化工进展 ›› 2019, Vol. 38 ›› Issue (05): 2212-2221.DOI: 10.16085/j.issn.1000-6613.2018-1311
用于质子交换膜燃料电池的高温无机质子传导材料研究进展
- 厦门大学能源学院,福建 厦门 361102
-
收稿日期:2018-06-25修回日期:2018-12-06出版日期:2019-05-05发布日期:2019-05-05 -
通讯作者:刘运权 -
作者简介:<named-content content-type="corresp-name">王颖锋</named-content>(1994—),男,硕士研究生,研究方向为燃料电池。E-mail: <email>wangyingfeng666@163.com</email>。|刘运权,教授,博士生导师,研究方向为制氢与燃料电池。E-mail:<email>yq_liu@xmu.edu.cn</email>。 -
基金资助:福建省科技厅重点项目(2016H6024)
Progress in high temperature inorganic proton conduction materials used for proton exchange membrane fuel cells
Yingfeng WANG( ),Kai LI,Shuirong LI,Duo WANG,Yueyuan YE,Yunquan LIU(
),Kai LI,Shuirong LI,Duo WANG,Yueyuan YE,Yunquan LIU( )
)
- College of Energy, Xiamen University, Xiamen 361102, Fujian, China
-
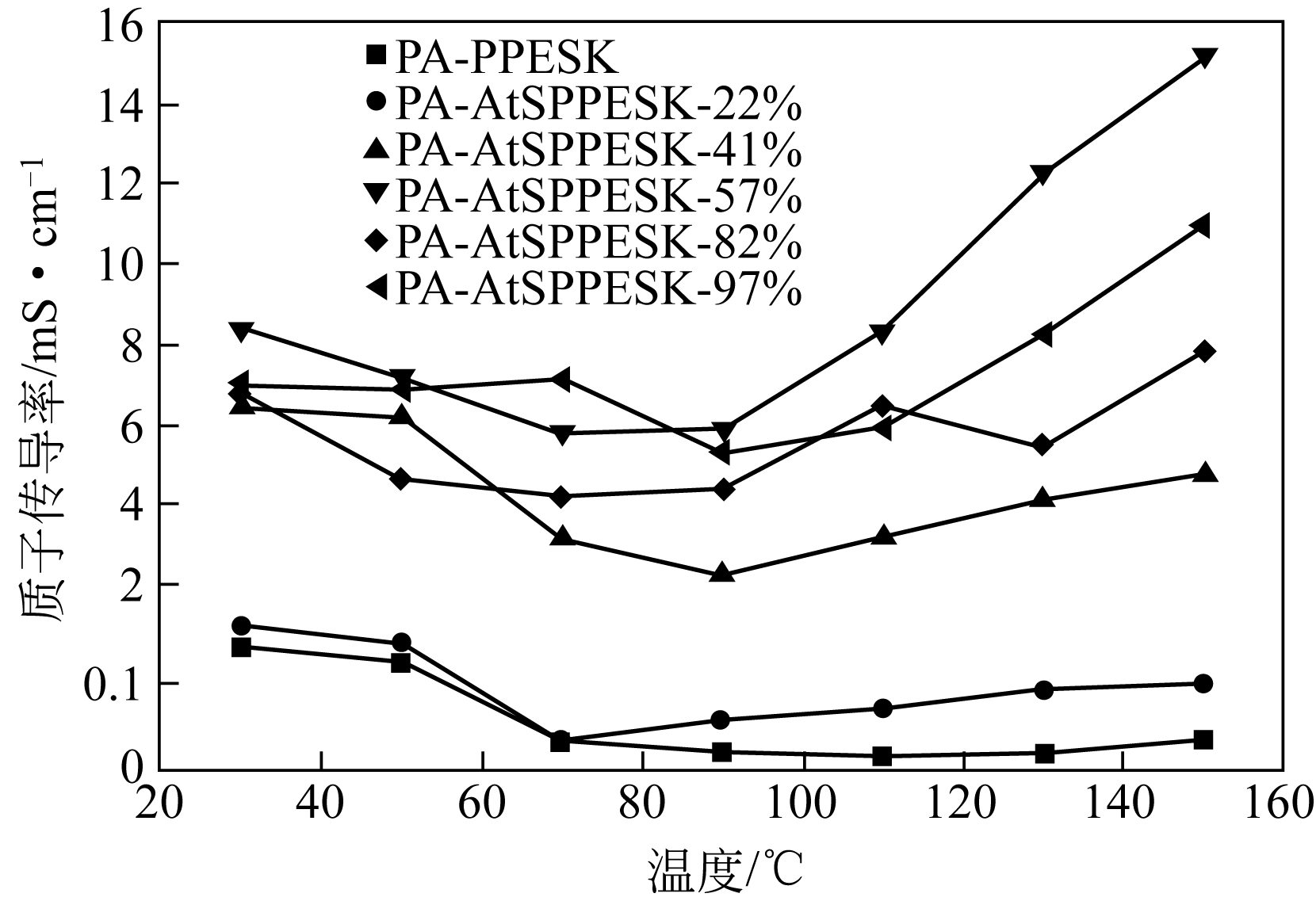
Received:2018-06-25Revised:2018-12-06Online:2019-05-05Published:2019-05-05 -
Contact:Yunquan LIU
摘要:
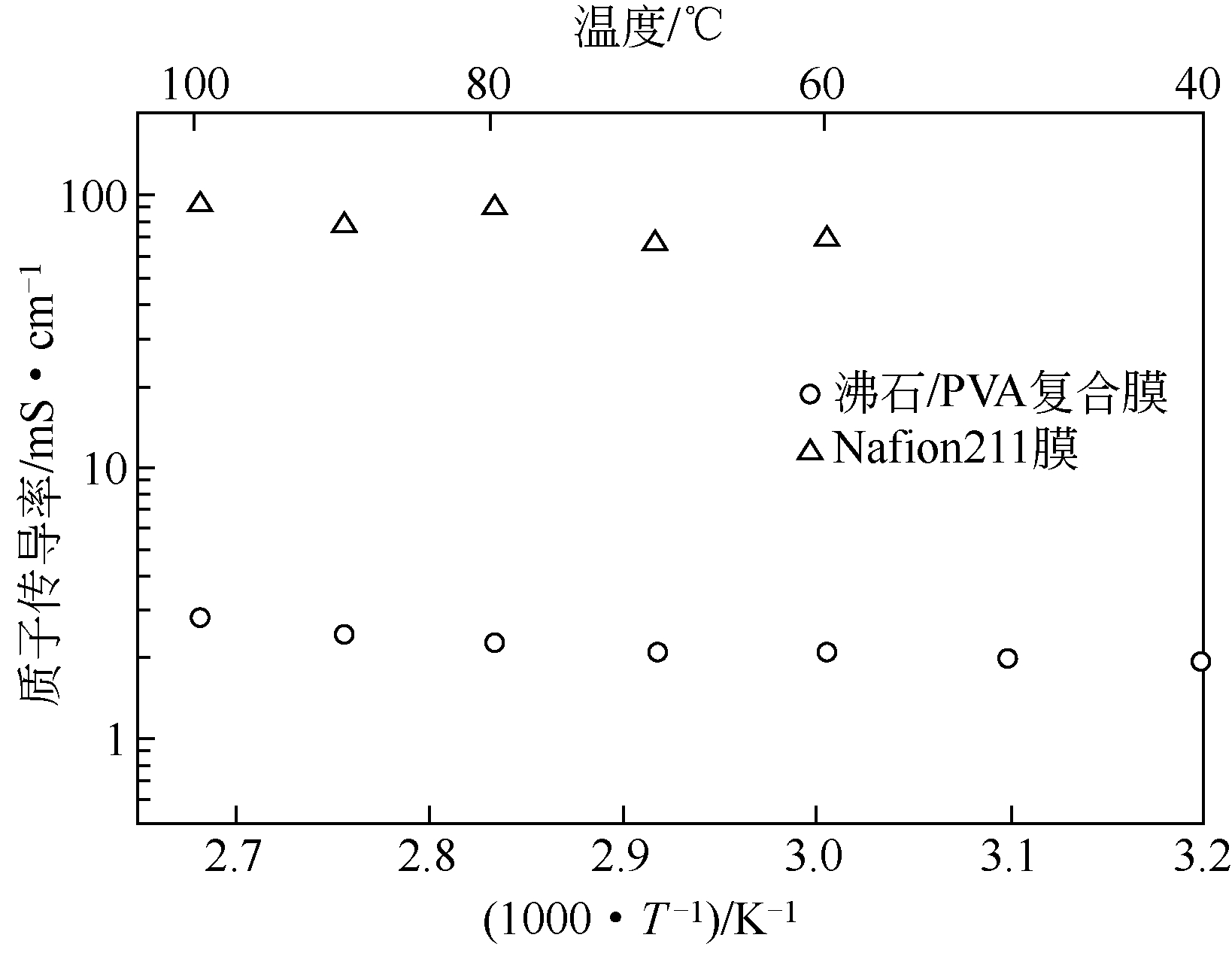
质子交换膜是质子交换膜燃料电池(PEMFC)的核心部件,其主要作用是传导质子。无机质子传导材料作为一种新型的质子传导介质,近年来逐渐引起了人们的关注。本文主要介绍了小分子磷酸、无机沸石材料、固体酸和无机氧化物陶瓷材料等几种高温无机质子传导材料,并对它们的性能和特点进行了评述。主要结论如下:小分子磷酸质子传导率高,但是容易泄露;无机沸石材料化学稳定性好,但质子传导率尚有提高的空间;无机氧化物陶瓷材料力学性能和化学温度性能均很好,但质子传导率相对较低;固体酸质子传导率优异,高温稳定性也好,是最有希望在PEMFC中获得推广应用的材料。
中图分类号:
引用本文
王颖锋, 李凯, 李水荣, 王夺, 叶跃元, 刘运权. 用于质子交换膜燃料电池的高温无机质子传导材料研究进展[J]. 化工进展, 2019, 38(05): 2212-2221.
Yingfeng WANG, Kai LI, Shuirong LI, Duo WANG, Yueyuan YE, Yunquan LIU. Progress in high temperature inorganic proton conduction materials used for proton exchange membrane fuel cells[J]. Chemical Industry and Engineering Progress, 2019, 38(05): 2212-2221.
| 膜类型 | 拉伸强度/MPa | 断裂拉伸率/% |
|---|---|---|
| PBI | 133±7.09 | 65±17 |
| PBI/DBpX | 122±11.5 | 79±17 |
| PBI/BADGE | 114±2.25 | 26±13 |
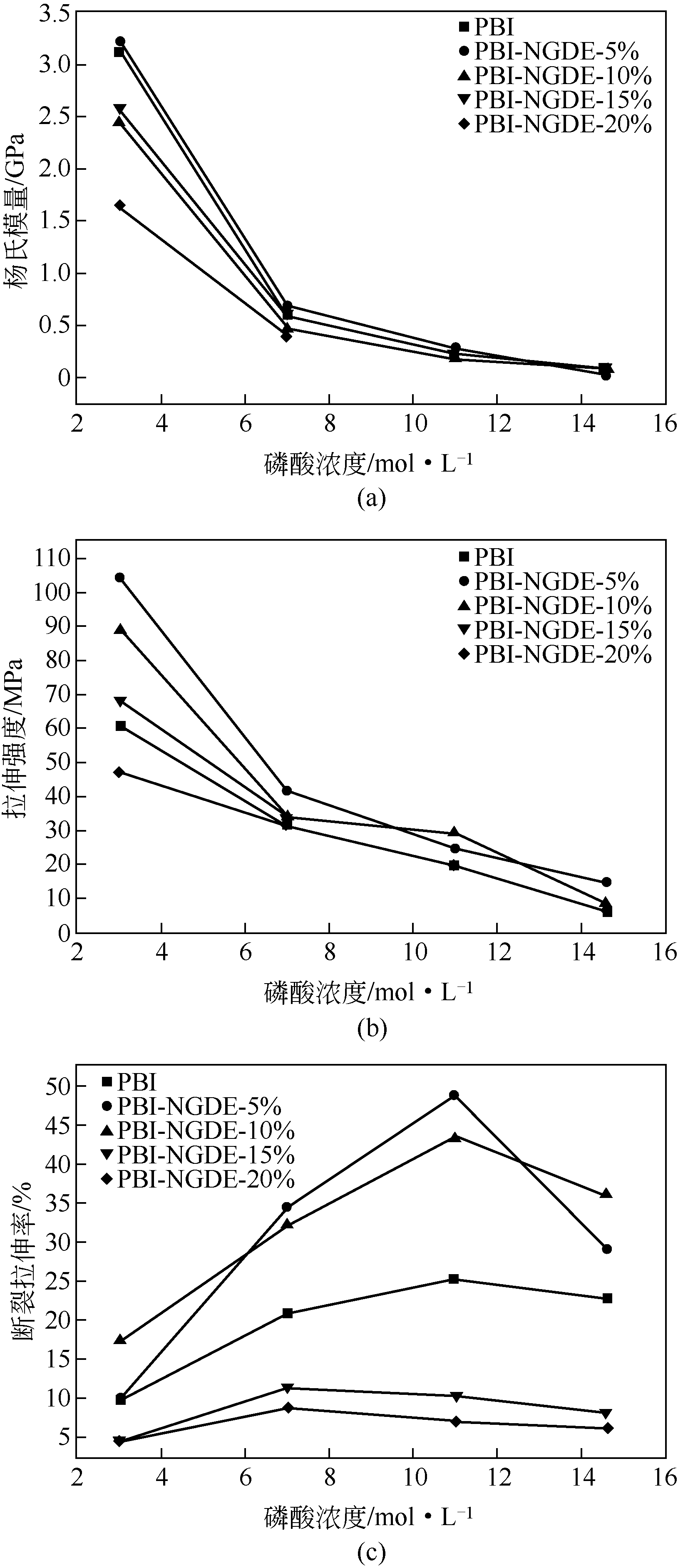
表1 PBI、PBI/DBpX和PBI/BADGE膜力学性能测试结果
| 膜类型 | 拉伸强度/MPa | 断裂拉伸率/% |
|---|---|---|
| PBI | 133±7.09 | 65±17 |
| PBI/DBpX | 122±11.5 | 79±17 |
| PBI/BADGE | 114±2.25 | 26±13 |
| 膜类型 | 质子传导率/S·cm-1 | 酸泄露率/% | 酸掺杂/% | 激活能量/kJ·mol-1 | ||
|---|---|---|---|---|---|---|
| 140℃ | 165℃ | 180℃ | ||||
| PBI | 0.0794 | 0.1030 | 0.1439 | 85 | 13.9±0.4 | 26 |
| PBI/BADGE | 0.0541 | 0.0590 | 0.0666 | 73 | 10.3±0.2 | 11 |
| PBI/EGDE | 0.0468 | 0.0682 | 0.0972 | 80 | 10.2±0.3 | 32 |
| PBI/TPA | 0.0631 | 0.0891 | 0.1172 | 82 | 12.7±0.5 | 28 |
| PBI/DBpX | 0.0711 | 0.1009 | 0.1513 | 80 | 15±0.4 | 32 |
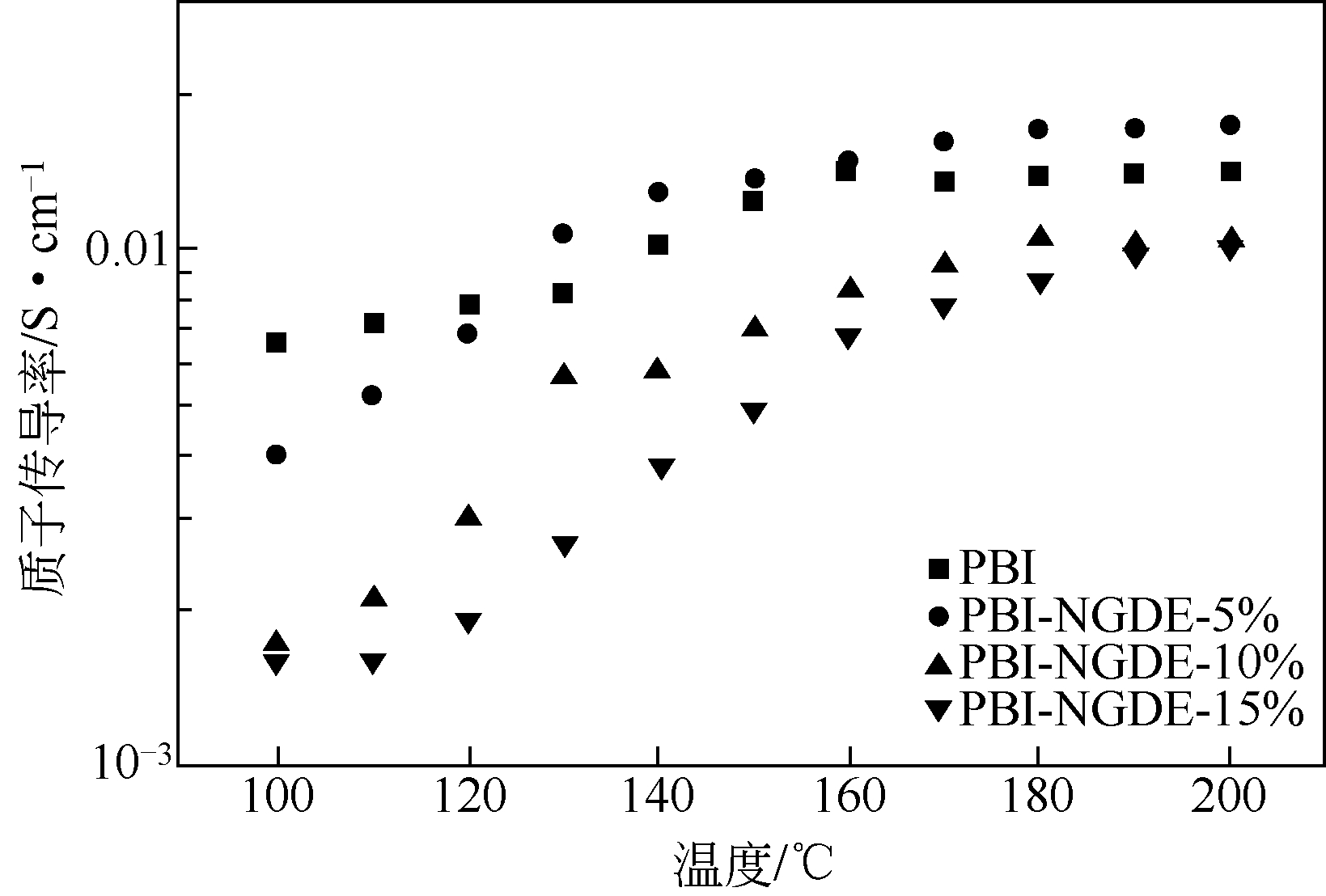
表2 PBI、PBI/BADGE、PBI/EGDE、PBI/TPA和PBI/DBpX膜的质子传导率、磷酸流失率、磷酸掺杂率和活化能
| 膜类型 | 质子传导率/S·cm-1 | 酸泄露率/% | 酸掺杂/% | 激活能量/kJ·mol-1 | ||
|---|---|---|---|---|---|---|
| 140℃ | 165℃ | 180℃ | ||||
| PBI | 0.0794 | 0.1030 | 0.1439 | 85 | 13.9±0.4 | 26 |
| PBI/BADGE | 0.0541 | 0.0590 | 0.0666 | 73 | 10.3±0.2 | 11 |
| PBI/EGDE | 0.0468 | 0.0682 | 0.0972 | 80 | 10.2±0.3 | 32 |
| PBI/TPA | 0.0631 | 0.0891 | 0.1172 | 82 | 12.7±0.5 | 28 |
| PBI/DBpX | 0.0711 | 0.1009 | 0.1513 | 80 | 15±0.4 | 32 |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试温度/℃ | |
|---|---|---|---|---|---|
| PBI/DBpX[ | 0.1513 | 180℃ | 0.123 | 165 | |
| PA-doped PESB[ | 0.0730 | 160℃ | 0.427 | 160 | |
| PA-doped PBI[ | 0.1230 | 室温下,100%相对湿度 | N/A | N/A | |
| Phosphoric acid doped MTZPAEK[ | 0.0510 | 190℃ | 0.061 | 160 | |
表3 近几年有关PA掺杂PBI的一些研究进展情况
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试温度/℃ | |
|---|---|---|---|---|---|
| PBI/DBpX[ | 0.1513 | 180℃ | 0.123 | 165 | |
| PA-doped PESB[ | 0.0730 | 160℃ | 0.427 | 160 | |
| PA-doped PBI[ | 0.1230 | 室温下,100%相对湿度 | N/A | N/A | |
| Phosphoric acid doped MTZPAEK[ | 0.0510 | 190℃ | 0.061 | 160 | |
| 样品 | 厚度/nm | 拉伸强度/MPa | 延伸率/% | 杨氏模量/MPa |
|---|---|---|---|---|
| ANA/NF | 0.15 | 18.29±0.54 | 304.67±22.01 | 279.17±7.32 |
| MOR/NF | 0.18 | 16.60±0.32 | 225.00±17.44 | 311.57±2.94 |
| NF | 0.15 | 21.52±0.83 | 205.00±25.15 | 276.81±5.05 |
| NF117 | 0.18 | 26.65±1.22 | 359.33±34.21 | 236.42±7.66 |
表4 喷雾法制备的复合膜的力学性能
| 样品 | 厚度/nm | 拉伸强度/MPa | 延伸率/% | 杨氏模量/MPa |
|---|---|---|---|---|
| ANA/NF | 0.15 | 18.29±0.54 | 304.67±22.01 | 279.17±7.32 |
| MOR/NF | 0.18 | 16.60±0.32 | 225.00±17.44 | 311.57±2.94 |
| NF | 0.15 | 21.52±0.83 | 205.00±25.15 | 276.81±5.05 |
| NF117 | 0.18 | 26.65±1.22 | 359.33±34.21 | 236.42±7.66 |
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试条件 |
|---|---|---|---|---|
| 磺化介孔硅材料(S-MCM-41)[ | 6.94×10-3 | N/A | N/A | |
| SPVA-MOR[ | 5.20×10-2 | 80℃下100% 相对湿度 | 3.5×10-3 | 25℃/甲醇浓度1mol?L-1 |
| Tri@MS-PrNH2-1[ | 8.34×10-3 | 120℃ | N/A | N/A |
| MCM-48[ | 4.80×10-3 | 140℃ | N/A | N/A |
表5 近几年来无机沸石材料的应用情况
| 材料类型 | 质子传导率/S·cm-1 | 测试条件 | 最大功率密度/W·cm-2 | 测试条件 |
|---|---|---|---|---|
| 磺化介孔硅材料(S-MCM-41)[ | 6.94×10-3 | N/A | N/A | |
| SPVA-MOR[ | 5.20×10-2 | 80℃下100% 相对湿度 | 3.5×10-3 | 25℃/甲醇浓度1mol?L-1 |
| Tri@MS-PrNH2-1[ | 8.34×10-3 | 120℃ | N/A | N/A |
| MCM-48[ | 4.80×10-3 | 140℃ | N/A | N/A |
| 膜类型 | 25℃下质量 损失率/% | 80℃下质量 损失率/% |
|---|---|---|
| SPEEK | 1.68 | 2.38 |
| SPEEK/HPW | 2.07 | 28.93 |
| SPEEK/HPW-PANI-1 | 2.00 | 28.61 |
| SPEEK/HPW-PANI-2 | 1.94 | 28.41 |
| SPEEK/HPW-PANI-3 | 1.73 | 27.51 |
| SPEEK/HPW-PANI-4 | 1.55 | 21.45 |
| SPEEK/HPW-PANI-5 | 1.29 | 19.84 |
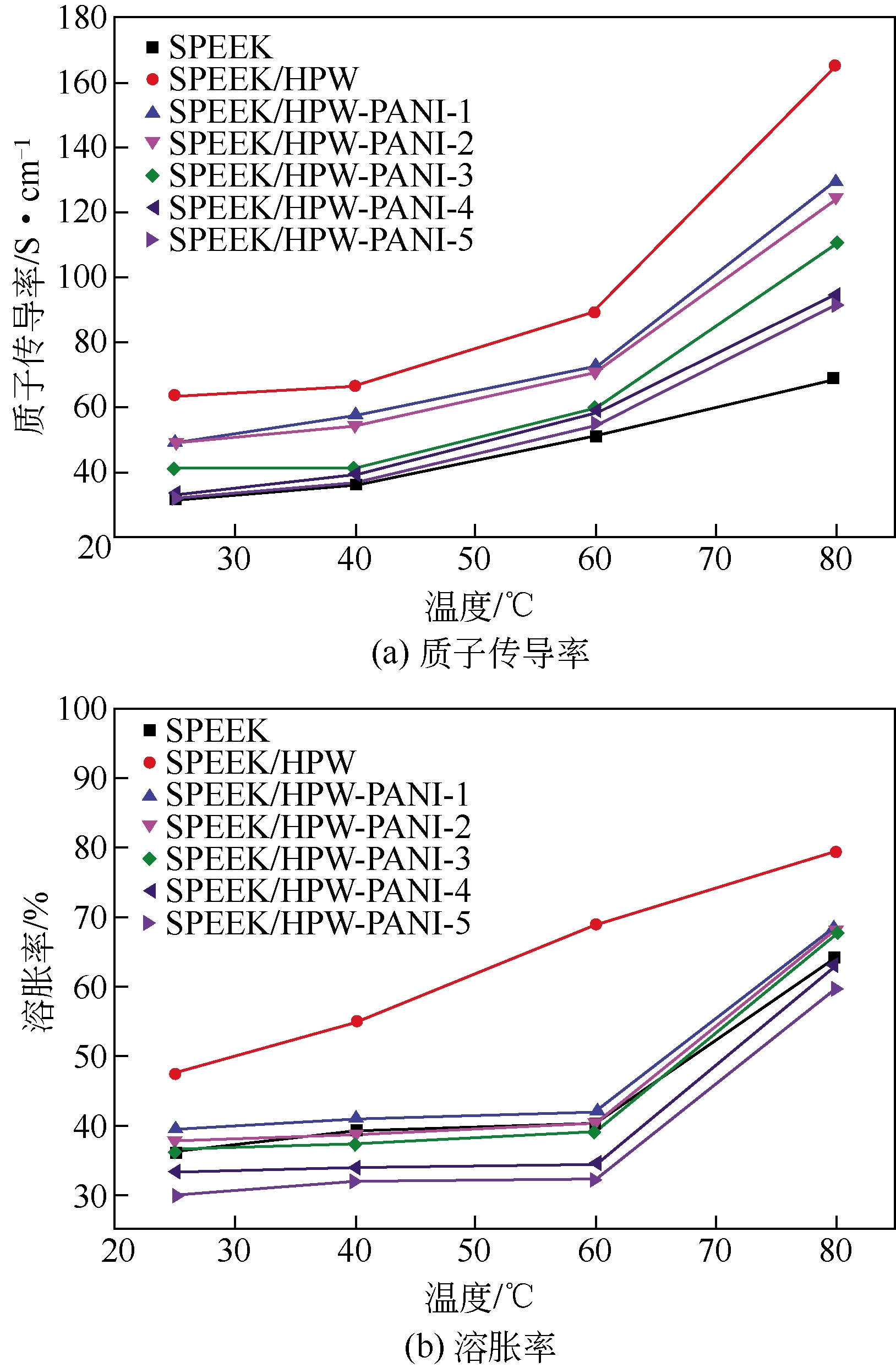
表6 SPEEK、SPEEK/HPW和SPEEK/HPW-PANI两种膜泡在不同温度水中,20天后的质量损失率
| 膜类型 | 25℃下质量 损失率/% | 80℃下质量 损失率/% |
|---|---|---|
| SPEEK | 1.68 | 2.38 |
| SPEEK/HPW | 2.07 | 28.93 |
| SPEEK/HPW-PANI-1 | 2.00 | 28.61 |
| SPEEK/HPW-PANI-2 | 1.94 | 28.41 |
| SPEEK/HPW-PANI-3 | 1.73 | 27.51 |
| SPEEK/HPW-PANI-4 | 1.55 | 21.45 |
| SPEEK/HPW-PANI-5 | 1.29 | 19.84 |
| 材料类型 | 质子传导率/ S·cm-1 | 测试条件 | 最大功率密度/ W·cm-2 | 测试条件 |
|---|---|---|---|---|
| SPEEK/HPW@MIL101[ | 0.2720 | 65℃,相对湿度100% | 0.383 | 相对湿度100%,60℃ |
| SPEEK/HPW/g-C3N4 [ | 0.2490 | 浸泡在60℃的水中48h | N/A | N/A |
| HPW/meso-SiO2/CSPEEK[ | 0.0019 | 相对湿度30%,120℃ | N/A | N/A |
| smpCTS/HPW[ | 0.0290 | 在80℃下 | 0.016 | 80℃/甲醇浓度2mol/L |
表7 近3年关于掺杂杂多酸无机材料膜的一些情况
| 材料类型 | 质子传导率/ S·cm-1 | 测试条件 | 最大功率密度/ W·cm-2 | 测试条件 |
|---|---|---|---|---|
| SPEEK/HPW@MIL101[ | 0.2720 | 65℃,相对湿度100% | 0.383 | 相对湿度100%,60℃ |
| SPEEK/HPW/g-C3N4 [ | 0.2490 | 浸泡在60℃的水中48h | N/A | N/A |
| HPW/meso-SiO2/CSPEEK[ | 0.0019 | 相对湿度30%,120℃ | N/A | N/A |
| smpCTS/HPW[ | 0.0290 | 在80℃下 | 0.016 | 80℃/甲醇浓度2mol/L |
| 1 | AHADI M , TAM M, STUMPER J , et al . Thermal conductivity of catalyst layer of polymer electrolyte membrane fuel cells: part 1-experimental study[J]. J. Power Sources, 2017, 354: 207-214. |
| 2 | CHANG Guanjun , SHANG Zhenfang , YANG Li . Hydrogen bond cross-linked sulfonated poly(iminoether ether ketone) (PIEEK) for fuel cell membranes[J]. J. Power Sources,2015,282:401-408. |
| 3 | ARAYA S S , ZHOU F , LISO V , et al . A comprehensive review of PBI-based high temperature PEM fuel cells[J]. Int. J. Hydrogen. Energy, 2016, 41:21310-21344. |
| 4 | DANG Jingchuan , ZHAO Liping , ZHANG Jie , et al . Imidazole microcapsules toward enhanced phosphoric acid loading of polymer electrolyte membrane for anhydrous proton conduction[J]. J. Membrane Science,2018,545:88-89. |
| 5 | MELCHIOR J P , MAJER G , KREUER K D .Why do proton conducting polybenzimidazole phosphoric acid membranes perform well inhigh-temperature PEM fuel cells?[J]. Phys. Chem. Chem.Phys.,2017,19:601-612. |
| 6 | DIPPEL T , KREUER K D , LASSÈGUES J C , et al . Proton conductivity in fused phosphoric acid; A1H/31P PFG-NMR and QNS study[J]. Solid State Ionics,1993,61:41-46. |
| 7 | VILCIAUSKAS L , TUCKERMAN M E , BESTER G , et al . The mechanism of proton conduction in phosphoric acid[J]. Nat. Chem., 2012,4:461-466. |
| 8 | LI Qingfeng , David AILI , HJULER Hans Aage, et al . High temperature polymer electrolyte membrane fuel cells[M]. Switzerland: Springer Cham., 2016:169-215. |
| 9 | YUAN Sen , GUO Xiaoxia , AILI D , et al . Poly(imide benzimidazole)s for high temperature polymer electrolyte membrane fuel cells[J]. J. Membranes Science, 2014, 454(59):351-358. |
| 10 | PAN Jiefeng , XU Tongwen , WU liang , et al . Proton exchange membrane from tetrazole-based poly(phthalazinone ether sulfone ketone) for high-temperature fuel cells[J]. International Journal of Hydrogen Energy,2016,41(28):12337-12346. |
| 11 | WANG Shuang , ZHANG Gang , NA Hui ,et al . Novel epoxy-based cross-linked polybenzimidazole for high temperature proton exchange membrane fuel cells[J]. Int.J. Hydrogen Energy, 2011,36(14):8412-8421. |
| 12 | CHE Quantong , YUE Jie . Polymerized imidazolium ionic liquids crosslinking sulfonated poly(ether ether ketone)(SPEEK) for high-temperature proton exchange membrane[J]. RSC Advances,2016,6(113):111729-111738. |
| 13 | OZDEMIR Y , OZKAN N , DEVRIM Y . Fabrication and characterization of cross-linked polybenzimidazole based membranes for high temperature pem fuel cells[J]. Electrochimica Acta,2017,245:1-13. |
| 14 | WANG Kaili , CHANG Guanjun , YANG Li , et al . Phosphoric acid-doped poly(ether sulfone benzotriazole) for high-temperature proton exchange membrane fuel cell applications[J]. Journal of Membrane Science,2018,549(74):23-27. |
| 15 | JAHANGIRI S , ARAVI I , OZDEN-YENIGUN E , et al . Fabrication and optimization of proton conductive polybenzimidazole electrospun nanofiber membranes[J]. Polym. Adv. Technol.,2018,29(1):594-602. |
| 16 | ZHAO Chengji , BU Fanzhe , ZHANG Yurong , et al . 1,2,4-Triazole functionalized poly(arylene ether ketone) for high temperature proton exchange membrane with enhanced oxidative stability[J]. Journal of Membrane Science,2018,545(43):167-175. |
| 17 | YOON M , KIM K, NATARAJAN S , et al . Proton conduction in metal-organic frameworks and related modularly built porous solids[J]. Angew. Chem. Int. Ed.,2013,52(10):2688-2700. |
| 18 | XU Feng , MU Shichun . Nanoceramic oxide hybrid electrolyte membranes for proton exchange membrane fuel cells[J]. Journal of Nanoscience and Nanotechnology,2014,14(2):1169-1180. |
| 19 | SHENDEROVICH I G , BUNTKOWSKY G , SCHREIBER A , et al . Pyridine-15N-A mobile NMR sensor for surface acidity and surface defects of mesoporous silica[J]. J.Phys.Chem.B, 2003,107(43):11924-11939. |
| 20 | FRANKE M E , SIMON U . Solvate-supported proton transport in zeolites[J]. Chem. Phys. Chem.,2004,5(4):465-472. |
| 21 | ARICO A S , ANTONUCCI P L , GIORDANO N , et al . Ionic conductivity in heteropolyacid-tin mordenite composite electrolytes[J]. Materials Letters, 1995,24(6):399-405. |
| 22 | BORDUIN R , LI W . Fabrication of foamed polyethersulfone-zeolite mixed matrix membranes for polyethsulfone-zeolite mixed marixmembranes for polymer electrolyte membrane fuel cell humidification[J]. Journal of Manufactuing Science and Engineering-Transactions of the Asme,2017,139:021004(1-7). |
| 23 | NISHIHARA M , TERAYAMA Y , HAJI T , et al . Proton-conductive nano zeolite-PVA composite film as a new water-absorbing electrolyte for water electrolysis[J]. Express Polymer Letters ,2018,12(3):256-264. |
| 24 | DATRINDADE L G , PEREIRA E C . SPEEK/zeolite/ionic-liquid anhydrous polymer membranes for fuel-cell applications[J]. Eur. J. Inor. Chem, 2017,17(17):2369-2376. |
| 25 | PRAPAINAINAR P , DU Z H , KONGKACHUICHAY P , et al . Mordenite/nafion and analcime/nafion composite membranes prepared by spray method for improved direct methanol fuel cell performance[J]. Applied Surface Science,2017,421(Part A):24-41. |
| 26 | GAHLOT S , SHARMA P P , KULSHRESTHA V , et al . Nanoporous composite proton exchange membranes: high conductivity and thermal stability[J]. Colloids and Surfaces A,2018,542:8-14. |
| 27 | UCTUG F G , NIJEM J . Effect of polymer sulfonation on the proton conductivity and fuel cell performance of polyvinylalcohol-mordenite direct methanol fuel cell membranes[J]. Asia-Pac. J.Chem. Eng.,2017,12(5):682-693. |
| 28 | WU Zhenzhen , LI Juan , ZHANG Xianming . Heterogeneous hybrid of propyl amino functionalized MCM-41 and 1H-1,2,4-triazole for high efficient intermediate temperature proton conductor[J]. RSC Advances,2017,7(82):52321-52326. |
| 29 | DVOYASHKINA N , WARK M , FREUDE D , et al . Proton mobility in sulfonic acid functionalized mesoporous materials studied by MAS PFG NMR diffusometry and impedance spectroscopy[J]. Microporous and Mesoporous Materials,2018,255:140-147. |
| 30 | Mohammad NORSYAHIDA , Bakar Mohamad ABU , Amir H Kadhum ABDUL , et al . A review on synthesis and characterization of solid acid materials for fuel cell applications[J]. Journal of Power Sources,2016,322:77-92. |
| 31 | A Boysen DANE , Uda TETSUYA , R I Chisholm CALUM , et al . High-performance solid acid fuel cells through humidity stabilization[J]. Science,2004,303(5654):68-70. |
| 32 | SOSSINA M H , DANE A B , CALUM R I C , et al . Solid acids as fuel cell electrolytes[J]. Nature,2001,410(6831):910-913. |
| 33 | OH S Y, KAWAMURA G , MUTO H , et al . Anhydrous protic conduction of mechanochemically synthesized CsHSO4-azole-derived composites[J]. Electrochimica Acta,2012,75:11-19. |
| 34 | DANG Dai , ZHAO Bote , CHEN Dongchang , et al . A durable polyvinyl butyral-CsH2PO4 composite electrolyte for solid acid fuel cells[J]. Journal of Power Sources,2017,359:1-6. |
| 35 | CHAI Zhanli , SUO Quanyu , WANG Hui , et al . Mesoporous lanthanum phosphate nanostructurescontaining H3PO4 as superior electrolyte for PEM fuelcells[J]. RSC Advances, 2013,3(44):21928-21935. |
| 36 | NAKAMURA O , KODAMA T , OGINO I , et al . High-conductivity solid proton conductors: dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals[J]. Chemistry Letters,1979,8(1):17-18. |
| 37 | 孙偲祎,魏梅林 .基于杂多酸和菲咯啉/联喹啉类金属配合物的复合物的结构及性能研究[D].新乡:河南师范大学,2017. |
| SUN Siwei , WEI Meilin . Structures and properties of the composites based on heteropoly acids and phenanthroline/biquinoline metal coordination compounds[D]. Xinxiang:Henan Normal University,2017. | |
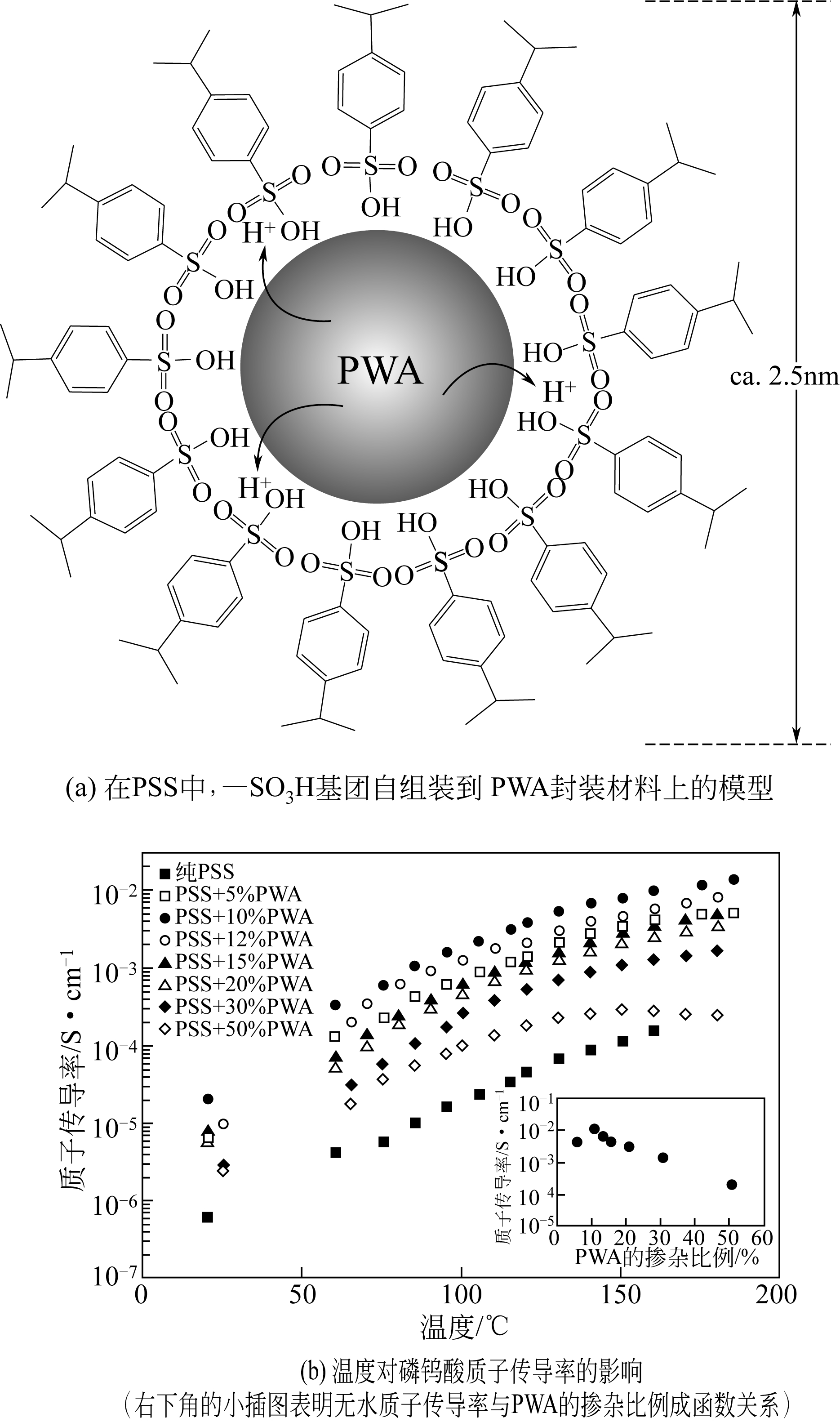
| 38 | YAMADA M , HONMA I . Heteropolyacid-encapsulated self-assembled materials for anhydrous proton-conducting electrolytes[J]. J.Phys.Chem.B, 2006,110(41):20486-20490. |
| 39 | LU Shanfu , XU Xin , XIANG Yan , et al . A self-anchored phosphotungstic acid hybrid proton exchange membrane achieved via one-step synthesis[J]. Adv. Energy Mater.,2014,4(17):1400842. |
| 40 | BOSE A B , GOPU S , LI W . Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes[J]. Journal of Power Source,2014,263:217-222. |
| 41 | REN Suzhen , XU Meiling , HAO Ce , et al . Effects of microstructural functional polyaniline layers on SPEEK/HPW proton exchange membranes[J]. Journal of Applied Polymer Science,2014,131:41033(1-8). |
| 42 | ZHANG Bei , CAO Ying , WU Hong , et al . Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications[J]. Electrochimica Acta,2017,240:186-194. |
| 43 | ZHOU Qiong , DONG Cuicui , WANG Qian , et al . Influence of alkaline 2D carbon nitride nanosheets as fillers for anchoring HPW and improving conductivity of SPEEK nanocomposite membranes[J]. Int.J. of Hydrogen Energy,2017,42:10317-10328. |
| 44 | SONG J-M , H-S WOO ,SOHNJ-Y, et al . 12HPW/meso-SiO2 nanocomposite CSPEEK membranes for proton exchange membrane fuel cells[J]. Journal of Industrial and Engineering Chemistry,2016,36263:132-138. |
| 45 | LU Shanfu , WU Qiuxia , WANG Haining , et al . Novel methanol-blocking proton exchange membrane achieved via self-anchoring phosphotungstic acid into chitosan membrane with submicro-pores[J]. Journal of Membrane Science,2016,500:203-210. |
| 46 | 伍志鲲 .铌酸催化剂在有机合成中的应用[D].长沙:湖南 师范大学,2001. |
| WU Zhikun . Application of niobic acids in organic synthesis [D]. Changsha:Hunan Normal University, 2001. | |
| 47 | 王一荻,臧宏瑛 .基于铌氢氧化合物复合材料的制备及其质子传导性能研究[D].长春:东北师范大学,2017. |
| WANG Yidi , ZANG Hongying . Preparation of niobium hydroxides-based composites and their proton conductivity[D]. Changchun:Northeast Normal University,2017. | |
| 48 | CHAI Zhanli , DONG Dehua , WANG Cheng , et al . Nanoporous niobium phosphate electrolyte membrane for low temperature fuel cell[J]. Journal of Membrane Science, 2010,356(1/2):147-153. |
| 49 | DUAN Chuancheng , TONG Jianhua , SHANG Meng , et al . Readily processed protonic ceramic fuel cells with high performance at low temperatures[J]. Science,2015,349(6254):1321-1326. |
| 50 | DUAN C C , KEE R J, ZHU HY , et al . Highly durable, coking and sulfur tolerant fuel-flexible protonic ceramic fuel cells[J]. Nature,2018,557(7704):217-221. |
| [1] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [2] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [3] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [4] | 马哲杰, 张文励, 赵炫凯, 李平. PEMFC阴极催化层氧传质阻力影响的研究进展[J]. 化工进展, 2023, 42(6): 2860-2873. |
| [5] | 蒋博龙, 崔艳艳, 史顺杰, 常嘉城, 姜楠, 谭伟强. 过渡金属Co3O4/ZnO-ZIF氧还原催化剂Co/Zn-ZIF模板法制备及其产电性能[J]. 化工进展, 2023, 42(6): 3066-3076. |
| [6] | 于海强, 郭泉忠, 杜克勤, 汪川. 脉冲电沉积PbO2涂层在PEMFC不锈钢双极板上的应用[J]. 化工进展, 2023, 42(2): 917-924. |
| [7] | 高帷韬, 殷屺男, 涂自强, 龚繁, 李阳, 徐宏, 王诚, 毛宗强. 金属有机框架材料中的质子传导及其在质子交换膜中的应用[J]. 化工进展, 2022, 41(S1): 260-268. |
| [8] | 胡兵, 徐立军, 何山, 苏昕, 汪继伟. 碳达峰与碳中和目标下PEM电解水制氢研究进展[J]. 化工进展, 2022, 41(9): 4595-4604. |
| [9] | 陈哲坤, 潘伟童, 姚顶松, 丁路, 王辅臣. 质子交换膜燃料电池微孔层浆液微观结构与流变性[J]. 化工进展, 2022, 41(7): 3808-3815. |
| [10] | 潘文政, 纪志永, 汪婧, 李淑明, 黄智辉, 郭小甫, 刘杰, 赵颖颖, 袁俊生. 微生物燃料电池处理偶氮含盐废水的产电性能和降解过程[J]. 化工进展, 2022, 41(6): 3306-3313. |
| [11] | 张东, 张瑞, 张彬, 安周建, 雷彻. 基于质子交换膜燃料电池的冷热电联产系统研究进展[J]. 化工进展, 2022, 41(3): 1608-1621. |
| [12] | 高帷韬, 雷一杰, 张勋, 胡晓波, 宋平平, 赵卿, 王诚, 毛宗强. 质子交换膜燃料电池研究进展[J]. 化工进展, 2022, 41(3): 1539-1555. |
| [13] | 陈诗雨, 许志成, 杨婧, 徐浩, 延卫. 微生物燃料电池在废水处理中的研究进展[J]. 化工进展, 2022, 41(2): 951-963. |
| [14] | 冯占雄, 汪云, 马强, 张创, 王诚. 连续管道微波技术制备Pt/C催化剂及其氧还原性能[J]. 化工进展, 2022, 41(12): 6377-6384. |
| [15] | 万年坊. 质子交换膜水电解制氢膜电极研究进展[J]. 化工进展, 2022, 41(12): 6385-6394. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||