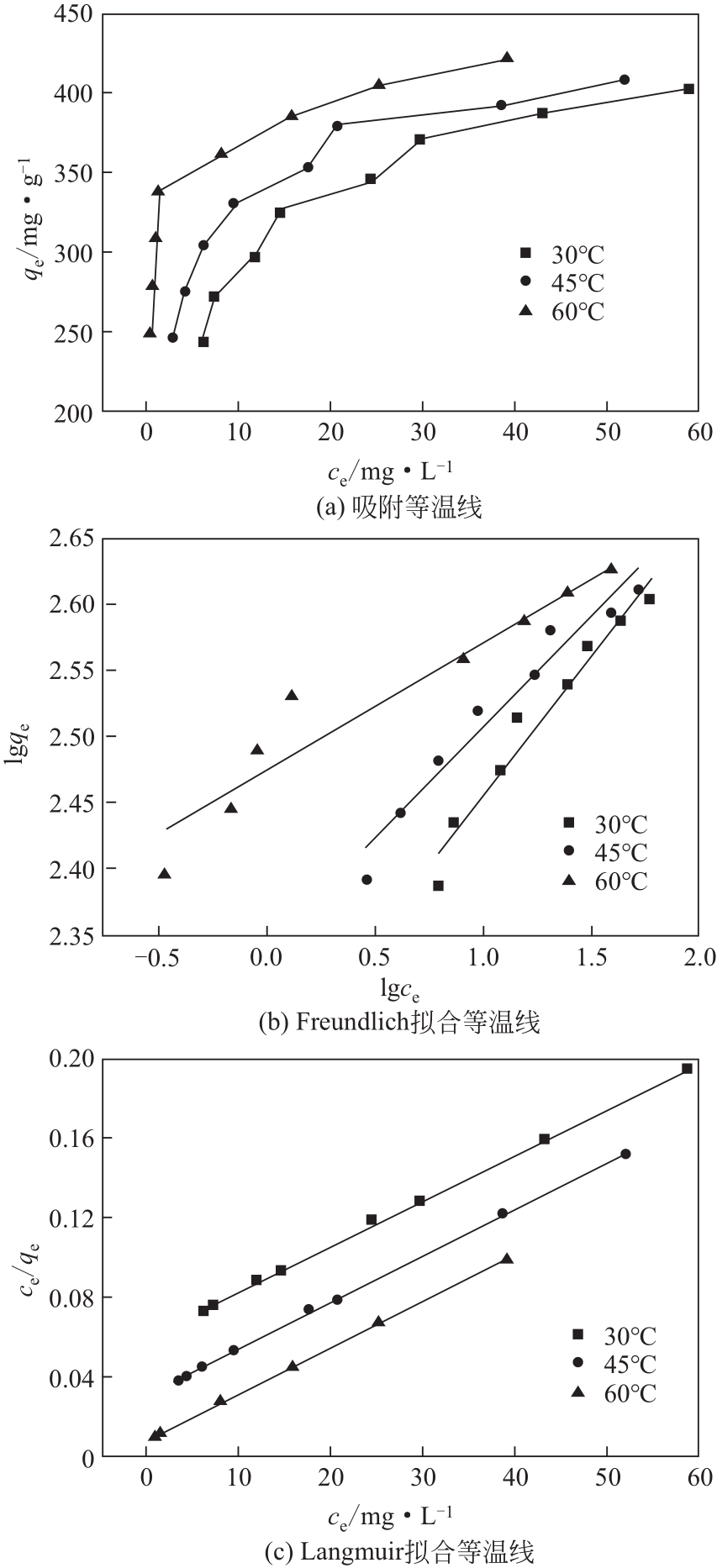
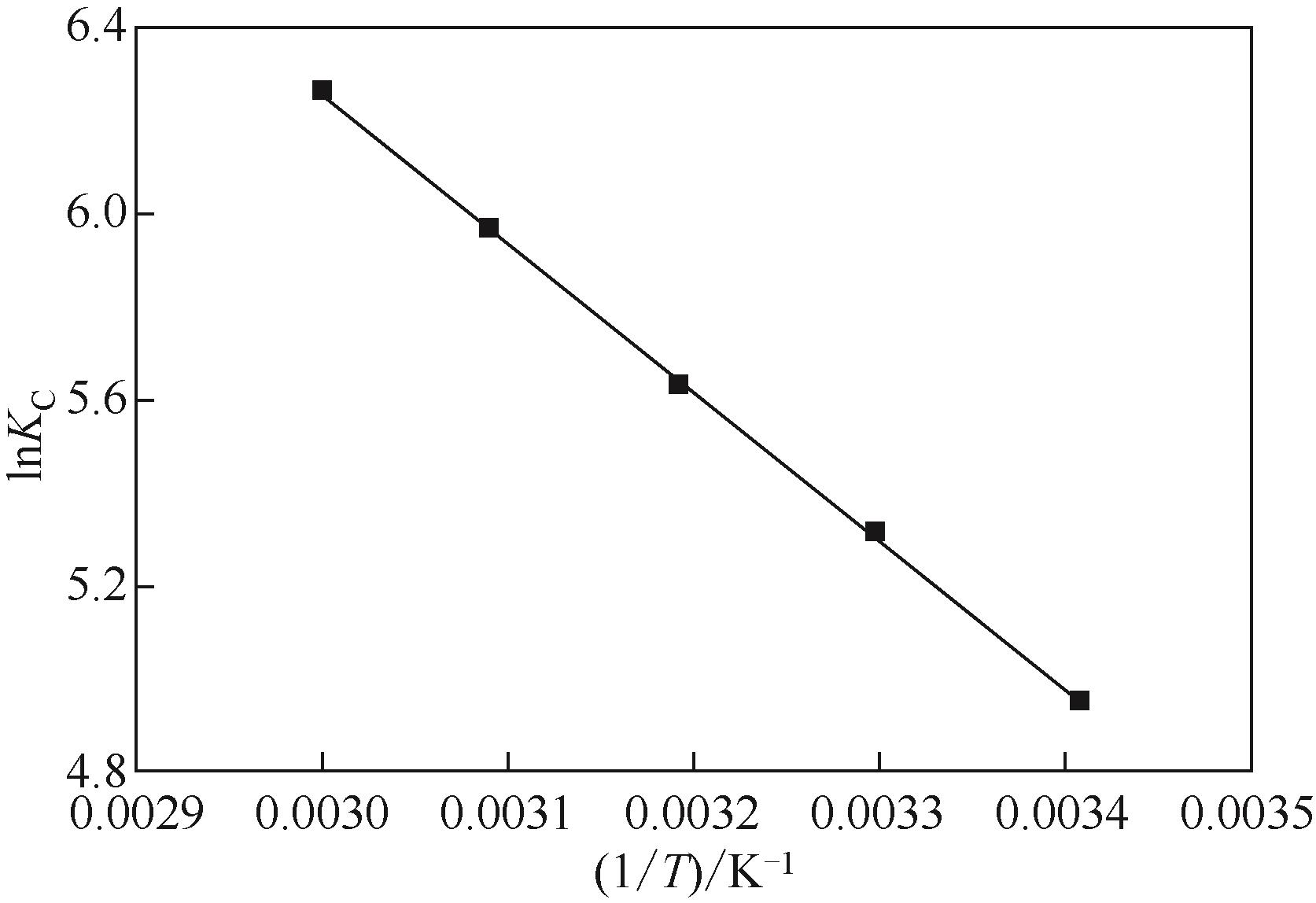
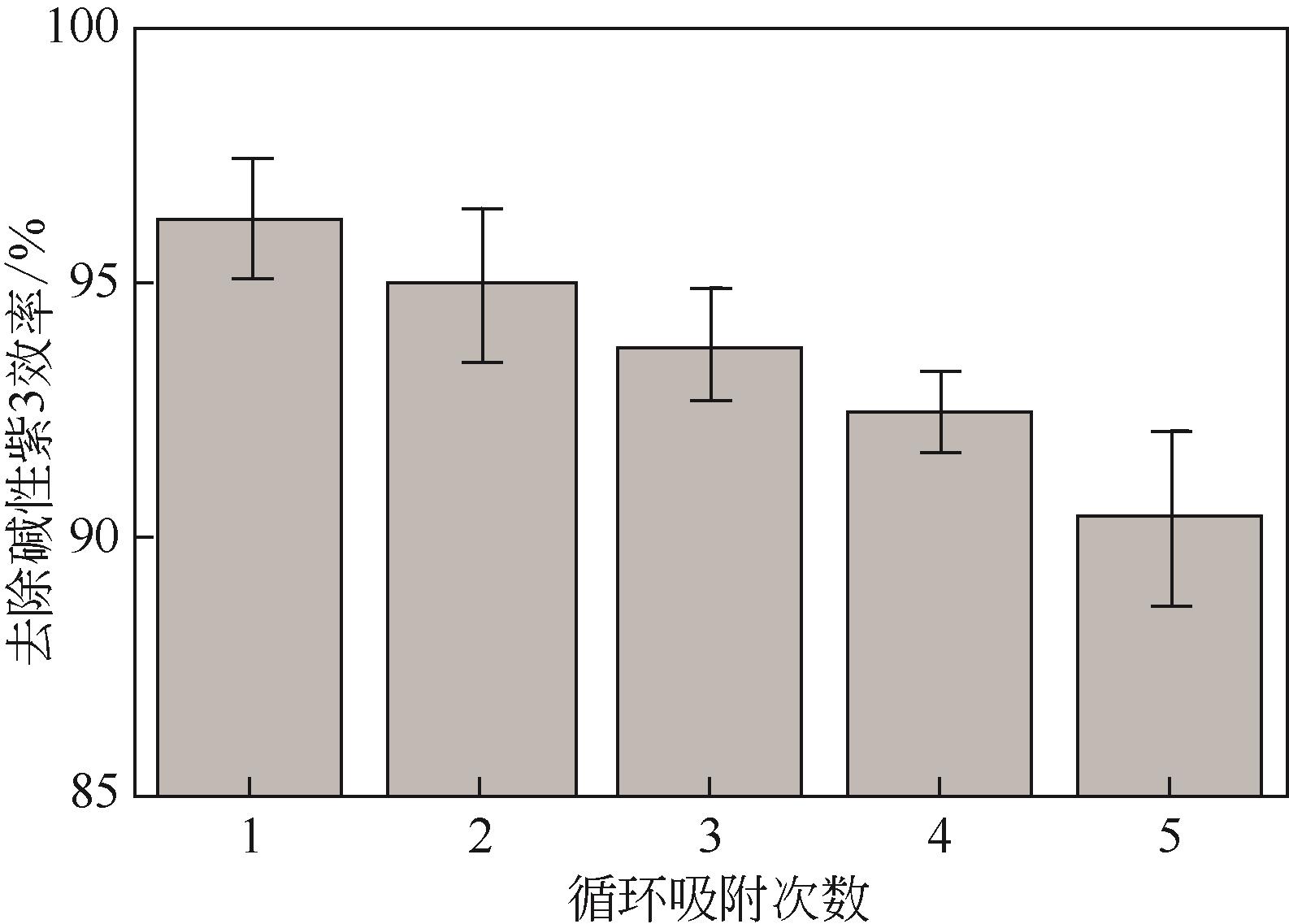
| 1 |
KARIMIFARD S, ALAVI MOGHADDAM M R. Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review[J]. Science of the Total Environment, 2018, 640/641: 772-797.
|
| 2 |
LI W, MU B N, YANG Y Q. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology[J]. Bioresource Technology, 2019, 277: 157-170.
|
| 3 |
ZHANG P, LO I, O'CONNOR D, et al. High efficiency removal of methylene blue using SDS surface-modified ZnFe2O4 nanoparticles[J]. Journal of Colloid and Interface Science, 2017, 508: 39-48.
|
| 4 |
THAKUR S, PANDEY S, AROTIBA O A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue[J]. Carbohydrate Polymers, 2016, 153: 34-46.
|
| 5 |
LIU C, OMER A M, OUYANG X K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: isotherm and kinetic studies[J]. International Journal of Biological Macromolecules, 2018, 106: 823-833.
|
| 6 |
LIU C, ZHU G L, SONG S X, et al. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector[J]. Minerals Engineering, 2019, 131: 385-391.
|
| 7 |
ZHU Y, FAN W H, ZHOU T T, et al. Removal of chelated heavy metals from aqueous solution: a review of current methods and mechanisms[J]. Science of the Total Environment, 2019, 678: 253-266.
|
| 8 |
宋晓东, 程昌敬, 余海溶. 阴离子聚电解质修饰磁性氧化石墨烯对碱性品红的吸附性能[J]. 化工进展, 2016, 35(9): 2973-2981.
|
|
SONG Xiaodong, CHENG Changjing, YU Hairong. Adsorption performance of an anionic polyelectrolyte-functionalized magnetic graphene oxide for basic fuchsin[J]. Chemical Industry and Engineering Progress, 2016, 35(9): 2973-2981.
|
| 9 |
BAI H Y, ZHAO Y L, ZHANG X, et al. Correlation of exfoliation performance with interlayer cations of montmorillonite in the preparation of two-dimensional nanosheets[J]. Journal of the American Ceramic Society, 2019, 102(7): 3908-3922.
|
| 10 |
ZHAO Y L, KANG S C, QIN L, et al. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: dye degradation by the synergistic effect of adsorption and photo-Fenton reaction[J]. Chemical Engineering Journal, 2020, 379: 122322.
|
| 11 |
FARROKHI-RAD M, MOHAMMADALIPOUR M, SHAHRABI T. Electrophoretically deposited halloysite nanotubes coating as the adsorbent for the removal of methylene blue from aqueous solution[J]. Journal of the European Ceramic Society, 2018, 38(10): 3650-3659.
|
| 12 |
LI Y H, DU Q J, LIU T H, et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes[J]. Chemical Engineering Research and Design, 2013, 91(2): 361-368.
|
| 13 |
KYZAS G Z, FU J, MATIS K A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters[J]. Materials, 2013, 6(11): 5131-5158.
|
| 14 |
杜夕铭, 卢钧, 陈泉源. 氧化石墨对活性红X-3B的吸附特性及再生性能[J]. 水处理技术, 2019, 45(8): 35-39, 44.
|
|
DU Ximing, LU Jun, CHEN Quanyuan. Adsorption characteristics of reactive red X-3B by graphite oxide and its regeneration[J]. Technology of Water Treatment, 2019, 45(8): 35-39, 44.
|
| 15 |
王希越, 明明, 连丽丽, 等. 磁性氧化石墨烯纳米粒子的制备及其对大红染料的去除[J]. 应用化工, 2019, 48(10): 2324-2327.
|
|
WANG Xiyue, MING Ming, LIAN Lili, et al. Preparation of magnetic graphene oxide nanoparticles and its application for red dye removal[J]. Applied Chemical Industry, 2019, 48(10): 2324-2327.
|
| 16 |
LIU L, ZHANG Y R, HE Y J, et al. Preparation of montmorillonite-pillared graphene oxide with increased single- and co-adsorption towards lead ions and methylene blue[J]. RSC Advances, 2015, 5(6): 3965-3973.
|
| 17 |
ZHANG Z L, LUO H J, JIANG X L, et al. Synthesis of reduced graphene oxide-montmorillonite nanocomposite and its application in hexavalent chromium removal from aqueous solutions[J]. RSC Advances, 2015, 5(59): 47408-47417.
|
| 18 |
王志明, 吕宪俊, 渠美云. 膨润土提纯工艺研究[J]. 现代矿业, 2013, 29(3): 26-29, 50.
|
|
WANG Zhiming, Xianjun LYU, QU Meiyun. Research on purification technology of montmorillonite[J]. Modern Mining, 2013, 29(3): 26-29, 50.
|
| 19 |
MUNIR M, AHMAD M, SAEED M, et al. Sustainable production of bioenergy from novel non-edible seed oil (Prunus cerasoides) using bimetallic impregnated montmorillonite clay catalyst[J]. Renewable and Sustainable Energy Reviews, 2019, 109: 321-332.
|
| 20 |
WANG J Q, HAN Z D. The combustion behavior of polyacrylate ester/graphite oxide composites[J]. Polymers for Advanced Technologies, 2006, 17(4): 335-340.
|
| 21 |
TYAGI B, CHUDASAMA C D, JASRA R V. Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2006, 64(2): 273-278.
|
| 22 |
ZHANG X L, CHENG L P, WU X P, et al. Activated carbon coated palygorskite as adsorbent by activation and its adsorption for methylene blue[J]. Journal of Environmental Sciences, 2015, 33: 97-105.
|
| 23 |
BRADDER P, LING S K, WANG S B, et al. Dye adsorption on layered graphite oxide[J]. Journal of Chemical & Engineering Data, 2011, 56(1): 138-141.
|
| 24 |
TEKIN N, ŞAFAKLI A, BINGÖL D. Process modeling and thermodynamics and kinetics evaluation of Basic Yellow 28 adsorption onto sepiolite[J]. Desalination and Water Treatment, 2015, 54(7): 2023-2035.
|
| 25 |
DERAKHSHAN Z, BAGHAPOUR M A, RANJBAR M, et al. Adsorption of methylene blue dye from aqueous solutions by modified pumice stone: kinetics and equilibrium studies[J]. Health Scope, 2013, 2(3): 136-144.
|
 ), 程宁2(
), 程宁2( ), 杨育兵1, 卢凯玲1, 罗应1, 易慧1
), 杨育兵1, 卢凯玲1, 罗应1, 易慧1
 ), CHENG Ning2(
), CHENG Ning2( ), YANG Yubing1, LU Kailing1, LUO Ying1, YI Hui1
), YANG Yubing1, LU Kailing1, LUO Ying1, YI Hui1