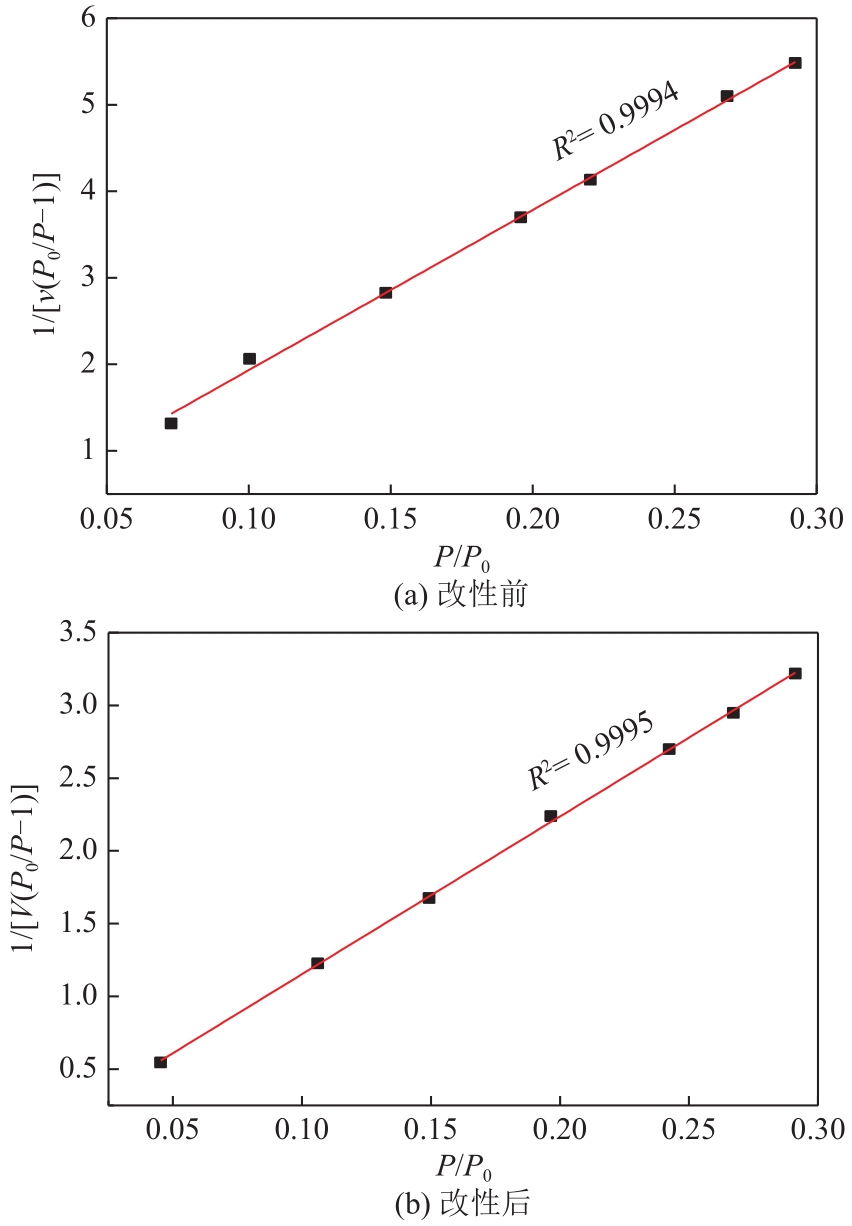
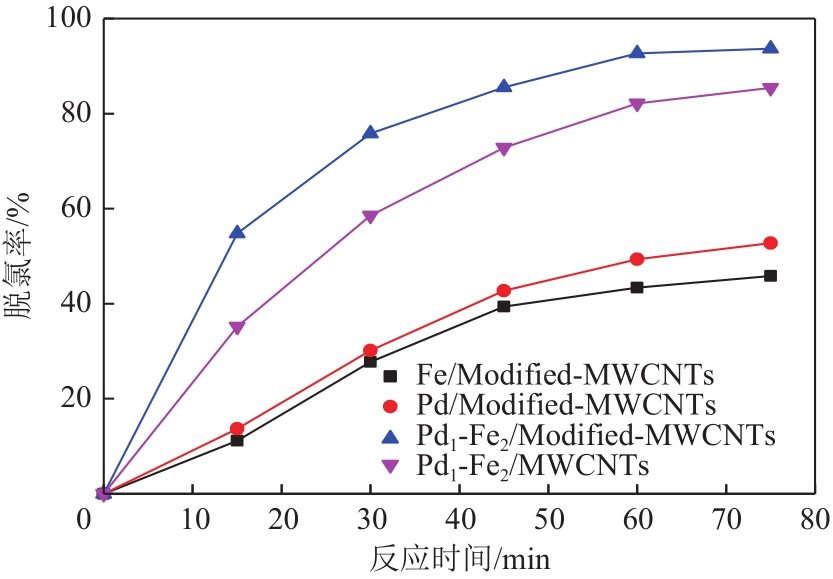
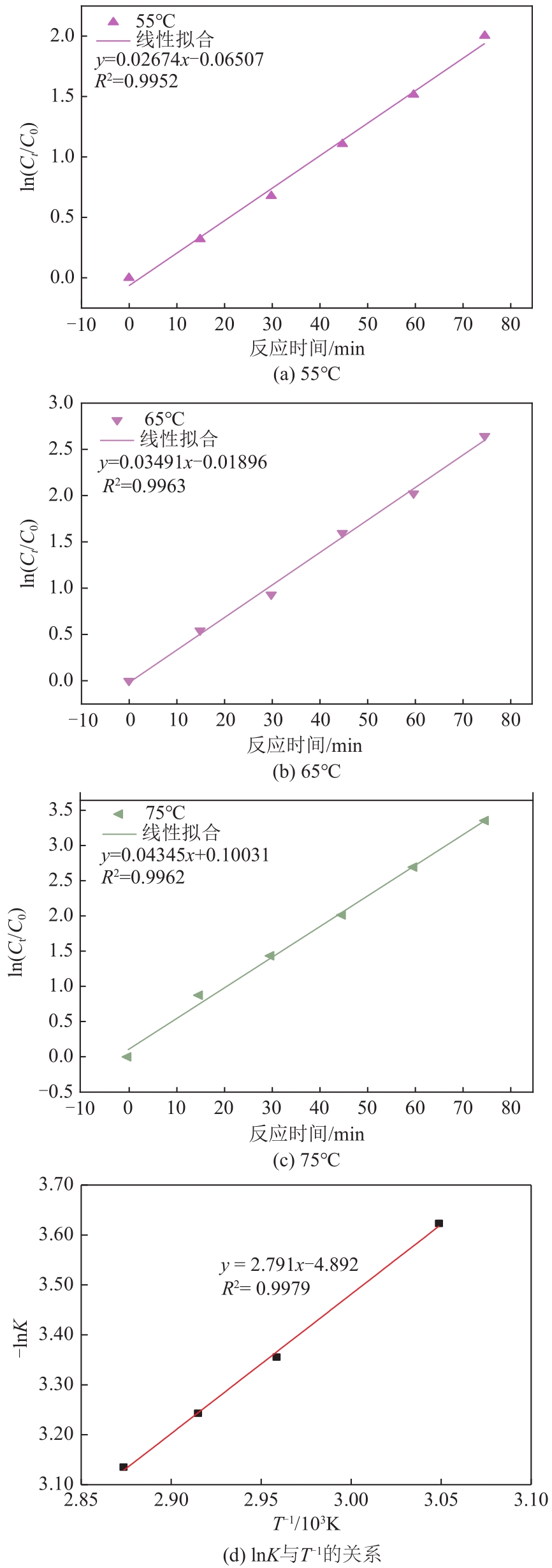
漂白紫胶常用于制药及食品行业,但制备过程中加成到其双键结构的结合氯对其使用领域和性能有负面影响,漂白紫胶中结合氯的脱除研究是紫胶高值利用研究的热点。本文以改性多壁碳纳米管为载体,采用超声强化、液相还原法制备出负载型Pd-Fe双金属催化剂,并用以漂白紫胶的催化加氢脱氯研究,以XRD、XPS、SEM、HRTEM对催化剂进行分析表征。结果表明,制备出的催化剂活性成分粒径为6.32nm,晶格参数为2.27Å;催化剂加氢脱氯反应属于取代反应,过程遵循一级反应动力学,反应的活化能值为23.20kJ/mol;55℃、65℃和75℃对应的反应速率常数分别为0.0267min-1、0.0349min-1和0.0435min-1;在最佳的脱氯反应条件下,制备得到的催化剂用于加氢脱除漂白紫胶中结合氯时,脱氯效率达到93.68%,氯质量分数由脱氯前的2.42%降至0.14%。
中图分类号:
引用本文
葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894.
GE Weitong, LIAO Yalong, LI Mingyuan, JI Guangxiong, XI Jiajun. Preparation and dechlorination kinetics of Pd-Fe/MWCNTs bimetallic catalyst[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1885-1894.
| 金属类型 | Pd金属质量分数 /% | Fe金属质量分数 /% | 粒径 /nm | 晶格参数 /Å |
|---|---|---|---|---|
| Pd-Fe | 5.0 | 5.3 | 6.36 | 2.27 |
表1 经XRD计算得到的粒径与晶格参数值
| 金属类型 | Pd金属质量分数 /% | Fe金属质量分数 /% | 粒径 /nm | 晶格参数 /Å |
|---|---|---|---|---|
| Pd-Fe | 5.0 | 5.3 | 6.36 | 2.27 |
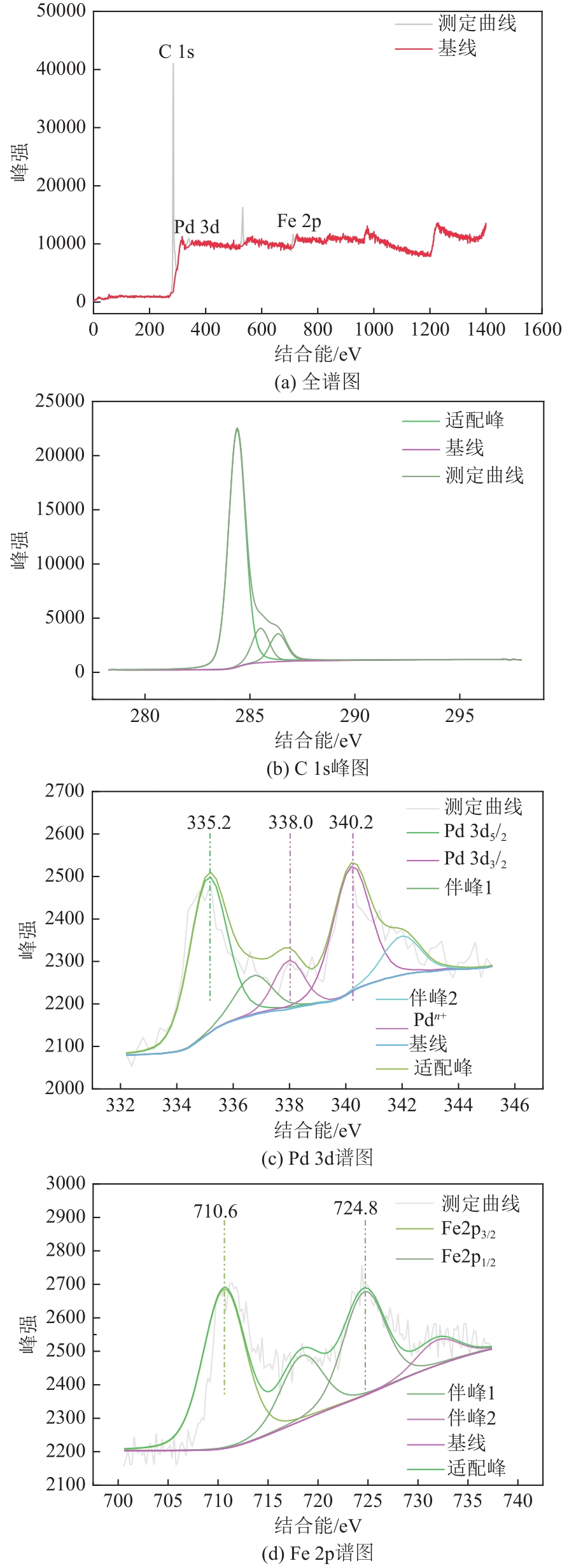
| 化学态 | 结合能/eV |
|---|---|
| Pd 3d3/2 | 340.2 |
| Pd 3d5/2 | 335.2 |
| Fe 2p3/2 | 710.6 |
| Fe 2p1/2 | 724.8 |
表2 Pd 3d信号与Fe 2p信号对应的结合能
| 化学态 | 结合能/eV |
|---|---|
| Pd 3d3/2 | 340.2 |
| Pd 3d5/2 | 335.2 |
| Fe 2p3/2 | 710.6 |
| Fe 2p1/2 | 724.8 |
| 项目 | 碳纳米管官能团含量/mmol·g-1 | ||
|---|---|---|---|
| 羧基(—COOH) | 羰基(C | 羟基(—OH) | |
| 改性前 | 0.17 | 1.35 | 4.37 |
| 改性后 | 0.49 | 2.47 | 7.26 |
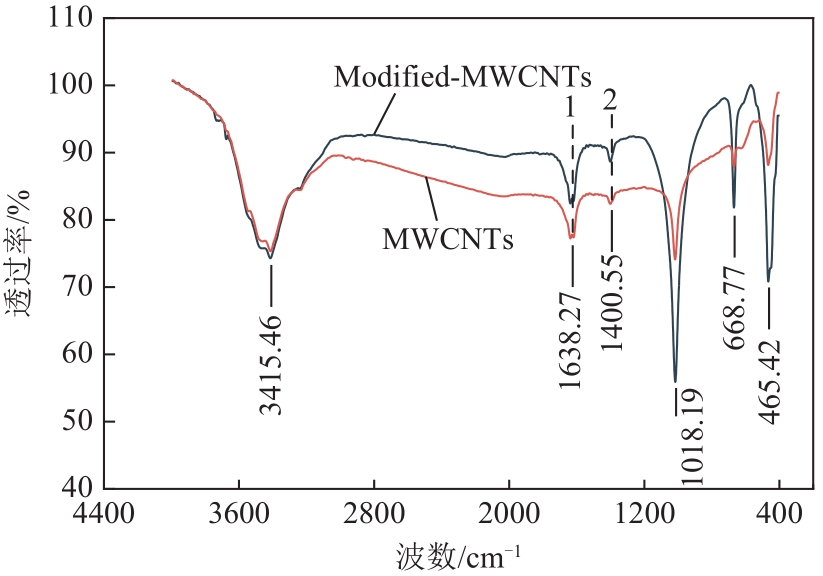
表3 改性前后碳纳米管含氧官能团变化
| 项目 | 碳纳米管官能团含量/mmol·g-1 | ||
|---|---|---|---|
| 羧基(—COOH) | 羰基(C | 羟基(—OH) | |
| 改性前 | 0.17 | 1.35 | 4.37 |
| 改性后 | 0.49 | 2.47 | 7.26 |
| 1 | AL-GOUSOUS J, PENNING M, LANGGUTH P. Molecular insights into shellac film coats from different aqueous shellac salt solutions and effect on disintegration of enteric-coated soft gelatin capsules[J]. International Journal of Pharmaceutics, 2015, 484(1/2): 283-291. |
| 2 | 廖亚龙, 彭金辉, 刘中华. 国内外紫胶深加工技术现状及趋势[J]. 林业科学, 2007, 43(7): 93-100. |
| LIAO Yalong, PENG Jinhui, LIU Zhonghua. National and international seedlac processing development and its trend[J]. Scientia Silvae Sinicae, 2007, 43(7): 93-100. | |
| 3 | REMADEVI O K, SIDDIQUI M Z, H Cet al NAGAVENI. Efficacy of shellac-based varnishes for protection of wood against termite, borer and fungal attack[J]. Journal of the Indian Academy of Wood Science, 2015, 12(1): 9-14. |
| 4 | JO W S, SONG H Y, SONG N B, et al. Quality and microbial safety of ‘Fuji’ apples coated with carnauba-shellac wax containing lemongrass oil[J]. LWT-Food Science and Technology, 2014, 55(2): 490-497. |
| 5 | PHAECHAMUD T, SETTHAJINDALERT O. Antimicrobial in situ forming gels based on bleached shellac and different solvents[J]. Journal of Drug Delivery Science and Technology, 2018, 46: 285-293. |
| 6 | WANG Xia, YU Dengguang, LI Xiaoyan, et al. Electrospun medicated shellac nanofibers for colon-targeted drug delivery[J]. International Journal of Pharmaceutics, 2015, 490(1/2): 384-390. |
| 7 | PHAECHAMUD T, MAHADLEK J, CHUENBARN T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment[J]. Materials & Design, 2016, 89: 294-303. |
| 8 | PHAECHAMUD T, SENARAT S, PUYATHORN N, et al. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and-microparticle[J]. International Journal of Biological Macromolecules, 2019, 135: 1261-1272. |
| 9 | LIAO Yalong, ZHOU Juan, HUANG Feirong, et al. Effects of combined chlorine on physicochemical properties and structure of shellac[J]. Pakistan Journal of Pharmaceutical Sciences, 2015, 28(1 ): 329-334. |
| 10 | LIAO Yalong, ZHOU Juan, HUANG Feirong. Process and kinetic mechanism of elimination of chlorine combined in molecule of bleached shellac[J]. Tropical Journal of Pharmaceutical Research, 2015, 14(11): 1953-1960. |
| 11 | 廖亚龙, 杨功舜, 周娟. 消除法制备低氯漂白紫胶及机理[J]. 材料工程, 2016, 44(4): 59-64. |
| LIAO Yalong, YANG Gongshun, ZHOU Juan. Preparation and mechanism of low chlorine shellac by means of elimination method[J]. Journal of Materials Engineering, 2016, 44(4): 59-64. | |
| 12 | LIAO Yalong, CHAI Xijuan, XU Fuchang. Preparation of low chlorine shellac by catalytic hydrogenation with Pd/C[J]. Advanced Materials Research, 2010, 152/153: 372-376. |
| 13 | LIAO Yalong, XU Fuchang, LI Dongbo. Preparation of low chlorine shellac from seedlac by dechlorination process using Pd/Fe binary metal as catalyst[J]. Advanced Materials Research, 2009, 79/80/81/82: 1879-1882. |
| 14 | ZHANG Yu, LIAO Yalong, SHI Gongchu, et al. Preparation, characterization, and catalytic performance of Pd-Ni/AC bimetallic nano-catalysts[J]. Green Processing and Synthesis, 2020, 9(1): 760-769. |
| 15 | ZHANG Yu, WANG Yiyang, LIAO Yalong, et al. Preparation, characterization and dechlorination property of nano Pd-Ni/γ-Al2O3 bimetallic catalyst[J]. Functional Materials Letters, 2019, 12(6): 1951003. |
| 16 | LIAO Yalong, WANG Wei, ZHANG Yu, et al. Preparation, characterization and catalytic hydrodechlorination property for bleached shellac of Pd-Ni@SiO2 bimetallic nano-catalyst[J]. Reaction Kinetics, Mechanisms and Catalysis, 2020, 130(2): 1043-1061. |
| 17 | LIAO Yalong, WANG Yiyang, ZHANG Yu. Preparation and catalytic hydrodechlorination property of nano bimetallic catalyst Pd-Ni/γ-Al2O3-SiO2 [J]. Catalysts, 2022, 12(4): 370. |
| 18 | ZHAO Ming, ABE K, YAMAURA S I, et al. Fabrication of Pd-Ni-P metallic glass nanoparticles and their application as highly durable catalysts in methanol electro-oxidation[J]. Chemistry of Materials, 2014, 26(2): 1056-1061. |
| 19 | CHENG Honghui, CHEN Gang, ZHANG Yao, et al. Boosting low-temperature de/re-hydrogenation performances of MgH2 with Pd-Ni bimetallic nanoparticles supported by mesoporous carbon[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10777-10787. |
| 20 | ZAFARI R, ABDOUSS M, ZAMANI Y. Application of response surface methodology for the optimization of light olefins production from CO hydrogenation using an efficient catalyst[J]. Fuel, 2019, 237: 1262-1273. |
| 21 | KOSKUN Y, ŞAVK A, ŞEN B, et al. Highly sensitive glucose sensor based on monodisperse palladium nickel/activated carbon nanocomposites[J]. Analytica Chimica Acta, 2018, 1010: 37-43. |
| 22 | SEN B, KUZU S, DEMIR E, et al. Monodisperse palladium-nickel alloy nanoparticles assembled on graphene oxide with the high catalytic activity and reusability in the dehydrogenation of dimethylamine-borane[J]. International Journal of Hydrogen Energy, 2017, 42(36): 23276-23283. |
| 23 | ŞEN B, AYGÜN A, OKYAY T O, et al. Monodisperse palladium nanoparticles assembled on graphene oxide with the high catalytic activity and reusability in the dehydrogenation of dimethylamine-borane[J]. International Journal of Hydrogen Energy, 2018, 43(44): 20176-20182. |
| 24 | TAN Ling, LI Ting, ZHOU Juan, et al. Liquid-phase hydrogenation of N-nitrosodimethylamine over Pd-Ni supported on CeO2-TiO2: The role of oxygen vacancies[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 558: 211-218. |
| 25 | KIM K S, GOSSMANN A F, WINOGRAD N. X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode[J]. Analytical Chemistry, 1974, 46(2): 197-200. |
| 26 | KUMAR G, BLACKBURN J R, ALBRIDGE R G, et al. Photoelectron spectroscopy of coordination compounds. Ⅱ. Palladium complexes[J]. Inorganic Chemistry, 1972, 11(2): 296-300. |
| 27 | CHANG S L, ANDEREGG J W, THIEL P A. Surface oxidation of an Al-Pd-Mn quasicrystal, characterized by X-ray photoelectron spectroscopy[J]. Journal of Non-Crystalline Solids, 1996, 195(1/2): 95-101. |
| 28 | HE Zhiqiao, JIAN Qiwei, TANG Juntao, et al. Improvement of electrochemical reductive dechlorination of 2,4-dichlorophenoxyacetic acid using palladium catalysts prepared by a pulsed electrodeposition method[J]. Electrochimica Acta, 2016, 222: 488-498. |
| 29 | JIANG Tao, HUAI Qiang, GENG Tong, et al. Catalytic performance of Pd-Ni bimetallic catalyst for glycerol hydrogenolysis[J]. Biomass and Bioenergy, 2015, 78: 71-79. |
| 30 | CHEN Huan, LI Ting, JIANG Fang, et al. Enhanced catalytic reduction of N-nitrosodimethylamine over bimetallic Pd-Ni catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2016, 421: 167-177. |
| 31 | TAN B J, KLABUNDE K J, SHERWOOD P M A. X-ray photoelectron spectroscopy studies of solvated metal atom dispersed catalysts. Monometallic iron and bimetallic iron-cobalt particles on alumina[J]. Chemistry of Materials, 1990, 2(2): 186-191. |
| 32 | 王艳丽, 张明旭, 李本侠, 等. 软模板法六边形纳米Pd粒子的超声制备与表征[J]. 无机化学学报, 2011, 27(10): 1914-1918. |
| WANG Yanli, ZHANG Mingxu, LI Benxia, et al. Hexagonal nano-Pd particles: Sonochemical preparation by soft-template method and characterization[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(10): 1914-1918. | |
| 33 | 金永丽, 张祥, 张捷宇, 等. 稳恒磁场对Fe形核生长取向的影响[J]. 钢铁钒钛, 2019, 40(6): 129-134, 148. |
| JIN Yongli, ZHANG Xiang, ZHANG Jieyu, et al. Effect of static magnetic field on nucleation and growth orientation of Fe atomic[J]. Iron Steel Vanadium Titanium, 2019, 40(6): 129-134, 148. | |
| 34 | 李荣柱, 马艳红, 卢成. BET法测定氧化铝比表面积的研究[J]. 轻金属, 2020(9): 13-16. |
| LI Rongzhu, MA Yanhong, LU Cheng. Study on the specific surface area determination of alumina by BET method[J]. Light Metals, 2020(9): 13-16. | |
| 35 | 冒爱琴, 王华, 谈玲华, 等. 活性炭表面官能团表征进展[J]. 应用化工, 2011, 40(7): 1266-1270. |
| MAO Aiqin, WANG Hua, TAN Linghua, et al. Research progress in characterization of functional groups on activated carbon[J]. Applied Chemical Industry, 2011, 40(7): 1266-1270. | |
| 36 | ZHOU Juan, WU Ke, WANG Wenjuan, et al. Pd supported on boron-doped mesoporous carbon as highly active catalyst for liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol[J]. Applied Catalysis A: General, 2014, 470: 336-343. |
| 37 | DIAZ E, MOHEDANO A F, CASAS J A, et al. Analysis of the deactivation of Pd, Pt and Rh on activated carbon catalysts in the hydrodechlorination of the MCPA herbicide[J]. Applied Catalysis B: Environmental, 2016, 181: 429-435. |
| 38 | MAHY J G, TASSEROUL L, TROMME O, et al. Hydrodechlorination and complete degradation of chlorinated compounds with the coupled action of Pd/SiO2 and Fe/SiO2 catalysts: Towards industrial catalyst synthesis conditions[J]. Journal of Environmental Chemical Engineering, 2019, 7(2): 103014. |
| 39 | ORDÓÑEZ S, DÍEZ F V, SASTRE H. Catalytic hydrodechlorination of chlorinated olefins over a Pd/Al2O3 catalyst: Kinetics and inhibition phenomena[J]. Industrial & Engineering Chemistry Research, 2002, 41(3): 505-511. |
| 40 | GÓMEZ-SAINERO L M, SEOANE X L, FIERRO J L G, et al. Liquid-phase hydrodechlorination of CCl4 to CHCl3 on Pd/carbon catalysts: Nature and role of Pd active species[J]. Journal of Catalysis, 2002, 209(2): 279-288. |
| 41 | BAEZA J A, CALVO L, GILARRANZ M A, et al. Catalytic behavior of size-controlled palladium nanoparticles in the hydrodechlorination of 4-chlorophenol in aqueous phase[J]. Journal of Catalysis, 2012, 293: 85-93. |
| 42 | ZHANG Weixian, WANG Chuanbao, LIEN H L. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles[J]. Catalysis Today, 1998, 40(4): 387-395. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [10] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [11] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [12] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [13] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [14] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [15] | 毛善俊, 王哲, 王勇. 基团辨识加氢:从概念到应用[J]. 化工进展, 2023, 42(8): 3917-3922. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||