化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2380-2388.DOI: 10.16085/j.issn.1000-6613.2018-1879
溶析结晶在医药领域的研究进展
- 1. 天津大学化工学院,天津 300350
2. 中国石化扬子石油化工有限公司南京研究院,江苏 南京 210048
-
收稿日期:2018-09-18修回日期:2018-11-18出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:孙海龙,王靖涛 -
作者简介:<named-content content-type="corresp-name">黄炎</named-content>(1993—),男,硕士研究生,研究方向为结晶。E-mail:<email>hyahut@163.com</email>。 -
基金资助:国家自然科学基金(21576185,21376162)
Progress in antisolvent crystallization in pharmaceutical field
Yan HUANG1( ),Hailong SUN2(
),Hailong SUN2( ),Zichao MENG1,Zhongli TANG1,Jingtao WANG1(
),Zichao MENG1,Zhongli TANG1,Jingtao WANG1( )
)
- 1. School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China
2. Nanjing Research Institute of Sinopec Yangzi Petrochemical Company, Nanjing 210048,Jiangsu,China
-
Received:2018-09-18Revised:2018-11-18Online:2019-05-05Published:2019-05-05 -
Contact:Hailong SUN,Jingtao WANG
摘要:
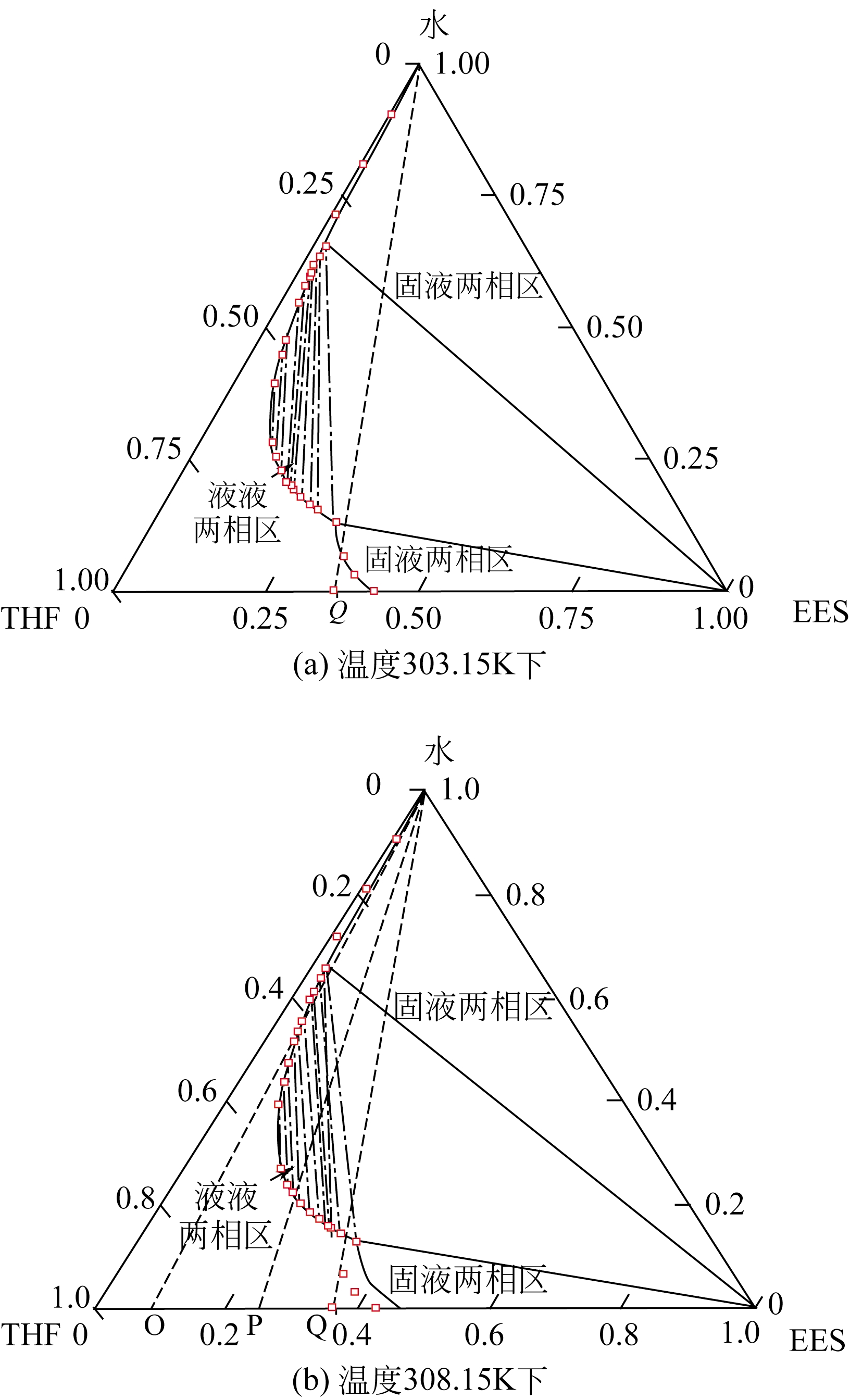
结晶作为一种传统的分离和提纯工艺,广泛运用于医药、化工、材料等领域。随着对结晶工艺的深入研究和对晶体产品质量越来越高的要求,结晶不再仅仅用于物质的分离和提纯,更重要的是根据产品功能的需要,制备特定结构的晶体。作为结晶的重要组成部分,溶析结晶因其操作简单、能耗相对较低、适用于热敏性物质等优势受到了广泛的关注。本文从溶析结晶相较于其他溶液结晶的不同点出发,重点介绍了溶析结晶热力学、溶析结晶动力学和工艺过程的研究,以及与溶析结晶相关的超临界流体技术和球形结晶技术。溶析结晶热力学关注了溶解度的测定方法和如何通过相图来确定合适的操作条件;溶析结晶动力学,详细描述了间歇、连续溶析结晶动力学模型的建立;工艺过程的研究,包括溶析剂与含有待结晶物质混合、结晶过程的控制和优化。同时本文对溶析结晶目前存在的问题进行了总结,并对未来的发展作了展望。
中图分类号:
引用本文
黄炎, 孙海龙, 孟子超, 唐忠利, 王靖涛. 溶析结晶在医药领域的研究进展[J]. 化工进展, 2019, 38(05): 2380-2388.
Yan HUANG, Hailong SUN, Zichao MENG, Zhongli TANG, Jingtao WANG. Progress in antisolvent crystallization in pharmaceutical field[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2380-2388.
| 1 | 丁绪淮, 谈遒 . 工业结晶[M]. 北京: 化学工业出版社, 1985: 294. |
| DING X H , TAN Q . Industrial crystallization[M]. Beijing: Chemical Industry Press, 1985:294. | |
| 2 | 龚俊波 . 工业结晶科学与技术专辑[J]. 化学工业与工程, 2018, 35(3): 1. |
| GONG J B . Industrial crystallization science and technology[J]. Chemical Industry and Engineering, 2018, 35(3): 1. | |
| 3 | 王静康//时钧, 汪家鼎, 余国琮, 等. . 结晶[M]. |
| 化学工程手册2版. 北京: 化学工业出版社, 1996:10-11. | |
| WANG J K //SHI J , WANG J D , YU G C , et al. . Crystallization[M]. | |
| Chemical engineering manual 2nd ed. Beijing: Chemical Industry Press, 1996:10-11. | |
| 4 | THORAT A A , DALVI S V . Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: recent developments and future perspective[J]. Chemical Engineering Journal, 2012, 181-182:1-34. |
| 5 | MULLER R H , KECK C M . Twenty years of drug nanocrystals: where are we, and where do we go[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2012, 80(1): 1-3. |
| 6 | KURUP M , R A R . Antisolvent crystallization a novel approach to bioavailability enhancement[J]. European Journal of Biomedical and Pharmaceutical Sciences, 2016, 3(3): 230-234. |
| 7 | MULLIN J W . Crystallization[M]. 4th. Woburn: Butterworth-Heinemann, 2001: 294. |
| 8 | WANG Z R , ZHOU G Y , DONG J J , et al . Measurement and correlation of the solubility of antipyrine in ten pure and water + ethanol mixed solvents at temperature from (288.15 to 328.15) K[J]. Journal of Molecular Liquids, 2018,268: 256-265. |
| 9 | LI K L , WU S G , XU S J , et al . Oiling out and polymorphism control of pyraclostrobin in cooling crystallization[J]. Industrial & Engineering Chemistry Research, 2016,55: 11631-11637. |
| 10 | LONG B , XIA Y , DENG Z , et al . Understanding the enhanced solubility of 1,3-benzenedicarboxylic acid in polar binary solvents of (acetone + water) at various temperatures[J]. The Journal of Chemical Themodynamics, 2017,105: 105-111. |
| 11 | LI S , JIANG L , QIU J , et al . Solubility and solution thermodynamics of the δ form of l‑citrulline in water + ethanol binary solvent mixtures[J]. Journal of Chemical & Engineering Data, 2016,61: 264-271. |
| 12 | ZHANG Q , HU Y , YANG Y , et al . Thermodynamic models for determination of the solubility of the the (1∶1) complex of (urea + l‑malic acid) in (methanol+acetonitrile) binary solvent mixtures binary solvent mixtures[J]. Journal of Chemical & Engineering Data, 2015,60: 1608-1613. |
| 13 | WANG J , XU A , XU R . Determination and correlation of terephthaldialdehyde solubility in (ethanol, isopropanol, ethyl acetate, isopentanol)+N,N-dimethylformamide mixed solvents at temperatures from 273.15K to 318.15K[J]. The Journal of Chemical Themodynamics, 2017,105: 327-336. |
| 14 | EGHRARY S H , ZARGHAMI R , MARTINEZ F , et al . Solubility of 2-butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl ketone hydrochloride (amiodarone HCl) in ethanol + water and N-methyl-2-pyrrolidone + water mixtures at various temperatures[J]. Journal of Chemical & Engineering Data, 2012,57: 1544-1550. |
| 15 | CRESTANI C E , BERNARDO A , COSTA C B B , et al . Fructose solubility in mixed (ethanol + water) solvent: experimental data and comparison among different thermodynamic models[J]. Journal of Chemical & Engineering Data, 2013,58(11): 3039-3045. |
| 16 | LI X , YIN Q , ZHANG M , et al . Process design for antisolvent crystallization of erythromycin ethylsuccinate in oiling-out system[J]. Industrial & Engineering Chemistry Research, 2016,55(27): 7484-7492. |
| 17 | KUDO S , TAKIYAMA H . Solubility determination for carbamazepine and saccharin in methanol/water mixed solvent: basic data for design of cocrystal production by antisolvent crystallization[J]. Journal of Chemical & Engineering Data, 2018, 63: 451-458. |
| 18 | GRANBERG R A , DUCREUX C , GRACIN S , et al . Primary nucleation of paracetamol in acetone-water mixtures[J]. Chemical Engineering Science, 2001, 56(7): 2305-2313. |
| 19 | LINDENBERG C , VICUM L , et al . Antisolvent precipitation of PDI 747: kinetics of particle formation and growth[J]. Crystal Growth & Design, 2007, 7(9): 1653-1661. |
| 20 | LUO Y , WU G , SUN B . Antisolvent crystallization of biapenem: estimation of growth and nucleation kinetics[J]. Journal of Chemical & Engineering Data, 2013, 58(3): 588-597. |
| 21 | NONOYAMA N , HANAKI K , YABUKI Y , et al . Constant supersaturation control of antisolvent-addition batch crystallization[J]. Organic Process Research & Development, 2006,10(4): 727-732. |
| 22 | TRIFKOVIC M , SHEIKHZADEH M , ROHANI S . Kinetics estimation and single and multi-objective optimization of a seeded, anti-solvent, isothermal batch crystallizer[J]. Industrial & Engineering Chemistry Research, 2018, 47: 1586-1595. |
| 23 | GARG M , ROY M, CHOKSHI P , et al . Process development in the QbD paradigm: mechanistic modeling of antisolvent crystallization for production of pharmaceuticals[J]. Crystal Growth & Design, 2018, 18(6): 3352-3359. |
| 24 | SCHALL J M , MANDUR J S , BRAATZ R D , et al . Nucleation and growth kinetics for combined cooling and antisolvent crystallization in a mixed-suspension, mixed-product removal system: estimating solvent dependency[J]. Crystal Growth & Design, 2018,18: 1560-1570. |
| 25 | HU J , NG W K, DONG Y , et al . Continuous and scalable process for water-redispersible nanoformulation of poorly aqueous soluble APIs by antisolvent precipitation and spray-drying[J]. International Journal of Pharmaceutics, 2011, 404(1/2): 198-204. |
| 26 | DOUROUMIS D , SCHELER S , FAHR A . Using a modified shepards method for optimization of a nanoparticulate cyclosporine a formulation prepared by a static mixer technique[J]. Journal of Pharmaceutical Sciences, 2008, 97(2): 919-930. |
| 27 | HU T , CHIOU H , CHAN H K , et al . Preparation of inhalable salbutamol sulphate using reactive high gravity controlled precipitation[J]. Journal of Pharmaceutical Sciences, 2008, 97(2): 944-949. |
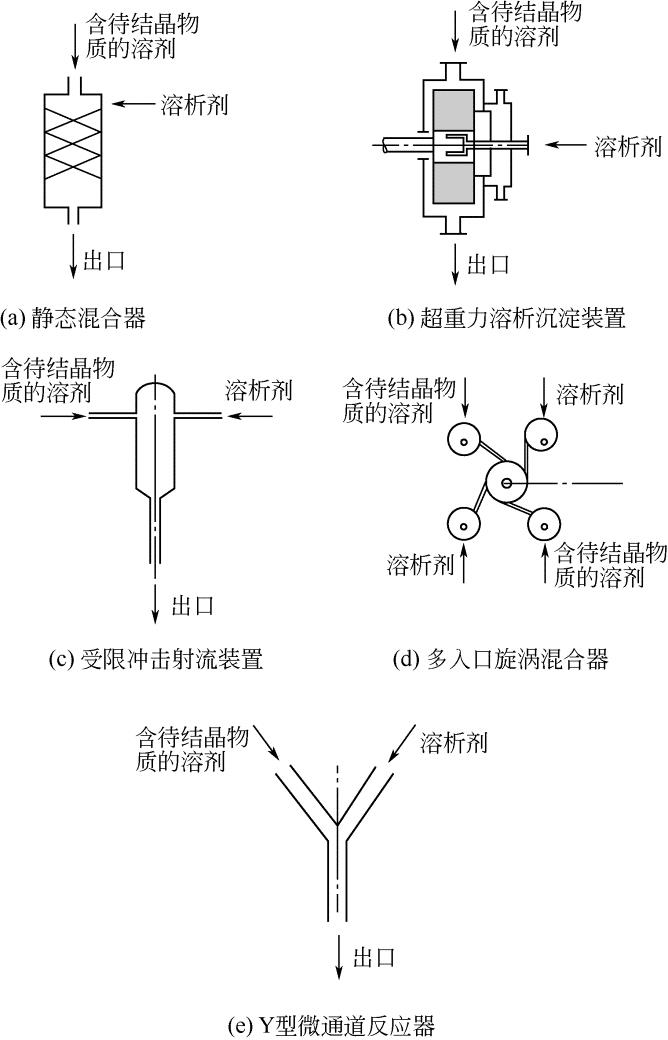
| 28 | JOHNSON B K , PRUD'HOMME R K . Chemical processing and micromixing in confined impinging jets[J]. AIChE Journal, 2003, 49(9): 2264-2282. |
| 29 | LIU Y , CHENG C , PRUD HOMME R K , et al . Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation[J]. Chemical Engineering Science, 2008, 63(11): 2829-2842. |
| 30 | DEMELLO A J . Control and detection of chemical reactions in microfluidic systems[J]. Nature, 2006, 442(7101): 394-402. |
| 31 | PANAGIOTOU T , MESITE S V , FISHER R J . Production of norfloxacin nanosuspensions using microfluidics reaction technology through solvent/antisolvent crystallization[J]. Industrial & Engineering Chemistry Research, 2009, 48(4): 1761-1771. |
| 32 | WANG J , ZHANG Q , ZHOU Y , et al . Microfluidic synthesis of amorphous cefuroxime axetil nanoparticles with size-dependent and enhanced dissolution rate[J]. Chemical Engineering Journal, 2010, 162(2): 844-851. |
| 33 | LIU Z , HUANG Y , JIN Y , et al . Mixing intensification by chaotic advection inside droplets for controlled nanoparticle preparation[J]. Chemical Engineering Science, 2010, 9(4): 773-786. |
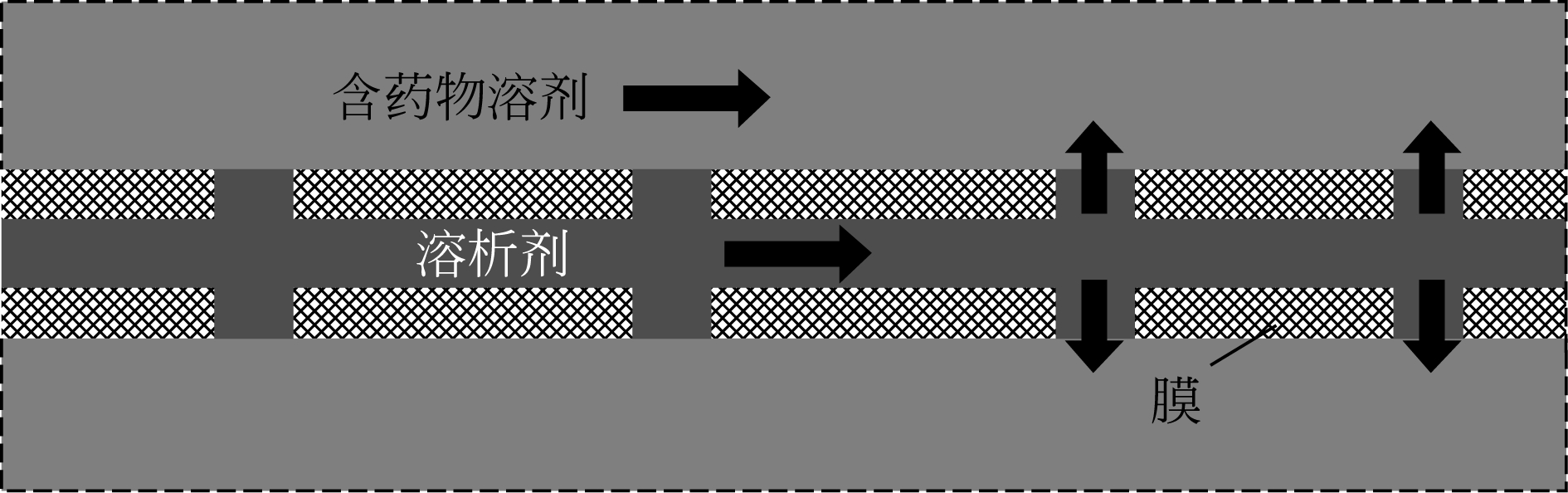
| 34 | FERN J , OHSAKI S , WATANO S , et al . Continuous synthesis of nano-drug particles by antisolvent crystallization using a porous hollow-fiber membrane module[J]. International Journal of Pharmaceutical, 2018, 543(1/2): 139-150. |
| 35 | OTHMAN R , VLADISAVLJEVI G T , SIMONE E , et al . Preparation of microcrystals of piroxicam monohydrate by antisolvent precipitation via microfabricated metallic membranes with ordered pore arrays[J]. Crystal Growth & Design, 2017, 17(12): 6692-6702. |
| 36 | TIERNEY T B , RASMUSON K C , HUDSON S P , et al . Size and shape control of micron-sized salicylic acid crystals during antisolvent crystallization[J]. Organic Process Research & Development, 2017, 21(11): 1732-1740. |
| 37 | JIANG S , HORST J H TER , JANSENS P J . Concomitant polymorphism of ο-aminobenzoic acid in antisolvent crystallization[J]. Crystal Growth & Design, 2008, 1(8): 37-43. |
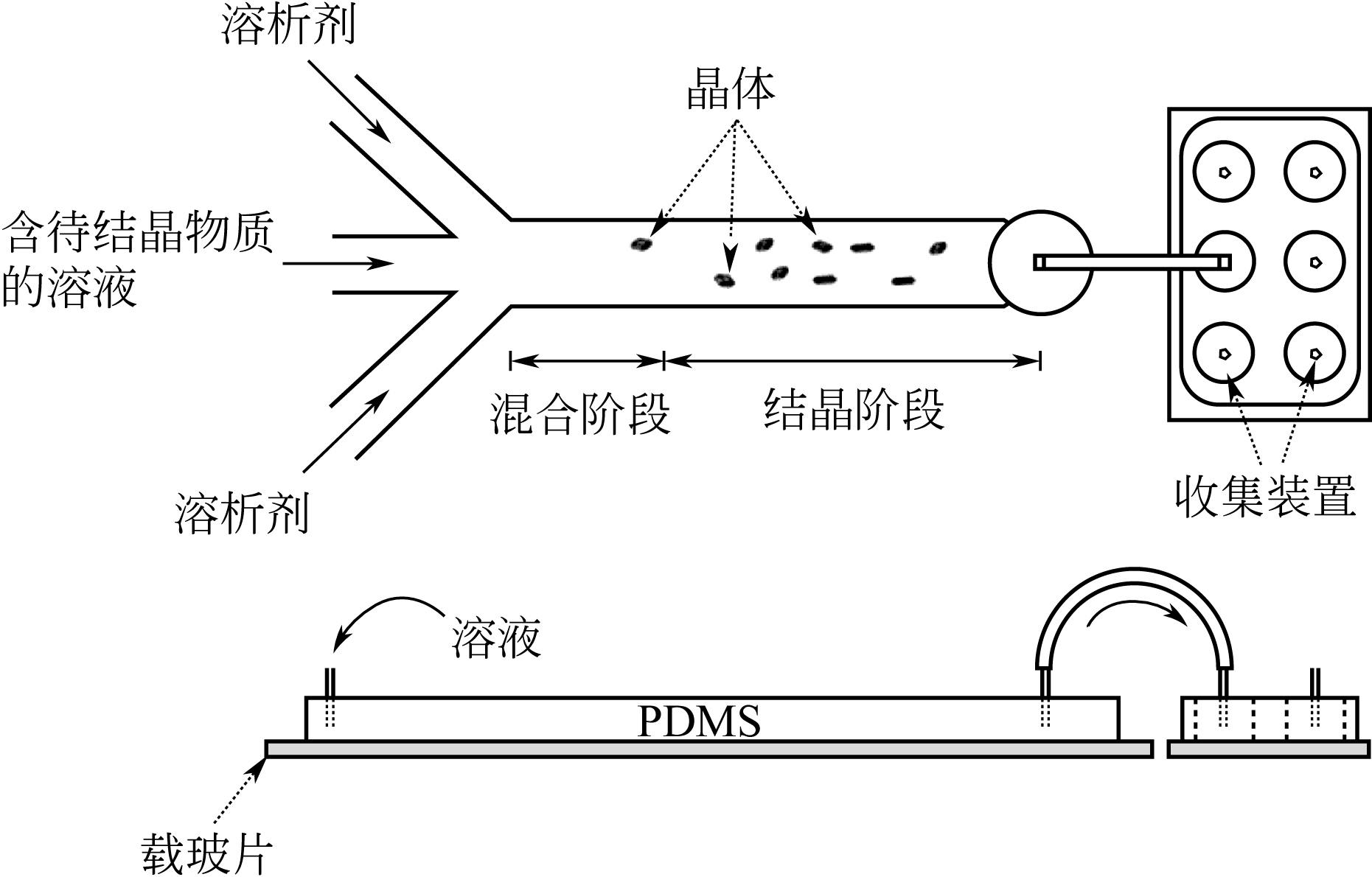
| 38 | BHAMIDI V , LEE S H, HE G , et al . Antisolvent crystallization and polymorph screening of glycine in microfluidic channels using hydrodynamic focusing[J]. Crystal Growth & Design, 2015, 15(7): 3299-3306. |
| 39 | FERGUSON S , MORRIS G , HAO H , Characterization of the anti-solvent batch, plug flow and MSMPR crystallization of benzoic acid [J]. Chemical Engineering Science, 2013, 104: 44-54. |
| 40 | NAGY Z K , FUJIWARA M , BRAATZ R D . Modelling and control of combined cooling and antisolvent crystallization processes[J]. Journal of Process Control, 2008, 18(9): 856-864. |
| 41 | RAWLINGS J B , MILLER S M , WITKOWSKI W R , et al . Model identification and control of solution crystallization processes: a review[J]. Industrial & Engineering Chemistry Research, 1993, 32(7): 1275-1296. |
| 42 | YANG Y , NAGY Z K . Model-based systematic design and analysis approach for unseeded combined cooling and antisolvent crystallization (CCAC) systems[J]. Crystal Growth & Design, 2014, 14(2): 687-698. |
| 43 | RIDDER B J , MAJUMDER A , NAGY Z K , et al . Population balance model-based multiobjective optimization of a multisegment multiaddition (MSMA) continuous plug-flow antisolvent crystallizer [J]. Industrial & Engineering Chemistry Research, 2014, 53: 4387-4397. |
| 44 | GYULAI O , KOVACS A , SOVANY T , et al . Optimization of the critical parameters of the spherical agglomeration crystallization method by the application of the quality by design approach[J]. Materials (Basel), 2018, 11(4): 1-16. |
| 45 | WANG J , LAKERVELD R . Integrated solvent and process design for continuous crystallization and solvent recycling using PC-SAFT[J]. AIChE Journal, 2018, 64(4): 1205-1216. |
| 46 | PATHAK P , MEZIANI M J , DESAI T , et al . Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing[J]. The Journal of Supercritical Fluids, 2006, 37(3): 279-286. |
| 47 | ESFANDIARI N . Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide[J]. The Journal of Supercritical Fluids, 2015, 100: 129-141. |
| 48 | FONTANA F , FIGUEIREDO P , ZHANG P , et al . Production of pure drug nanocrystals and nano co-crystals by confinement methods[J]. Advanced Drug Delivery Reviews, 2018, 131: 3-21. |
| 49 | JUNG J , PERRUT M . Particle design using supercritical fluids: literature and patent survey[J]. The Journal of Supercritical Fluids, 2001, 20(3): 179-219. |
| 50 | STECKEL H , PICHERT L , MULLER B W . Influence of process parameters in the ASES process on particle properties of budesonide for pulmonary delivery[J]. European Journal of Pharmaceutical and Biopharmaceutical, 2004, 57(3): 507-512. |
| 51 | SINHA B , MULLER R H , MOSCHWITZER J P , et al . Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size[J]. International Journal of Pharmaceutics, 2013, 453(1): 126-141. |
| 52 | CIOU J , WANG B , SU C , et al . Measurement of solid solubility of warfarin in supercritical carbon dioxide and recrystallization study using supercritical antisolvent process[J]. Advanced Powder Technology, 2018, 29(3): 479-487. |
| 53 | MANNA L , BANCHERO M . Solubility of tolbutamide and chlorpropamide in supercritical carbon dioxide[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1745-1751. |
| 54 | CAMPARDELLI R , REVERCHON E , MARCO I DE , et al . Dependence of SAS particle morphologies on the ternary phase equilibria[J]. The Journal of Supercritical Fluids, 2017, 130: 273-281. |
| 55 | ESFANDIARI N , GHOREISHI S M . Optimal thermodynamic conditions for ternary system[J]. Journal of the Taiwan Institute of Chemical Engineers, 2015, 50: 31-36. |
| 56 | VATANARA A , ROUHOLAMINI NAJAFABADI A , GILANI K , et al . A Plackett-Burman design for screening of the operation variables in the formation of salbutamol sulphate particles by supercritical antisolvent[J]. The Journal of Supercritical Fluids, 2007, 40(1): 111-116. |
| 57 | MIGUEL F , MARTÍN A , MATTEA F , et al . Precipitation of lutein and co-precipitation of lutein and poly-lactic acid with the supercritical anti-solvent process[J]. Chemical Engineering and Processing, 2008, 47(9): 1594-1602. |
| 58 | PARK S , YEO S . Recrystallization of caffeine using gas antisolvent process[J]. The Journal of Supercritical Fluids, 2008, 47(1): 85-92. |
| 59 | JAFARI D , NOWEE S M , NOIE S H , et al . A kinetic modeling of particle formation by gas antisolvent process: precipitation of aspirin[J]. Journal of Dispersion Science and Technology, 2017, 5(38): 677-685. |
| 60 | ESFANDIARI N , GHOREISHI S M . Kinetic modeling of the gas antisolvent[J]. Chemical Engineering & Technology, 2014, 1(37): 73-80. |
| 61 | ESFANDIARI N , GHOREISHI S M . Kinetics modeling of ampicillin nanoparticles synthesis via supercritical gas antisolvent process[J]. The Journal of Supercritical Fluids, 2013, 81: 119-127. |
| 62 | KAWASHIMA Y , NIWA T , HANDA T , et al . Preparation of controlled-release microspheres of ibuprofen with acrylic polymers by a novel quasi-emulsion solvent diffusion method[J]. Journal of Pharmaceutical Sciences, 1989, 78(1): 68-72. |
| 63 | KOVA I B , VRE ER F, PLANIN EK O , et al . Spherical crystallization of drugs[M]//Advanced topics in crystallization. Yitzhak Mastai, Croatia: IntechOpen, 2012: 1-14. |
| 64 | TAHARA K , MAHONY M O , MYERSON A S , et al . Continuous spherical crystallization of albuterol sulfate with solvent recycle system[J]. Crystal Growth & Design, 2015, 15(10): 5149-5156. |
| 65 | JITKAR S , THIPPARABOINA R , CHAVAN R B , et al . Spherical agglomeration of platy crystals: curious case of etodolac[J]. Crystal Growth & Design, 2016, 16(7): 4034-4042. |
| 66 | ZHOU X , ZHANG Q , XU R , et al . A novel spherulitic self-assembly strategy for organic explosives: modifying the hydrogen bonds by polymeric additives in emulsion crystallization[J]. Crystal Growth & Design, 2018, 18(4): 2417-2423. |
| [1] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [4] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [5] | 刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103. |
| [6] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [7] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [8] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [9] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [10] | 曾天续, 张永显, 严渊, 刘宏, 马娇, 党鸿钟, 吴新波, 李维维, 陈永志. 羟胺对硝化菌活性及其动力学参数的影响[J]. 化工进展, 2023, 42(6): 3272-3280. |
| [11] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [12] | 马润梅, 杨海超, 李正大, 李双喜, 赵祥, 章国庆. 表面强化镀层对高速轴承腔密封端面变形及摩擦磨损影响分析[J]. 化工进展, 2023, 42(4): 1688-1697. |
| [13] | 张成松, 张静, 龚斌, 李明洋, 袁佳新, 李宏业. 自吸射流柔性搅拌桨振动特性[J]. 化工进展, 2023, 42(4): 1728-1738. |
| [14] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [15] | 贺山明, 潘界昌, 徐国钻, 李文君, 梁勇. 粗钨酸钠溶液亚铁盐沉淀法除铬、钒的热力学分析及实验验证[J]. 化工进展, 2023, 42(4): 2171-2179. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||