化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4571-4579.DOI: 10.16085/j.issn.1000-6613.2021-1963
聚合离子液体的合成及其吸附脱硫性能
- 河北科技大学化学与制药工程学院,河北 石家庄 050000
-
收稿日期:2021-09-13修回日期:2022-01-20出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:张娟 -
作者简介:单清雯(1997—),女,硕士研究生,研究方向为吸附脱硫。E-mail:814251749@qq.com 。 -
基金资助:国家自然科学基金(21106032);河北省自然科学基金(B2021103012)
Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization
SHAN Qingwen( ), ZHANG Juan(
), ZHANG Juan( ), WANG Yajuan, LIU Wenqiang
), WANG Yajuan, LIU Wenqiang
- College of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050000, Hebei, China
-
Received:2021-09-13Revised:2022-01-20Online:2022-08-25Published:2022-08-22 -
Contact:ZHANG Juan
摘要:
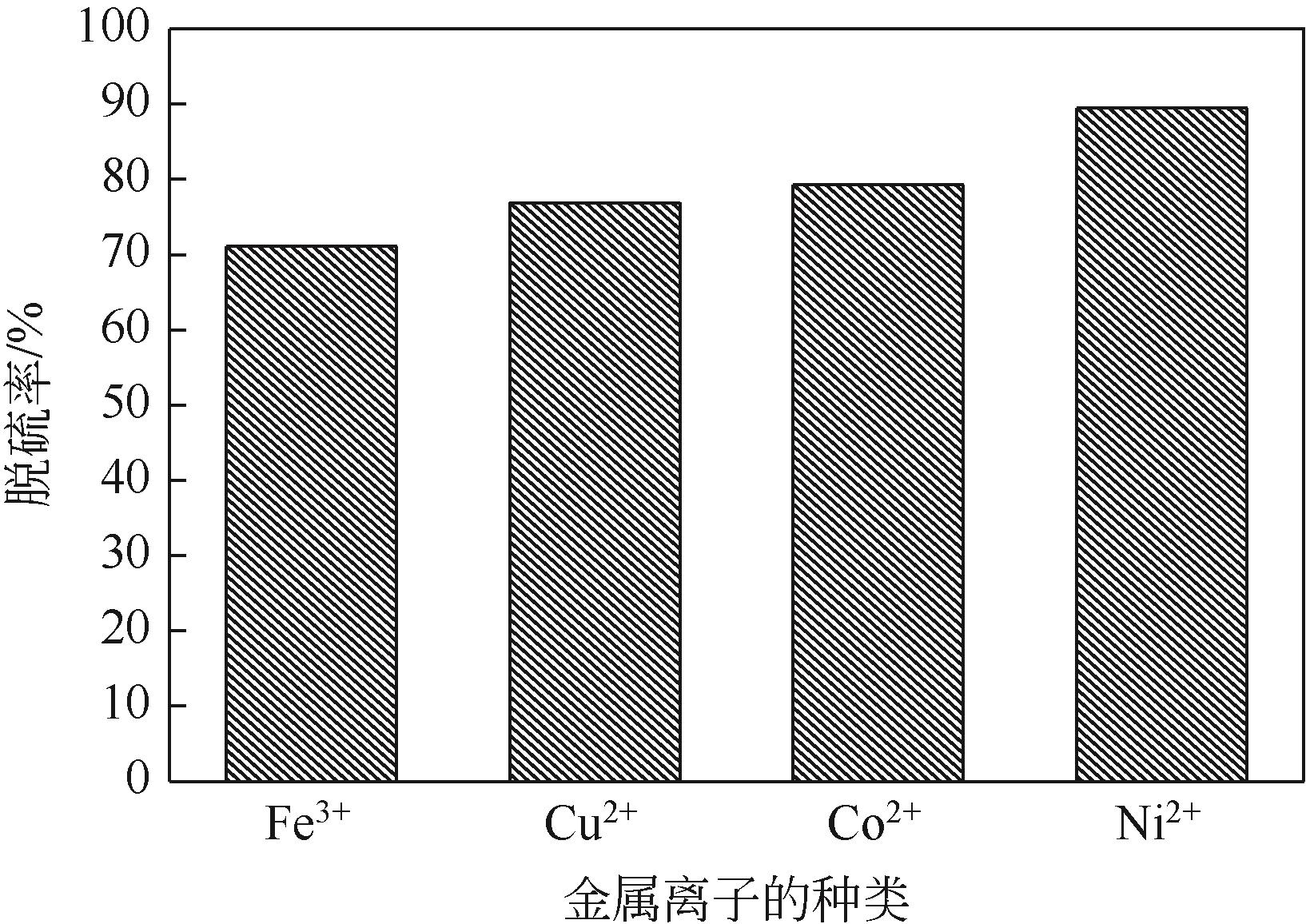
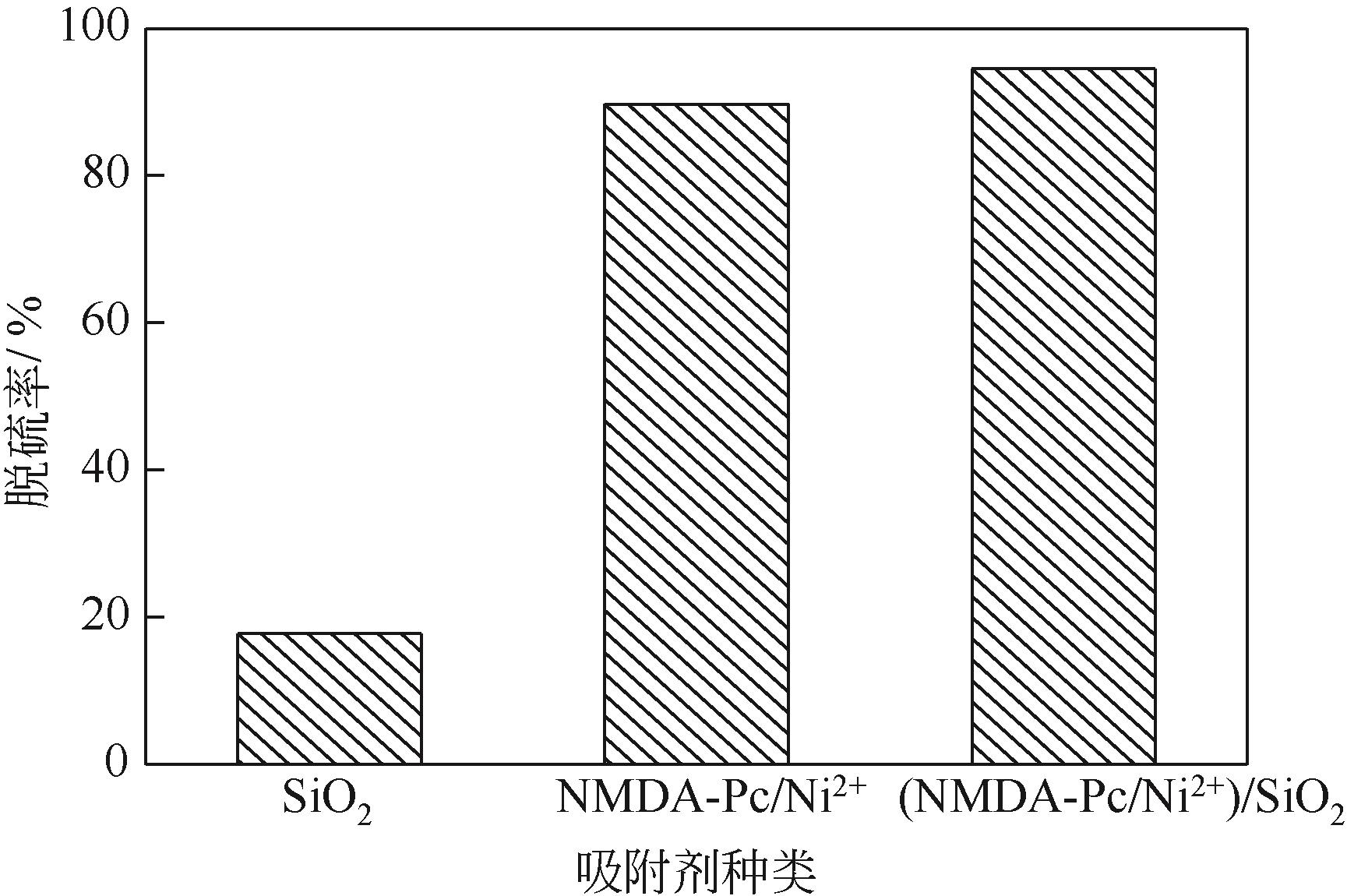
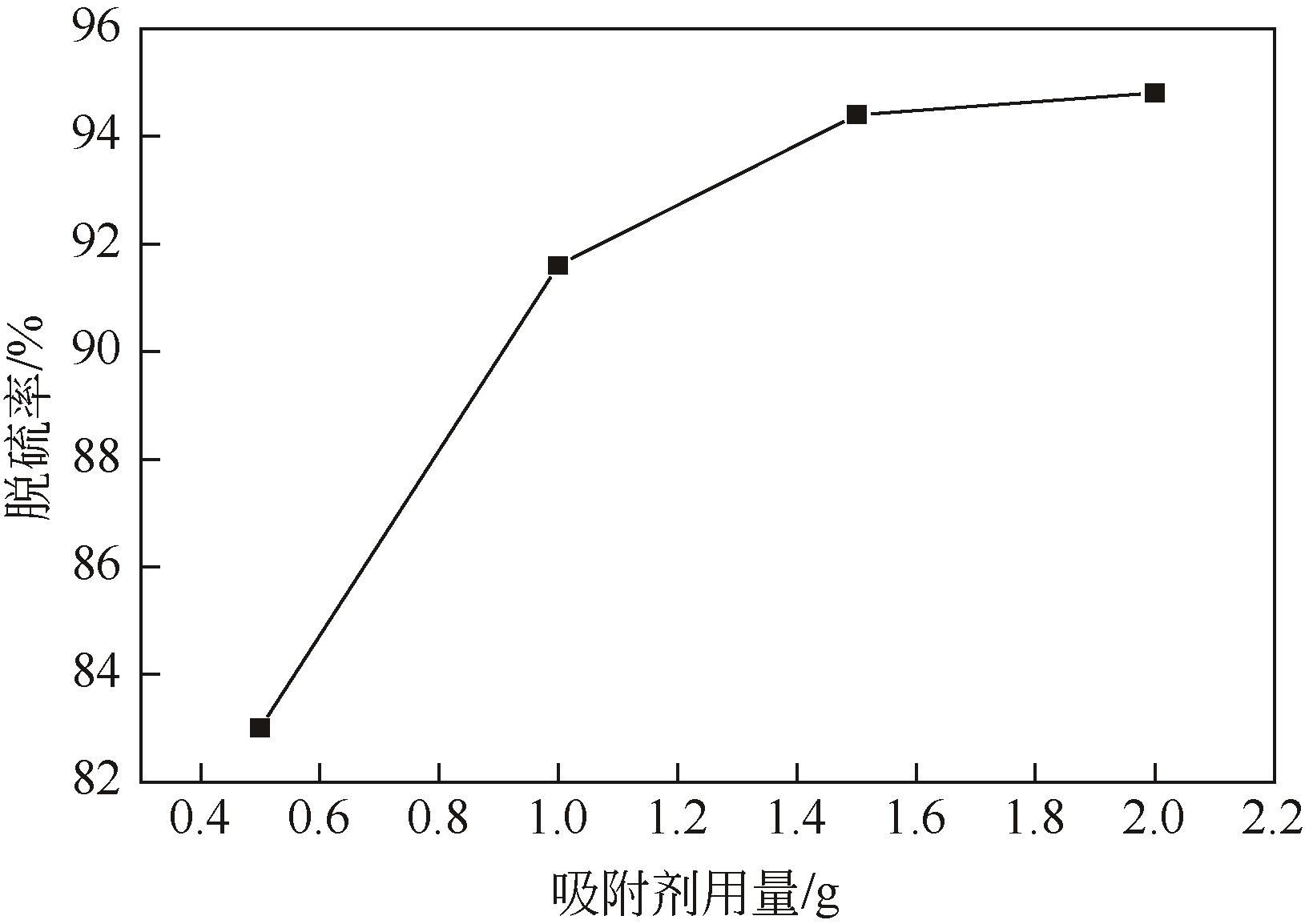
以N-甲基二烯丙基胺与金属酞菁合成的功能化离子液体为单体、硅胶球为载体,在载体表面聚合制备成硅胶球负载的聚合功能化离子液体吸附材料[(NMDA-Pc/Ni2+)/SiO2]。本研究采用红外光谱、X射线衍射、扫描电镜、偏光显微镜对其进行表征。考察了吸附剂在常压室温下对二苯并噻吩(DBT)的吸附脱硫性能。结果表明,(NMDA-Pc/Ni2+)/SiO2的吸附脱硫性能最好。最佳吸附条件为吸附剂用量为1.5g/10mL模型油,吸附时间为20min,DBT的最大吸附量为6.198mg/g。该吸附剂对DBT的吸附行为遵循Freundlich 吸附等温模型和拟二级动力学模型。以甲醇洗涤再生,重复使用5次后,吸附性能没有明显降低。烯烃和芳烃都会影响吸附剂的吸附脱硫效果,但芳烃对DBT选择性吸附的影响小于烯烃。吸附剂对不同的硫化合物也有良好的吸附作用,去除顺序为:二苯并噻吩>苯并噻吩>噻吩。
中图分类号:
引用本文
单清雯, 张娟, 王亚娟, 刘文强. 聚合离子液体的合成及其吸附脱硫性能[J]. 化工进展, 2022, 41(8): 4571-4579.
SHAN Qingwen, ZHANG Juan, WANG Yajuan, LIU Wenqiang. Synthesis of polymeric ionic liquid and its performance on adsorption desulfurization[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4571-4579.
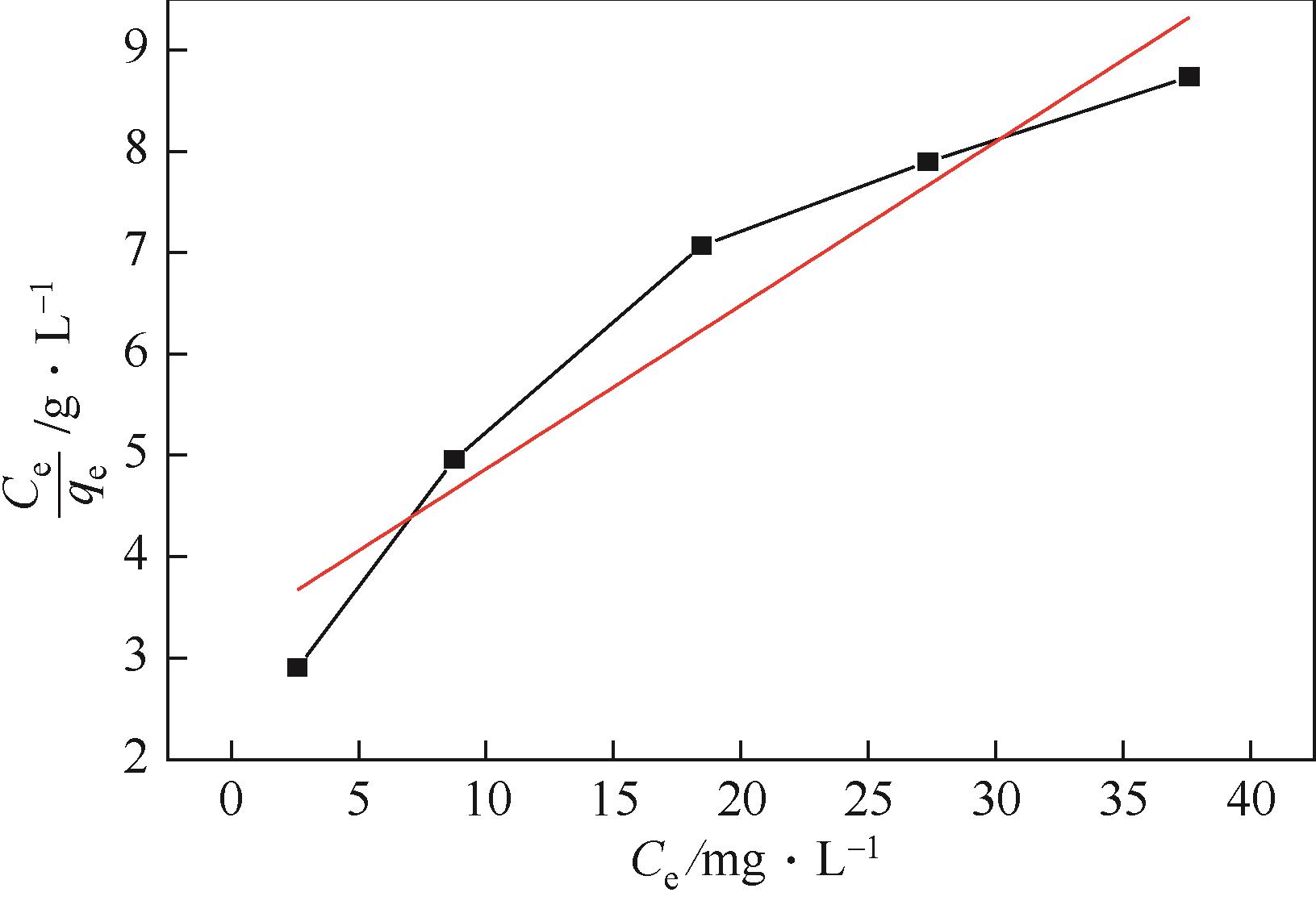
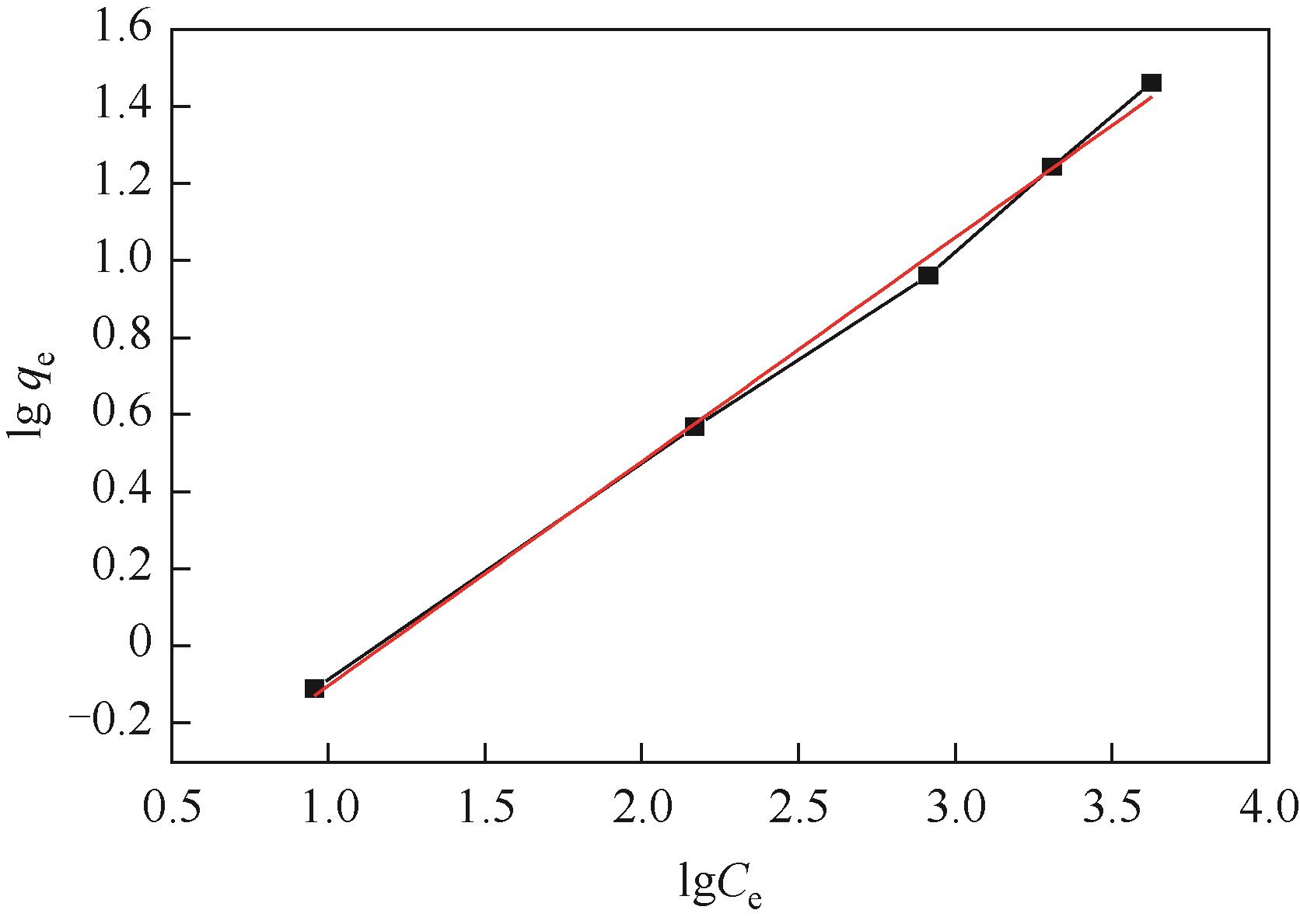
| 等温吸附模型 | 参数 | 数值 |
|---|---|---|
| Langmuir | qm/mg·g-1 | 6.198 |
| KL | 20.156 | |
| R2 | 0.89416 | |
| Freundlich | KF | 0.5048 |
| 1/n | 0.581 | |
| R2 | 0.99632 |
表1 吸附平衡等温线参数
| 等温吸附模型 | 参数 | 数值 |
|---|---|---|
| Langmuir | qm/mg·g-1 | 6.198 |
| KL | 20.156 | |
| R2 | 0.89416 | |
| Freundlich | KF | 0.5048 |
| 1/n | 0.581 | |
| R2 | 0.99632 |
| 吸附剂 | 硫化物 种类 | 脱硫量 /mg·g-1 | 参考 文献 |
|---|---|---|---|
| Ni/ZSM-5 | DBT | 0.496 | [ |
| 过渡金属化合物负载的硅胶体系 | DBT | 0.5184 | [ |
| Ni-Ce/Al2O3-SiO2 | DBT | 3.97 | [ |
| Cu(Ⅰ)-Y 分子筛 | DBT | 4.925 | [ |
| (NMDA-Pc/Ni2+)/SiO2 | DBT | 6.196 | 本实验 |
表2 不同吸附剂对DBT的吸附能力对比
| 吸附剂 | 硫化物 种类 | 脱硫量 /mg·g-1 | 参考 文献 |
|---|---|---|---|
| Ni/ZSM-5 | DBT | 0.496 | [ |
| 过渡金属化合物负载的硅胶体系 | DBT | 0.5184 | [ |
| Ni-Ce/Al2O3-SiO2 | DBT | 3.97 | [ |
| Cu(Ⅰ)-Y 分子筛 | DBT | 4.925 | [ |
| (NMDA-Pc/Ni2+)/SiO2 | DBT | 6.196 | 本实验 |
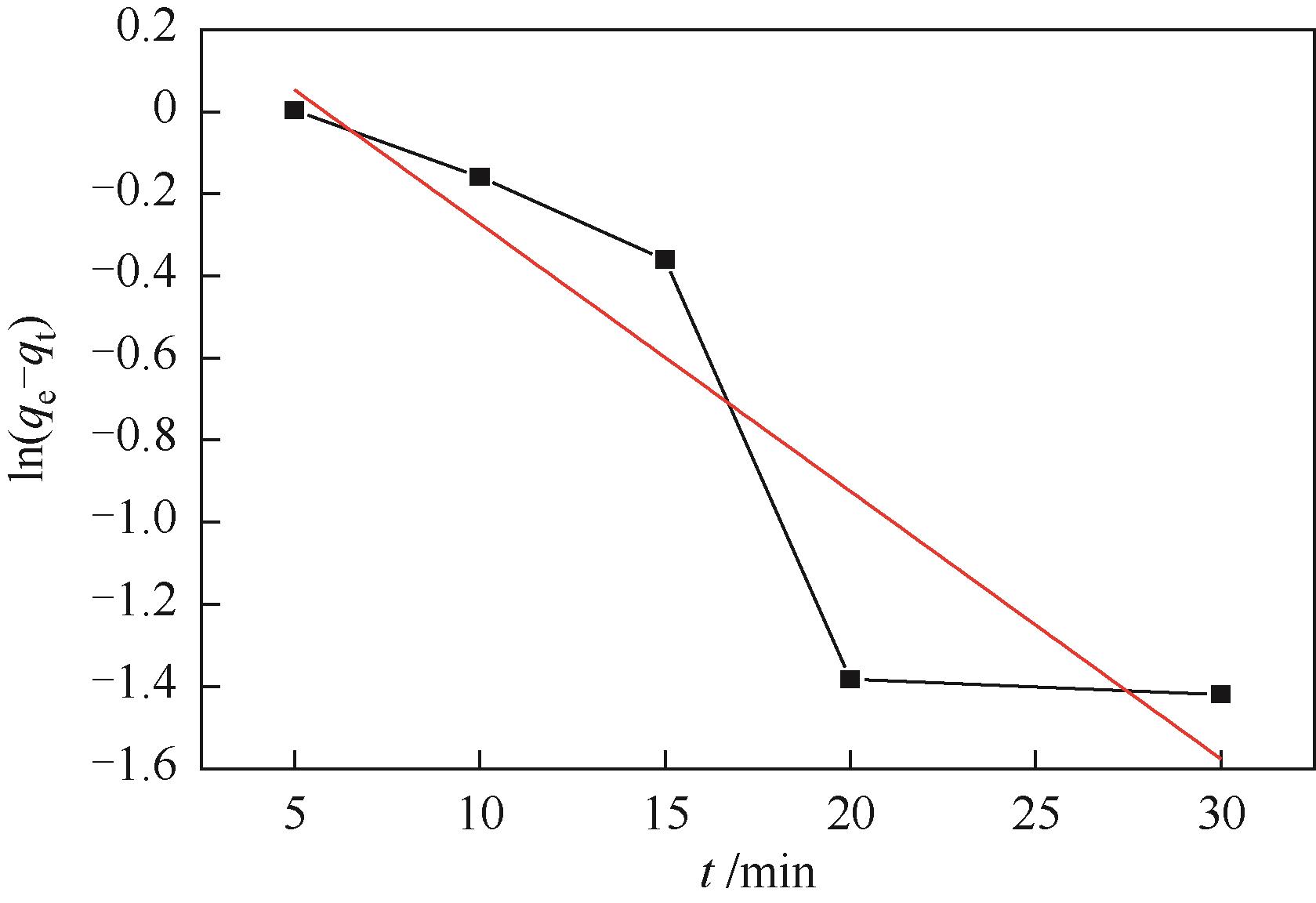
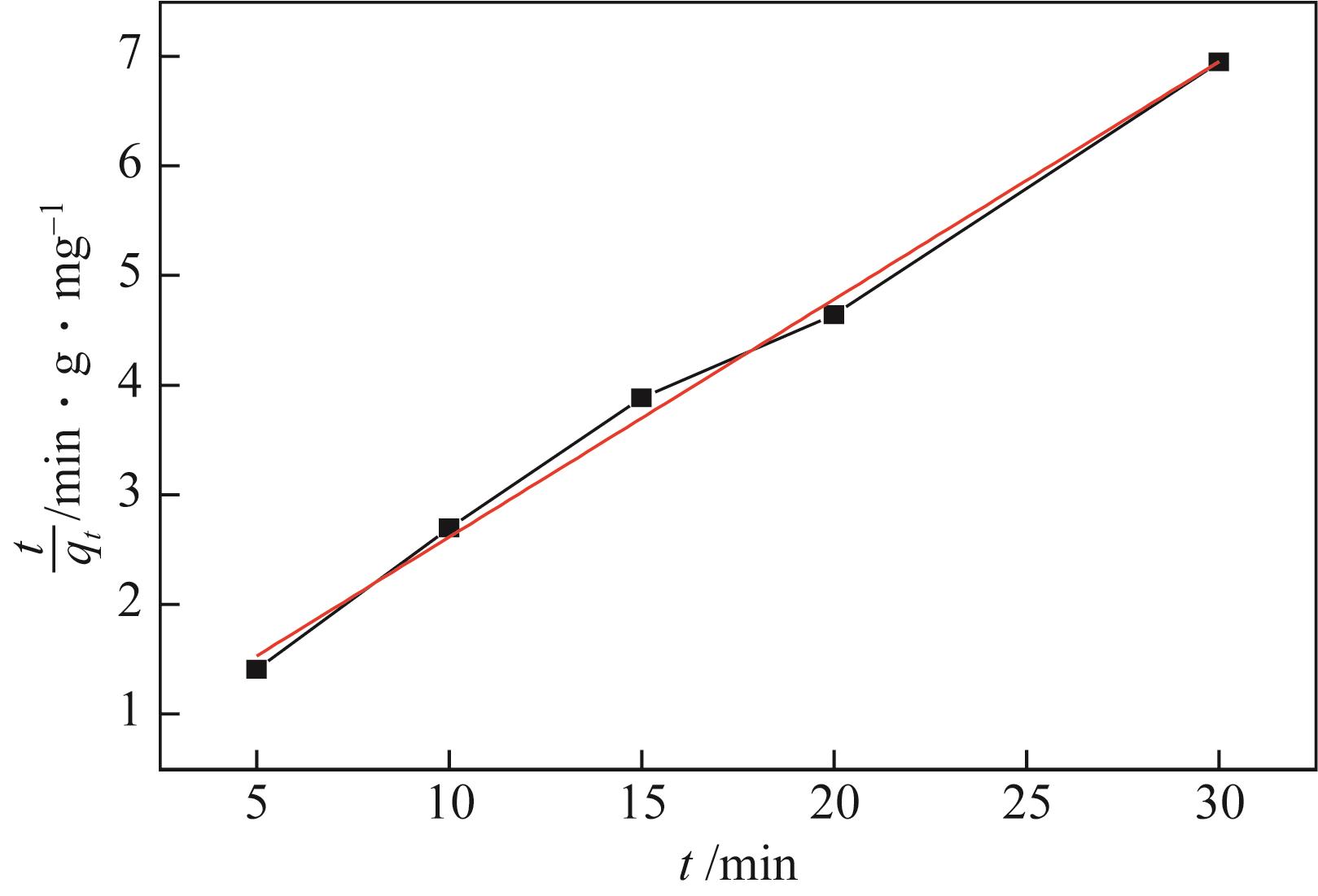
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学 | k1/min-1 | 0.06523 |
| qe,cal/mg·g-1 | 1.4619 | |
| R2 | 0.78241 | |
| 拟二级动力学 | k2/min-1 | 0.1063 |
| qe,cal/mg·g-1 | 4.6081 | |
| R2 | 0.99416 |
表3 拟一级动力学和拟二级动力学的数据
| 模型 | 参数 | 数值 |
|---|---|---|
| 拟一级动力学 | k1/min-1 | 0.06523 |
| qe,cal/mg·g-1 | 1.4619 | |
| R2 | 0.78241 | |
| 拟二级动力学 | k2/min-1 | 0.1063 |
| qe,cal/mg·g-1 | 4.6081 | |
| R2 | 0.99416 |
| 1 | WU Jianxiang, GAO Yilong, ZHANG Wei, et al. Deep desulfurization by oxidation using an active ionic liquid-supported Zr metal-organic framework as catalyst[J]. Applied Organometallic Chemistry, 2015, 29(2): 96-100. |
| 2 | SURYAWANSHI N B, BHANDARI V M, SOROKHAIBAM L G, et al. Investigating adsorptive deep desulfurization of fuels using metal-modified adsorbents and process intensification by acoustic cavitation[J]. Industrial & Engineering Chemistry Research, 2019, 58(18): 7593-7606. |
| 3 | 张健, 韩磊, 刘树伟, 等. 大孔Ni-Mo/Al2O3催化剂上重馏分油加氢脱硫生产低硫船用燃料油[J]. 石油化工, 2021, 50(2): 117-122. |
| ZHANG Jian, HAN Lei, LIU Shuwei, et al. Hydrodesulfurization of heavy distillate oil over macroporous Ni-Mo/Al2O3 catalyst with the aim of producing low-sulfur marine fuel[J]. Petrochemical Technology, 2021, 50(2): 117-122. | |
| 4 | FOX E B, LIU Zhongwen, LIU Zhaotie. Ultraclean fuels production and utilization for the twenty-first century: advances toward sustainable transportation fuels[J]. Energy & Fuels, 2013, 27(11): 6335-6338. |
| 5 | CHANDRA SRIVASTAVA V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels[J]. RSC Adv., 2012, 2(3): 759-783. |
| 6 | 刘璇, 崔颖娜, 尹静梅, 等. 金属有机骨架材料在吸附脱硫领域的应用[J]. 化工进展, 2020, 39(8): 3163-3176. |
| LIU Xuan, CUI Yingna, YIN Jingmei, et al. Application of meta-organic frameworks materials in adsorptive desulfurization[J]. Chemical Industry and Engineering Progress, 2020, 39(8): 3163-3176. | |
| 7 | 张娟, 任腾杰, 胡颜荟, 等. MCM-41分子筛负载金属酞菁在氧化脱硫反应中的催化性能[J]. 化工学报, 2014, 65(8): 3012-3018. |
| ZHANG Juan, REN Tengjie, HU Yanhui, et al. Catalytic performance of metal phthalocyanine loaded on MCM-41 molecular sieve in oxidation desulfurization[J]. CIESC Journal, 2014, 65(8): 3012-3018. | |
| 8 | 易成高, 于寒颖, 赵欢, 等. 石油和天然气生物脱硫技术分析和展望[J]. 石油化工, 2010, 39(6): 681-687. |
| YI Chenggao, YU Hanying, ZHAO Huan, et al. Technique analysis and prospect of biologic desulphurization process for crude oil and natural gas[J]. Petrochemical Technology, 2010, 39(6): 681-687. | |
| 9 | 张娟, 李俊盼, 任腾杰, 等. [C n mim]Br/FeCl3型离子液体萃取脱除二苯并噻吩[J]. 化工学报, 2013, 64(10): 3647-3651. |
| ZHANG Juan, LI Junpan, REN Tengjie, et al. Extraction desulfurization of dibenzothiophene with [C3~8mim]Br/FeCl3 ionic liquids[J]. CIESC Journal, 2013, 64(10): 3647-3651. | |
| 10 | 刘卉, 高金森, 赵亮. 吸附脱除噻吩类硫化物机理的研究进展[J]. 石油化工, 2010, 39(9): 1059-1065. |
| LIU Hui, GAO Jinsen, ZHAO Liang. Advances in adsorptive desulfurization mechanism for thiophene-type sulfide[J]. Petrochemical Technology, 2010, 39(9): 1059-1065. | |
| 11 | WANG Sihua, ZU Yun, QIN Yucai, et al. Fabrication of effective desulfurization species active sites in the CeY zeolites and the adsorption desulfurization mechanisms[J]. Journal of Fuel Chemistry and Technology, 2020, 48( 1): 52-62. |
| 12 | BAGHERI M, MASOOMI M Y, MORSALI A. High organic sulfur removal performance of a cobalt based metal-organic framework[J]. Journal of Hazardous Materials, 2017, 331: 142-149. |
| 13 | BLANCO-BRIEVA G, CAMPOS-MARTIN J M, AL-ZAHRANI S M, et al. Effectiveness of metal-organic frameworks for removal of refractory organo-sulfur compound present in liquid fuels[J]. Fuel, 2011, 90(1): 190-197. |
| 14 | 肖永厚, 朱科润, 董晓莹, 等. 燃油选择性吸附脱硫的多孔材料研究进展[J]. 化工进展, 2020, 39(6): 2241-2250. |
| XIAO Yonghou, ZHU Kerun, DONG Xiaoying, et al. Research progress on porous materials for desulfurization of fuel by selective adsorption[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2241-2250. | |
| 15 | 杨诗, 蔡阳, 李长平, 等. 磷钨酸负载锆基金属有机骨架PTA@MOF-808的制备及其吸附脱硫性能[J]. 化工学报, 2021, 72(3): 1722-1731. |
| YANG Shi, CAI Yang, LI Changping, et al. Preparation of phosphotungstic acid loaded Zr-based metal-organic framework PTA@MOF-808 and its adsorption desulfurization performance[J]. CIESC Journal, 2021, 72(3): 1722-1731. | |
| 16 | ZHANG Junheng, CHEN Shiyuan, HE Qianjun, et al. Toughening benzoxazines with hyperbranched polymeric ionic liquids: effect of cations and anions[J]. Reactive and Functional Polymers, 2018, 133: 37-44. |
| 17 | 李春喜, 熊佳丽, 孟洪, 等. 从ILs到PILs: 聚合离子液体介孔材料的制备性质及结构调控方法[J]. 化工进展, 2014, 33(8): 1941-1950. |
| LI Chunxi, XIONG Jiali, MENG Hong, et al. From ILs to PILs: synthesis and structure tuning of poly ionic liquids mesoporous materials[J]. Chemical Industry and Engineering Progress, 2014, 33(8): 1941-1950. | |
| 18 | 冯建朋, 张香平, 尚大伟, 等. 离子液体中电化学还原CO2研究评述与展望[J]. 化工学报, 2018, 69(1): 69-75. |
| FENG Jianpeng, ZHANG Xiangping, SHANG Dawei, et al. Review and prospect of CO2 electro-reduction in ionic liquids[J]. CIESC Journal, 2018, 69(1): 69-75. | |
| 19 | CHEN Yuanzhe, ZHANG Fengwei, FANG Yiyun, et al. Phosphotungstic acid containing ionic liquid immobilized on magnetic mesoporous silica rod catalyst for the oxidation of dibenzothiophene with H2O2 [J]. Catalysis Communications, 2013, 38: 54-58. |
| 20 | 孙爽, 李未康, 张娟, 等. 聚合离子液体的吸附分离应用研究进展[J]. 现代化工, 2017, 37(6): 38-42. |
| SUN Shuang, LI Weikang, ZHANG Juan, et al. Research progress on application of polymeric ionic liquids in adsorption separation[J]. Modern Chemical Industry, 2017, 37(6): 38-42. | |
| 21 | QIAN Wenjing, TEXTER J, YAN Feng. Frontiers in poly(ionic liquid)s: syntheses and applications[J]. Chemical Society Reviews, 2017, 46(4): 1124-1159. |
| 22 | 李春喜, 许慧慧, 朱学习, 等. 吡啶基聚合离子液体的制备及其对油中噻吩硫的吸附性能[J]. 化工学报, 2016, 67(7): 2880-2886. |
| LI Chunxi, XU Huihui, ZHU Xuexi, et al. Synthesis of pyridinium based polymerized ionic liquid and its adsorptive desulfurization performance for thiophenic sulfurs from oil[J]. CIESC Journal, 2016, 67(7): 2880-2886. | |
| 23 | ZHANG Juan, SUN Shuang, BIAN Yuhang, et al. Adsorptive desulfurization of metal phthalocyanine functionalized poly-ionic liquids grafted to silica gel[J]. Fuel, 2018, 220: 513-520. |
| 24 | BI Wentao, ZHU Tao, PARK D W, et al. Sorption of carbon dioxide by ionic liquid-based sorbents[J]. Asia-Pacific Journal of Chemical Engineering, 2012, 7(1): 86-92. |
| 25 | HAN Peng, ZHANG Hongming, QIU Xuepeng, et al. Palladium within ionic liquid functionalized mesoporous silica SBA-15 and its catalytic application in room-temperature Suzuki coupling reaction[J]. Journal of Molecular Catalysis A: Chemical, 2008, 295(1/2): 57-67. |
| 26 | SAHOO S, KUMAR P, LEFEBVRE F, et al. Oxidative kinetic resolution of alcohols using chiral Mn-salen complex immobilized onto ionic liquid modified silica[J]. Applied Catalysis A: General, 2009, 354(1/2): 17-25. |
| 27 | ZHAO Dishun, ZHANG Juan, DUAN Erhong, et al. Adsorption equilibrium and kinetics of dibenzothiophene from n-octane on bamboo charcoal[J]. Applied Surface Science, 2008, 254(10): 3242-3247. |
| 28 | SRIVASTAV A, SRIVASTAVA V C. Adsorptive desulfurization by activated alumina[J]. Journal of Hazardous Materials, 2009, 170(2/3): 1133-1140. |
| 29 | MITCHELL L A, LEVAN M D. Development of adsorption equilibrium relations for mixtures from pure component isotherms and Henry’s law behavior with components in excess[J]. Industrial & Engineering Chemistry Research, 2014, 53(40): 15531-15537. |
| 30 | KUNDU S, GUPTA A K. Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization[J]. Chemical Engineering Journal, 2006, 122(1/2): 93-106. |
| 31 | SARDA K K, BHANDARI A, PANT K K, et al. Deep desulfurization of diesel fuel by selective adsorption over Ni/Al2O3 and Ni/ZSM-5 extrudates[J]. Fuel, 2012, 93: 86-91. |
| 32 | MA Xiaoliang, SUN Lu, SONG Chunshan. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications[J]. Catalysis Today, 2002, 77(1/2): 107-116. |
| 33 | XU Xinhai, ZHANG Shuyang, LI Peiwen, et al. Equilibrium and kinetics of Jet-A fuel desulfurization by selective adsorption at room temperatures[J]. Fuel, 2013, 111: 172-179. |
| 34 | HERNÁNDEZ-MALDONADO A J, YANG R T. Desulfurization of commercial liquid fuels by selective adsorption via π-complexation with Cu( Ⅰ )-Y zeolite[J]. Industrial & Engineering Chemistry Research, 2003, 42(13): 3103-3110. |
| 35 | CHENG Bei, LE Yao, CAI Weiquan, et al. Synthesis of hierarchical Ni(OH)2 and NiO nanosheets and their adsorption kinetics and isotherms to Congo red in water[J]. Journal of Hazardous Materials, 2011, 185(2/3): 889-897. |
| [1] | 盛维武, 程永攀, 陈强, 李小婷, 魏嘉, 李琳鸽, 陈险峰. 微气泡和微液滴双强化脱硫反应器操作分析[J]. 化工进展, 2023, 42(S1): 142-147. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [10] | 王尚彬, 欧红香, 薛洪来, 曹海珍, 王钧奇, 毕海普. 黄原胶和纳米二氧化硅对无氟泡沫性能的影响[J]. 化工进展, 2023, 42(9): 4856-4862. |
| [11] | 朱传强, 茹晋波, 孙亭亭, 谢兴旺, 李长明, 高士秋. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| [12] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [13] | 李伯耿, 罗英武, 刘平伟. 聚合物产品工程研究内容与方法的思考[J]. 化工进展, 2023, 42(8): 3905-3909. |
| [14] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [15] | 王云刚, 焦健, 邓世丰, 赵钦新, 邵怀爽. 冷凝换热与协同脱硫性能实验分析[J]. 化工进展, 2023, 42(8): 4230-4237. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||