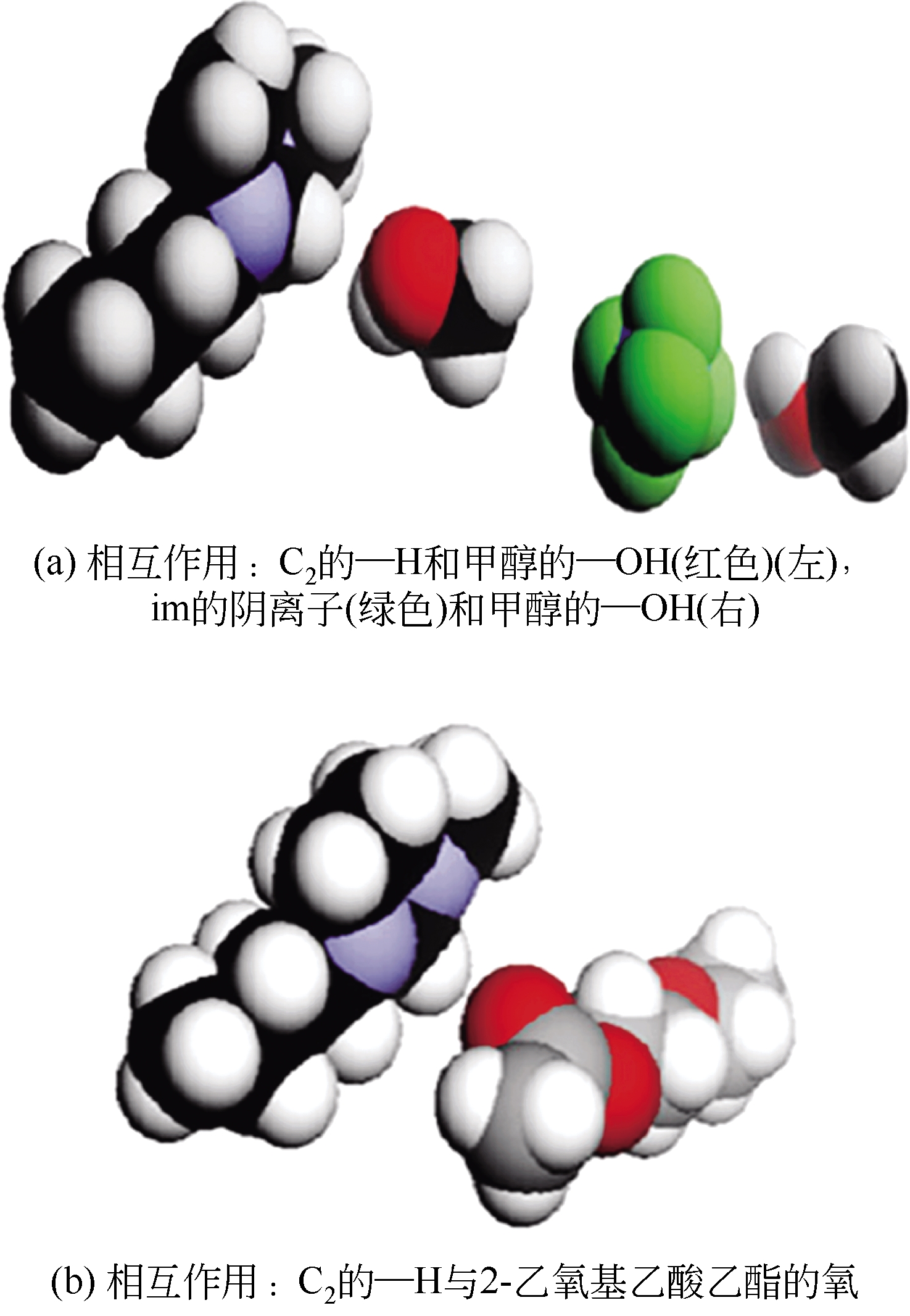
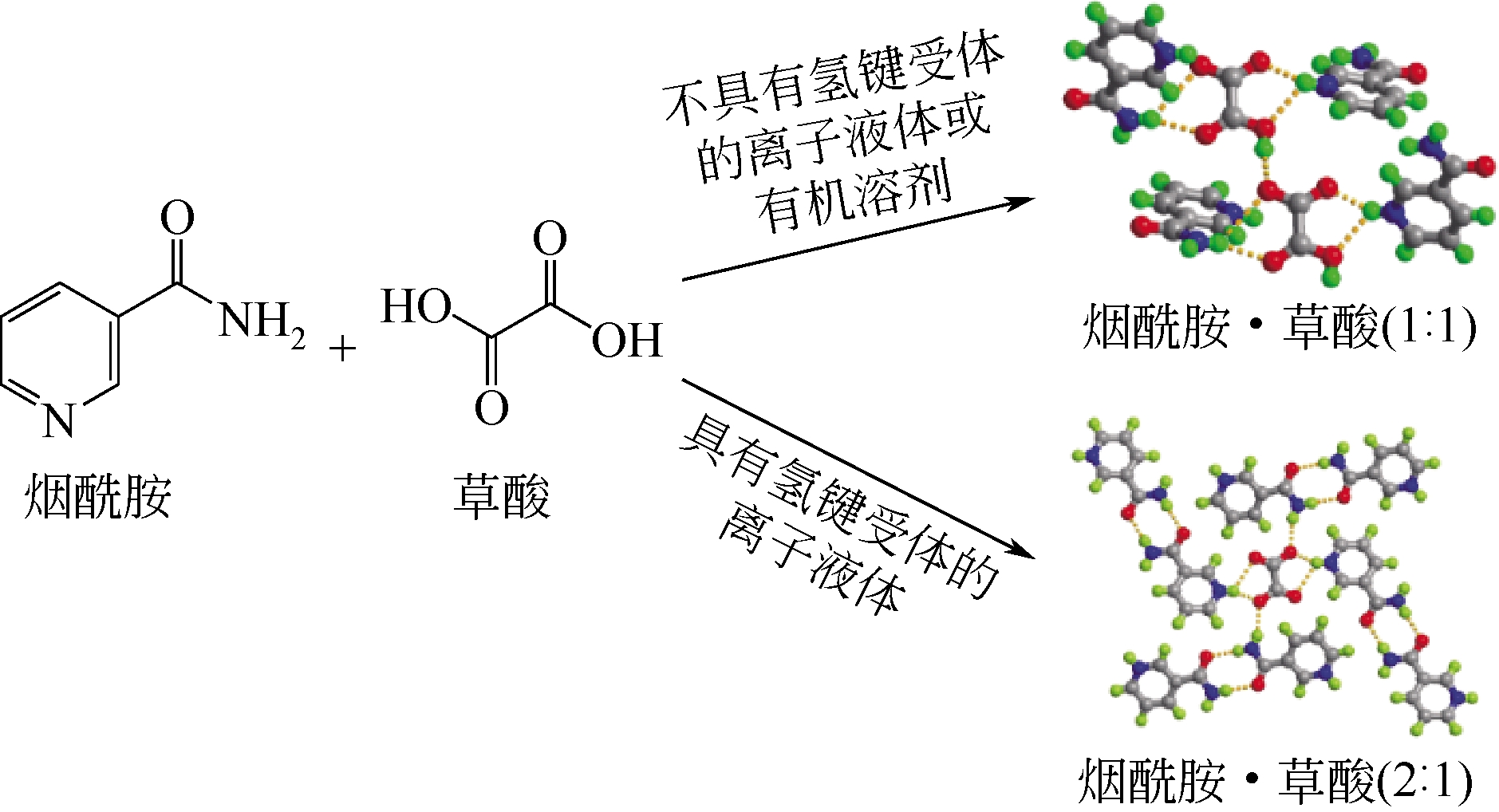
化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2389-2401.DOI: 10.16085/j.issn.1000-6613.2018-1482
离子液体在药物晶体工程中的应用
尚泽仁1,2,3( ),胡卫国1,2,3,4,汤伟伟1,2,3,贾丽娜1,2,3,李鸣晨1,2,3,赵燕晓1,2,3,魏宁1,2,3,龚俊波1,2,3(
),胡卫国1,2,3,4,汤伟伟1,2,3,贾丽娜1,2,3,李鸣晨1,2,3,赵燕晓1,2,3,魏宁1,2,3,龚俊波1,2,3( )
)
- 1. 天津大学化工学院国家工业结晶工程技术研究中心,天津 300072
2. 天津化学化工协同创新中心,天津 300072
3. 现代药物传递及功能高效化天津市重点实验室,天津 300072
4. 华北制药股份有限公司,河北 石家庄 050015
-
收稿日期:2018-07-17修回日期:2019-01-03出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:龚俊波 -
作者简介:<named-content content-type="corresp-name">尚泽仁</named-content>(1994—),男,硕士研究生,研究方向为药物晶体工程。E-mail:<email>shangzeren@tju.edu.cn</email>。 -
基金资助:国家自然科学基金(21676179、91634117、81361140344);国家科技重大专项(2017ZX07402003、2017ZX09101001);天津市科技计划(15JCZDJC33200)
Application of ionic liquids in pharmaceutical crystal engineering
Zeren SHANG1,2,3( ),Weiguo HU1,2,3,4,Weiwei TANG1,2,3,Lina JIA1,2,3,Mingchen LI1,2,3,Yanxiao ZHAO1,2,3,Ning WEI1,2,3,Junbo GONG1,2,3(
),Weiguo HU1,2,3,4,Weiwei TANG1,2,3,Lina JIA1,2,3,Mingchen LI1,2,3,Yanxiao ZHAO1,2,3,Ning WEI1,2,3,Junbo GONG1,2,3( )
)
- 1. National Engineering Research Center of Industry Crystallization Technology,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072, China
2. Collaborative Innovation Center of Chemical Science and Engineering Tianjin,Tianjin 300072, China
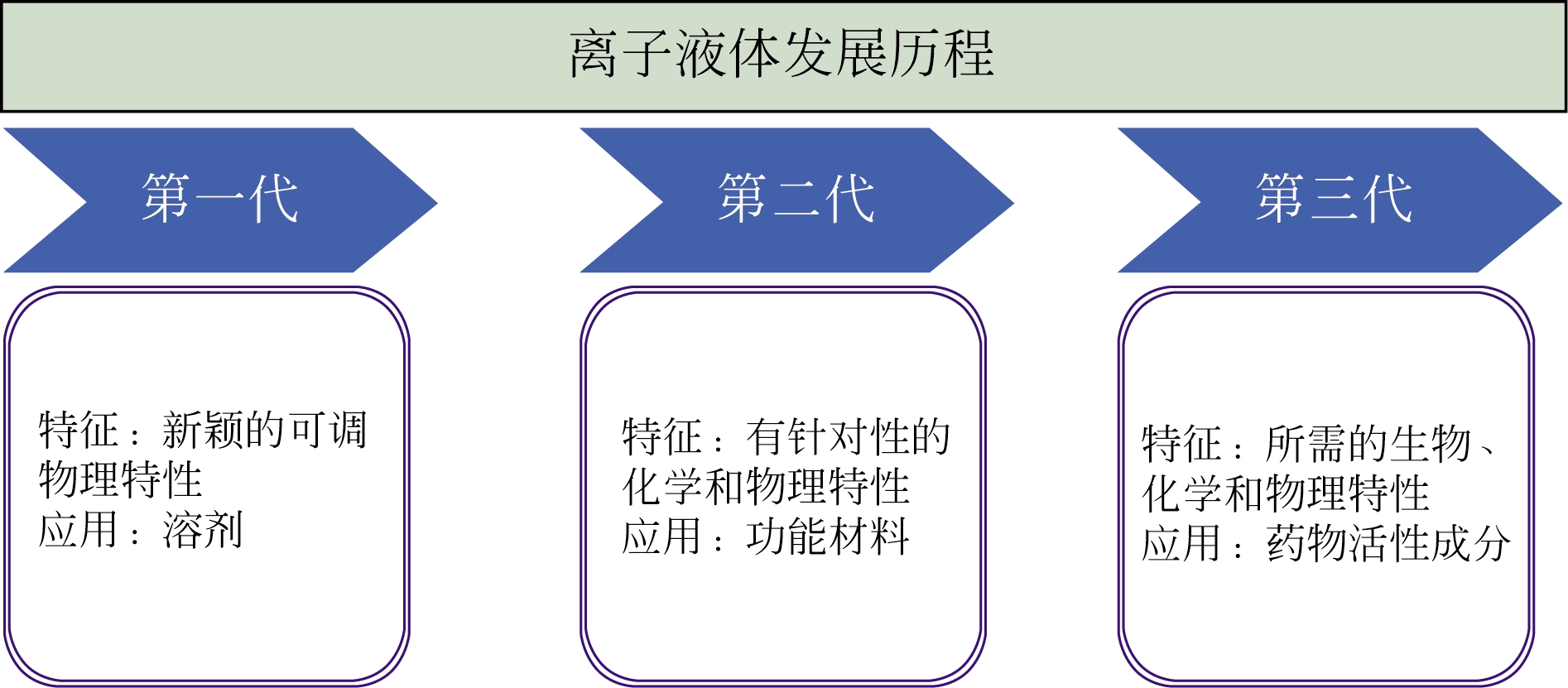
3. Key Laboratory of Modern Drug Delivery and High Efficiency in Tianjin University,Tianjin 300072, China
4. North China Pharmaceutical Company Ltd. ,Shijiazhuang 050015,Hebei,China
-
Received:2018-07-17Revised:2019-01-03Online:2019-05-05Published:2019-05-05 -
Contact:Junbo GONG
摘要:
近年来,使用离子液体替代传统溶剂参与调节结晶过程中的各种相互作用以获得新颖的晶体结构是药物晶体工程的研究前沿。研究人员在离子液体体系中得到了使用传统溶剂无法制得的药物晶型或晶习并进行了相应机理的初步探索。针对离子液体在药物晶体工程中的应用,本文从离子液体的组成和性质及其在药物增溶、药物晶型及晶习的调控和药物共晶及盐的制备几方面展开,首次综述了相关研究成果并基于红外光谱、分子动力学模拟等手段从相互作用的角度对机理进行分析,其中氢键和范德华力相互作用起到了重要影响。最后针对该领域现存的问题如离子液体的选择处于盲筛阶段,相应机理研究缺乏和应用范围不广泛,指出建立系统的离子液体选择标准和深入研究机理是未来的主要研究方向。
中图分类号:
引用本文
尚泽仁, 胡卫国, 汤伟伟, 贾丽娜, 李鸣晨, 赵燕晓, 魏宁, 龚俊波. 离子液体在药物晶体工程中的应用[J]. 化工进展, 2019, 38(05): 2389-2401.
Zeren SHANG, Weiguo HU, Weiwei TANG, Lina JIA, Mingchen LI, Yanxiao ZHAO, Ning WEI, Junbo GONG. Application of ionic liquids in pharmaceutical crystal engineering[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2389-2401.
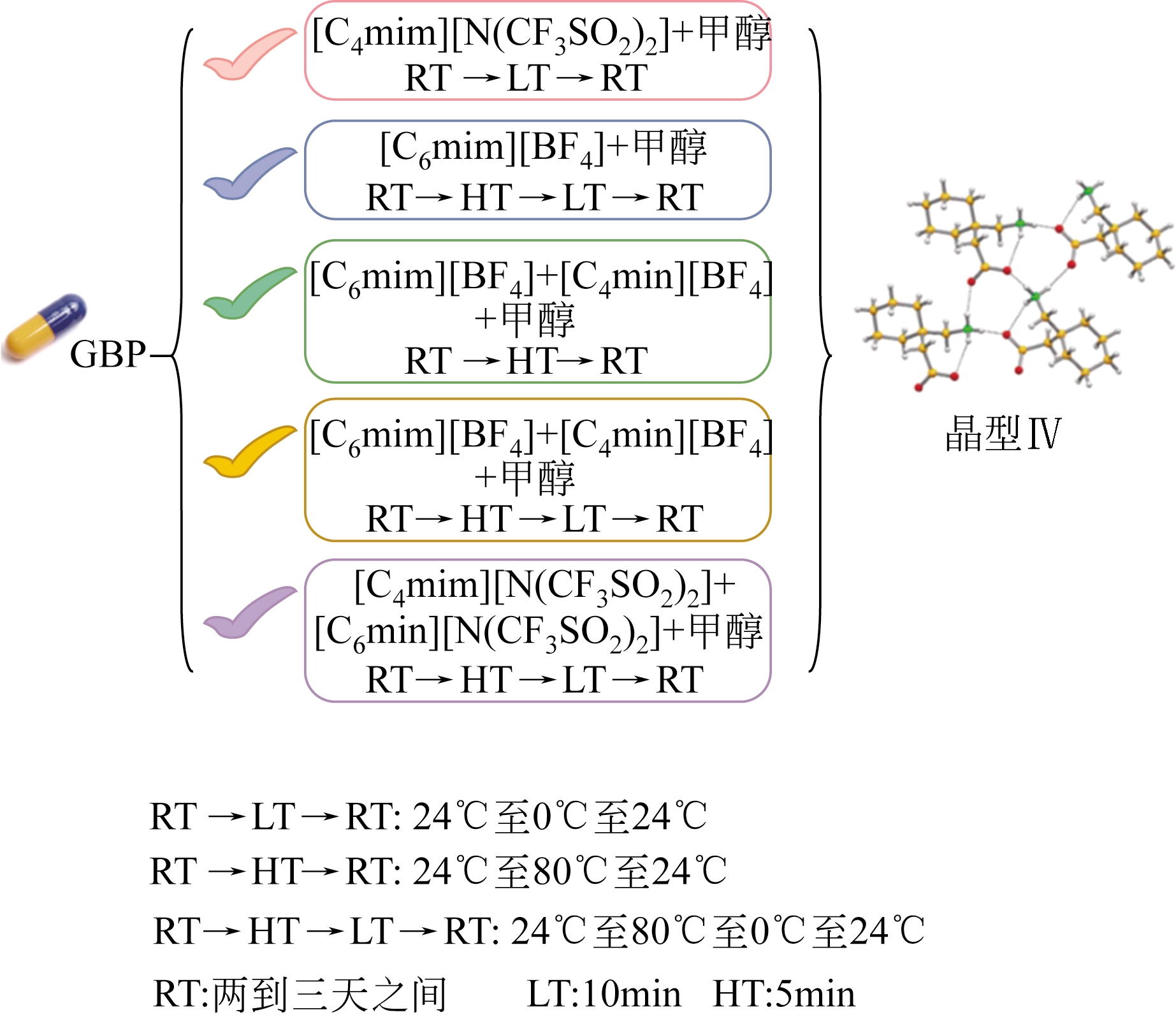
| 药物名称 | 晶型 | 临床疗效 | 文献 |
|---|---|---|---|
| 甲苯达唑 | 晶型A,晶型B,晶型C | 晶型C > 晶型A | [ |
| 法莫替丁 | 晶型A,晶型B,晶型C,晶型D | 晶型B > 晶型A | [ |
| 棕榈氯霉素 | 晶型A,晶型B,晶型C | 晶型B和晶型C > 晶型A | [ |
| 尼群地平 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅰ | [ |
| 利福定 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅱ | [ |
表1 部分多晶型药物的临床疗效比较
| 药物名称 | 晶型 | 临床疗效 | 文献 |
|---|---|---|---|
| 甲苯达唑 | 晶型A,晶型B,晶型C | 晶型C > 晶型A | [ |
| 法莫替丁 | 晶型A,晶型B,晶型C,晶型D | 晶型B > 晶型A | [ |
| 棕榈氯霉素 | 晶型A,晶型B,晶型C | 晶型B和晶型C > 晶型A | [ |
| 尼群地平 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅰ | [ |
| 利福定 | 晶型Ⅰ,晶型Ⅱ,晶型Ⅲ,晶型Ⅳ | 晶型Ⅳ > 晶型Ⅱ | [ |
| 名称 | 英文缩写 | CAS # | 结构 |
|---|---|---|---|
| 1-丁基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C4mim][N(CF3SO2)2] | 174899-83-3 |  |
| 1-己基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C6mim][N(CF3SO2)2] | 382150-50-7 |  |
| 1-丁基-3-甲基咪唑四氟硼酸盐 | [C4mim][BF4] | 174501-65-6 |  |
| 1-己基-3-甲基咪唑四氟硼酸盐 | [C6mim][BF4] | 244193-50-8 |  |
| 1-烯丙基-3-乙基咪唑四氟硼酸盐 | [aeim][BF4] | 945732-42-3 |  |
| 1-丁基-2,3-二甲基咪唑四氟硼酸盐 | [bdmim][BF4] | 402846-78-0 |  |
| 1,3-二烯丙基咪唑四氟硼酸盐 | [aaim][BF4] | 852699-06-0 |  |
| 1-乙基-3-甲基咪唑硫酸乙酯盐 | [emim][EtSO4] | 342573-75-5 |  |
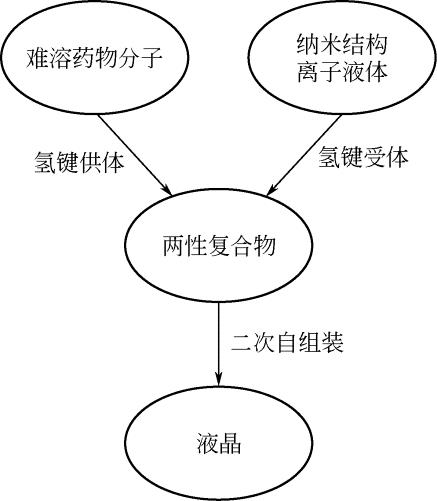
| 1-丁基-3-甲基咪唑六氟磷酸盐 | [bmim][PF6] | 174501-64-5 |  |
| 1-乙基-3-甲基咪唑甲基磷酸酯盐 | [emim][CH3O(H)PO2] | 81994-80-1 |  |
| 1-己基-3-甲基咪唑六氟磷酸盐 | [hmim][PF6] | 304680-35-1 |  |
| 1-乙基-3-甲基咪唑乙酸盐 | [C2mim][OAc] | 143314-17-4 |  |
| 1-丁基-3-甲基咪唑溴盐 | [C4mim]Br | 85100-77-2 |  |
| 三己基(十四烷基)鏻双(草酸根)硼酸盐 | [P6,6,6,14][BOB] | 880497-87-0 |  |
表2 用作药物结晶介质的典型离子液体名称和结构
| 名称 | 英文缩写 | CAS # | 结构 |
|---|---|---|---|
| 1-丁基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C4mim][N(CF3SO2)2] | 174899-83-3 |  |
| 1-己基-3-甲基咪唑双三氟甲磺酰亚胺盐 | [C6mim][N(CF3SO2)2] | 382150-50-7 |  |
| 1-丁基-3-甲基咪唑四氟硼酸盐 | [C4mim][BF4] | 174501-65-6 |  |
| 1-己基-3-甲基咪唑四氟硼酸盐 | [C6mim][BF4] | 244193-50-8 |  |
| 1-烯丙基-3-乙基咪唑四氟硼酸盐 | [aeim][BF4] | 945732-42-3 |  |
| 1-丁基-2,3-二甲基咪唑四氟硼酸盐 | [bdmim][BF4] | 402846-78-0 |  |
| 1,3-二烯丙基咪唑四氟硼酸盐 | [aaim][BF4] | 852699-06-0 |  |
| 1-乙基-3-甲基咪唑硫酸乙酯盐 | [emim][EtSO4] | 342573-75-5 |  |
| 1-丁基-3-甲基咪唑六氟磷酸盐 | [bmim][PF6] | 174501-64-5 |  |
| 1-乙基-3-甲基咪唑甲基磷酸酯盐 | [emim][CH3O(H)PO2] | 81994-80-1 |  |
| 1-己基-3-甲基咪唑六氟磷酸盐 | [hmim][PF6] | 304680-35-1 |  |
| 1-乙基-3-甲基咪唑乙酸盐 | [C2mim][OAc] | 143314-17-4 |  |
| 1-丁基-3-甲基咪唑溴盐 | [C4mim]Br | 85100-77-2 |  |
| 三己基(十四烷基)鏻双(草酸根)硼酸盐 | [P6,6,6,14][BOB] | 880497-87-0 |  |
| 名称英文缩写 | 熔点/°C | 黏度25℃/Pa?s | 亲水/疏水 |
|---|---|---|---|
| [C4mim][N(CF3SO2)2] | -4.9 | 0.0511 | 亲水 |
| [C6mim][N(CF3SO2)2] | -10.5 | 0.0705 | 亲水 |
| [C4mim][BF4] | -17.0 | 0.0949 | 亲水 |
| [C6mim][BF4] | -81.0 | 0.1456 | 疏水 |
| [aeim][BF4] | — | — | 亲水 |
| [bdmim][BF4] | 39.7 | 0.6500 | 亲水 |
| [aaim][BF4] | 4.3 | — | 亲水 |
| [emim][EtSO4] | -65.0 | 0.0962 | 亲水 |
| [bmim][PF6] | 10.9 | 0.2740 | 疏水 |
| [emim][CH3O(H)PO2] | — | 0.1564 ① | 亲水 |
| [hmim][PF6] | -80.0 | 0.4830 | 疏水 |
| [C2mim][OAc] | < -20.0 | 0.1285 | 亲水 |
| [C4mim]Br | 72.9 | 0.0570 ② | 亲水 |
| [P6,6,6,14][BOB] | — | 0.6150 ① | 亲水 |
表3 用作药物结晶介质的典型离子液体的性质
| 名称英文缩写 | 熔点/°C | 黏度25℃/Pa?s | 亲水/疏水 |
|---|---|---|---|
| [C4mim][N(CF3SO2)2] | -4.9 | 0.0511 | 亲水 |
| [C6mim][N(CF3SO2)2] | -10.5 | 0.0705 | 亲水 |
| [C4mim][BF4] | -17.0 | 0.0949 | 亲水 |
| [C6mim][BF4] | -81.0 | 0.1456 | 疏水 |
| [aeim][BF4] | — | — | 亲水 |
| [bdmim][BF4] | 39.7 | 0.6500 | 亲水 |
| [aaim][BF4] | 4.3 | — | 亲水 |
| [emim][EtSO4] | -65.0 | 0.0962 | 亲水 |
| [bmim][PF6] | 10.9 | 0.2740 | 疏水 |
| [emim][CH3O(H)PO2] | — | 0.1564 ① | 亲水 |
| [hmim][PF6] | -80.0 | 0.4830 | 疏水 |
| [C2mim][OAc] | < -20.0 | 0.1285 | 亲水 |
| [C4mim]Br | 72.9 | 0.0570 ② | 亲水 |
| [P6,6,6,14][BOB] | — | 0.6150 ① | 亲水 |
| 晶型 | 结晶方法 | 溶剂 | 文献 |
|---|---|---|---|
| 晶型Ⅰ无水物(C20H32N5O8P) | 冷却结晶 | 丙酮+二正丁醚 | [ |
| 晶型Ⅱ二水合物(C20H32N5O8P·2H2O) | 溶析结晶 | 甲醇/水 | [ |
| 晶型Ⅲ溶剂化物(C20H32N5O8P·CH3OH) | 蒸发结晶 | 甲醇 | [ |
| 晶型Ⅳ盐型(C20H32N5O8P·C4H4O4) | 冷却结晶 | 异丙醇+富马酸 | [ |
| 晶型Ⅴ无水物(C20H32N5O8P) | 喷雾干燥 | 乙醇 | [ |
| 晶型Ⅵ一水合物(C20H32N5O8P·H2O) | 蒸发结晶 | 二氯甲烷+甲醇+水蒸气 | [ |
| 晶型Ⅶ溶剂化物(C20H32N5O8P·C4H10O) | 溶析结晶 | 正丁醇/己烷 | [ |
| 晶型Ⅷ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅸ半水合物(C20H32N5O8P·0.5H2O) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅹ无水物(C20H32N5O8P) | 溶析结晶 | 二氯甲烷/正己烷 | [ |
| 晶型Ⅺ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/[bdmim][BF4] | [ |
表4 阿德福韦酯具有的晶型
| 晶型 | 结晶方法 | 溶剂 | 文献 |
|---|---|---|---|
| 晶型Ⅰ无水物(C20H32N5O8P) | 冷却结晶 | 丙酮+二正丁醚 | [ |
| 晶型Ⅱ二水合物(C20H32N5O8P·2H2O) | 溶析结晶 | 甲醇/水 | [ |
| 晶型Ⅲ溶剂化物(C20H32N5O8P·CH3OH) | 蒸发结晶 | 甲醇 | [ |
| 晶型Ⅳ盐型(C20H32N5O8P·C4H4O4) | 冷却结晶 | 异丙醇+富马酸 | [ |
| 晶型Ⅴ无水物(C20H32N5O8P) | 喷雾干燥 | 乙醇 | [ |
| 晶型Ⅵ一水合物(C20H32N5O8P·H2O) | 蒸发结晶 | 二氯甲烷+甲醇+水蒸气 | [ |
| 晶型Ⅶ溶剂化物(C20H32N5O8P·C4H10O) | 溶析结晶 | 正丁醇/己烷 | [ |
| 晶型Ⅷ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅸ半水合物(C20H32N5O8P·0.5H2O) | 溶析结晶 | [aeim][BF4]/水 | [ |
| 晶型Ⅹ无水物(C20H32N5O8P) | 溶析结晶 | 二氯甲烷/正己烷 | [ |
| 晶型Ⅺ无水物(C20H32N5O8P) | 溶析结晶 | [aeim][BF4]/[bdmim][BF4] | [ |
| 1 | SCHMIDT G M J . Photodimerization in the solid state[J]. Pure and Applied Chemistry,1971, 27(4): 647-678. |
| 2 | BRAGA D . Crystal engineering, where from? where to?[J]. Chemical Communications, 2003, 39(22): 2751-2754. |
| 3 | HILFIKER R . Polymorphism:in the pharmaceutical industry[M]. Hoboken:Wiley, 2006: 1-15. |
| 4 | CHEN J , SARMA B , EVANS J M B , et al . Pharmaceutical crystallization[J]. Crystal Growth & Design, 2011, 11(4): 887-895. |
| 5 | SARMA B , CHEN J , HIS H Y, et al . Solid forms of pharmaceuticals:polymorphs, salts and cocrystals[J]. Korean Journal of Chemical Engineering, 2011, 28(2): 315-322. |
| 6 | SUN C C , GRANT D J . Improved tableting properties of p-hydroxybenzoic acid by water of crystallization: a molecular insight[J]. Pharmaceutical Research, 2004, 21(2): 382-386. |
| 7 | CHANG S Y , SUN C C . Superior plasticity and tabletability of theophylline monohydrate[J]. Molecular Pharmaceutics, 2017, 14(6): 2047-2055. |
| 8 | CHAROENLARP P , WAIKAGUL J , MUENNOO C , et al . Efficacy of single-dose mebendazole, polymorphic forms A and C, in the treatment of hookworm and trichuris infections[J]. Southeast Asian Journal of Tropical Medicine & Public Health, 1993, 24(4): 712-716. |
| 9 | 唐素芳 . 药物多晶型的研究及其对药效和理化性质的影响[J]. 天津药学, 2002, 14(2): 12-14. |
| TANG S F . Research on pharmaceutical polymorphs and the influence of efficacy[J]. Tianjin Pharmacy, 2002, 14(2): 12-14. | |
| 10 | 张伟国, 刘昌孝 . 多晶型药物的生物利用度研究概况[J]. 天津药学, 2007, 19(2): 59-61. |
| ZHANG W G , LIU C X . The bioavailability research of pharmaceutical polymorphs[J]. Tianjin Pharmacy, 2007, 19(2): 59-61. | |
| 11 | DU G H , LV Y , ZHAO Y , et al . New nitrendipine crystal used for treating cardiovascular and cerebrovascular diseases:CN101544596[P]. 2009-09-30. |
| 12 | 马廷升, 朱兰翠 . 药物生物利用度影响因素的动态分析[J]. 怀化学院学报, 2006, 25(2): 76-77. |
| MA T S, ZHU L C . Study on the effective factors of bioavailability[J]. Journal of Huaihua University, 2006, 25(2): 76-77. | |
| 13 | DESIRAJU G R . Crystal engineering: a holistic view[J]. Angewandte Chemie: International Edition, 2007, 46(44): 8342-8356. |
| 14 | GAO Z , ROHANI S , GONG J , et al . Recent developments in the crystallization process: toward the pharmaceutical industry[J]. Engineering, 2017, 3(3): 343-353. |
| 15 | WU S , CHEN M , LI K , et al . Solvent penetration mediated phase transformation for the preparation of aggregated particles with well-defined shape [J]. Cryst. Eng. Comm., 2016, 18(48): 9223-9226. |
| 16 | TANG W , ZHANG M , MO H , et al . Higher-order self-assembly of benzoic acid in solution[J]. Crystal Growth & Design, 2017, 17(10): 5049-5053. |
| 17 | GAO Z , ALTIMIMI F , GONG J , et al . Ultrasonic irradiation and seeding to prevent metastable liquid-liquid phase separation and intensify crystallization [J]. Crystal Growth & Design, 2018, 18(4): 2628-2635. |
| 18 | REICHERT W M , HOLBREY J D , VIGOUR K B , et al . Approaches to crystallization from ionic liquids: complex solvents-complex results, or, a strategy for controlled formation of new supramolecular architectures?[J]. Chemical Communications, 2006, 42(46): 4767-4779. |
| 19 | HARDACRE C , BOWRON D T , HOLBREY J D , et al . Structure of molten 1, 3-dimethylimidazolium chloride using neutron diffraction[J]. The Journal of Chemical Physics, 2003, 118(1): 273-278. |
| 20 | HOUGH W L , SMIGLAK M , RODRIGUEZ H , et al . The third evolution of ionic liquids:active pharmaceutical ingredients[J]. New Journal of Chemistry, 2007, 31(8): 1429-1436. |
| 21 | WELTON T . Room-temperature ionic liquids: solvents for synthesis and catalysis[J]. Chemical Reviews, 1999, 99(8): 2071-2084. |
| 22 | HALLETT J P , WELTON T . Room-temperature ionic liquids:solvents for synthesis and catalysis. 2 [J]. Chemical Reviews, 2011, 111(5): 3508-3576. |
| 23 | AHMED E , BRETERNITZ J , GROH M F , et al . Ionic liquids as crystallisation media for inorganic materials[J]. Cryst. Eng. Comm., 2012, 14(15): 4874-4885. |
| 24 | MEINDERSMA G W , HAAN A B DE . In ionic liquids uncoiled[M]. 8th ed. Hoboken:Wiley, 2013:119. |
| 25 | CHEN X , JI Y , WANG J . Improvement on the crystallization of lysozyme in the presence of hydrophilic ionic liquid[J]. Analyst, 2010, 135(9): 2241-2248. |
| 26 | JUDGE R A , TAKAHASHI S , LONGENECKER K L . The effect of ionic liquids on protein crystallization and X-ray diffraction resolution[J]. Crystal Growth & Design, 2009, 9(8): 3463-3469. |
| 27 | LI X , XU X , FENG Y D J , et al . The crystallization of lysozyme in the system of ionic liquid [BMIm][BF4]-water[J]. Crystal Research and Technology, 2008, 43(10): 1062-1068. |
| 28 | BARZEGAR M , HABIBI-YANGJEH A , BEHBOUDNIA M . Template-free preparation and characterization of nanocrystalline ZnO in aqueous solution of [EMIM][EtSO4] as a low-cost ionic liquid using ultrasonic irradiation and photocatalytic activity[J]. Journal of Physics and Chemistry of Solids, 2009, 70(10): 1353-1358. |
| 29 | BARZEGAR M , HABIBI-YANGJEH A , BEHBOUDNIA M . Ultrasonic-assisted preparation and characterization of CdS nanoparticles in the presence of a halide-free and low-cost ionic liquid and photocatalytic activity[J]. Journal of Physics and Chemistry of Solids, 2010, 71(9): 1393-1397. |
| 30 | ZHAO Y , CHEN Z , WANG H , et al . Crystallization control of CaCO3 by ionic liquids in aqueous solution[J]. Crystal Growth & Design, 2009, 9(11): 4984-4986. |
| 31 | AN J H , KIM J M, CHANG S M , et al . Application of ionic liquid to polymorphic design of pharmaceutical ingredients[J]. Crystal Growth & Design, 2010, 10(7): 3044-3050. |
| 32 | AN J H , KIM W S . Antisolvent crystallization using ionic liquids as solvent and antisolvent for polymorphic design of active pharmaceutical ingredient [J]. Crystal Growth & Design, 2013, 13(1): 31-39. |
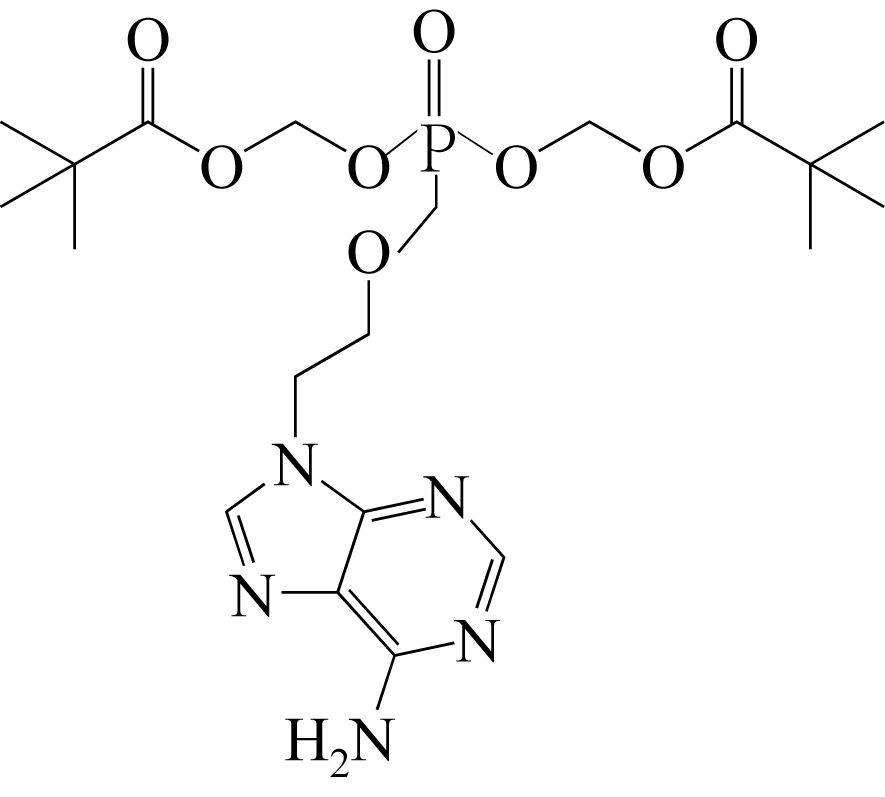
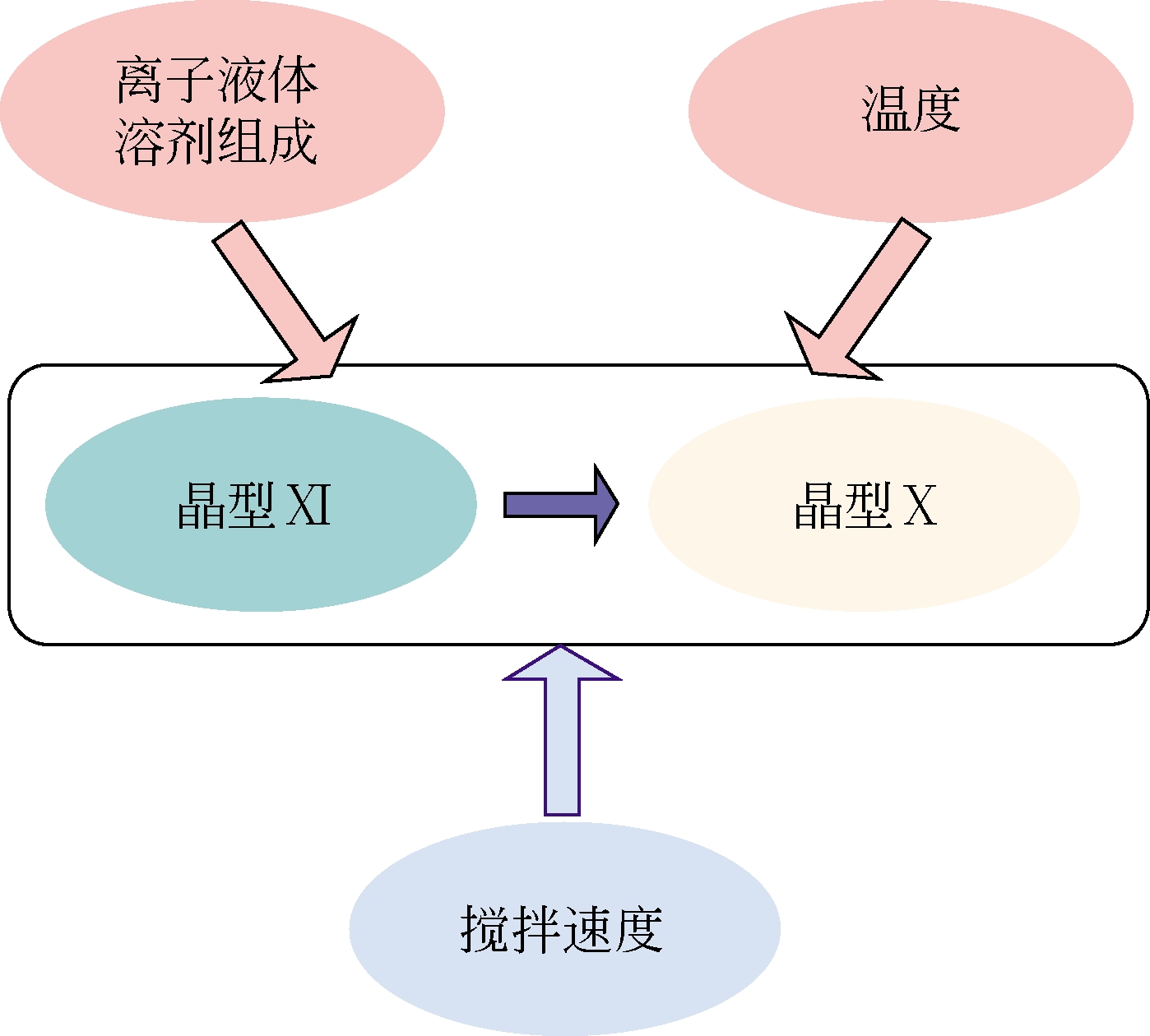
| 33 | AN J H , JIN F , KIM H S, et al . Application of ionic liquid to polymorphic transformation of anti-viral/HIV drug adefovir dipivoxil[J]. Archives of Pharmacal Research, 2016, 39(5): 646-659. |
| 34 | MARTINS I C B , GOMES J R B , DUARTE M T , et al . Understanding polymorphic control of pharmaceuticals using imidazolium-based ionic liquid mixtures as crystallization directing agents[J]. Crystal Growth & Design, 2017, 17(2): 428-432. |
| 35 | AN J H , JIN F , KIM H S, et al . Investigation of the polymorphic transformation of the active pharmaceutical ingredient clopidogrel bisulfate using the ionic liquid AEImBF4 [J]. Crystal Growth & Design, 2016, 16(4): 1829-1836. |
| 36 | SMITH K B , BRIDSON R H , LEEKE G A . Crystallisation control of paracetamol from ionic liquids[J]. Cryst. Eng. Comm., 2014, 16(47): 10797-10803. |
| 37 | HA G S, KIM J H . Effect of an ionic liquid on vancomycin crystallization[J]. Korean Journal of Chemical Engineering, 2015, 32(4): 576-582. |
| 38 | KARTHIKA S , RADHAKRISHNAN T K , KALAICHELVI P . The role of hydrogen bonding propensity in tuning the morphology of crystals obtained from imidazolium based ionic liquids[J]. Journal of Crystal Growth, 2017, 463:168-175. |
| 39 | VICOSA A , LETOURNEAU J J , ESPITALIER F , et al . An innovative antisolvent precipitation process as a promising technique to prepare ultrafine rifampicin particles[J]. Journal of Crystal Growth, 2012, 342(1): 80-87. |
| 40 | AZEVEDO JACQUELINE R DE , ESPITALIER F , LETOURNEAU J J , et al . Antisolvent crystallization of a cardiotonic drug in ionic liquids: effect of mixing on the crystal properties[J]. Journal of Crystal Growth, 2017, 472:29-34. |
| 41 | GOLOVANOV D G , LYSSENKO K A , ANTIPIN M Y , et al . Cocrystal of an ionic liquid with organic molecules as a mimic of ionic liquid solution[J]. Crystal Growth & Design, 2005, 5(1): 337-340. |
| 42 | TITI H M , KELLEY S P , EASTON M E , et al . Formation of ionic co-crystals of amphoteric azoles directed by the ionic liquid co-former 1-ethyl-3-methylimidazolium acetate[J]. Chemical Communications, 2017, 53(61): 8569-8572. |
| 43 | SHIMPI M R , VELAGA S P , SHAH F U , et al . Pharmaceutical crystal engineering using ionic liquid anion-solute interactions[J]. Crystal Growth & Design, 2017, 17(4): 1729-1734. |
| 44 | KANDULA R K , VEPURI S B , DEVARAJEGOWDA H C , et al . Crystallization of R-(+)-atenolol hydrochloride from racemic ionic liquid - a selective double decomposition green reaction[J]. Journal of Molecular Structure, 2018, 1169: 39-45. |
| 45 | BYRN S R , PFEIFFER R R , STOWELL J G . Solid-state chemistry of drugs[M]. 2nd ed. West Lafayette:SSCI, 1999: 233-247. |
| 46 | STOIMENOVSKI J , MACFARLANE D R , BICA K , et al . Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper[J]. Pharmaceutical Research, 2010, 27(4): 521-526. |
| 47 | CHERUKUVADA S , NANGIA A . Polymorphism in an API ionic liquid:ethambutol dibenzoate trimorphs[J]. Cryst. Eng. Comm., 2012, 14(23): 7840-7843. |
| 48 | KUMAR V , PARMAR V S , MALHOTRA S V . Enhanced solubility and selective benzoylation of nucleosides in novel ionic liquid[J]. Tetrahedron Letters, 2007, 48(5): 809-812. |
| 49 | MONIRUZZAMAN M , TAHARA Y , TAMURA M , et al . Ionic liquid-assisted transdermal delivery of sparingly soluble drugs[J]. Chemical Communications, 2010, 46(9): 1452-1454. |
| 50 | JIANG Y , LIANG M , SVEJKAR D , et al . Albumin-micelles via a one-pot technology platform for the delivery of drugs[J]. Chemical Communications, 2014, 50(48): 6394-6397. |
| 51 | WINTER K DE , VERLINDEN K , KREN V , et al . Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: balancing acceptor solubility and enzyme stability[J]. Green Chemistry, 2013, 15(7): 1949-1955. |
| 52 | USUKI T , YASUDA N , YOSHIZAWA-FUJITA M , et al . Extraction and isolation of shikimic acid from ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose[J]. Chemical Communications, 2011, 47(38): 10560-10562. |
| 53 | DAI Y , SPRONSEN J VAN , WITKAMP G J , et al . Natural deep eutectic solvents as new potential media for green technology[J]. Analytica Chimica Acta, 2013, 766(5): 61-68. |
| 54 | SINTRA T E , SHIMIZU K , VENTURA S P M , et al . Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes[J]. Physical Chemistry Chemical Physics, 2018, 20(3): 2094-2103. |
| 55 | SMITH K B , BRIDSON R H , LEEKE G A . Solubilities of pharmaceutical compounds in ionic liquids[J]. Journal of Chemical & Engineering Data, 2011, 56(5): 2039-2043. |
| 56 | JIN W , YANG Q , ZHANG Z , et al . Self-assembly induced solubilization of drug-like molecules in nanostructured ionic liquids[J]. Chemical Communications, 2015, 51(67): 13170-13173. |
| 57 | JIA L , XU S , LIU S , et al . Polymorphs of daidzein and intermolecular interaction effect on solution crystallization[J]. Cryst. Eng. Comm., 2017, 19(47): 7146-7153. |
| 58 | HILDEN J L , REYES C E , KELM M J , et al . Capillary precipitation of a highly polymorphic organic compound[J]. Crystal Growth & Design, 2003, 3(6): 921–926. |
| 59 | LANG M D , GRZESIAK A L , MATZGER A J . The use of polymer heteronuclei for crystalline polymorph selection[J]. Journal of the American Chemical Society, 2002, 124(50): 14834–14835. |
| 60 | ARIMILLI M N , LEE T T K, MANES L V , et al . Nucleotide analog compositions:WO9904774A2[P]. 1999-02-04. |
| 61 | WANG G , LU X , LIU Q , et al . A new crystal form of adefovir dipivoxil and its composition:WO2004043972A1[P]. 2004-05-27. |
| 62 | GALIMI S , VECCHIO E , PIZZOCZRO R . Adefovir dipivoxil crystalline monohydrate form:WO2009015892A1[P]. 2009-02-05. |
| 63 | MIN Y S , CHOUL L H . DH-type crystalline form of adefovir dipivoxil, preparing method thereof, and pharmaceutical composition for antiviral agent comprising the same:WO2010110506A1[P]. 2010-09-30. |
| 64 | AN J H , CHOI G J , KIM W S . Polymorphic and kinetic investigation of adefovir dipivoxil during phase transformation[J]. International Journal of Pharmaceutics, 2012, 422(1): 185-193. |
| 65 | DAVEY R J , DENT G , MUGHAL R K , et al . Concerning the relationship between structural and growth synthons in crystal nucleation:solution and crystal chemistry of carboxylic acids as revealed through IR spectroscopy[J]. Crystal Growth & Design, 2006, 6(8): 1788-1796. |
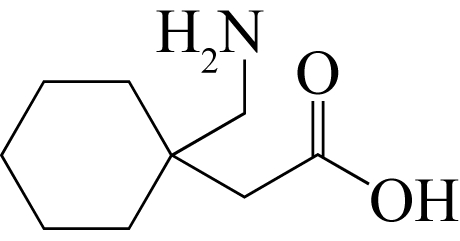
| 66 | IBERS J A . Gabapentin and gabapentin monohydrate[J]. Acta Crystallographica Section C, 2001, 57(5): 641-643. |
| 67 | REECE H A , LEVENDIS D C . Polymorphs of gabapentin[J]. Acta Crystallographica Section C, 2008, 64(3): o105-o108. |
| 68 | BRAGA D , GREPIONI F , MAINI L , et al . Polymorphic gabapentin:thermal behaviour, reactivity and interconversion of forms in solution and solid-state[J]. New Journal of Chemistry, 2008, 32(10): 1788-1795. |
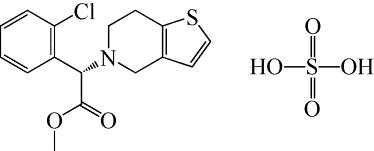
| 69 | KORADLA V , CHAWLA G , BANSAL A . Qualitative and quantitative analysis of clopidogrel bisulphate polymorphs[J]. Acta Pharmaceutica, 2004, 54(3): 193-204. |
| 70 | KIM H J, KIM K J . Quantitative study on polymorphic form in solution crystallization of clopidogrel hydrogen sulfate[J]. Industrial & Engineering Chemistry Research, 2009, 48(24): 11133-11139. |
| 71 | QU H M , MUNK T M , CORNETT C , et al . Influence of temperature on solvent-mediated anhydrate-to-hydrate transformation kinetics[J]. Pharmaceutical Research, 2011, 28(2): 364-373. |
| 72 | MCCABE W L , SMITH J C , HARRIOTT P . Unit operations of chemical engineering[M]. 7th ed. New York:McGraw Hill, 2005:11-15. |
| 73 | DONG Y , NG W K, SHEN S , et al . Preparation and characterization of spironolactone nanoparticles by antisolvent precipitation[J]. International Journal of Pharmaceutics, 2009, 375(1): 84-88. |
| 74 | FINNIE S D , RISTIC R I , SHERWOOD J N , et al . Morphological and growth rate distributions of small self-nucleated paracetamol crystals grown from pure aqueous solutions[J]. Journal of Crystal Growth, 1999, 207(4): 308-318. |
| 75 | YU Z Q , TAN R B H , CHOW P S . Effects of operating conditions on agglomeration and habit of paracetamol crystals in anti-solvent crystallization [J]. Journal of Crystal Growth, 2005, 279(3): 477-488. |
| 76 | LEE T, KUO C S, CHEN Y H . Solubility, polymorphism, crystallinity, and crystal habit of acetaminophen and ibuprofen:by initial solvent screening[J]. Pharmaceutical Technology, 2006, 30(10): 72-92. |
| 77 | SMITH K B . Crystallisation of active pharmaceutical ingredients using ionic liquids[D]. Birmingham:University of Birmingham, 2015. |
| 78 | HUNT PATRICIA A , KIRCHNER B , WELTON T . Characterising the electronic structure of ionic liquids:an examination of the 1-butyl-3-methylimidazolium chloride ion pair[J]. Chemistry:A European Journal, 2006, 12(26): 6762-6775. |
| 79 | HECQ J , DELEERS M , FANARA D , et al . Preparation and in vitro/in vivo evaluation of nano-sized crystals for dissolution rate enhancement of ucb-35440-3, a highly dosed poorly water-soluble weak base[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2006, 64(3): 360-368. |
| 80 | KRAUSE K P , MULLER R H . Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation[J]. International Journal of Pharmaceutics, 2001, 214(1): 21-24. |
| 81 | WANG Z , CHEN J F , LE Y , et al . Preparation of ultrafine beclomethasone dipropionate drug powder by antisolvent precipitation[J]. Industrial & Engineering Chemistry Research, 2007, 46(14): 4839-4845. |
| 82 | RASENACK N , HARTENHAUER H , MULLER B W . Microcrystals for dissolution rate enhancement of poorly water-soluble drugs[J]. International Journal of Pharmaceutics, 2003, 254(2): 137-145. |
| 83 | ZHANG H X , WANG J X , ZHANG Z B , et al . Micronization of atorvastatin calcium by antisolvent precipitation process[J]. International Journal of Pharmaceutics, 2009, 374(1): 106-113. |
| 84 | MIZUUCHI H , JAITELY V , MURDAN S , et al . Room temperature ionic liquids and their mixtures:potential pharmaceutical solvents[J]. European Journal of Pharmaceutical Sciences, 2008, 33(4): 326-331. |
| 85 | DUGGIRALA N K , PERRY M L , ALMARSSON O , et al . Pharmaceutical cocrystals:along the path to improved medicines[J]. Chemical Communications, 2016, 52(4): 640-655. |
| 86 | AAKEROY C B , FASULO M E , DESPER J . Cocrystal or salt:does it really matter?[J]. Molecular Pharmaceutics, 2007, 4(3): 317-322. |
| 87 | ALMARSSON O , ZAWOROTKO M J . Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines?[J]. Chemical Communications, 2004, 40(17): 1889-1896. |
| 88 | CLARE SPEAKMAN J . Acid salts of carboxylic acids, crystals with some“very short”hydrogen bonds[C]// Structure and Bonding.Glasgow. 1972. |
| 89 | BRAGA D , GREPIONI F , MAINI L , et al . From unexpected reactions to a new family of ionic co-crystals:the case of barbituric acid with alkali bromides and caesium iodide[J]. Chemical Communications, 2010, 46(41): 7715-7717. |
| 90 | KELLEY S P , NARITA A , HOLBREY J D , et al . Understanding the effects of ionicity in salts, solvates, co-crystals, ionic co-crystals, and ionic liquids, rather than nomenclature, is critical to understanding their behavior[J]. Crystal Growth & Design, 2013, 13(3): 965-975. |
| 91 | ETTER M C . Hydrogen bonds as design elements in organic chemistry[J]. The Journal of Physical Chemistry, 1991, 95(12): 4601-4610. |
| 92 | WALSH R D B , BRADNER M W , FLEISCHMAN S , et al . Crystal engineering of the composition of pharmaceutical phases[J]. Chemical Communications, 2003, 39(2): 186-187. |
| 93 | JOHANSSON K M , IZGORODINA E I , FORSYTH M , et al . Protic ionic liquids based on the dimeric and oligomeric anions:[(AcO) x H x -1][J]. Physical Chemistry Chemical Physics, 2008, 10(20): 2972-2978. |
| 94 | BLAGDEN N , MATAS M DE , GAVAN P T , et al . Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates[J]. Advanced Drug Delivery Reviews, 2007, 59(7): 617-630. |
| [1] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [2] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [3] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [4] | 陈钰, 刘冲, 邱于荟, 贲梓欣, 牟天成. 离子液体和低共熔溶剂绿色回收废旧锂离子电池的研究进展[J]. 化工进展, 2022, 41(S1): 485-496. |
| [5] | 华渠成, 段庆华. 离子液体极压抗磨剂的研究进展[J]. 化工进展, 2022, 41(S1): 331-339. |
| [6] | 韩明阳, 乔慧, 付佳铭, 马泽雯, 王妍, 欧阳嘉. 非水溶剂预处理木质纤维原料研究进展[J]. 化工进展, 2022, 41(8): 4086-4097. |
| [7] | 单清雯, 张娟, 王亚娟, 刘文强. 聚合离子液体的合成及其吸附脱硫性能[J]. 化工进展, 2022, 41(8): 4571-4579. |
| [8] | 阮佳纬, 叶香珠, 陈立芳, 漆志文. 离子液体和低共熔溶剂催化二氧化碳合成有机碳酸酯的研究进展[J]. 化工进展, 2022, 41(3): 1176-1186. |
| [9] | 倪清, 来锦波, 彭东岳, 管翠诗, 龙军. 离子液体萃取分离烃类化合物的研究进展[J]. 化工进展, 2022, 41(2): 619-627. |
| [10] | 鲁泽平, 裴新华, 薛誉, 张晓光, 胡燚. 甜菜碱类离子液体化学修饰猪胰脂肪酶提升其酶学性能[J]. 化工进展, 2022, 41(11): 6045-6052. |
| [11] | 徐波, 蒋国斌, 于劲磊, 胡金燕, 赵靓, 徐炳科. 不同表面活性剂对单相微乳液特性的影响[J]. 化工进展, 2021, 40(S1): 350-356. |
| [12] | 陈亚举, 任清刚, 周贤太, 纪红兵. 多孔有机聚合物催化二氧化碳合成环状碳酸酯研究进展[J]. 化工进展, 2021, 40(7): 3564-3583. |
| [13] | 白瑞兵, 王均凤, 王道广, 张延强. 离子液体基萃取体系用于卤水中锂分离的研究进展[J]. 化工进展, 2021, 40(6): 3224-3238. |
| [14] | 刘铮, 刘燃, 花儿, 冀健龙. 水分含量对正己胺-Tf2N型质子化离子液体物理化学性质的影响[J]. 化工进展, 2021, 40(4): 2270-2277. |
| [15] | 张文林, 刘雪娇, 马青查, 杨双丞, 张永康, 李春利. 高镍锂离子电池三元材料NCM电解质的应用[J]. 化工进展, 2021, 40(4): 2175-2187. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||