化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2611-2628.DOI: 10.16085/j.issn.1000-6613.2023-2067
• 催化与材料技术 • 上一篇
光辅助直接甲醇燃料电池阳极催化剂的研究进展
方峣1,2,3( ), 刘雷2, 高志华1,2,3, 黄伟1,2,3, 左志军1,2,3(
), 刘雷2, 高志华1,2,3, 黄伟1,2,3, 左志军1,2,3( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学化学工程与技术学院,山西 太原 030024
3.太原理工大学煤科学与技术教育部重点实验室,山西 太原 030024
-
收稿日期:2023-11-28修回日期:2024-03-11出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:左志军 -
作者简介:方峣(1995—),男,博士研究生,研究方向为锂硫电池和燃料电池的理论研究。E-mail:932866749@qq.com。 -
基金资助:国家自然科学基金(22078214);山西省科技创新人才团队专项资金(202204051001009);中央引导地方科技发展资金(YDZJSX2022A013)
Advances in anode catalysts for photo-assisted direct methanol fuel cells
FANG Yao1,2,3( ), LIU Lei2, GAO Zhihua1,2,3, HUANG Wei1,2,3, ZUO Zhijun1,2,3(
), LIU Lei2, GAO Zhihua1,2,3, HUANG Wei1,2,3, ZUO Zhijun1,2,3( )
)
- 1.State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
3.Key Laboratory of Coal Science and Technology of Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
-
Received:2023-11-28Revised:2024-03-11Online:2024-05-15Published:2024-06-15 -
Contact:ZUO Zhijun
摘要:
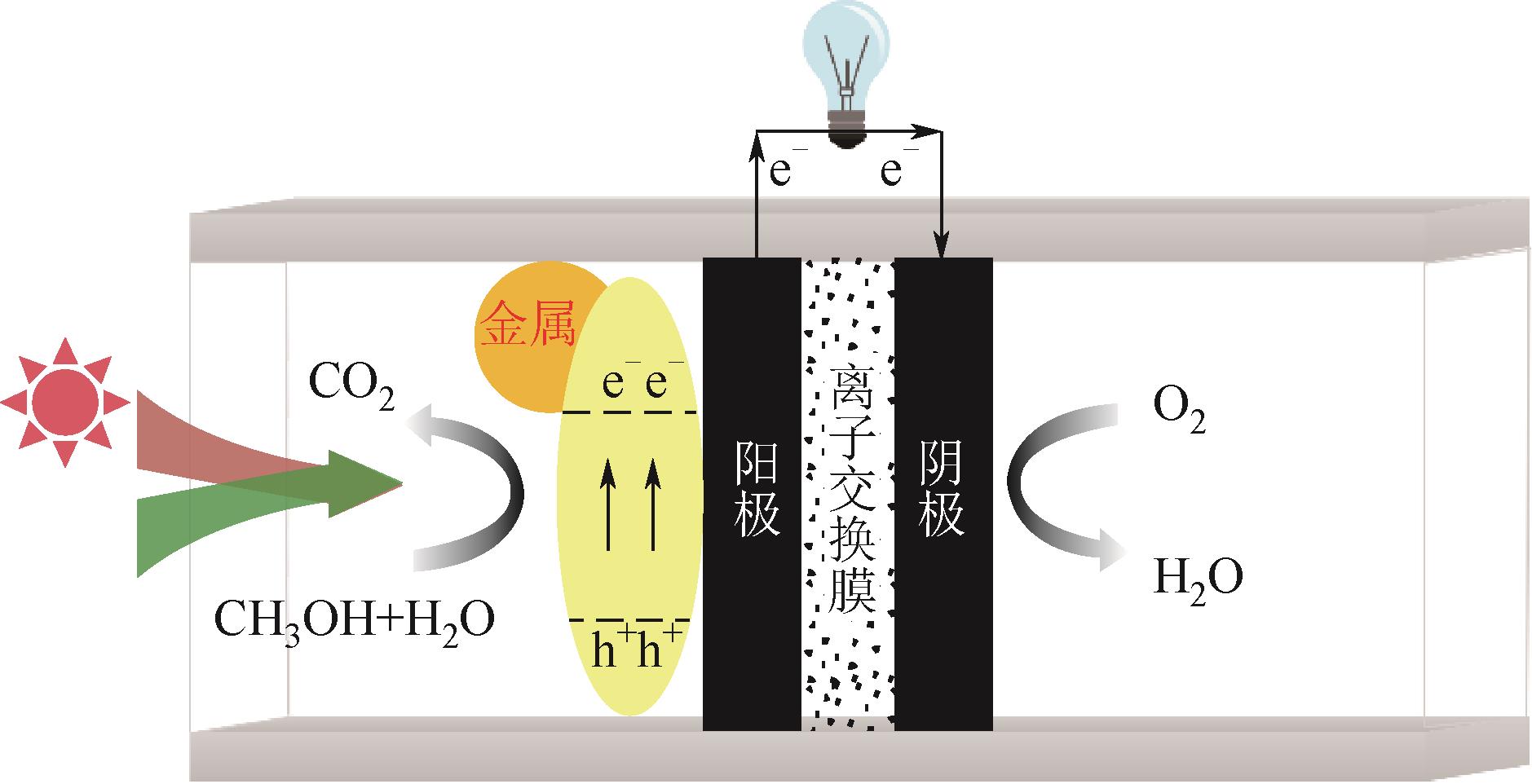
直接甲醇燃料电池(DMFCs)因其能够在较低工作温度下提供清洁、可持续能源方面的潜力而备受关注,但阳极缓慢的甲醇氧化反应(MOR)限制了其商业化应用。光辅助直接甲醇燃料电池(PMFCs)能利用光电耦合机制加速MOR过程,实现甲醇的化学能高效转化为电能,为开发高效、可持续的能源转换系统带来了巨大希望。本文首先简要回顾了PMFCs的背景、意义和在能源研究中的价值。从PMFCs的基本结构组成和酸碱介质下甲醇光电催化氧化的基本路径出发,总结了PMFCs阳极光电催化剂的研究进展。重点针对不同类型的PMFCs阳极光电催化剂,讨论光辅助下的MOR增强机制,如催化剂的尺寸效应、晶面效应、吸光性能、导电性以及表面等离激元共振(SPR)效应等方面的影响。PMFCs未来需要重点关注的研究方向是深入理解PMFCs的光电耦合机制和PMFCs阳极催化剂的构效关系,以及解决实际二电极系统下PMFCs光阳极的设计问题。
中图分类号:
引用本文
方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628.
FANG Yao, LIU Lei, GAO Zhihua, HUANG Wei, ZUO Zhijun. Advances in anode catalysts for photo-assisted direct methanol fuel cells[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2611-2628.
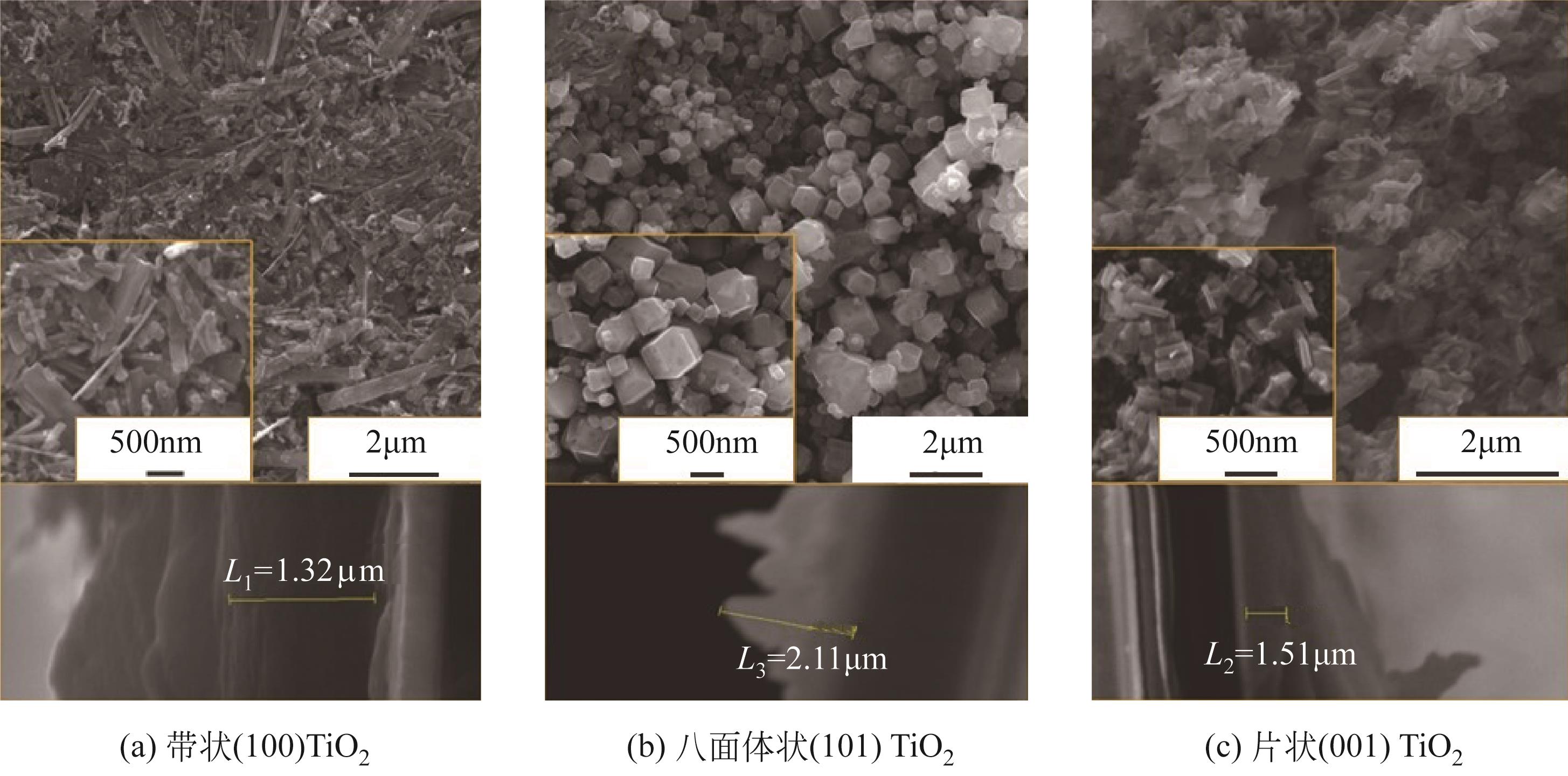
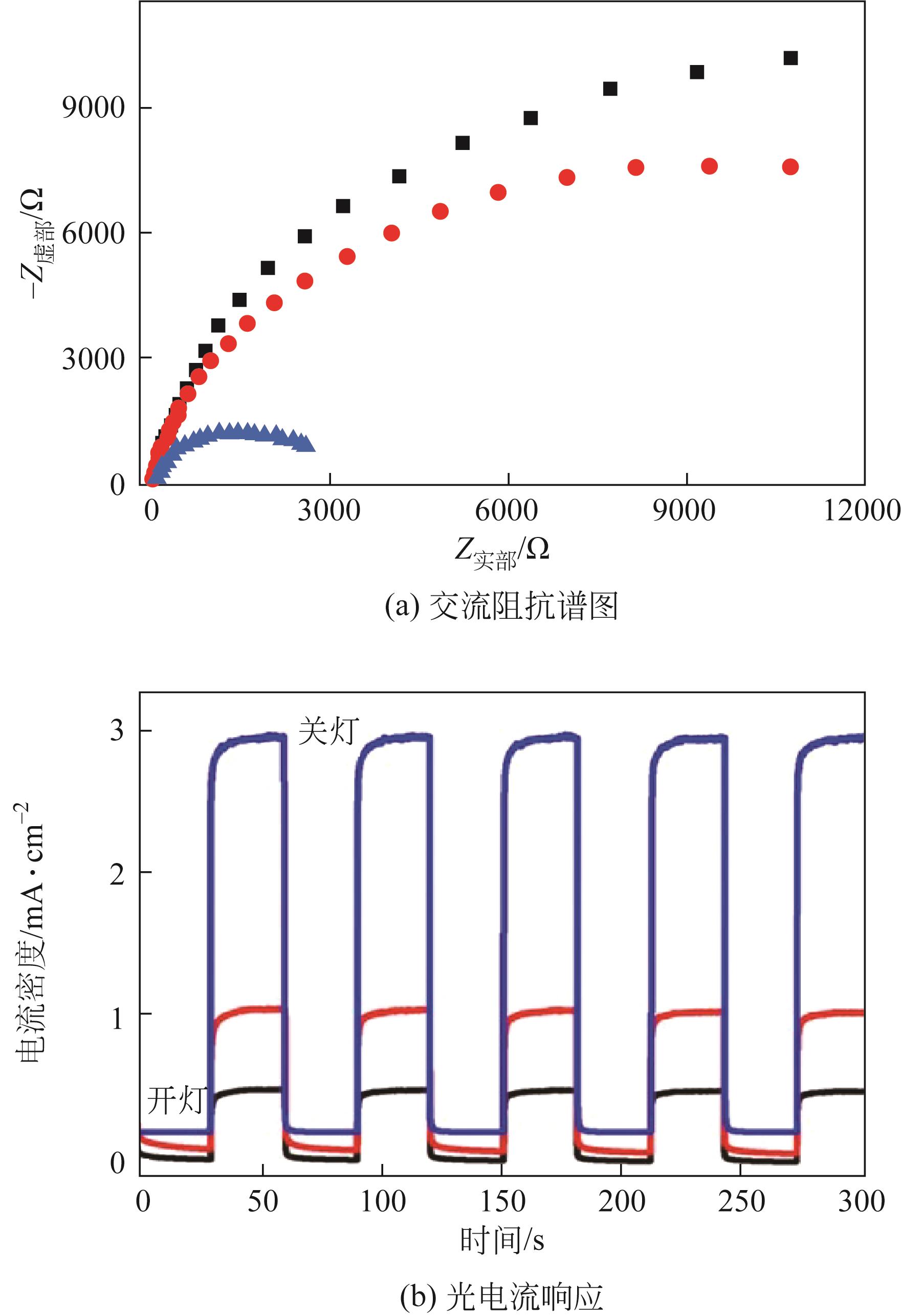
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-TiO2 | — | — | — | 1.48 | [ |
| Pt3-CD2/TiO2 | 0.35V vs. Ag/AgCl | 2.02mA/cm2 | 2.5mA/cm2 | 1.24 | [ |
| PtRu-TiO2 | 0.38V vs. RHE | 18.1mA | 21.4mA | 1.18 | [ |
| Pt(Cu)-TiO2 | -0.15V vs. SCE | — | — | 1.66 | [ |
| G-TNRs | -0.4V vs. SCE | 0.2μA/cm2 | 4.9μA/cm2 | 24.5 | [ |
| Pt-TNRs | -0.6V vs. Hg/HgO | 1.86mA/mgPt | 5.32mA/mgPt | 2.86 | [ |
| Pt-TNTs | — | — | — | 2.6 | [ |
| PtNi-TNTs | — | 24.3mA/cm2 | 37.5mA/cm2 | 1.54 | [ |
| PtNi/C-TNTs | 0.6V vs. Ag/AgCl | 108mA/cm2 | 123mA/cm2 | 1.13 | [ |
| Pt-TNTs | 0.4V vs. Ag/AgCl | 357.4mA/mgPt | 525mA/mgPt | 1.47 | [ |
| Pt-TNTs/RGO | — | 1.36mA/cm2 | 4.4mA/cm2 | 3.24 | [ |
| Pt-TiO2/GNs | — | 820mA/mgPt | 1354mA/mgPt | 1.65 | [ |
| Pt-RGO/TiO2 | — | 363.9mA/mgPt | 507.4mA/mgPt | 1.39 | [ |
| Pt-TiO2/G-PV | — | 1214mA/mgPt | 3293mA/mgPt | 2.71 | [ |
| Pt-GNs/TiO2 | -0.373V vs. Hg/HgO | 3368mA/mgPt | 4715mA/mgPt | 1.4 | [ |
| TiO2-MWCNT | — | 0.51mA/cm2 | 4.5mA/cm2 | 8.8 | [ |
| Pt-TiO2@C | 0.18V vs. RHE | 1.15mA/cm2 | 2.86mA/cm2 | 2.5 | [ |
| Pt-TiO2/SiO2 | -0.6V vs. SCE | 0.3mA/cm2 | 0.95mA/cm2 | 3.17 | [ |
| Pt-MXene-TiO2 | 0.33V vs. SCE | 703.31mA/mgPt | 2750.4mA/mgPt | 3.9 | [ |
表1 TiO2基催化剂MOR光电催化性能
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-TiO2 | — | — | — | 1.48 | [ |
| Pt3-CD2/TiO2 | 0.35V vs. Ag/AgCl | 2.02mA/cm2 | 2.5mA/cm2 | 1.24 | [ |
| PtRu-TiO2 | 0.38V vs. RHE | 18.1mA | 21.4mA | 1.18 | [ |
| Pt(Cu)-TiO2 | -0.15V vs. SCE | — | — | 1.66 | [ |
| G-TNRs | -0.4V vs. SCE | 0.2μA/cm2 | 4.9μA/cm2 | 24.5 | [ |
| Pt-TNRs | -0.6V vs. Hg/HgO | 1.86mA/mgPt | 5.32mA/mgPt | 2.86 | [ |
| Pt-TNTs | — | — | — | 2.6 | [ |
| PtNi-TNTs | — | 24.3mA/cm2 | 37.5mA/cm2 | 1.54 | [ |
| PtNi/C-TNTs | 0.6V vs. Ag/AgCl | 108mA/cm2 | 123mA/cm2 | 1.13 | [ |
| Pt-TNTs | 0.4V vs. Ag/AgCl | 357.4mA/mgPt | 525mA/mgPt | 1.47 | [ |
| Pt-TNTs/RGO | — | 1.36mA/cm2 | 4.4mA/cm2 | 3.24 | [ |
| Pt-TiO2/GNs | — | 820mA/mgPt | 1354mA/mgPt | 1.65 | [ |
| Pt-RGO/TiO2 | — | 363.9mA/mgPt | 507.4mA/mgPt | 1.39 | [ |
| Pt-TiO2/G-PV | — | 1214mA/mgPt | 3293mA/mgPt | 2.71 | [ |
| Pt-GNs/TiO2 | -0.373V vs. Hg/HgO | 3368mA/mgPt | 4715mA/mgPt | 1.4 | [ |
| TiO2-MWCNT | — | 0.51mA/cm2 | 4.5mA/cm2 | 8.8 | [ |
| Pt-TiO2@C | 0.18V vs. RHE | 1.15mA/cm2 | 2.86mA/cm2 | 2.5 | [ |
| Pt-TiO2/SiO2 | -0.6V vs. SCE | 0.3mA/cm2 | 0.95mA/cm2 | 3.17 | [ |
| Pt-MXene-TiO2 | 0.33V vs. SCE | 703.31mA/mgPt | 2750.4mA/mgPt | 3.9 | [ |
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-ZnO@CC | — | 2.58mA/cm2 | 3.86mA/cm2 | 1.5 | [ |
| Pt-ZnO/KB | — | 432mA/mgPt | 964mA/mgPt | 2.23 | [ |
| Pt-ZnO | -0.796V vs. SCE | 427.5mA/mgPt | 547.96mA/mgPt | 1.28 | [ |
| Pt(Pd)/ZnO/GNs | Pt 0.38V vs. Ag/AgCl | 957.3mA/mgPt | 1935.5mA/mgPt | 2.02 | [ |
| Pd -0.63V vs. Ag/AgCl | 513.2mA/mgPt | 818.3mA/mgPt | 1.59 |
表2 ZnO基催化剂MOR光电催化性能
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-ZnO@CC | — | 2.58mA/cm2 | 3.86mA/cm2 | 1.5 | [ |
| Pt-ZnO/KB | — | 432mA/mgPt | 964mA/mgPt | 2.23 | [ |
| Pt-ZnO | -0.796V vs. SCE | 427.5mA/mgPt | 547.96mA/mgPt | 1.28 | [ |
| Pt(Pd)/ZnO/GNs | Pt 0.38V vs. Ag/AgCl | 957.3mA/mgPt | 1935.5mA/mgPt | 2.02 | [ |
| Pd -0.63V vs. Ag/AgCl | 513.2mA/mgPt | 818.3mA/mgPt | 1.59 |
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-WO3/Gr | 0.4V vs. SCE | — | — | 1.2 | [ |
| Pt-2D Fe2O3 | — | 167.8mA/mgPt | 太阳光 389.1mA/mgPt | 2.32 | [ |
| 可见光 218.7mA/mgPt | 1.30 | ||||
| Pt-Cu2O/GNs | 0.48V vs. Ag/AgCl | 1321.9mA/mgPt | 1800.6mA/mgPt | 1.36 | [ |
| Pd-Cu2O/GNs | -0.50V vs. Ag/AgCl | 718mA/mgPt | 899.5mA/mgPt | 1.25 | |
| Pt/SnO2/GNs | — | 670.3mA/mgPt | 1103mA/mgPt | 1.64 | [ |
| PtPd/SnO2/GNs | 0.174V vs. Ag/AgCl | 7718mA/mgPt | 10029mA/mgPt | 1.3 | [ |
| Pt-Bi2WO6/RGO | — | 936mA/mgPt | 1800mA/mgPt | 1.92 | [ |
| CQDs-Pt@Bi2WO6/FTO | — | 0.73mA/cm2 | 0.89mA/cm2 | 1.21 | [ |
| Pt-BiOBr (001) | — | 251.1mA/mgPt | 太阳光 1321.1mA/mgPt | 5.2 | [ |
| 可见光 594.3mA/mgPt | 2.4 | ||||
| Pt-g-C3N4 | 酸性:— | 157.7mA/mgPt | 520.4mA/mgPt | 3.3 | [ |
| 碱性:-0.5V vs. SCE | 49.89mA/mgPt | 91.3mA/mgPt | 1.83 | ||
| Pt/N-g-C3N4/KB | — | 1700mA/mgPt | 2800mA/mgPt | 1.63 | [ |
| Pt-Fe2P | -0.6V vs. SCE | 521.1mA/mgPt | 2430mA/mgPt | 4.7 | [ |
| Pt-CdS QDs | — | 1.33mA/cm2 | 4.08mA/cm2 | 3.1 | [ |
表3 其他单一半导体催化剂的MOR光电催化性能
| 催化剂 | 甲醇起始氧化电位 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Pt-WO3/Gr | 0.4V vs. SCE | — | — | 1.2 | [ |
| Pt-2D Fe2O3 | — | 167.8mA/mgPt | 太阳光 389.1mA/mgPt | 2.32 | [ |
| 可见光 218.7mA/mgPt | 1.30 | ||||
| Pt-Cu2O/GNs | 0.48V vs. Ag/AgCl | 1321.9mA/mgPt | 1800.6mA/mgPt | 1.36 | [ |
| Pd-Cu2O/GNs | -0.50V vs. Ag/AgCl | 718mA/mgPt | 899.5mA/mgPt | 1.25 | |
| Pt/SnO2/GNs | — | 670.3mA/mgPt | 1103mA/mgPt | 1.64 | [ |
| PtPd/SnO2/GNs | 0.174V vs. Ag/AgCl | 7718mA/mgPt | 10029mA/mgPt | 1.3 | [ |
| Pt-Bi2WO6/RGO | — | 936mA/mgPt | 1800mA/mgPt | 1.92 | [ |
| CQDs-Pt@Bi2WO6/FTO | — | 0.73mA/cm2 | 0.89mA/cm2 | 1.21 | [ |
| Pt-BiOBr (001) | — | 251.1mA/mgPt | 太阳光 1321.1mA/mgPt | 5.2 | [ |
| 可见光 594.3mA/mgPt | 2.4 | ||||
| Pt-g-C3N4 | 酸性:— | 157.7mA/mgPt | 520.4mA/mgPt | 3.3 | [ |
| 碱性:-0.5V vs. SCE | 49.89mA/mgPt | 91.3mA/mgPt | 1.83 | ||
| Pt/N-g-C3N4/KB | — | 1700mA/mgPt | 2800mA/mgPt | 1.63 | [ |
| Pt-Fe2P | -0.6V vs. SCE | 521.1mA/mgPt | 2430mA/mgPt | 4.7 | [ |
| Pt-CdS QDs | — | 1.33mA/cm2 | 4.08mA/cm2 | 3.1 | [ |
| 催化剂 | 光源 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| p-n异质结 | |||||
| Ni(OH)2/TiO2 NTs | 500W Xe lamp | 23.8mA/cm2 | 28.3mA/cm2 | 1.2 | [ |
| Pt-CuI/TiO2 | 500W Xe lamp | 936mA/mg | 3772mA/mg | 4.0 | [ |
| Ⅱ型异质结 | |||||
| Pt-WO3-TiO2 | 8W UV light(λ=265nm) | 0.35mA/cm2 | 2.2mA/cm2 | 6.3 | [ |
| Ni-TiO2/PHI | 365nm LED | — | 11.0mA/cm2 | — | [ |
| Pt-CdS/MoS2 | 150W Xe lamp(λ>400nm) | 1.13mA/cm2 | 4.56mA/cm2 | 4.0 | [ |
| Pt/g-C3N4/CdS | 150W Xe lamp(λ>420nm) | 16.9mA/mg | 127.7mA/mg | 7.4 | [ |
| Pt-Bi2WO6/MoS2 | 300W Xe lamp | 1829mA/mg | 2743mA/mg | 1.5 | [ |
| Pt-Bi2WO6/Cu2S | 500W Xe lamp(λ>420nm) | 1854.5mA/mg | 5538mA/mg | 3.0 | [ |
| Pt-BiOI/MoS2 | 150W Xe lamp(λ>420nm) | 222.3mA/mg | 1007.2mA/mg | 4.5 | [ |
| Pt-BiVO4/Bi2O3 | 50W Xe lamp(λ>320nm) | 281.4mA/mg | 506.6mA/mg | 1.8 | [ |
| CoS x /NiS x | 50W Xe lamp | 28.5A/g | 30.4A/g | 1.065 | [ |
| Z型异质结 | |||||
| Si NWs@ZnO | 500W Xe lamp | 67.0μA/cm2 | 100.5μA/cm2 | 1.5 | [ |
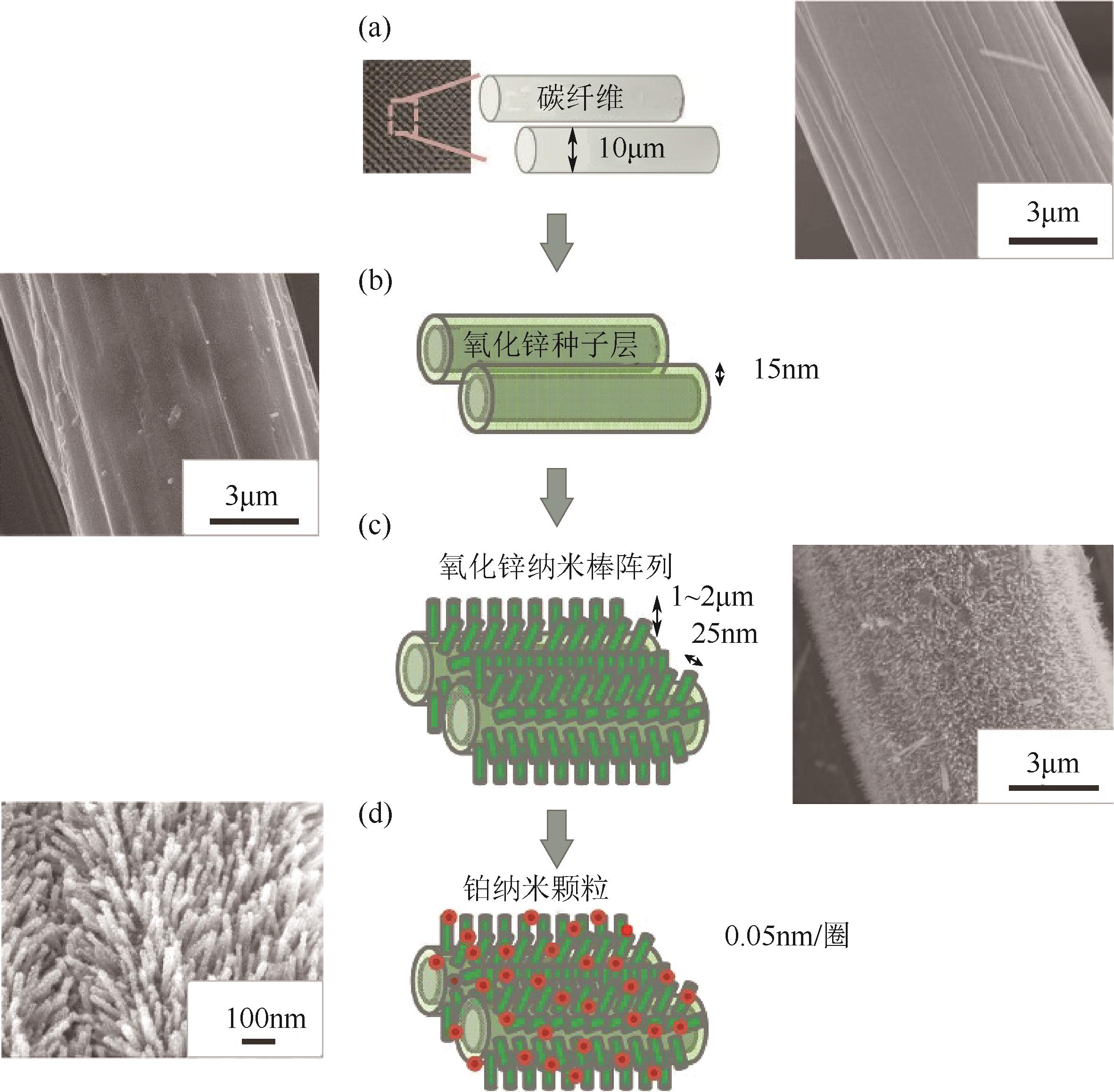
表4 半导体异质结催化剂的MOR光电催化性能
| 催化剂 | 光源 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| p-n异质结 | |||||
| Ni(OH)2/TiO2 NTs | 500W Xe lamp | 23.8mA/cm2 | 28.3mA/cm2 | 1.2 | [ |
| Pt-CuI/TiO2 | 500W Xe lamp | 936mA/mg | 3772mA/mg | 4.0 | [ |
| Ⅱ型异质结 | |||||
| Pt-WO3-TiO2 | 8W UV light(λ=265nm) | 0.35mA/cm2 | 2.2mA/cm2 | 6.3 | [ |
| Ni-TiO2/PHI | 365nm LED | — | 11.0mA/cm2 | — | [ |
| Pt-CdS/MoS2 | 150W Xe lamp(λ>400nm) | 1.13mA/cm2 | 4.56mA/cm2 | 4.0 | [ |
| Pt/g-C3N4/CdS | 150W Xe lamp(λ>420nm) | 16.9mA/mg | 127.7mA/mg | 7.4 | [ |
| Pt-Bi2WO6/MoS2 | 300W Xe lamp | 1829mA/mg | 2743mA/mg | 1.5 | [ |
| Pt-Bi2WO6/Cu2S | 500W Xe lamp(λ>420nm) | 1854.5mA/mg | 5538mA/mg | 3.0 | [ |
| Pt-BiOI/MoS2 | 150W Xe lamp(λ>420nm) | 222.3mA/mg | 1007.2mA/mg | 4.5 | [ |
| Pt-BiVO4/Bi2O3 | 50W Xe lamp(λ>320nm) | 281.4mA/mg | 506.6mA/mg | 1.8 | [ |
| CoS x /NiS x | 50W Xe lamp | 28.5A/g | 30.4A/g | 1.065 | [ |
| Z型异质结 | |||||
| Si NWs@ZnO | 500W Xe lamp | 67.0μA/cm2 | 100.5μA/cm2 | 1.5 | [ |
| 催化剂 | 光源 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Au-CA | 模拟太阳光100mW/cm2 | 115.86μA/μg | 248.9μA/μg | 2.2 | [ |
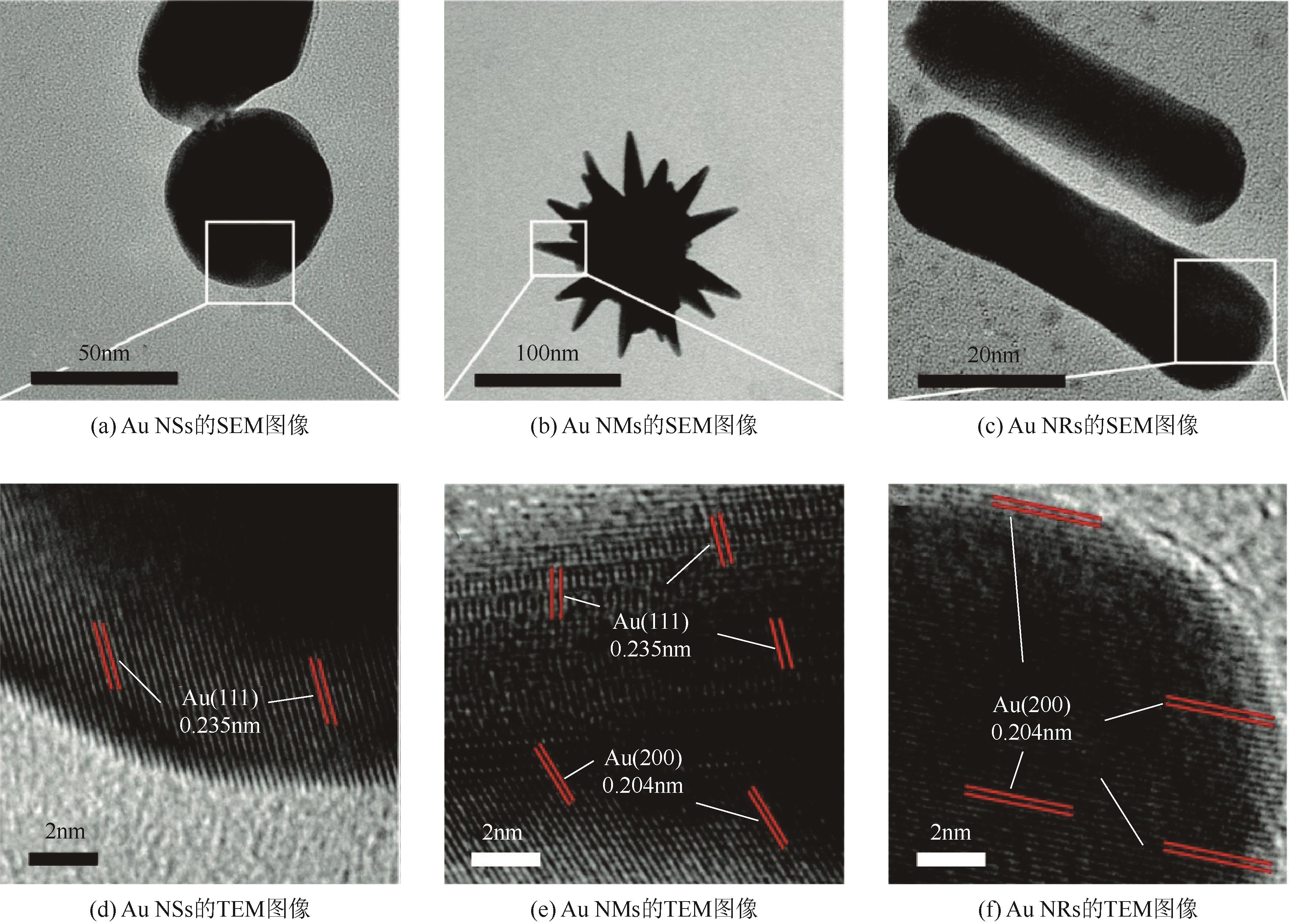
| Au NSs | 300W Xe lamp | 0.71mA/mg | 1.65mA/mg | 2.3 | [ |
| Au NMs | 2.50mA/mg | 7.98mA/mg | 3.2 | ||
| Au NRs | 4.08mA/mg | 12.50mA/mg | 3.1 | ||
| NPG | 300W Xe lamp | — | 531μA/cm2 | >2 | [ |
| Pt-Au NDs | 300W Xe lamp(>420nm) | 0.79mA/cm2 | 2.61mA/cm2 | 3.3 | [ |
| AgPt-tipped Au NSs | 300W Xe lamp(>420nm) | 2.74A/mg | 4.0A/mg | 1.5 | [ |
| PtNiCu alloy | 300W Xe lamp(>420nm) | — | 3.3A/mgPt | — | [ |
| Pt-Ag/GNs | 15W UV lamp | 838.3mA/mg | 1842.4mA/mg | 2.2 | [ |
| Au-6T/SG/Cu | 565nm LED | 169.4μA/μg | 288μA/μg | 1.7 | [ |
| PtIn/3D-GNs | 300W Xe lamp | 612.3mA/mg | 1570.7mA/mg | 2.6 | [ |
| Au-DNFs/TiN | 模拟太阳光150W(>400nm) | 476.51μA/cm2 | 565.84μA/cm2 | 1.2 | [ |
| Au-DNFs/Si | 423.48μA/cm2 | 514.93μA/cm2 | 1.2 | ||
| Ag/TiO2 | 模拟太阳光100mW/cm2 | — | 1mA/cm2 | — | [ |
| Pd/TiO2 | 模拟太阳光100mW/cm2 | 可见光0.132mA/cm2 | 可见光0.353mA/cm2 | 2.7 | [ |
| 紫外光0.132mA/cm2 | 紫外光0.562mA/cm2 | 4.3 | |||
| Au/TNT | Xe lamp 165mW/cm2 | 53.27mA/mg | 189.51mA/mg | 3.6 | [ |
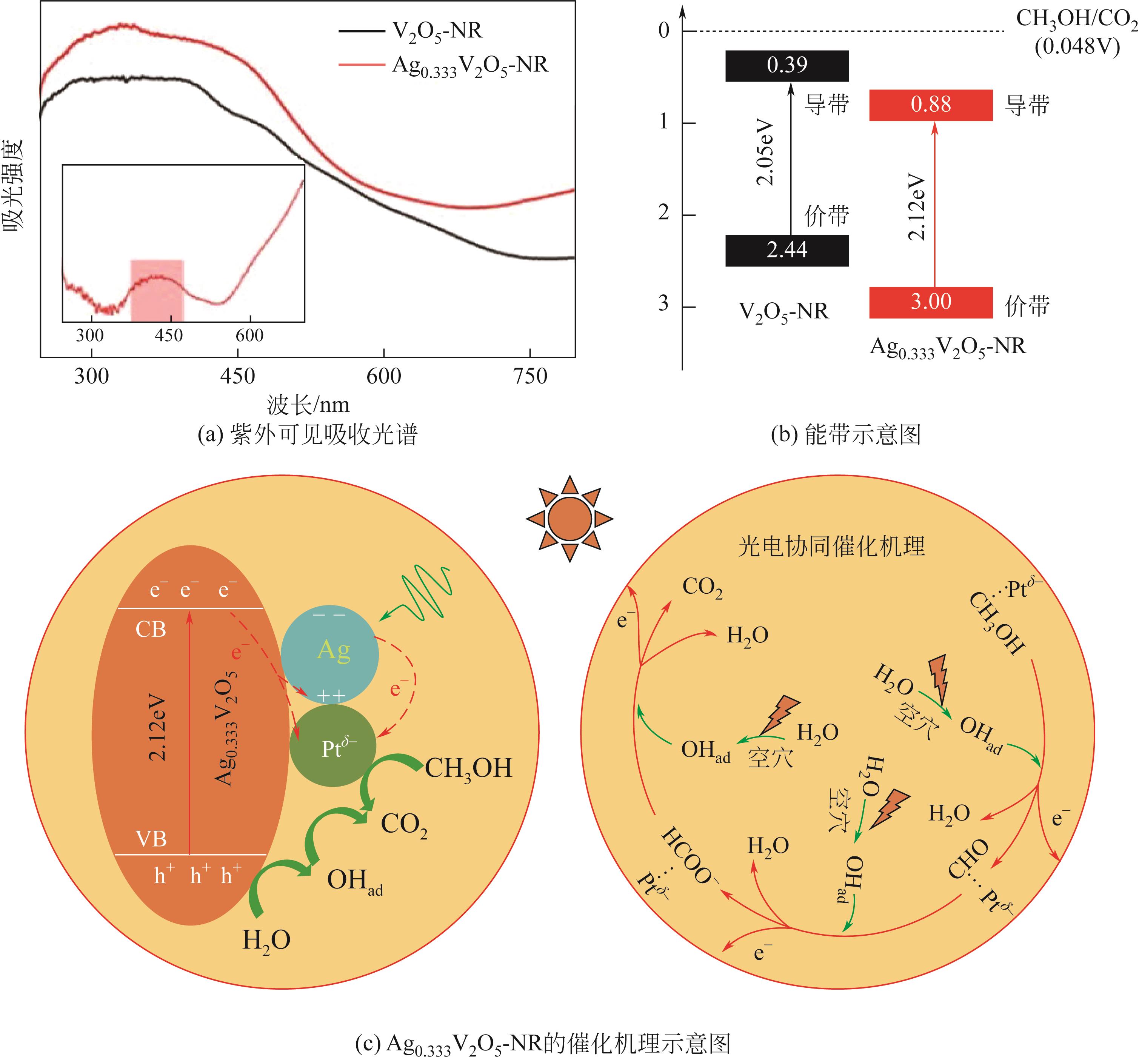
| Pt/Ag0.333V2O5-NR | Xe lamp | 5.32mA/cm2 | 10.73mA/cm2 | 2.0 | [ |
表5 等离激元金属催化剂的MOR光电催化性能
| 催化剂 | 光源 | 电流强度(暗态) | 电流强度(光照) | 增强因子 | 参考文献 |
|---|---|---|---|---|---|
| Au-CA | 模拟太阳光100mW/cm2 | 115.86μA/μg | 248.9μA/μg | 2.2 | [ |
| Au NSs | 300W Xe lamp | 0.71mA/mg | 1.65mA/mg | 2.3 | [ |
| Au NMs | 2.50mA/mg | 7.98mA/mg | 3.2 | ||
| Au NRs | 4.08mA/mg | 12.50mA/mg | 3.1 | ||
| NPG | 300W Xe lamp | — | 531μA/cm2 | >2 | [ |
| Pt-Au NDs | 300W Xe lamp(>420nm) | 0.79mA/cm2 | 2.61mA/cm2 | 3.3 | [ |
| AgPt-tipped Au NSs | 300W Xe lamp(>420nm) | 2.74A/mg | 4.0A/mg | 1.5 | [ |
| PtNiCu alloy | 300W Xe lamp(>420nm) | — | 3.3A/mgPt | — | [ |
| Pt-Ag/GNs | 15W UV lamp | 838.3mA/mg | 1842.4mA/mg | 2.2 | [ |
| Au-6T/SG/Cu | 565nm LED | 169.4μA/μg | 288μA/μg | 1.7 | [ |
| PtIn/3D-GNs | 300W Xe lamp | 612.3mA/mg | 1570.7mA/mg | 2.6 | [ |
| Au-DNFs/TiN | 模拟太阳光150W(>400nm) | 476.51μA/cm2 | 565.84μA/cm2 | 1.2 | [ |
| Au-DNFs/Si | 423.48μA/cm2 | 514.93μA/cm2 | 1.2 | ||
| Ag/TiO2 | 模拟太阳光100mW/cm2 | — | 1mA/cm2 | — | [ |
| Pd/TiO2 | 模拟太阳光100mW/cm2 | 可见光0.132mA/cm2 | 可见光0.353mA/cm2 | 2.7 | [ |
| 紫外光0.132mA/cm2 | 紫外光0.562mA/cm2 | 4.3 | |||
| Au/TNT | Xe lamp 165mW/cm2 | 53.27mA/mg | 189.51mA/mg | 3.6 | [ |
| Pt/Ag0.333V2O5-NR | Xe lamp | 5.32mA/cm2 | 10.73mA/cm2 | 2.0 | [ |
| 1 | 赵顺炜, 王耀琼, 高焕方, 等. 金属化合物作直接甲醇燃料电池阳极催化剂载体的研究进展[J]. 化工进展, 2017, 36(3):965-972. |
| ZHAO Shunwei, WANG Yaoqiong, GAO Huanfang, et al. Resent progress in metal compound supports of anode catalyst for direct methanol fuel cell[J]. Chemical Industry and Engineering Progress, 2017, 36(3): 965-972. | |
| 2 | WANG Jianmei, ZHANG Bingxing, GUO Wei, et al. Toward electrocatalytic methanol oxidation reaction: Longstanding debates and emerging catalysts[J]. Advanced Materials, 2023, 35(26): e2211099. |
| 3 | 丁鑫, 张栋铭, 焦纬洲, 等. 直接甲醇燃料电池阳极催化剂研究进展[J]. 化工进展, 2021, 40(9): 4918-4930. |
| DING Xin, ZHANG Dongming, JIAO Weizhou, et al. Research progress of anode catalysts for direct methanol fuel cells[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4918-4930. | |
| 4 | GONG Liyuan, YANG Zhiyuan, LI Kui, et al. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell[J]. Journal of Energy Chemistry, 2018, 27(6): 1618-1628. |
| 5 | TIAN Hao, YU Yanhui, WANG Qian, et al. Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction[J]. International Journal of Hydrogen Energy, 2021, 46(61): 31202-31215. |
| 6 | ANTOLINI Ermete. Photo-assisted methanol oxidation on Pt-TiO2 catalysts for direct methanol fuel cells: A short review[J]. Applied Catalysis B: Environmental, 2018, 237: 491-503. |
| 7 | DREW Kristine, GIRISHKUMAR G, VINODGOPAL K, et al. Boosting fuel cell performance with a semiconductor photocatalyst: TiO2/Pt-Ru hybrid catalyst for methanol oxidation[J]. The Journal of Physical Chemistry B, 2005, 109(24): 11851-11857. |
| 8 | HOSSEINI Mir Ghasem, MOMENI Mohamad Mohsen. UV-cleaning properties of Pt nanoparticle-decorated titania nanotubes in the electro-oxidation of methanol: An anti-poisoning and refreshable electrode[J]. Electrochimica Acta, 2012, 70: 1-9. |
| 9 | HOSSEINI Mir Ghasem, MOMENI Mohamad Mohsen. Evaluation of the performance of platinum nanoparticle-titanium oxide nanotubes as a new refreshable electrode for formic acid electro-oxidation[J]. Fuel Cells, 2012, 12(3): 406-414. |
| 10 | AHMED Amira Y, KANDIEL Tarek A, IVANOVA Irina, et al. Photocatalytic and photoelectrochemical oxidation mechanisms of methanol on TiO2 in aqueous solution[J]. Applied Surface Science, 2014, 319: 44-49. |
| 11 | LIN Chunting, HUANG Hung Ji, YANG Jr-Jung, et al. A simple fabrication process of Pt-TiO2 hybrid electrode for photo-assisted methanol fuel cells[J]. Microelectronic Engineering, 2011, 88(8): 2644-2646. |
| 12 | BORA Anindita, MOHAN Kiranjyoti, DOLEY Simanta, et al. Broadening the sunlight response region with carbon dot sensitized TiO2 as a support for a Pt catalyst in the methanol oxidation reaction[J]. Catalysis Science & Technology, 2018, 8(16): 4180-4192. |
| 13 | POLO André S, SANTOS M C, DE SOUZA Rodrigo F B, et al. Pt-Ru-TiO2 photoelectrocatalysts for methanol oxidation[J]. Journal of Power Sources, 2011, 196(2): 872-876. |
| 14 | IVANOV Svetlozar, MINTSOULI Ioanna, GEORGIEVA Jenia, et al. Platinized titanium dioxide electrodes for methanol oxidation and photo-oxidation[J]. Journal of Electrochemical Science and Engineering, 2012, 2(4): 155-169. |
| 15 | LI Xinyuan, WANG Guowen, JING Lin, et al. A photoelectrochemical methanol fuel cell based on aligned TiO2 nanorods decorated graphene photoanode[J]. Chemical Communications, 2016, 52(12): 2533-2536. |
| 16 | HU Sujuan, WANG Baoling, JU Haidong, et al. Photo-assisted electrocatalytic methanol oxidation based on an efficient 1D-TiO2 nanorods arrays support electrode[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 80: 533-539. |
| 17 | WANG Y Q, WEI Z D, GAO B, et al. The electrochemical oxidation of methanol on a Pt/TNTs/Ti electrode enhanced by illumination[J]. Journal of Power Sources, 2011, 196(3): 1132-1135. |
| 18 | HE Huichao, XIAO Peng, ZHOU Ming, et al. Preparation of well-distributed Pt-Ni nanoparticles on/into TiO2NTs by pulse electrodeposition for methanol photoelectro-oxidation[J]. Catalysis Communications, 2011, 16(1): 140-143. |
| 19 | HE Huichao, XIAO Peng, ZHOU Ming, et al. PtNi alloy nanoparticles supported on carbon-doped TiO2 nanotube arrays for photo-assisted methanol oxidation[J]. Electrochimica Acta, 2013, 88: 782-789. |
| 20 | ZHANG Jianbo, SU Nan, HU Xiulan, et al. Facile synthesis of Pt nanoparticles supported on anatase TiO2 nanotubes with good photo-electrocatalysis performance for methanol[J]. RSC Advances, 2017, 7(89): 56194-56203. |
| 21 | ZHAI Chunyang, ZHU Mingshan, Duan BIN, et al. Visible-light-assisted electrocatalytic oxidation of methanol using reduced graphene oxide modified Pt nanoflowers-TiO2 nanotube arrays[J]. ACS Applied Materials & Interfaces, 2014, 6(20): 17753-17761. |
| 22 | YE Lingting, LI Zhongshui, ZHANG Lian, et al. A green one-pot synthesis of Pt/TiO2/Graphene composites and its electro-photo-synergistic catalytic properties for methanol oxidation[J]. Journal of Colloid and Interface Science, 2014, 433: 156-162. |
| 23 | WANG Caiqin, JIANG Fengxing, YUE Ruirui, et al. Enhanced photo-electrocatalytic performance of Pt/RGO/TiO2 on carbon fiber towards methanol oxidation in alkaline media[J]. Journal of Solid State Electrochemistry, 2014, 18(2): 515-522. |
| 24 | ODETOLA Christopher, TREVANI Liliana N, EASTON E Bradley. Photo enhanced methanol electrooxidation: Further insights into Pt and TiO2 nanoparticle contributions[J]. Applied Catalysis B: Environmental, 2017, 210: 263-275. |
| 25 | ZHANG Jianbo, HU Xiulan, ZHU Faquan, et al. Simple synthesized Pt/GNs/TiO2 with good mass activity and stability for methanol oxidation[J]. Nanotechnology, 2017, 28(50): 505603. |
| 26 | MOHAMED Mohamed Mokhtar, Salah EID, EL-ETRE A Y. Methanol photo-oxidation at graphene and carbon nanotubes modified TiO2 nanosheets electrocatalysts[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2017, 338: 37-48. |
| 27 | LI Wei, BAI Yang, LI Fujun, et al. Core-shell TiO2/C nanofibers as supports for electrocatalytic and synergistic photoelectrocatalytic oxidation of methanol[J]. Journal of Materials Chemistry, 2012, 22(9): 4025-4031. |
| 28 | FAN X L, ZHANG C X, XUE H R, et al. Fabrication of SiO2 incorporated ordered mesoporous TiO2 composite films as functional Pt supports for photo-electrocatalytic methanol oxidation[J]. RSC Advances, 2015, 5(96): 78880-78888. |
| 29 | SUN Yue, ZHOU Yunjie, LIU Yan, et al. A photoactive process cascaded electrocatalysis for enhanced methanol oxidation over Pt-MXene-TiO2 composite[J]. Nano Research, 2020, 13(10): 2683-2690. |
| 30 | MIKRUT Paweł, KOBIELUSZ Marcin, MACYK Wojciech. Spectroelectrochemical characterization of euhedral anatase TiO2 crystals-implications for photoelectrochemical and photocatalytic properties of {001} {100} and {101} facets[J]. Electrochimica Acta, 2019, 310: 256-265. |
| 31 | MIKRUT Paweł, MITORAJ Dariusz, BERANEK Radim, et al. Facet-dependent activity of tailored anatase TiO2 crystals in photoanodes for photocatalytic fuel cells[J]. Applied Surface Science, 2021, 566: 150662. |
| 32 | PLAÇA L F, VITAL P S, GOMES L E, et al. Black TiO2 photoanodes for direct methanol photo fuel cells[J]. ACS Applied Materials & Interfaces, 2023, 15(37): 43259-43271. |
| 33 | YU Yingjian, YANG Jiangxia, WANG Xianlu, et al. Atomic Fe sites doped two-dimensional (001) TiO2 nanosheets as an effective photo-response support in methanol electro-oxidation[J]. Surfaces and Interfaces, 2021, 25: 101231. |
| 34 | KHAN Mohammad Ehtisham, MOHAMMAD Akbar, YOON Taeho. State-of-the-art developments in carbon quantum dots (CQDs): Photo-catalysis, bio-imaging, and bio-sensing applications[J]. Chemosphere, 2022, 302: 134815. |
| 35 | Jae-Kyung OH, LEE Young-Woo, PARK Kyung-Won. Improved photo-catalytic activity of single-crystalline TiO2 nanowires surrounded by Pt cube nanoparticles[J]. Journal of Industrial and Engineering Chemistry, 2013, 19(4): 1391-1394. |
| 36 | GRASSER Jordan A, STOVER Benjamin K, MUGGLI Darrin S. Synthesis factors that impact TiO2 nanotube activity during gas-phase photocatalytic oxidation of methanol[J]. Chemical Engineering Communications, 2013, 200(3): 337-350. |
| 37 | DÍAZ-REAL J A, DUBED-BANDOMO G C, GALINDO-DE-LA-ROSA J, et al. Evaluation of transferable TiO2 nanotube membranes as electrocatalyst support for methanol photoelectrooxidation[J]. Applied Catalysis B: Environmental, 2018, 222: 18-25. |
| 38 | ADÁN C, MARUGÁN J, SÁNCHEZ E, et al. Understanding the effect of morphology on the photocatalytic activity of TiO2 nanotube array electrodes[J]. Electrochimica Acta, 2016, 191: 521-529. |
| 39 | MENA Esperanza, MARTÍN DE VIDALES María José, MESONES Sandra, et al. Influence of anodization mode on the morphology and photocatalytic activity of TiO2-NTs array large size electrodes[J]. Catalysis Today, 2018, 313: 33-39. |
| 40 | TANG H, PRASAD K, SANJINÈS R, et al. Electrical and optical properties of TiO2 anatase thin films[J]. Journal of Applied Physics, 1994, 75(4): 2042-2047. |
| 41 | AHMAD Haslina, KAMARUDIN Siti Kartom, MINGGU Lorna Jeffery, et al. Enhancing methanol oxidation with a TiO2-modified semiconductor as a photo-catalyst[J]. International Journal of Hydrogen Energy, 2017, 42(14): 8986-8996. |
| 42 | ÖZGÜR Ü, ALIVOV Ya I, LIU C, et al. A comprehensive review of ZnO materials and devices[J]. Journal of Applied Physics, 2005, 98(4): 41301-41301-103. |
| 43 | Agnieszka KOŁODZIEJCZAK-RADZIMSKA, JESIONOWSKI Teofil. Zinc oxide-from synthesis to application: A review[J]. Materials, 2014, 7(4): 2833-2881. |
| 44 | SU Chung-Yi, HSUEH Yang-Chih, Chi-Chung KEI, et al. Fabrication of high-activity hybrid Pt@ZnO catalyst on carbon cloth by atomic layer deposition for photoassisted electro-oxidation of methanol[J]. The Journal of Physical Chemistry C, 2013, 117(22): 11610-11618. |
| 45 | HU Xiulan, GE Chao, SU Nan, et al. Solution plasma synthesis of Pt/ZnO/KB for photo-assisted electro-oxidation of methanol[J]. Journal of Alloys and Compounds, 2017, 692: 848-854. |
| 46 | KANTI BERA Kamal, CHAKRABORTY Malay, KANTI BERA Shyamal, et al. Synthesis of different Pt-ZnO binary composites for synergistic photo-electrocatalytic oxidation of methanol in alkali[J]. ChemistrySelect, 2021, 6(25): 6586-6596. |
| 47 | LI Zhongshui, YE Lingting, LEI Fengling, et al. Enhanced electro-photo synergistic catalysis of Pt (Pd)/ZnO/graphene composite for methanol oxidation under visible light irradiation[J]. Electrochimica Acta, 2016, 188: 450-460. |
| 48 | GEORGIEVA J, SOTIROPOULOS S, VALOVA E, et al. Methanol oxidation and photo-oxidation at Pt/WO3 electrocatalysts on graphite substrates[J]. Journal of Electroanalytical Chemistry, 2014, 727: 135-140. |
| 49 | HU Sujuan, JIANG Lei, WANG Baoling, et al. Enhanced electrocatalytic methanol oxidation properties by photo-assisted Fe2O3 nanoplates[J]. International Journal of Hydrogen Energy, 2019, 44(26): 13214-13220. |
| 50 | YE Lingting, LI Zhongshui, ZHANG Xiaofeng, et al. One-step microwave synthesis of Pt (Pd)/Cu2O/GNs composites and their electro-photo-synergistic catalytic properties for methanol oxidation[J]. Journal of Materials Chemistry A, 2014, 2(48): 21010-21019. |
| 51 | LEI Fengling, LI Zhongshui, YE Lingting, et al. One-pot synthesis of Pt/SnO2/GNs and its electro-photo-synergistic catalysis for methanol oxidation[J]. International Journal of Hydrogen Energy, 2016, 41(1): 255-264. |
| 52 | YANG Bingqian, YU Yawei, ZHANG Jianbo, et al. Facile synthesis of PtPd/SnO2 nanocatalysts with good photo-electrocatalytic property[J]. Applied Surface Science, 2019, 471(31): 263-272. |
| 53 | GAO Haifeng, YUAN Chen, HE Zhilong, et al. Enhanced electrocatalytic oxidation of methanol on Pt-decorated Bi2WO6/graphene nanosheets with visible light assistance[J]. Energy Technology, 2020, 8(9): 2000210. |
| 54 | ZHENG Huajun, NIU Ping, ZHAO Zhefei. Carbon quantum dot sensitized Pt@Bi2WO6/FTO electrodes for enhanced photoelectro-catalytic activity of methanol oxidation[J]. RSC Advances, 2017, 7(43): 26943-26951. |
| 55 | HU Sujuan, JIANG Lei, TU Yujiao, et al. An efficient photo-assisted BiOBr nanoplates support for electrocatalyst for methanol oxidation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 86: 113-119. |
| 56 | ZHU Mingshan, ZHAI Chunyang, SUN Mingjuan, et al. Ultrathin graphitic C3N4 nanosheet as a promising visible-light-activated support for boosting photoelectrocatalytic methanol oxidation[J]. Applied Catalysis B: Environmental, 2017, 203: 108-115. |
| 57 | CHENG Jiexu, HU Xiulan, ZHANG Jianbo, et al. Fabrication of a composite of platinum, N-g-C3N4 and Ketjen Black for photo-electrochemical methanol oxidation[J]. Journal of Materials Science, 2017, 52(14): 8444-8454. |
| 58 | XU Zhaofen, HU Jiayue, DONG Haojie, et al. Near-infrared light-assisted methanol oxidation reaction over the ferrous phosphide[J]. Journal of Colloid and Interface Science, 2022, 626: 599-607. |
| 59 | ZHAI Chunyang, ZHU Mingshan, PANG Fenzhi, et al. High efficiency photoelectrocatalytic methanol oxidation on CdS quantum dots sensitized Pt electrode[J]. ACS Applied Materials & Interfaces, 2016, 8(9): 5972-5980. |
| 60 | DALAPATI Goutam Kumar, SHARMA Himani, GUCHHAIT Asim, et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review[J]. Journal of Materials Chemistry A, 2021, 9(31): 16621-16684. |
| 61 | KULESZA Pawel J, FAULKNER Larry R. Electrocatalytic properties of bifunctional Pt/W(Ⅵ, Ⅴ) oxide microstructures electrodeposited on carbon substrates[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1989, 259(1/2): 81-98. |
| 62 | TSEUNG A C C, CHEN K Y. Hydrogen spill-over effect on Pt/WO3 anode catalysts[J]. Catalysis Today, 1997, 38(4): 439-443. |
| 63 | 孙凌波, 胡明忠, 梁明明, 等. 铋系半导体光催化剂研究进展[J]. 化工进展, 2022, 41(9): 4813-4830. |
| SUN Lingbo, HU Mingzhong, LIANG Mingming, et al. Research progress of bismuth-based semiconductor photocatalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4813-4830. | |
| 64 | VINODGOPAL K, KAMAT P V. Enhanced rates of photocatalytic degradation of an azo dye using SnO2/TiO2 coupled semiconductor thin films[J]. Environmental Science & Technology, 1995, 29(3): 841-845. |
| 65 | RANJIT K T, Synthesis VISWANATHAN B., characterization and photocatalytic properties of iron-doped TiO 2 catalysts[J]. Journal of Photochemistry and Photobiology A: Chemistry, 1997, 108(1): 79-84. |
| 66 | HE Huichao, XIAO Peng, ZHOU Ming, et al. Boosting catalytic activity with a p-n junction: Ni/TiO2 nanotube arrays composite catalyst for methanol oxidation[J]. International Journal of Hydrogen Energy, 2012, 37(6): 4967-4973. |
| 67 | SUN Mingjuan, HU Jiayue, ZHAI Chunyang, et al. A p-n heterojunction of CuI/TiO2 with enhanced photoelectrocatalytic activity for methanol electro-oxidation[J]. Electrochimica Acta, 2017, 245: 863-871. |
| 68 | WANG Tao, TANG Jing, WU Shichao, et al. Preparation of ordered mesoporous WO3-TiO2 films and their performance as functional Pt supports for synergistic photo-electrocatalytic methanol oxidation[J]. Journal of Power Sources, 2014, 248: 510-516. |
| 69 | BLASKIEVICZ S F, SANTOS H L S, TEIXEIRA I F, et al. Nickel-modified polymeric carbon nitride for improving TiO2-based photoanode: Photoelectrocatalytical evaluation and mechanistical insights[J]. Materials Today Nano, 2022, 18: 100192. |
| 70 | ZHAI Chunyang, SUN Mingjuan, ZHU Mingshan, et al. Insights into photo-activated electrode for boosting electrocatalytic methanol oxidation based on ultrathin MoS2 nanosheets enwrapped CdS nanowires[J]. International Journal of Hydrogen Energy, 2017, 42(8): 5006-5015. |
| 71 | HU Jiayue, YU Chaokai, ZHAI Chunyang, et al. 2D/1D heterostructure of g-C3N4 nanosheets/CdS nanowires as effective photo-activated support for photoelectrocatalytic oxidation of methanol[J]. Catalysis Today, 2018, 315: 36-45. |
| 72 | ZHANG Hongmin, HE Jie, ZHAI Chunyang, et al. 2D Bi2WO6/MoS2 as a new photo-activated carrier for boosting electrocatalytic methanol oxidation with visible light illumination[J]. Chinese Chemical Letters, 2019, 30(12): 2338-2342. |
| 73 | YUAN Chen, GAO Haifeng, XU Qiyan, et al. Pt decorated 2D/3D heterostructure of Bi2WO6 nanosheet/Cu2S snowflake for improving electrocatalytic methanol oxidation with visible-light assistance[J]. Applied Surface Science, 2020, 521: 146431. |
| 74 | ZHANG Hongmin, ZHAI Chunyang, YANG Peizhou, et al. In situ growth of BiOI/MoS2 heterostructure as Pt supports for visible light-assisted electrocatalytic methanol oxidation reaction[J]. Energy Technology, 2020, 8(1): 1900731. |
| 75 | WANG Gong, XIE Xingming, CUI Xuejing, et al. Photoinduced Pt/BiVO4/Bi2O3 heterostructures for methanol oxidation and new insights on the photo-/electrocatalysis coupling mechanism[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(11): 4271-4281. |
| 76 | WANG Feng, WU Lisha, QIAN Lei, et al. Noble metal-free CoS x /NiS x heterojunction for photo-assisted methanol electrooxidation[J]. ChemElectroChem, 2022, 9(19): e202200909. |
| 77 | XIE Dongxue, HU Sujuan, TENG Daihui, et al. Non-noble Si NWs@ZnO core-shell heterojunction anode enables a photo-assisted mirco direct methanol fuel cell[J]. Chemical Engineering Journal, 2023, 457: 141310. |
| 78 | Jingxiang LOW, YU Jiaguo, JARONIEC Mietek, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. |
| 79 | MI Yang, WENG Yuxiang. Band alignment and controllable electron migration between rutile and anatase TiO2 [J]. Scientific Reports, 2015, 5: 11482. |
| 80 | BARD Allen J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors[J]. Journal of Photochemistry, 1979, 10(1): 59-75. |
| 81 | YU Jiaguo, WANG Shuhan, Jingxiang LOW, et al. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air[J]. Physical Chemistry Chemical Physics: PCCP, 2013, 15(39): 16883-16890. |
| 82 | YANG Minquan, XU Yijun, LU Wanheng, et al. Self-surface charge exfoliation and electrostatically coordinated 2D hetero-layered hybrids[J]. Nature Communications, 2017, 8: 14224. |
| 83 | WANG Xuehua, WANG Xianghu, HUANG Jianfeng, et al. Interfacial chemical bond and internal electric field modulated Z-scheme Sv-ZnIn2S4/MoSe2 photocatalyst for efficient hydrogen evolution[J]. Nature Communications, 2021, 12: 4112. |
| 84 | LIU Yaxing, CHEN Fuyi, WANG Qiao, et al. Surface plasmon-enhanced activity and stability for methanol oxidation on gold caviar-like assembly under solar light[J]. Journal of Materials Chemistry A, 2018, 6(22): 10515-10524. |
| 85 | SUI Ning, GAO Hongxu, ZHU Jiacheng, et al. Shape- and size-dependences of gold nanostructures on the electrooxidation of methanol under visible light irradiation[J]. Nanoscale, 2019, 11(39): 18320-18328. |
| 86 | WANG Zhili, DU Jing, ZHANG Yongzheng, et al. Free-standing nanoporous gold for direct plasmon enhanced electro-oxidation of alcohol molecules[J]. Nano Energy, 2019, 56: 286-293. |
| 87 | CHEN Guanying, SUN Mingjuan, LI Juan, et al. Plasmonic hot electron transfer in anisotropic Pt-Au nanodisks boosts electrochemical reactions in the visible-NIR region[J]. Nanoscale, 2019, 11(40): 18874-18880.[PubMed] |
| 88 | WEI Yan, ZHANG Xiao, LIU Zhiguo, et al. Site-selective modification of AgPt on multibranched Au nanostars for plasmon-enhanced hydrogen evolution and methanol oxidation reaction in visible to near-infrared region[J]. Journal of Power Sources, 2019, 425: 17-26. |
| 89 | WEI Yan, ZHANG Xiao, LIU Yingjie, et al. Ternary PtNiCu self-assembled nanocubes for plasmon-enhanced electrocatalytic hydrogen evolution and methanol oxidation reaction in visible light[J]. Electrochimica Acta, 2020, 349: 136366. |
| 90 | XU Shuhong, YE Lingting, LI Zhongshui, et al. Facile synthesis of bimetallic Pt-Ag/graphene composite and its electro-photo-synergistic catalytic properties for methanol oxidation[J]. Catalysts, 2016, 6(9): 144. |
| 91 | LIU Yaxing, CHEN Fuyi, WANG Qiao, et al. Plasmonic-enhanced catalytic activity of methanol oxidation on Au-graphene-Cu nanosandwiches[J]. Nanoscale, 2019, 11(18): 8812-8824. |
| 92 | YE Yixiang, WANG Yanli, LI Zhongshui, et al. One-pot synthesis of PtIn/3D-GNs composites with high alloying degree for electro-photo synergistic catalysis[J]. New Journal of Chemistry, 2019, 43(43): 17023-17032. |
| 93 | SHIAO Ming-Hua, LIN Chunting, HUANG Hung Ji, et al. Novel gold dendritic nanoflowers deposited on titanium nitride for photoelectrochemical cells[J]. Journal of Solid State Electrochemistry, 2018, 22(10): 3077-3084. |
| 94 | Su Pei LIM, PANDIKUMAR Alagarsamy, HUANG Nay Ming, et al. Silver/titania nanocomposite-modified photoelectrodes for photoelectrocatalytic methanol oxidation[J]. International Journal of Hydrogen Energy, 2014, 39(27): 14720-14729. |
| 95 | XIONG Yunjie, ZOU Liangliang, PAN Qingguang, et al. Photo-electro synergistic catalysis: Can Pd be active for methanol electrooxidation in acidic medium?[J]. Electrochimica Acta, 2018, 278: 210-218. |
| 96 | LI Pingping, WANG Jinshu, ZU Guannan, et al. Synergetic catalytic properties of gold nanoparticles planted on transparent titanium dioxide nanotube array bed[J]. Materials Chemistry and Physics, 2018, 217: 437-444. |
| 97 | XIE Xingming, FENG Jianguang, CUI Xuejing, et al. Plasmon-enhanced photocatalysis coupling electrocatalysis steering methanol oxidation toward a CO-free dominant pathway[J]. ACS Catalysis, 2021, 11(21): 13160-13168. |
| 98 | Lance KELLY K, CORONADO Eduardo, ZHAO Lin lin, et al. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment[J]. The Journal of Physical Chemistry B, 2003, 107(3): 668-677. |
| 99 | ATWATER Harry A, POLMAN Albert. Plasmonics for improved photovoltaic devices[J]. Nature Materials, 2010, 9(3): 205-213. |
| 100 | 周利, 王取泉. 等离激元共振能量转移与增强光催化研究进展[J]. 物理学报, 2019, 68(14): 118-132. |
| ZHOU Li, WANG Ququan. Plasmon resonance energy transfer and research progress in plasmon-enhanced photocatalysis[J]. Acta Physica Sinica, 2019, 68(14): 118-132. |
| [1] | 周安宁, 江雨寒, 刘墨宣, 赵伟, 李振. 电解煤浆制氢过程中煤阶及矿物的影响与煤结构演化研究进展[J]. 化工进展, 2024, 43(5): 2294-2310. |
| [2] | 吴达, 蒋淑娇, 魏强, 袁胜华, 杨刚, 张成. 能源转型中渣油高效利用技术的研究进展[J]. 化工进展, 2024, 43(5): 2343-2353. |
| [3] | 桂鑫, 陈汇勇, 白柏杨, 贾永梁, 马晓迅. Mo掺杂改性NiC/Al-MCM-41的芘催化加氢性能[J]. 化工进展, 2024, 43(5): 2386-2395. |
| [4] | 丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448. |
| [5] | 李思, 陶艺月, 肖振翀, 张亮, 李俊, 朱恂, 廖强. 热再生电池堆-二氧化碳电化学还原池系统耦合特性[J]. 化工进展, 2024, 43(5): 2568-2575. |
| [6] | 段翔, 田野, 董文威, 宋松, 李新刚. 苯酐合成的反应网络及催化反应机制研究现状与展望[J]. 化工进展, 2024, 43(5): 2587-2599. |
| [7] | 王冰, 王磊, 黄欣茹, 袁红鹏, 赖小娟, 李朋. 一种耐酸耐碱高强树脂的合成及性能[J]. 化工进展, 2024, 43(4): 1992-2000. |
| [8] | 丁嘉, 吴文琦, 李鹏程. 两电子水氧化反应抑制掺硼金刚石电极氧化有机物过程中氯酸盐和高氯酸盐的生成[J]. 化工进展, 2024, 43(4): 2183-2190. |
| [9] | 刘若璐, 汤海波, 何翡翡, 罗凤盈, 王金鸽, 杨娜, 李洪伟, 张锐明. 液态有机储氢技术研究现状与展望[J]. 化工进展, 2024, 43(4): 1731-1741. |
| [10] | 王红妍, 马子然, 李歌, 马静, 赵春林, 周佳丽, 王磊, 彭胜攀. 燃煤耦合可再生燃料烟气多污染物协同催化脱除研究进展[J]. 化工进展, 2024, 43(4): 1783-1795. |
| [11] | 陈家一, 高帷韬, 阴亚楠, 王诚, 欧阳鸿武, 毛宗强. 电化学沉积法制备质子交换膜燃料电池催化剂[J]. 化工进展, 2024, 43(4): 1796-1809. |
| [12] | 吴晨赫, 刘彧旻, 杨昕旻, 崔记伟, 姜韶堃, 叶金花, 刘乐全. 粉体光催化全水分解技术研究进展[J]. 化工进展, 2024, 43(4): 1810-1822. |
| [13] | 刘雨蓉, 王兴宝, 李文英. 分子筛负载Pt催化剂酸性位点调控及对蒽深度加氢性能的影响[J]. 化工进展, 2024, 43(4): 1832-1839. |
| [14] | 郭潇东, 毛玉娇, 刘相洋, 邱丽, 于峰, 闫晓亮. Ni/Sm2O3-CeO2/Al2O3催化剂氧空位对二氧化碳低温甲烷化的影响[J]. 化工进展, 2024, 43(4): 1840-1850. |
| [15] | 高飞, 刘志松, 潘珂珂, 刘敏敏, 代斌, 但建明, 于锋. 蛭石基FeCeO x 催化剂及CO选择性催化还原NO[J]. 化工进展, 2024, 43(4): 1851-1862. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||